Abstract
Background and Aim:
The process of laryngoscopy and endotracheal intubation is associated with intense sympathetic activity, which may precipitate intra-operative complications. Taking the advantage of dexmedetomidine's good bioavailability and rapid absorption through nasal mucosa; we contemplated this study to evaluate the effects of nebulised dexmedetomidine as a premedication in blunting the haemodynamic response to laryngoscopy and tracheal intubation.
Methods:
This prospective, randomised, comparative study was conducted in 100 American Society of Anesthesiologists (ASA) I, II patients. The primary outcome was to evaluate the effects of dexmedetomidine nebulisation in blunting the stress response to laryngoscopy and intubation. The secondary outcome was to study its adverse effects. The study population was divided randomly into two groups. Control group C (n = 50) received nebulisation with 5 ml of normal saline and group D (n = 50) received 1 μg/kg dexmedetomidine 5 ml 10 min before induction in sitting position.
Results:
Demographics were comparable. Following laryngoscopy and intubation, systolic (SBP), diastolic (DBP) and mean arterial pressure (MAP), response entropy (RE) and state entropy (SE) were markedly increased in the control group whereas in group D there was a fall in SBP (at 1 min-126.64 ± 26.37; P 0.01, 5 min-109.50 ± 16.83; P 0.02, 10 min-106.94 ± 17.01; P 0.03), DBP (at 1 min-83.18 ± 17.89; P 0.001, 5 min-66.40 ± 13.88; P 0.001, 10 min- 62.56 ± 14.91; P 0.01) and MAP (at 1 min-99.68 ± 19.22; P 0.001, 5 min- 84.08 ± 13.66; P 0.003, 10 min- 81.74 ± 14.79; P 0.008), RE and SE which was statistically significant (P 0.002). There was a dose sparing effect of propofol in group D; sedation score was comparable.
Conclusion:
Nebulised dexmedetomidine effectively blunts the stress response to laryngoscopy and intubation with no adverse effects.
Key words: Dexmedetomidine, entropy, intubation, laryngoscopy, premedication, sedation
INTRODUCTION
Direct laryngoscopy and intubation are noxious stimuli and are associated with transient, unpredictable and variable haemodynamic changes. This response occurs within 30 sec after intubation and lasts less than 10 min.[1] The consequences of laryngoscopy and intubation may precipitate ischaemia, arrhythmias, cerebrovascular stroke, pulmonary oedema, increase in intracranial pressure in the vulnerable group.[2] Till date, numerous drugs and various routes have been tried to attenuate this stress response such as opioids, vasodilators, beta-blockers, calcium channel blockers, intravenous lignocaine, topical sprays, volatile agents, α2 agonists but none of the agents proved to be ideal.[3]
Dexmedetomidine, a selective alpha2-adrenoceptor agonist, is short-acting and has sedative, hypnotic, anxiolytic, analgesic, anti-sialagogue, sympatholytic properties and promotes cardiac, respiratory and neurological stability.[4] Dexmedetomidine has the potential to produce bradycardia and hypotension when administered as a bolus; in a way to circumvent this problem, nebulisation route was chosen. Moreover, nebulised dexmedetomidine has a bioavailability of 65% through the nasal mucosa and 82% through the buccal mucosa.[5,6] Nebulised drug administration may be preferred over intranasal administration, as it avoids transient nasal irritation, cough, vocal cord irritation or laryngospasm.[7]
In this study, we hypothesised that nebulised dexmedetomidine will blunt the intubation response due to its rapid absorption and good bioavailability. Hence, this study was contemplated; the first of its kind in an attempt to investigate, its role in attenuating the stress response to laryngoscopy and intubation.
The primary aim of this study was to evaluate the role of nebulised dexmedetomidine as a premedication in attenuating the stress response to laryngoscopy and intubation. The secondary aim was to study any adverse effects of the drug such as cough, bradycardia, hypotension and dose-sparing effect of propofol and sedation.
METHODS
This study has institutional ethics committee approval bearing EC/NIMS/1991/2017 and also registered in the clinical trial registry of India with CTRI/2018/07/014837.
This was a prospective, randomised, controlled study, which recruited 100 adult patients. The inclusion criteria were patients who were American Society of Anesthesiologists (ASA) physical status I and II aged between 18 and 60 years, with normal airway belonging to both the genders undergoing elective surgery under general anaesthesia with endotracheal intubation. Patients who were not consenting for the study, predicted airway difficulty, pregnancy, renal failure,uncontrolled hypertension, seizure disorders, patient on anti-depressants/anti-psychotics, patients with a poor cardiopulmonary reserve and with body mass index (BMI) <30 kg/m2 were excluded from the study. They were randomised into two groups C and D using simple randomisation and closed envelope method according to the computer-generated table of random numbers. Group C (control n-50) received 5 ml of normal saline and group D (study group n-50) received 5 ml of dexmedetomidine at a dose of 1 μg/kg as nebulisation. The study drug was prepared by the anaesthesiology technician who was not involved in the study.
A day prior to the surgery, a preoperative visit was made and a detailed history and clinical examination of the patient was done. All patients were explained about the study protocol and the consent was obtained for the same. Dexmedetomidine at a dose of 1 μg/kg (mixed with saline to a total volume of 5 ml) nebulisation was administered to Group D (study group) with a nebuliser face mask and a continuous flow of 100% oxygen at 6 L/min for 10 min before induction of anaesthesia in sitting position and the control group received nebulisation with normal saline according to the group assigned. Baseline sedation score was noted and patients were premedicated with injection glycopyrrolate (0.02 mg/kg), fentanyl 2 μg/kg, induced with propofol (1 to 2 mg/kg) titrated to the loss of verbal response and the amount of drug administered was noted, and atracurium as a muscle relaxant in the dose of 0.5 mg/kg, to facilitate intubation with an aim to maintain both response entropy (RE) and state entropy (SE) around 40–60. Direct laryngoscopy (appropriate size Macintosh blade) and intubation were done using an appropriate sized endotracheal tube by an experienced consultant anaesthesiologist and patient was connected to the ventilator. The patient was undisturbed for a period of 10 min after intubation for noting the vital parameters like heart rate (HR) blood pressure (systolic (SBP), diastolic (DBP) and mean arterial pressure (MAP), pulse oximetry (SpO2), RE and SE, by an anaesthesia resident doctor not involved in the study at the following time points: baseline (Tb), after nebulisation (after neb), post-intubation at 1, 5 and 10 min (T1, T5 and T10) and study ends here. All the patients were administered with inj. paracetamol 1 gram IV intraoperatively. Once the surgical procedure was done, the residual neuromuscular blockade was reversed with inj glycopyrrolate and neostigmine, the patient's trachea was extubated after meeting the extubation criteria and shifted to post anaesthesia care unit.
Statistical analysis was performed using International Business Machine Statistical Package for Social Sciences (IBM SPSS) version 20. The statistical analysis for comparison of continuous variables between the groups was performed using analysis of variance (ANOVA) and a two-tailed significance of P < 0.05 was considered as a significant difference. Bonferroni post-hoc analysis was performed for variables with a significant difference between the groups. The comparison of categorical variables between the groups was performed using the Chi-square test or Fisher exact test when the expected cell values were <5. A two-tailed P value of <0.05 was considered as a significant difference between the groups.
The sample size was calculated using Statistical Software G Power 3.1.9.2. In a study by Sale H K et al.[8] intravenous lignocaine was compared with dexmedetomidine for blunting the intubation response. The effect size was calculated from this study taking into consideration the difference in the mean of mean arterial pressure from baseline (91.00 ± 7.80) and 1 min after intubation (80.50 ± 7.09). With this effect size 0.6 and a power of 90% and an alpha error of 0.05 the sample size was calculated to be 98 (49 patients in each group) and a total of 100 patients were recruited for the study, but there were no dropouts in our study.
RESULTS
Consort chart of 100 patients, who underwent surgery under general anaesthesia, was studied [Figure 1]. The data were collected, tabulated, analysed and the following observations were made. Demographic data were comparable between the groups [Table 1].
Figure 1.
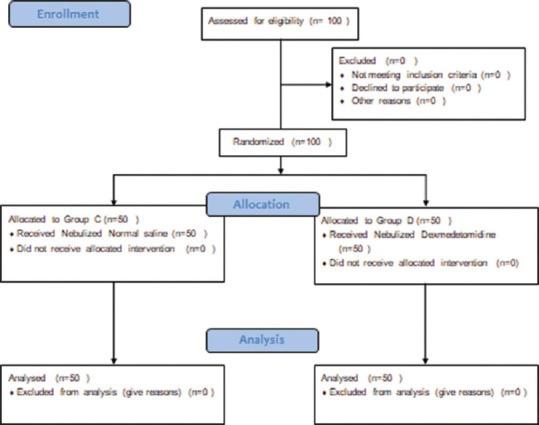
CONSORT CHART
Table 1.
Demographic data
| Parameters | Group C mean±SD | Group D mean±SD |
|---|---|---|
| Age; yrs | 40.66±11.55 | 37.16±11.63 |
| Gender, M/F% | M: 26 (52%) F: 24 (48%) | M: 21 (42%) F: 29 (58%) |
| Weight Kg | 63.44±10.25 | 60.84±12.98 |
| ASA grade | I: 50 (100%) II: 0 (0%) | I: 48 (96%) II: 2 (4%) |
The primary outcome of the study was to evaluate changes in MAP values after as a component of haemodynamic stress response to laryngoscopy and intubation with nebulised dexmedetomidine. MAP after nebulisation and immediately after intubation was comparable in both groups. The MAP values after intubation were lower in group D, which was statistically significant with mean, standard deviation (SD) and P values of 99.68 ± 19.22; P 0.001 at 1 min, 84.08 ± 13.66; P 0.003 at 5 min and 81.74 ± 14.79; P 0.008 at 10 min [Figure 2].
Figure 2.
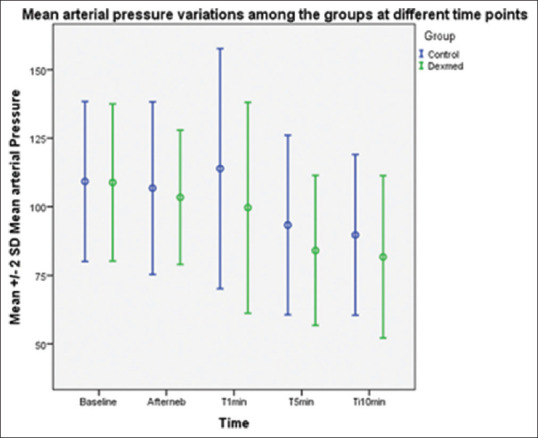
Mean blood pressure after nebulisation and immediately after intubation were comparable in both groups. The reduction in the mean arterial blood pressure at 1, 5 and 10 min after intubation were significantly lower in the dexmedetomidine group in a statistically highly significant manner
Within the group, comparison was statistically significant (P 0.03) when compared to the baseline value. The difference in HR between the two groups at various time intervals following laryngoscopy and intubation was comparable but not statistically significant with P 0.990.
SBP values after nebulisation and immediately after intubation were comparable in both groups. The SBP values at 1, 5 and 10 min after intubation were lower in group D in a statistically significant manner with P values of 0.01, 0.02, 0.03, respectively [Figure 3]. DBP values after nebulisation and immediately after intubation were comparable in both groups. The DBP values following laryngoscopy and intubation at 1, 5 and 10 min were lower in group D, which was statistically significant with P values of 0.001, 0.001, 0.01, respectively [Figure 4].
Figure 3.
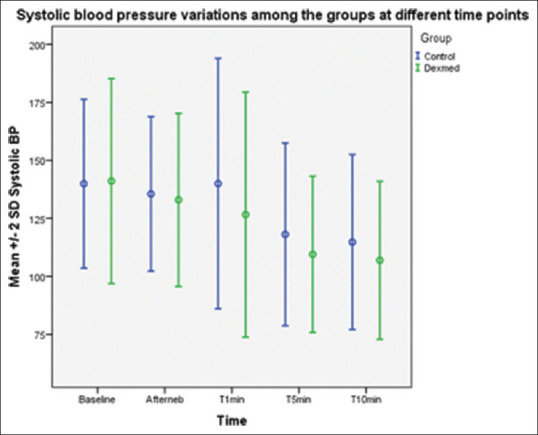
Systolic blood pressure after nebulisation and immediately after intubation were comparable in both groups. The reduction in the systolic blood pressure at 1, 5 and 10 min after intubation were significantly lower in the group D in a statistically highly significant manner with P < 0.05
Figure 4.
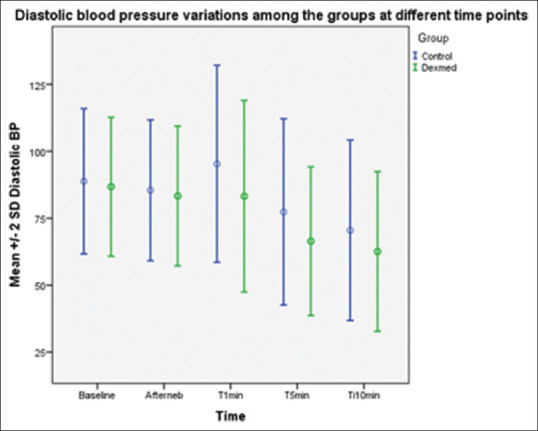
Diastolic blood pressure after nebulisation and immediately after intubation were comparable in both groups. The reduction in the diastolic blood pressure following laryngoscopy and intubation at 1, 5 and 10 min were significantly lower in the dexmedetomidine group in a statistically highly significant manner
There was a significant change in RE and SE following nebulisation between the two groups (P 0.002) [Table 2]. The difference between SPO2 in the two groups at various time intervals following laryngoscopy and intubation was not statistically significant (P 0.884). In group C, the mean dose of propofol for induction was 91 mg (1.45 mg/kg body weight) and in group D it was 70 mg (1.17 mg/kg body weight) showing reduction of 0.28 mg/kg body weight which was statistically significant (P 0.02). By comparing the median interquartile range between two groups for the sedation score, there was no statistically significant difference between the two groups (P 0.753).
Table 2.
Comparison of entropy in two groups
| Response Entropy (RE) and State Entropy (SE) | Group C (control) (Mean±SD) | Group D (dexmed) (Mean±SD) | P |
|---|---|---|---|
| Base RE | 98.90±0.953 | 98.26±1.72* | 0.240 |
| Neb RE | 97.36±1.675 | 94.90±4.40* | 0.002 |
| Ti1RE | 60.40±14.727 | 61.86±13.26* | 0.604 |
| Ti5RE | 66.28±14.479 | 63.26±11.17* | 0.246 |
| Ti10RE | 66.98±15.007 | 66.04±12.95* | 0.738 |
| Base SE | 89.44±2.215 | 89.00±1.92* | 0.292 |
| Neb SE | 88.40±2.356 | 86.02±3.87* | 0.002 |
| T1 SE | 56.88±14.014 | 57.40±12.51* | 0.845 |
| T 5SE | 62.82±13.638 | 60.26±10.89* | 0.302 |
| T10 SE | 63.22±14.787 | 62.50±12.53* | 0.793 |
*Within the group comparison was statistically significant (P<0.05) when compared to baseline value. There was significant change in RE and SE following nebulisation between the two groups (P<0.05)
DISCUSSION
This prospective randomised study is unique in the administration of dexmedetomidine through nebulised route—a noninvasive method for attenuation of intubation stress response by making use of its rapid onset and good bioavailability through the large surface area of the mucosa. Further nebulised drug administration avoids transient nasal irritation, cough, vocal cord irritation or laryngospasm over intranasal administration[5,6] and also transient adverse effects of bradycardia and hypotension by its intravenous route.
A combination of a calm sedated patient, without bradycardia and respiratory depression along with remarkable blunting of haemodynamic response at intubation is a novel response seen with the administration of dexmedetomidine by the nebulised route. There were no studies to similar effect as the present research in the adult population and to the best of our knowledge, our's is the first such study to evaluate the effects of nebulised dexmedetomidine in blunting haemodynamic stress response to intubation.
The authors found that nebulised dexmedetomidine was effective in blunting the haemodynamic response to laryngoscopy without any adverse effects. There was a statistically significant decrease in MAP at 1,5 and 10 min after intubation in group D [Figure 2], and also a statistically significant intra-group decline in MAP as compared to the baseline (P 0.03). Such a decrease in MAP can be attributed to dexmedetomidine's highly selective α2 agonistic action that causes a decrease in serum norepinephrine concentration thus leading to a dose-dependent decrease in arterial blood pressure.[4,9] A literature review in this regard revealed three randomised controlled trials (RCTs) where there was a remarkable decline in SBP with intranasal dexmedetomidine administration.[10,11,12] However, all these RCTs entailed the use of dexmedetomidine for sedation in the paediatric population.
Depending on the method of induction and in the absence of any specific measures to attenuate the haemodynamic response to laryngoscopy and intubation, it causes an increase in the HR ranging from 26% to 66% and the blood pressure from 36% to 45%.[13] The adverse haemodynamic responses can adversely affect the outcome of patients. Attenuation of such responses is of great importance to decrease perioperative morbidity and mortality.[14]
Dexmedetomidine is a highly selective α2 agonist with sedative, analgesic and anaesthetic sparing effects. It causes a decrease in serum norepinephrine concentration that leads to a dose-dependent decrease in arterial blood pressure and HR without side effects such as respiratory depression and post-operative nausea and vomiting. Nebulisation is an alternate method of drug delivery with higher bioavailability, greater ease of administration[5,6] and less effect on haemodynamics as compared to the intravenous (IV) route. Various drugs have been tried through nebulisation for sedation and blunting haemodynamic response such as lignocaine by Laurito et al.[15] and midazolam by Kaabachi et al.[16] Nebulised dexmedetomidine[7,17] before induction of anaesthesia was contemplated as it has a very short distribution half-life of 6 min and elimination half-time of 2 h without the adverse haemodynamic effects of IV dexmedetomidine. It also has an added advantage of avoiding bronchospasm over the commonly employed lignocaine. Zanaty and El Metainy[7] compared nebulised dexmedetomidine, nebulised ketamine and their combination. They concluded that the combination resulted in better sedation, smoother induction and more rapid recovery. Another study by Abdel-Ghaffar HS et al.[17] comparing nebulised dexmedetomidine, ketamine and midazolam found that nebulised dexmedetomidine provided more satisfactory sedation with shorter recovery time. But most of these studies were done in the paediatric population.
A dose of 1 μ/kg of dexmedetomidine was chosen in this study as it proved to be clinically effective both by the intranasal and IV routes in previous clinical studies.[7,18,19,20] The authors found a significant decrease in SBP, DBP and MAP at 1, 5 and 10 min after laryngoscopy and intubation in group D as compared to the control group. This significant decrease in BP from the baseline was due to the prevention of the stress response to laryngoscopy by dexmedetomidine.
In several studies,[21,22,23,24] dexmedetomidine given intravenously 10 min before induction was associated with adverse effects like bradycardia, hypotension, hypertension and respiratory depression. In this study, nebulised dexmedetomidine did not produce a significant change in HR at any time point throughout the study period. The absence of bradycardia could probably be explained by the omission of the IV bolus dose of the drug. This finding suggests that nebulised dexmedetomidine may be safer than IV dexmedetomidine in patients receiving beta-blockers or with a low basal heart rate.
Dexmedetomidine when given as a pre-medication acts on locus coeruleus to induce sedation and modulates nociceptive neurotransmission. Studies conducted in paediatric group[6,16] have reported significant sedation with dexmedetomidine. This could be due to the higher vascularity and increased surface area of the naso-oro-pharynx in children as compared to the adults. In our study, we achieved good sedation scores and a calm cooperative patient at induction as evidenced by a statistically significant reduction in the RE and SE in patients in group D. No decrease in entropy was noted in the saline group. No current literature reports sedation with nebulised dexmedetomidine in adults. It was also observed that there was a dose-sparing effect of propofol used for the induction of anaesthesia. In group D, the propofol used was 70 mg (1.17 mg/kg body weight) and in group C the mean dose was 91 mg (1.45 mg/kg body weight) showing reduction of 0.28 mg/kg body weight, which was statistically significant (P 0.02) a similar observation was made by Sharma et al.[25]
Our study has the following limitations. Cases with difficult airway were excluded and the time required for laryngoscopy and intubation was not taken into account. Also, the results cannot be extrapolated to high-risk patients with comorbidities. More RCTs are required to establish its safety and superiority over other routes for this purpose.
CONCLUSION
Nebulised dexmedetomidine effectively blunts the stress response to laryngoscopy and intubation and with no adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Low JM, Harvey JT, Prys-Roberts C, Dagnino J. Studies of anaesthesia in relation to hypertension: VII: Adrenergic responses to laryngoscopy. Br J Anaesth. 1986;585:471–7. doi: 10.1093/bja/58.5.471. [DOI] [PubMed] [Google Scholar]
- 2.Mahajon L, Kaur M, Gupta R, Aujila KS, Singh A, Kaur A. Attenuation of the pressor responses to laryngoscopy and endotracheal intubation with intravenous dexmedetomidine versus magnesium sulphate under bispectral index-controlled anaesthesia: A placebo-controlled prospective randomised trial. Indian J Anaesth. 2018;62:337–43. doi: 10.4103/ija.IJA_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan FA, Ullah H. Pharmacological agents for preventing morbidity associated with the haemodynamic response to tracheal intubation. Cochrane Database Syst Rev. 2013;7:CD004087. doi: 10.1002/14651858.CD004087.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason KP, Lerman J. Dexmedetomidine in children: Current knowledge and future applications. Anesth Analg. 2011;113:1129–42. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 6.Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56:691–3. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesth Analg. 2015;121:167–71. doi: 10.1213/ANE.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 8.Sale HK, Shendage VJ. Lignocaine and dexmedetomidine in attenuation of pressor response to laryngoscopy and intubation: A prospective study. Int J Sci Study. 2015;3:155–60. [Google Scholar]
- 9.Paranjpe JS. Dexmedetomidine: Expanding role in anesthesia. Med J DY Patil Univ. 2013;6:5–13. [Google Scholar]
- 10.Qiu J, Luo Z. The comparison of dexmedetomidine and ketamine for pediatric dental surgery: A meta-analysis of randomized controlled studies. Medicine. 2019;98:e51068. doi: 10.1097/MD.0000000000015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra PU, Thakur S, Singhal P, Chauhan D, Jayam C, Sood R, et al. Comparative evaluation of dexmedetomidine and midazolam-ketamine combination as sedative agents in pediatric dentistry: A double-blinded randomized controlled trial. Contemp Clin Dent. 2016;7:186–92. doi: 10.4103/0976-237X.183058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surendar MN, Pandey RK, Saksena AK, Kumar R, Chandra G. A comparative evaluation of intranasal dexmedetomidine, midazolam and ketamine for their sedative and analgesic properties: A triple blind randomized study. J Clin Pediatr Dent. 2014;38:255–61. doi: 10.17796/jcpd.38.3.l828585807482966. [DOI] [PubMed] [Google Scholar]
- 13.Singh H, Vichitvejpaisal P, Gaines GY, White PF. Comparative effects of lidocaine, esmolol, and nitroglycerin in modifying the haemodynamic response to laryngoscopy and intubation. J Clin Anaesth. 1995;7:5–8. doi: 10.1016/0952-8180(94)00013-t. [DOI] [PubMed] [Google Scholar]
- 14.Chraemmer-Jørgensen B, Hertel S, Strøm J, Høilund-Carlsen PF, Bjerre-Jepsen K. Catecholamine response to laryngoscopy and intubation. The influence of three different drug combinations commonly used for induction of anaesthesia. Anaesthesia. 1992;47:750–6. doi: 10.1111/j.1365-2044.1992.tb03252.x. [DOI] [PubMed] [Google Scholar]
- 15.Laurito CE, Bangham VL, Becker GL, Polek WV, Reigler FX, Vadenboncouer TR. Effects of aerosolised and/or intravenous lidocaine on haemodynamic responses to laryngoscopy and intubation in out-patients. Anesth Analg. 1988;67:389–92. [PubMed] [Google Scholar]
- 16.Kaabachi O, Ouezini R, Hajjej Z, Rais K, Koubaa W. Comparative study between mask nebulisation and oral administration of midazolam for premedication in children: 10AP3-4. Eur J Anaesthesiol. 2008;25:158–64. [Google Scholar]
- 17.Abdel-Ghaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. Br J Anesth. 2018;121:445–52. doi: 10.1016/j.bja.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Bajwa SJS, Kaur J, Singh A, Parmar S. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunisawa T, Nagata O, Nagashima M, Mitamura S, Ueno M, Suzuki A, et al. Dexmedetomidine suppresses the decrease in blood pressure during anesthetic induction and blunts the cardiovascular response to tracheal intubation. J Clin Anesth. 2009;21:194–9. doi: 10.1016/j.jclinane.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Niyogi S, Biswas A, Chakraborty I, Chakraborty S, Acharjee A. Attenuation of haemodynamic responses to laryngoscopy and endotracheal intubation with dexmedetomidine: A comparison between intravenous and intranasal route. Indian J Anaesth. 2019;63:915–23. doi: 10.4103/ija.IJA_320_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing hysterectomy. Anesthesiology. 1991;74:997–1001. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Jaakola ML, Ali-Melkkilä T, Kanto J. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. Br J Anaesth. 1992;68:570–5. doi: 10.1093/bja/68.6.570. [DOI] [PubMed] [Google Scholar]
- 23.Mowfi HA, Aldossary N, Ismail SA, Alqutiani J. Effect of Dexmedetomidine premedication on the intraocular pressure changes after succinylcholine and intubation. Br J Anaesth. 2008;100:485–9. doi: 10.1093/bja/aen020. [DOI] [PubMed] [Google Scholar]
- 24.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma N, Mehta N. Therapeutic efficacy of two different doses of dexmedetomidine on the hemodynamic response to intubation, the intubating conditions, and the effect on the induction dose of propofol: A randomized, double-blind, placebo-controlled study. Anesth Essays Res. 2018;12:566–71. doi: 10.4103/aer.AER_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


