Abstract
Background and Aims:
Hypotension is one of the most common side effects of spinal anaesthesia and preoperative volume status is one of the predictive variables for developing spinal-induced hypotension (SIH). Inferior venacaval ultrasound (IVCUS) is effective to assess fluid responsiveness in critical care patients. The aim of this study was to evaluate the IVCUS-guided volume optimisation prior to spinal anaesthesia to prevent SIH and requirement of vasopressors.
Methods:
Eighty patients undergoing inguinal hernia/hydrocele surgeries under spinal anaesthesia were randomised into group A consisting of an IVCUS-guided volume optimisation before spinal anaesthesia and group B with no IVCUS assessment. Unpaired t-test and Z test were used for statistical analysis. Pearson's correlation coefficient was used to find correlation. The primary outcome was relative risk reduction in the incidence of SIH between the groups. Secondary outcomes were the need for vasopressor drugs, the total volume of fluids required throughout procedure, and correlation between IVC collapsibility index (IVCCI) versus prespinal fluids, IVCCI versus baseline mean arterial pressure (MAP).
Results:
The relative risk reduction in the incidence of SIH was lower in group A compared to group B which was 40% (P = 0.002 CI = 95%). The SIH in group A was 20% and group B was 50%. There was decreased requirement of vasopressors in group A compared to group B. Total IV fluids given was more in group A. There was a positive correlation between IVCCI and pre-spinal fluids.
Conclusion:
IVCUS assessment reduces the SIH as well as requirement of vasopressor for hernia and hydrocele surgeries.
Key words: Hypotension, IVC collapsibility index, IVC ultrasound, spinal anaesthesia
INTRODUCTION
Spinal anaesthesia is frequently used in daily clinical practice. The most common side effects of spinal anaesthesia are hypotension, bradycardia, nausea, and vomiting.[1] The reduction in both systemic vascular resistance (SVR) and cardiac output contributes significantly to SIH. Patient's susceptibility to intraoperative hypotension can also be influenced by the preoperative volume status that may differ according to comorbidities, physical status, pre-operative medications, and fasting.[2] SIH is a common side effect with an incidence of 15.3–33% that may result in organ hypoperfusion and ischemic events.[3] To minimise haemodynamic impairment, preventive empiric volume loading is commonly performed in obstetric anaesthesia before giving spinal anaesthesia.[4] Furthermore, several studies of prophylactic volume loading to prevent SIH have provided inconsistent results. Therefore, blind volume preloading before spinal anaesthesia in non-obstetric patients is not regularly performed. However, fluid coload has been proved to lower the incidence of SIH and significantly decrease vasopressor requirements.[5] Consequently, the search for predictors of SIH is becoming mandatory to avoid blind volume loading and reserve it only for patients who are expected to develop SIH. Various parameters have been investigated recently to estimate the pre-operative intravascular volume status. Ultrasonographic determination of inferior vena cava (IVC) and inferior vena cava collapsibility index (IVCCI) was used in spontaneously breathing intensive care patients for assessment of intravascular volume and was reported to be reliable, noninvasive, and easy.[6] Recently, few studies were conducted for estimation of the IVCCI as a reliable predictor of arterial hypotension after induction of general anaesthesia.[2] The aim of this study was to evaluate the ultrasound-guided IVC volume optimisation to avert SIH in spontaneously breathing patients posted for hernia and hydrocele surgeries. The primary outcome was reduction in the incidence of SIH. Secondary outcomes were the requirement for administration of vasopressors after the procedure and correlation between IVCCI versus pre-spinal, IVC collapsibility versus baseline mean arterial blood pressure (MAP).
METHODS
This study was registered with Clinical Trials Registry—India (CTRI/2019/04/018601) prospectively. After approval of local ethical committee (SNMC/IECHSR/ 2018-19/A-80/1.1) and written informed consent 80 patients between 18 and 60 years of age American Society of Anesthesiology (ASA) physical status I–II undergoing inguinal hernia and hydrocele surgeries were included in to this prospective randomised controlled clinical trial. Study period was between February 2019 and July 2019. Patients who refused to give consent to the study, body mass index ≥40 kg/m2, patients who were scheduled for bilateral or recurrent inguinal hernia repair, obstetric surgeries, orthopedic surgeries, emergency surgeries, patients with intra-abdominal tumors, and hypersensitivity to local anaesthetic drugs were excluded from the study. Eighty patients were divided into two groups Group A (IVC group) and Group B (control group) with 40 patients in each group using a computer-generated randomisation. Concealment of group was done using sealed envelope technique. Preoperative fasting status was ensured before surgery. After shifting to operation theatre monitors like electrocardiogram (ECG), noninvasive blood pressure (NIBP) and pulse oximetry were attached and basal readings were noted. Intravenous (IV) 18 G cannula was secured. No pre-spinal anaesthesia IV fluid was given. For group A patients, ultrasound-guided IVC measurements were performed in supine position before spinal anaesthesia by an experienced anaesthesiologist using a curvilinear probe of 1–8 MHz GE LOGIQe portable ultrasound (LOGIQ e ultrasonography machine; GE Healthcare, California, USA) and the IVC diameter variation was measured during spontaneous breathing by an M-mode modality through the subcostal view. IVC collapsibility index was measured as IVCCI = (IVCmax - IVCmin)/IVCmax at 2 cm from the right atrium [Figure 1]. Patients having collapsibility index of ≥36% were considered as fluid responders and were given 500 ml of crystalloids over 15 min after which the IVC diameter variation was reassessed. The same fluid bolus, that is, 500 ml of crystalloids was given until a non-fluid responder pattern was observed during IVCUS. Thereafter, spinal anaesthesia was performed. For group B patients, spinal anaesthesia was performed without taking inferior venacaval measurements. The spinal anaesthesia procedure was standardised for both groups and performed in lateral decubitus position under all aseptic precautions at L3-L4 lumbar space using a 25G Quincke's spinal needle (B. Braun Medical SA, Melsungen, Germany).A standard dose of heavy bupivacaine 0.5% (15–18 mg depending on patient's constitution) was injected slowly with the needle orifice oriented cranially. After injection, patients were positioned supine and the degree of sensory block was assessed with the aim of a T6–T8 level block by using cold test. Significant hypotension was defined as a fall in systolic arterial blood pressure of 25% of the baseline value or MAP less than 60 mm of Hg. In group B patients with SIH were treated initially with 500 ml of crystalloids. If patients did not respond, ephedrine (5 mg) was administered. Group A patients were supplemented only with maintenance fluids and loss during intraoperative period and hypotension was treated only with vasopressor (ephedrine 5 mg) drug. If required incremental doses of ephedrine 5 mg given. No additional fluid bolus was given for intra operative hypotension. During the study period the following parameters were estimated-IVCCI in group A patients, total amount of vasopressors used in both the groups, total amount of fluids given in both the groups and correlation between collapsibility index versus pre-spinal fluids and collapsibility versus baseline MAP in group A patients. The following vitals like blood pressure, pulse rate, Spo2, and any other complications were noted.
Figure 1.
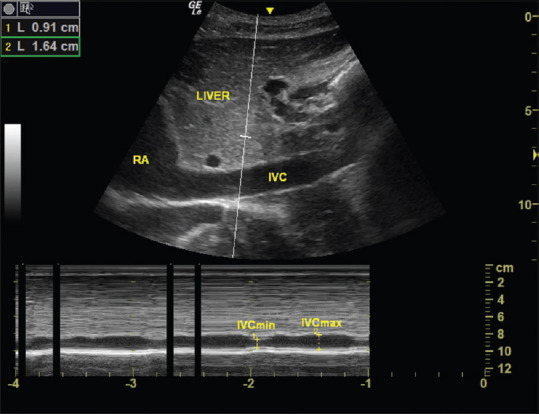
IVCUS and IVCCI measured during spontaneous breathing by an M-mode modality through the subcostal view at 2 cm from the right atrium
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences, IBM, SPSS statistics, USA) version 19. Sample size estimation was done using medcalc software at 95% confidence level and 95% power of the study. According to study conducted by Ceruti et al.,[7] the correlation of fluid and IVC was 0.56. Sample size estimated was 34 in each group. Considering additional 10% as dropouts the number was rounded off to 40 in each group. Formula used; N = ({Zα+ Zβ}/C)2 + 3 where C = 0.5 × In({1+r}/{1-r}). Continuous data were expressed as mean ± SD, categorical variables were expressed in the form of number and percentage. All continuous data were analysed by the unpaired t-test and Z test for proportion. Pearson's correlation coefficient was used to find correlation. Relative risk was used for calculating incidence of SIH in both groups. P value was considered statistically significant if less than 0.05 (95% confidence interval).
RESULTS
A total of 80 patients were included in the study. Eighty patients were enrolled for statistical analysis divided into 40 patients in each group and randomised into Group A and Group B respectively [Figure 2]. Upper level of sensory block was same in all patients (T6–T8). Patients in both the groups were of comparable demographic variables such as age, weight, height, and duration of surgery [Table 1]. The overall incidence rate of hypotension after spinal anaesthesia was 35% (28/80) the Group A showed a lower incidence 20% (8/40 patients) of arterial hypotension compared to control group 50% (20/40 patients) [Figure 3]. The relative risk reduction in the incidence of SIH was lower in group A compared to group B which was 40% (P = 0.002 CI = 95%). Out of 40 group A patients, 8 of them showed normal IVC collapsibility index (<36%) and remaining 32 patients showed <36%. Those 32 patients were further optimised by IVCCI guided fluid therapy. Number of patients requiring first dose of vasopressors in Group A was 20% (8/40) and in Group B was 50% (20/40) which was statistically significant (P = 0.002) [Table 2]. Number of patients requiring 2nd dose of vasopressors in Group A was nil and in Group B was 20% (8/40) which was statistically significant (P = 0.001). The relative risk between the groups was 40%. Total amount of intravenous fluids in group A was more 758.50 ± 304.35 ml (pre-spinal fluids-425 ± 241.52 ml + post-spinal fluids- 332.75 ± 202.84 ml) compared to group B 525.50 ± 265.46 ml (pre-spinal fluids-nil + post-spinal fluids-525.50 ± 265.46 ml) which was statistically significant (P = 0.001) [Table 2]. There was positive IVC correlation between collapsibility index and pre-spinal IV fluids (r2 = 0.795) which was statistically significant (P = 0.001) [Figure 4]. There was no IVC correlation between collapsibility index (>36%) and baseline MAP (r2 = 0082) [Figure 5].
Figure 2.
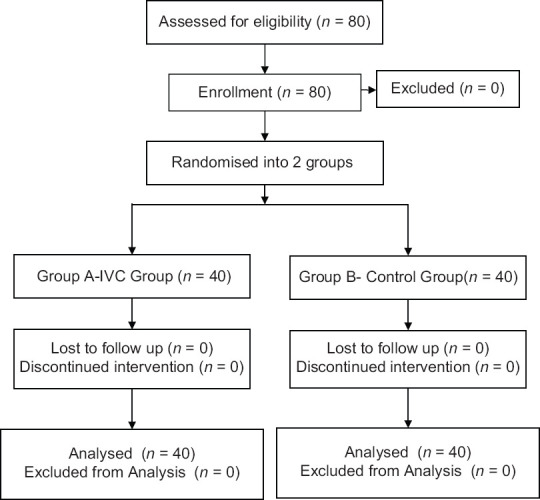
Consort chart
Table 1.
Demographic variables between Group A and Group B
| Group | n | Mean | SD | P | |
|---|---|---|---|---|---|
| Age | Group A | 40 | 43.48 | 13.424 | 0.558 |
| Group B | 40 | 45.23 | 13.161 | ||
| Duration of Surgery | Group A | 40 | 60.25 | 17.429 | 0.287 |
| Group B | 40 | 64.50 | 18.003 | ||
| Weight in Kgs | Group A | 40 | 66.52 | 5.91 | 000.685 |
| Group B | 40 | 67.1 | 6.82 | ||
| Height in cms | Group A | 40 | 168.57 | 5.35 | 0.528 |
| Group B | 40 | 169.35 | 5.66 |
Data presented as mean, SD – Standard Deviation
Figure 3.
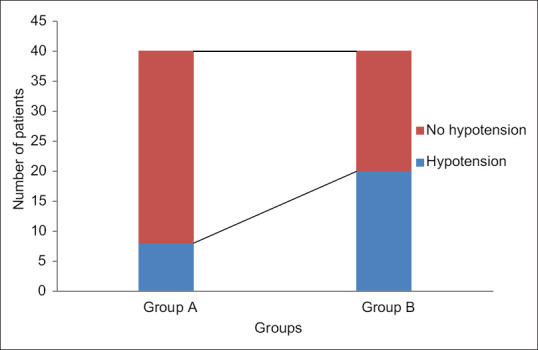
Incidence of hypotension between group A and group B
Table 2.
Vasopressors and intravenous fluids usage between the groups
| Parameters | Group A (n=40) | Group B (n=40) | P |
|---|---|---|---|
| Number of times vasopressors given | |||
| 1st Dose | 8/40 (20%) | 20/40 (50%) | 0.002 |
| 2nd Dose | Nil | 8/40 (20%) | 0.001 |
| Pre-spinal IV fluids (ml) | 425±241.523 | Nil | 0.001 |
| Total IV fluids (ml) | 758.50±304.349 | 525.50±265.465 | 0.001 |
Figure 4.
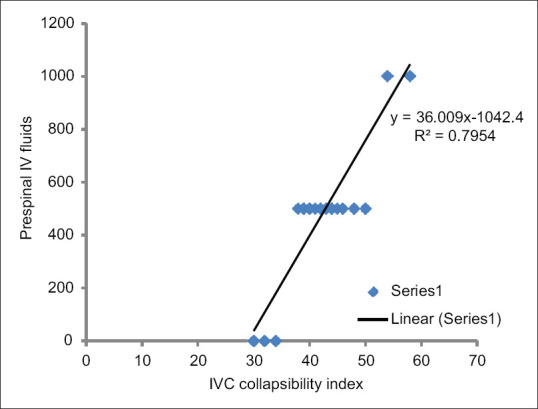
Correlation between collapsibility index versus prespinal IV fluids.
Figure 5.
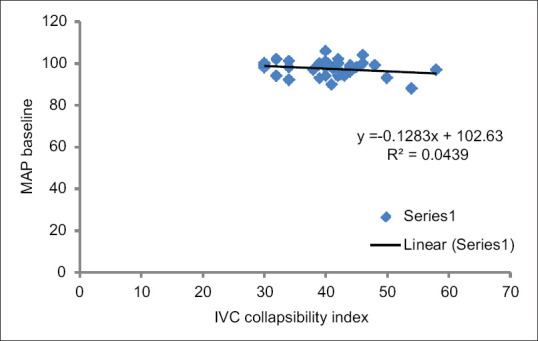
Correlation between collapsibility index versus baseline MAP
DISCUSSION
In the present study, we have demonstrated that the IVC-guided fluid optimisation by using USG before spinal anaesthesia leads to 40% reduction in the incidence of SIH as well as requirement of vasopressor during spinal anaesthesia. SIH may cause several adverse effects such as coronary ischemia and delirium.[8,9] Transient hypotension might have major impact in the outcome of patients with cardiovascular risk factors. However it is generally well tolerated by healthy patients leading only to light headedness, nausea, and vomiting.[10] Monk et al.[11] in his retrospective analysis showed an association between intraoperative hypotension and 30-day postoperative mortality in non-cardiac surgery. In spite of this evidence, fluids and vasopressor drugs used to prevent or treat SIH are often not administered following a patient-adapted and monitoring-based concept.[12] IVCUS assessment is a better tool compared to MAP, HR, and CVP measurements for induction in operating room area and used as a perioperative screening and monitoring tool.[2,13] In the present study, we identified fluid responders using IVCCI cutoff value <36% and performed volume optimisation before spinal anaesthesia. This cutoff value was taken from study done by Zhang et al.[14] in a systematic review where a total of eight studies involving 235 patients were analysed. The cutoff values of IVCCI varied across studies ranging from 12 to 40%. The sensitivity and specificity in the overall population were 0.76 (95% CI: 0.61-0.86) and 0.86 (95% CI: 0.69–0.95), respectively. We concluded that IVCCI was of great value in predicting fluid responsiveness. Zhang et al.[2] investigated the prediction of hypotension after induction of general anesthesia by preoperative IVCUS in a prospective study on 100 patients. Maximum IVC diameter and IVCCI were measured preoperatively. After regression analysis, they found that IVCCI was an independent predictor of hypotension with odds ratio 1.17 (1.09–1.26). IVCCI was also positively associated with a percentage decrease in MAP (regression coefficient, 0.27). They concluded that preoperative IVCCI measurement was a reliable predictor of hypotension after induction of general anaesthesia with a sensitivity of 78.6% and a specificity of 91.7% at a cutoff value of 43% and an AUC of 0.90. Szabo et al.[15] have studied the role of IVCCI in the prediction of hypotension associated with general anaesthesia in spontaneously breathing noncardiac surgical patients. They concluded that preoperatively detected IVCC ≥50% can predict post-induction hypotension with high specificity but low sensitivity. Salam ER,et al.[16] in his study concluded that preoperative IVCCI and IVC: Ao index are good predictors of the occurrence of post-spinal arterial hypotension. The present study concluded that ultrasound-guided IVC fluid optimisation before giving spinal anaesthesia led to 40% reduction in the incidence of SIH. Similar results were seen in a study done by Ceruti et al.[7] on 160 patients posted for surgeries under spinal anaesthesia. They used trans-thoracic ultrasound with 3s probe and they showed 35% reduction in SIH. In our study we performed subcostal transabdominal ultrasound using curvilinear probe for IVC measurements at 2 cm from the right atrium in patients posted for hernia and hydrocele surgeries as quoted in studies done by Pinsky et al. and Maciuliene et al.[17,18] reduction in IVC diameters and increase in IVCCI do not predict hypotension and bradycardia during SA in spontaneously breathing patients undergoing elective knee joint replacement surgery. This study results are contradicting to our study. This may be because IVC-guided fluid optimisation before giving spinal anaesthesia was not done by them and they measured time point IVC measurements between hypotensive versus non-hypotensive, bradycardia versus non-bradycardia patients. In the present, there was a reduced need for vasopressor drugs in group A compared to group B and also, total IV fluids given were more in group A. Our results correlate with Ceruti et al.[7] In our study we observed a positive IVC correlation between collapsibility index versus pre-spinal IV fluids (r2 = 0.795) and no correlation between collapsibility versus baseline MAP. Though study done by Zhang et al.[2] showed positive correlation between collapsibility index and MAP, Ceruti et al.[7] showed weak correlation between collapsibility versus pre-spinal fluids and no correlation between collapsibility versus MAP. These findings may be because we have done IVC-guided fluid optimisation before spinal anaesthesia which was one of the predictors for SIH.
In our study cases, the measurements of IVC collapsibility may have been compromised by the upward and downward movement of the diaphragm during the respiratory cycle which could have resulted in an underestimation of IVCCI.
Further studies are needed considering extreme age, cardiovascular compromised patients and other infra umbilical surgeries under spinal anaesthesia.
CONCLUSION
IVC-guided fluid optimisation by using USG before spinal anaesthesia leads to a reduction in the incidence of spinal induced hypotension as well as in the need for vasopressors during spinal anaesthesia. Also, there is positive correlation between IVCCI anaesthesia. Also, IVCCI and pre-spinal IV fluids in patients posted for hernia and hydrocele surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank Dr. Manjula, Associate Professor, Dept of Community Medicine, S N Medical College Bagalkot.
REFERENCES
- 1.Hartmann B, Junger A, Klasen J, Benson M, Jost A, Banzhaf A, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: An analysis with automated data collection. Anesth Analg. 2002;94:1521–9. doi: 10.1097/00000539-200206000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Critchley LA. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124:580–9. doi: 10.1097/ALN.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–16. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Singh J, Ranjit S, Shrestha S, Sharma R, Marahatta SB. Effect of preloading on hemodynamic of the patient undergoing surgery under spinal anaesthesia. Kathmandu Univ Med J. 2010;8:216–21. doi: 10.3126/kumj.v8i2.3562. [DOI] [PubMed] [Google Scholar]
- 5.Khan MU, Memon AS, Ishaq M, Aqil M. Preload versus coload and vasopressor requirement for the prevention of spinal anesthesia induced hypotension in nonobstetric patients. J Coll Physicians Surg Pak. 2015;25:851–5. [PubMed] [Google Scholar]
- 6.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–32. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 7.Ceruti S, Anselmi L, Minotti B, Franceschini D, Aguirre J, Borgeat A, et al. Prevention of arterial hypotension after spinal anaesthesia using vena cava ultrasound to guide fluid management. Br J Anaesth. 2018;120:101–8. doi: 10.1016/j.bja.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Kweon TD, Kim SY, Cho SA, Kim JH, Kang YR, Shin Y-S. Heart rate variability as a predictor of hypotension after spinal anesthesia in hypertensive patients. Korean J Anesthesiol. 2013;65:317–21. doi: 10.4097/kjae.2013.65.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 10.Volk T. [Complications of spinal anesthesia and how to avoid them] Anasthesiol Intensivmed Notfallmed Schmerzther. 2010;45:188–95. doi: 10.1055/s-0030-1249402. [DOI] [PubMed] [Google Scholar]
- 11.Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping STJ, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–19. doi: 10.1097/ALN.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 12.Meyhoff CS, Haarmark C, Kanters JK, Rasmussen LS. Is it possible to predict hypotension during onset of spinal anesthesia in elderly patients? J Clin Anesth. 2009;21:23–9. doi: 10.1016/j.jclinane.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Achar SK, Sagar MS, Shetty R, Kini G, Samanth J, Nayak C, et al. Respiratory variation in aortic flow peak velocity and inferior vena cava distensibility as indices of fluid responsiveness in anaesthetised and mechanically ventilated children. Indian J Anaesth. 2016;60:121–6. doi: 10.4103/0019-5049.176285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: Systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845–53. doi: 10.1016/j.ultrasmedbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Szabó M, Bozó A, Darvas K, Horváth A, Iványi ZD. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anesthesia: An observational study. BMC Anesthesiol. 2019;19:139:1–8. doi: 10.1186/s12871-019-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama ER, Elkashlan M. Pre-operative ultrasonographic evaluation of inferior vena cava collapsibility index and caval aorta index as new predictors for hypotension after induction of spinal anaesthesia: A prospective observational study. Eur J Anaesthesiol. 2019;36:297–302. doi: 10.1097/EJA.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 17.Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care. 2005;9:566–72. doi: 10.1186/cc3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mačiulienė A, Gelmanas A, Jaremko I, Tamošiūnas R, Smailys A, Macas A. Measurements of inferior vena cava diameter for prediction of hypotension and bradycardia during spinal anesthesia in spontaneously breathing patients during elective knee joint replacement surgery. Medicina (Kaunas) 2018;54:49. doi: 10.3390/medicina54030049. [DOI] [PMC free article] [PubMed] [Google Scholar]


