Abstract
Background:
Tumor budding (Bd) has been demonstrated to be a promising prognostic factor in many carcinomas and in gastric cancer. It may represent an optimal additional parameter that is helpful for risk stratification in gastric adenocarcinoma. Hence, the present research was designed to predict the survival outcomes of gastric cancer in Vietnam, applying the tumor budding criteria of the International Tumor Budding Consensus Conference (ITBCC) 2016.
Methods:
The present study was conducted on 109 gastric cancer patients who underwent surgery but did not receive neo-adjuvant chemotherapy from 2012 to 2015. The patients’ clinicopathological features were recorded. Bd was evaluated according to the 2016 ITBCC criteria and classified as Bd1 (0–4 buds), Bd2 (5–9 buds), and Bd3 (≥10 buds) grades, in addition to being categorized into 2 main Bd groups: low (<10 buds) and high (≥10 buds) Bd. Kaplan–Meier and log-rank models were applied to analyze survival proportions.
Results:
Of all the patients, 22.9% were classified as Bd1, 31.2% as Bd2, and 45.9% as Bd3 grades. Furthermore, 54.1% patients were categorized into the low and 45.9% into the high Bd groups. Patients with Bd1 and Bd2 grades (the low Bd group) exhibited the best prognosis, with 5-year overall survival (OS) rates of 85.7%, 90.8%, and90.3%, respectively. Patients with Bd3 grade (the high Bd group exhibited the worst prognosis, and none of them lived for 5 years (p < 0.001). Similar to OS rates, disease-free survival (DFS) rates markedly reduced from the Bd1 to Bd3 grade: Bd1, 95.0%; Bd2, 84.7%; and Bd3, 0% (p < 0.001).
Conclusion:
Patients with different gastric cancer Bd grades exhibited significantly different OS and DFS rates. The present study findings suggest that the ITBCC criteria can be used to stratify Bd for the treatment and prognosis of gastric cancer patients in Vietnam.
Keywords: tumor budding, prognosis, pathology, gastric cancer, clinical
Introduction
Tumor budding (Bd) is a novel prognostic marker that has garnered interest, especially in the treatment and prognosis of colorectal cancer.1 Single cells or clusters of up to 4 cells at the infiltrated margins of large intestinal carcinoma are termed Bd.1,2 Peritumoral budding can only be assessed in endoscopic or surgical resection specimens.3 Bd is associated with poorer clinicopathological features and outcomes. It is important to note that Bd can be quantified on histopathological slides to provide a Bd score that correlates with tumor aggressiveness.
The prognostic value of Bd has been established in many independent retrospective studies for many gastrointestinal tract cancers, such as esophageal cancer, colorectal cancer, and gastric cancer.4-6 Importantly, Bd is officially recognized as a complementary prognostic hallmark by the Union for International Cancer Control (UICC) for colorectal carcinoma and is listed by the European and Japanese guidelines for colorectal cancer screening and diagnosis as well as the guidelines of the European Society for Medical Oncology.7-9 Furthermore, Bd has recently been listed as a coreless data item by the European consensus conference colon and rectum (EURECCA), highlighting the increased use of this prognostic marker in clinical practice.10
Although much progress has been made in the diagnosis and treatment of cancers, gastric cancer remains the most common cause of death in Vietnam. To improve the survival of patients, making more suitable decisions on the adjuvant treatment after operating for gastric cancer is very important, especially in the early-stage cancer. Therefore, there is an essential need for the validation of treatment protocols that are applied in Vietnam for managing gastric cancer patients. Vietnam is a developing country, so it is crucial to choose tools for risk stratification that are eligible in terms of both expense and value in selecting the exact adjuvant therapy. The evaluation of Bd may be a good candidate, and this factor has not been used in Vietnam to identify risk groups. Hence, this research was designed to predict the survival outcomes of gastric cancer in Vietnam, applying the tumor budding criteria of the International Tumor Budding Consensus Conference (ITBCC) 2016.
Methods
This was a retrospective study including 109 patients with gastric cancer aged 28–80 years who underwent surgery from 2012 to 2015 at the National Cancer Hospital (NCH), Vietnam. NCH is the largest hospital specializing in oncological diagnosis, treatment, screening, prevention, and control in Vietnam. Only patients with treatment-naïve tumors were selected. Patients who presented with secondary or recurrent malignant tumors were excluded. The clinical information of the patients was extracted from their medical charts and records and was recorded; it included age, sex, tumor location, and date at initial diagnosis. All the patients were operated upon for tumor removal via total or partial gastrectomy, combined with regional lymph node dissection. The maximum diameter of resected tumors was calculated. pTNM staging of gastric cancer was applied using the criteria of the American Joint Committee on Cancer (AJCC, 7th edition).11 Tumor and lymph node specimens were obtained for histopathological examination.
After surgery, 58 of the patients were treated via adjuvant chemotherapy with XELOX and/or capecitabine regimens. The remaining 51 patients exhibited no indication for postoperative chemotherapy owing to early-stage cancer or refusal to receive the adjuvant treatment. All individual information was deleted or disguised in order to ensure patient anonymity.
Histopathology
All specimens were obtained in the operating room and then transferred to the pathology department. Samples were fixed in 10% neutral formalin for 24 h. Lymph node and tumor samples were then prepared using routine pathological techniques, such as hematoxylin and eosin (HE) staining. Experienced pathologists evaluated all histopathological features such as tumor size, histopathological type, and grade; nodal status; and deep, perineural infiltration, or peritumoral lymphovascular invasion (LVI). Histopathological types were classified according to the Lauren and 2010 WHO classifications.12 Additionally, histological grading was performed according to the 2010 WHO classification.12
Tumor Budding Categories
All patients were classified into Bd categories based on histopathological criteria. Bd was evaluated only on HE-stained slides. Many HE-stained slides of each patient were viewed by pathologists under a light microscope, and a representative HE-stained section with the deepest invasion was used for further analysis. Bd was analyzed independently by 2 experienced pathologists, and any disagreement was resolved by reinvestigation to reach a consensus. The assessment procedure proposed by the ITBCC 2016 was used.1 It was performed in 5 steps: the first step is defining the field (specimen) area for the 20× objective lens of the microscope based on the eyepiece field number diameter; the second step is selecting the HE-stained slide with the highest Bd grade at the invasive front; the third step is counting tumor buds in the selected “hotspot” (20× objective); and finally, dividing the Bd count by the normalization factor to determine the Bd count per 0.785mm2, selecting the Bd category based on Bd count, and indicating the absolute count per 0.785mm2.1
Bd was categorized according to the 3-grade system of the Japanese Society for Cancer of the Colon and Rectum13 as follows: low budding (Bd1), 0–4 tumor buds; intermediate budding (Bd2), 5–9 tumor buds; and high budding (Bd3), ≥10 tumor buds. Thereafter, patients with the 2 lowest Bd grades (Bd1 and Bd2) were combined to form the low Bd group, and those with Bd3 grade formed the high Bd group. In the present study, revaluation was needed to reach a consensus for 14.8% of all the patients because different pathologists assigned various Bd groups to the same slides in case of these patients. The difficulties in recognizing the area with most Bds and in distinguishing Bd, especially to small signet-ring cancer cells, from stromal or immune cells in certain cases were the major reasons for reassessment.
Follow-Up and Outcomes
Overall survival (OS) was defined as the period from the date of initial diagnosis to the day of death due to gastric cancer, or the last available time before losing follow-up.14 Patients would be censored if they did not die of gastric cancer. The dates of death were displayed on the death documents such as certificates were issued by the commune government in Vietnam. The relapse and the recurrent dates were shown by image analytical and/or morphological data. Patients would be censored until the date of death if they did not present with any relapse.14 Disease-free survival (DFS) was defined as the period from the date of gastric cancer surgery until the date of diagnosis of the recurrent gastric cancer, or GC-specific death, including locoregional and distant relapses.14
Statistical Analysis
All the patients were categorized into the different Bd groups using the criteria from the ITBCC 2016, list of Bd categories: Bd1, Bd2, and Bd3. The Bd1 and Bd2 grades were combined into the low Bd group and the Bd3 grade into the high Bd group. Pearson chi-square test, likelihood ratio, and Fisher’s exact tests were used to determine the clinicopathological differences between the Bd groups. The Kaplan–Meier model was used to investigate the 5-year OS and DFS according to the Bd1, Bd2, and Bd3 grades or the low and high Bd groups. Survival curves were compared by performing log-rank test. Differences were considered to be significant if p-value was <0.05. In multivariate analysis, Cox proportional hazards regression model was applied to determine hallmarks that were independently associated with OS and DFS. All analyses were conducted using the statistical software SPSS version 19.0.
Results
Baseline Clinicopathological Features and Tumor Budding Grades
The present study comprised 109 gastric cancer patients who underwent surgery for tumor removal. Table 1 shows their basic clinicopathological features. The patient’s age range was 28–80 years. Most patients were aged 50–59 years and >60 years (36.7% and 41.3%, respectively). The cancer was more common in male than in female patients (62.4% vs 37.6%). The antrum was the most common tumor location (79.8%). Regarding pathological features, most of the histopathological types were adenocarcinoma, NOS, and intestinal type (Lauren classification) (78.9% and 86.2%, respectively). Gastric adenocarcinoma was mostly moderately and poorly differentiated (41.9% and 50.0%, respectively). Less than half of the cases presented with negative regional lymph nodes (36.7%) and exhibited LVI and perineural infiltration (38.5% and 37.6%, respectively). Among the positive nodal group, 38.5% of the patients presented with up to 3 metastasized adjacent lymph nodes, counted in the highest proportion. Tumors invaded the lamina propria, muscularis mucosa, or submucosa at a low rate (0.9%). Such cases were similar to pathological stage I, and these tumors accounted for only 2.8% of all cases.
Table 1.
Baseline Clinicopathological Features in 109 Gastric Cancer Patients.
| Characteristics | No. of patients (%) | Characteristics | No. of patients (%) |
|---|---|---|---|
| Age group | Lymph node status | ||
| <30 | 1 (0.9) | Negative | 40 (36.7) |
| 30-39 | 11 (10.1) | 1-2 positive node(s) | 42 (38.5) |
| 40-49 | 12 (11.0) | 3-6 positive nodes | 17 (15.6) |
| 50-59 | 40 (36.7) | 7-15 positive nodes | 9 (8.3) |
| ≥60 | 45 (41.3) | > 15 positive nodes | 1 (0.9) |
| Sex | LVI | ||
| Male | 68 (62.4) | Present | 42 (38.5) |
| Female | 41 (37.6) | Perineural invasion | |
| Present | 41 (37.6) | ||
| Tumor location | Invasive depth | ||
| Antrum | 87 (79.8) | T1 | 1 (0.9) |
| Antrum—Lesser curvature | 7 (6.4) | T2 | 18 (16.5) |
| Fundus/Corpus | 5 (4.6) | T3 | 16 (14.7) |
| Corpus—Lesser curvature | 3 (2.8) | T4a | 50 (45.9) |
| Cardia | 3 (2.8) | T4b | 24 (22.0) |
| Pyloric Antrum | 3 (2.8) | ||
| Unknown | 1 (0.9) | ||
| Histopathological type | pTNM Stage | ||
| Adenocarcinoma, NOS | 86 (78.9) | I | 3 (2.8) |
| Signet ring cell | 16 (14.7) | IIA | 24 (22.0) |
| Mucinous | 7 (6.4) | IIB | 24 (22.0) |
| IIIA | 21 (19.3) | ||
| IIIB | 26 (23.9) | ||
| IIIC | 11 (10.1) | ||
| Differentiated grade | Tumor budding | ||
| Well-differentiated | 7 (8.1) | Grade I | 25 (22.9) |
| Moderately-differentiated | 36 (41.9) | Grade II | 34 (31.2) |
| Poorly-differentiated | 43 (50.0) | Grade III | 50 (45.9) |
| Lauren classification | Relapse | ||
| Intestinal type | 93 (85.3) | Present | 31 (28.4) |
| Diffuse type | 16 (14.7) | Recurrent pattern | 21 (75.0) |
| Distant metastasis |
A gradual increase in the Bd grade was observed, with 25 patients (22.9%) classified as low Bd, 34 (31.2%) as intermediate Bd, and 50 (45.9%) as high Bd. After a maximum follow-up period of 83 months, 28.4% patients were found to have recurrent tumor. Among the patients with cancer relapse, 67.7% presented with distant metastasis to organs such as the liver and lung.
Clinicopathological Features of Different Bd Grades
To assess the relationship between clinicopathological features and the Bd groups, all the patients were classified according to the 3 Bd grades: low, moderate, and high Bd (according to the ITBCC 2016 criteria). Table 2 displays the relationship between clinicopathological features and the Bd grades for GC. Patients aged >50 years were more common compared with other age groups in both the high and low Bd groups, nevertheless high Bd was observed at a lower rate than low Bd and was 34.0 and 36.0%, respectively, in patients aged 50–59 years and 60–69 years; meanwhile, low Bd was observed at rates of 36.7% and 41.3% in patients aged 50–59 and 60–69 years, respectively. However, the Bd grades were dependent on age, sex, and tumor location (p > 0.05). Significant differences were observed between the histopathological types according to the WHO classification, Lauren classification, and histological grading and Bd groups (p < 0.001). The high Bd grade showed a trend of being more common in patients with histopathological types that indicated poor prognosis. Regarding invasive features, patients with the high Bd grade showed the highest rates of LVI and perineural infiltration (54.0% and 68.0%, respectively). Similarly, recurrent cases were the most common in the high Bd group (56.0%), but they accounted for the smallest proportion of the low Bd tumors (5.1%). Characteristics of infiltration and relapse were found to significantly correlate with the Bd grades (p < 0.001). Conversely, no trends were observed in the Bd grades according to the pTNM stages (p > 0.05).
Table 2.
Associations of Tumor Budding and Clinicopathological Features in 109 Gastric Cancer Patients.
| Characteristics | No. of patients (%) | Tumor budding | p (χ2) | |
|---|---|---|---|---|
| Low 59 (54.1) | High 50 (45.9) | |||
| Age group | 0.382(a) | |||
| <30 | 1 (0.9) | 0 | 1 (2.0) | |
| 30-39 | 11 (10.1) | 4(6.8) | 7 (14.0) | |
| 40-49 | 12 (11.0) | 5 (8.5) | 7 (14.0) | |
| 50-59 | 40 (36.7) | 23 (39.0) | 17 (34.0) | |
| ≥60 | 45 (41.3) | 27 (45.8) | 18 (36.0) | |
| Sex | 0.384 | |||
| Male | 68 (62.4) | 39 (66.1) | 29 (58.0) | |
| Female | 41 (37.6) | 20 (33.9) | 21 (42.0) | |
| Lateral | 0.443(a) | |||
| Antrum | 87 (79.8) | 46 (78.0) | 41 (82.0) | |
| Antrum—Lesser curvature | 7 (6.4) | 3 (5.1) | 4 (8.0) | |
| Fundus/Corpus | 5 (4.6) | 4 (6.8) | 1 (2.0) | |
| Corpus—Lesser curvature | 3 (2.8) | 1 (1.7) | 2 (4.0) | |
| Cardia | 3 (2.8) | 1 (1.7) | 2 (4.0) | |
| Pyloric Antrum | 3 (2.8) | 1 (1.7) | 2 (4.0) | |
| Unknown | 1 (0.9) | 1 (1.7) | 0 | |
| Histopathological type | <0.001(a) | |||
| Adenocarcinoma, NOS | 86 (78.9) | 56 (94.9) | 30 (60.0) | |
| Signet ring cell | 16 (14.7) | 0 | 16 (32.0) | |
| Mucinous | 7 (6.4) | 3 (5.1) | 4 (8.0) | |
| Differentiated grade | 0.009(a) | |||
| Well-differentiated | 7 (8.1) | 7 (12.5) | 0 | |
| Moderately-differentiated | 36 (41.9) | 27 (48.2) | 9 (30.0) | |
| Poorly-differentiated | 43 (50.0) | 22 (39.3) | 21 (70.0) | |
| Lauren classification | <0.001 | |||
| Intestinal type | 93 (85.3) | 59 (100.0) | 34 (68.0) | |
| Diffuse type | 16 (14.7) | 0 | 16 (32.0) | |
| LVI | 0.003 | |||
| Absent | 67 (61.5) | 44 (74.6) | 23 (46.0) | |
| Present | 42 (38.5) | 15 (25.4) | 27 (54.0) | |
| Perineural invasion | <0.001 | |||
| Absent | 68 (62.4) | 52 (88.1) | 16 (32.0) | |
| Present | 41 (37.6) | 7 (11.9) | 34 (68.0) | |
| Relapse | <0.001 | |||
| Absent | 78 (71.6) | 56 (94.9) | 22 (44.0) | |
| Present | 31 (28.4) | 3 (5.1) | 28 (56.0) | |
| pT | 0.426(a) | |||
| T1 | 1 (0.9) | 1 (1.7) | 0 | |
| T2 | 18 (16.5) | 13 (22.0) | 5 (10.0) | |
| T3 | 16 (14.7) | 8 (13.6) | 8 (16.0) | |
| T4a | 50 (45.9) | 25 (42.4) | 25 (50.0) | |
| T4b | 24 (22.0) | 12 (20.3) | 12 (24.0) | |
| Lymph node status | <0.001 | |||
| Negative | 40 (36.7) | 28 (47.5) | 12 (24.0) | |
| 1-2 positive node(s) | 42 (38.5) | 20 (33.9) | 22 (44.0) | |
| 3-6 positive nodes | 17 (15.6) | 8 (13.6) | 9 (18.0) | |
| 7-15 positive nodes | 9 (8.3) | 3 (5.1) | 6 (12.0) | |
| > 15 positive nodes | 1 (0.9) | 0 | 1 (2.0) | |
| pTNM stage | 0.124 | |||
| I | 3 (2.8) | 2 (3.4) | 1 (2.0) | |
| IIA | 24 (22.0) | 19 (32.2) | 5 (10.0) | |
| IIB | 24 (22.0) | 12 (20.3) | 12 (24.0) | |
| IIIA | 21 (19.3) | 10 (16.9) | 11 (22.0) | |
| IIIB | 26 (23.9) | 11 (18.6) | 15 (30.0) | |
| IIIC | 11 (10.1) | 5 (8.5) | 6 (12.0) | |
(a): Fisher exact test.
Associations of Outcomes and Clinicopathological Characteristics
Table 3 shows the relationship between clinicopathological features and outcomes of GC. Regarding histopathological features, the death rate was highest in patients with poor histopathological categories according to the WHO classification, i.e. signet-ring cell carcinoma (11/16 cases: 68.8%) and mucinous adenocarcinoma (4/7 cases: 57.1%), and according to the Lauren classification, i.e. diffuse type (11/16 cases: 68.8%). Histopathological classifications were significantly associated with survival (p < 0.05). This difference was also illustrated in the relationship between clinicopathological characteristics and survival. The death rate was the highest in patients with stage IIIC gastric cancer (7/11 cases: 63.6%).
Table 3.
Associations of Outcomes and Clinicopathological Features in 109 Gastric Cancer Patients.
| Characteristics | No. of patients (%) | Outcome | p (χ2) | |
|---|---|---|---|---|
| Survival | Death | |||
| Age group | 0.501(a) | |||
| <30 | 1 (0.9) | 0 | 1 (2.6) | |
| 30-39 | 11 (10.1) | 7 (10.0) | 4 (10.3) | |
| 40-49 | 12 (11.0) | 6 (8.6) | 6 (15.4) | |
| 50-59 | 40 (36.7) | 28 (40.0) | 12 (30.8) | |
| ≥60 | 45 (41.3) | 29 (41.4) | 16 (41.0) | |
| Sex | 0.410 | |||
| Male | 68 (62.4) | 46 (65.7) | 22 (56.4) | |
| Female | 41 (37.6) | 24 (34.3) | 17 (43.6) | |
| Location | 0.775(a) | |||
| Antrum | 87 (79.8) | 56 (80.0) | 31 (79.5) | |
| Antrum—Lesser curvature | 7 (6.4) | 5 (7.1) | 2 (5.1) | |
| Fundus/Corpus | 5 (4.6) | 4 (5.7) | 1 (2.6) | |
| Corpus—Lesser curvature | 3 (2.8) | 1 (1.4) | 2 (5.1) | |
| Cardia | 3 (2.8) | 1 (1.4) | 2 (5.1) | |
| Pyloric antrum | 3 (1.8) | 1 (1.4) | 1 (5.1) | |
| Unknown | 1 (0.9) | 1 (1.4) | 0 | |
| Histopathological type | 0.003(a) | |||
| Adenocarcinoma, NOS | 86 (78.9) | 62 (88.6) | 24 (61.5) | |
| Signet ring cell | 16 (14.7) | 5 (7.1) | 11 (28.2) | |
| Mucinous | 7 (6.4) | 3 (4.3) | 4 (10.3) | |
| Histological grade | 0.533 | |||
| Well-differentiated | 7 (8.1) | 6 (9.7) | 1 (4.2) | |
| Moderately-differentiated | 36 (41.9) | 27 (43.5) | 9 (37.5) | |
| Poorly-differentiated | 43 (50.0) | 29 (46.8) | 14 (58.3) | |
| Lauren classification | 0.004 | |||
| Intestinal type | 93 (85.3) | 65 (92.9) | 28 (71.8) | |
| Diffuse type | 16 (14.7) | 5 (7.1) | 11 (28.2) | |
| Tumor Budding | <0.001 | |||
| Low | 59 (54.1) | 55 (78.6) | 4 (10.3) | |
| High | 50 (45.9) | 15 (21.4) | 35 (89.7) | |
| LVI | <0.001 | |||
| Absent | 67 (61.5) | 54 (77.1) | 13 (33.3) | |
| Present | 42 (38.5) | 16 (22.9) | 26 (66.7) | |
| Perineural invasion | <0.001 | |||
| Absent | 68 (62.4) | 55 (78.6) | 13 (33.3) | |
| Present | 41 (37.6) | 15 (21.4) | 26 (66.7) | |
| Lymph node status | 0.027(a) | |||
| Negative | 40 (36.7) | 32 (45.7) | 8 (20.5) | |
| 1-2 positive node(s) | 42 (38.5) | 26 (37.1) | 16 (41.0) | |
| 3-6 positive nodes | 17 (15.6) | 8 (11.4) | 9 (23.1) | |
| 7-15 positive nodes | 9 (8.3) | 4 (5.7) | 5 (12.8) | |
| > 15 positive nodes | 1 (0.9) | 0 | 1 (2.6) | |
| pTNM stage | 0.045(a) | |||
| I | 3 (2.8) | 3 (4.3) | 0 | |
| IIA | 24 (22.0) | 20 (28.6) | 4 (10.3) | |
| IIB | 24 (22.0) | 17 (24.3) | 7 (17.9) | |
| IIIA | 21 (19.3) | 12 (17.1) | 9 (23.1) | |
| IIIB | 26 (23.9) | 14 (20.0) | 12 (30.8) | |
| IIIC | 11 (10.1) | 4 (5.7) | 7 (17.9) | |
| Relapse | <0.001 | |||
| Absent | 78 (71.6) | 69 (88.5) | 9 (11.5) | |
| Present | 31 (28.4) | 1 (3.2) | 30 (96.8) | |
| Metastatic pattern | 0.75(a) | |||
| Locoregional metastasis | 7 (25.0) | 0 | 7 (100.0) | |
| Distant metastasis | 21 (75.0) | 1 (4.8) | 20 (95.2) | |
(a): Fisher exact test.
Regarding Bd grades, it was clear that survival was different across the grades. A gradual decrease in the death rate was observed from the high to low Bd grade. The death rate was the highest in the patients with Bd3 grade (89.7%), whereas patients with Bd1 grade exhibited the lowest death rate (2.6%). There was a significant difference among the patients with the 3 Bd grades and survival (p < 0.001). Patients in whom gastric cancer did not show LVI and perineural infiltration exhibited the highest survival (77.1% and 78.6%, respectively) (p < 0.001).
Additionally, a significant difference was observed between the Bd groups regarding recurrence (p < 0.001). Disease-free patients exhibited the highest survival rate (88.5%). Conversely, patients with recurrence exhibited the highest death rate (96.8%). Although the death rate of patients with distant metastasis was little lower than that of those with locoregional metastasis (95.2%and 100.0%, respectively), a significant difference was not found (p > 0.05).
Regarding age group, sex, tumor location, and histological grade, the relationship among these features and outcomes did not show a significant difference (p > 0.05).
In multivariate analysis, as shown in Table 4, Bd was found to be an independent prognostic marker with a hazard ratio of 20.899 and p < 0.001.
Table 4.
Mulitivariate Cox Regression Analysis of Clinicopathological Characteristics According to OS and DFS.
| OS | DFS | |||
|---|---|---|---|---|
| Hazard ratio | p value (Wald) | Hazard ratio | p value (Wald) | |
| Tumor budding (High vs Low) | 20.899 | <0.001 | 12.7 | <0.001 |
| Histological grade (G1 vs G2 or G3) | 0.234 | 0.239 | 0.352 | 0.371 |
| LVI (present vs absent) | 0.332 | 0.022 | 0.275 | 0.006 |
| Perineural invasion (Yes vs No) | 1.398 | 0.496 | 1.173 | 0.750 |
| pTNM (III vs I or II) | 0.456 | 0.112 | 0.381 | 0.049 |
Survival
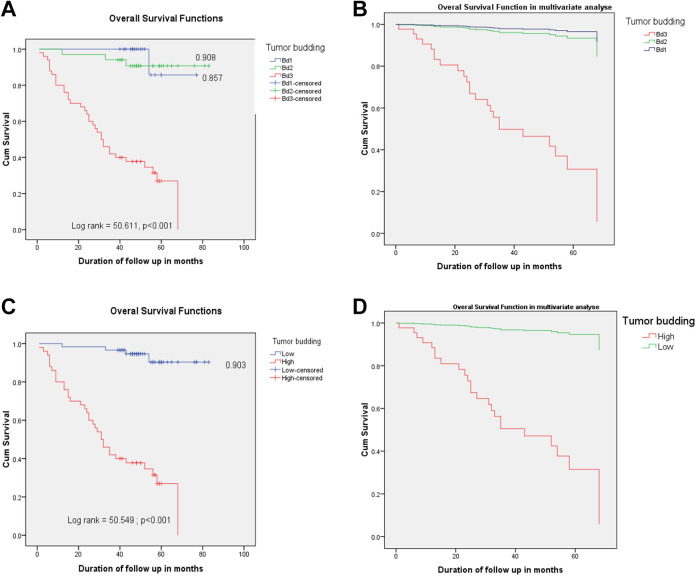
The 5-year OS of the patients who underwent surgery for gastric cancer was 43.02 ± 1.77 months. It was 73.71 ± 3.04 and 78.09 ± 2.76 months for the Bd1 and Bd2 groups, respectively, and 78.91 ± 2.00 months for the low Bd group (Bd1 and Bd2 combined); the 5-year OS of the Bd3/high Bd group was apparently less (36.69 ± 3.45 months). The patients with Bd1 and Bd2grades exhibited a better prognosis, with a 5-year OS rate of 85.7% and 90.8%, respectively; this rate was 90.3% in the low Bd group. Conversely, none of the patients in the Bd3/high Bd group exhibited 5-year OS. A significant difference was observed between the Bd groups according to their survival curves (p < 0.001) in both univariate and multivariate analyses (Figure 1A–D).
Figure 1.
Five-year relative overall survival of tumor budding categories for gastric cancer. In univariate and multivariate analysis, the log-rank test showed that there was a significant difference between the 3 curves (p < 0.001, A and B). In both analyses, the log-rank test also illustrated a difference was significant between the 2 Bd groups and OS (p < 0.001, C and D).
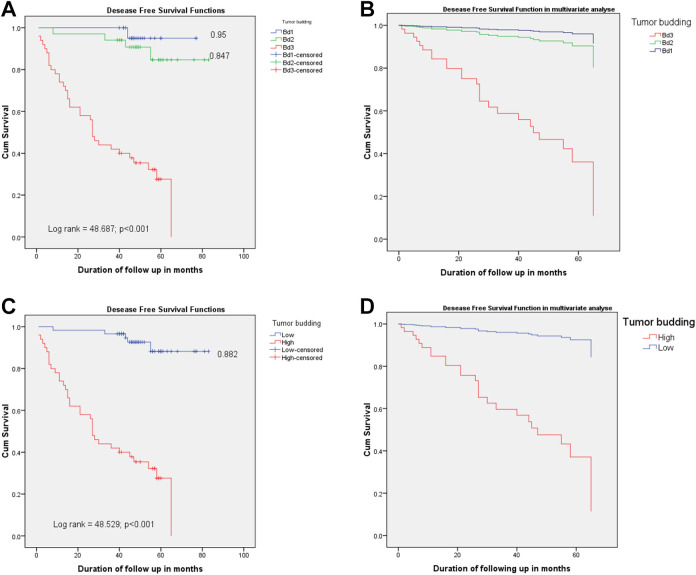
A significant difference was observed in the 5-year DFS rate between the Bd groups (p < 0.001) in both univariate and multivariate analyses (Figure 2A–D). The mean 5-year DFS was 41.88 ± 1.88 months; the mean survivals of the patients with Bd1 and Bd2 grades were 75.35 ± 1.61 and 76.29 ± 3.20 months, respectively, and the mean survival of the low Bd group was 78.07 ± 2.15 months. This result was similar to the 5-year OS rate of the Bd3/high Bd group, and the 5-year DFS of this group quickly decreased (33.87 ± 3.48 months). Five-year DFS rates of the patients with the Bd1 and Bd2 grades were 95.0% and 84.7%, respectively; this rate was 88.2% in the low Bd group. Conversely, all the patients in the Bd3/high Bd group died before the 5-year DFS, a finding similar to that for OS.
Figure 2.
Five-year relative disease free survival of the tumor budding degrees for gastric cancer. The log-rank test demonstrated that there is a significant difference between these 3 survival curves, in univariate and multivariate analysis (p < 0.001, A and B). Log-rank test exhibited a significant different association of DFS to 2 Bd categories in both analyses, as well (p < 0.001, C and D).
Discussion
Gastric cancer is the third common cause of cancer-related death in the world, and has high rate of recurrence after a potentially curative surgery; even early-stage gastric cancer patients die in many cases.15,16 Thus, finding novel markers to precisely estimate the pathological diagnosis and prognosis of gastric cancer is imperative. One such marker is Bd, the finding that underpins metastasis; it is defined as single cells or clusters of up to 4 cancer cells at the infiltrated margin. This phenomenon was not only observed in the region of the infiltrated front, where it has been previously described, but also in the tumor itself.2,4 Bd was demonstrated to be a promising prognostic hallmark in many carcinomas, such as large intestinal carcinoma; pancreatic ductal adenocarcinoma; squamous cell carcinoma of the tongue, pulmonary tissue, and laryngeal and esophageal tissue; breast cancer; gingival buccal complex squamous cell carcinoma; and gastric cancer.4,6,17-33 In 1949, Imai initially described this phenomenon in the Japanese medical literature in relation to gastric cancer.34 From to the pathological aspect, Bd is a phenotype encountered in various cancers, in which a number of finger-like projections of a primary cancer tissue invade into adjacent stroma, some of them eventually detach from the main tumor as small cell clusters.35 Bd is considered to be the first step in the distant metastasis as budding cells are thought to migrate through the extracellular matrix, infiltrating into lymphovascular architectures and forming settlements of metastatic cancer cells in lymph nodes and at distant locations.8 Regarding the biological significance of Bd, researchers have demonstrated that this phenomenon may be involved in epithelial–mesenchymal transition (EMT), thereby increasing malignant cell migration and invasion.36,37 EMT is a polystep dynamic cellular phenomenon in which an epithelial cell converts to a mesenchymal phenotype and acquires migratory and invasive features that are typical of mesenchymal cells.38,39 Aberrant activation of EMT is considered to be the hallmark of metastastic cancer.39-41 The relationship between Bd and EMT has been investigated in various cancers. However, most EMT processes in Bd are incomplete, suggesting that tumor buds undergo partial EMT.35,42 The malignant cells that undergo EMT have been shown to exhibit increased invasive and metastatic capabilities, which might explain why patients with high Bd exhibit worse outcomes than those with low Bd.36
Bd may represent an optimal complementary hallmark that is helpful for risk stratification in gastric adenocarcinoma. The UICC has officially recognized Bd as an additional independent prognostic parameter in colorectal carcinoma.43 It is speculated that Bd is an important indicator of outcomes, providing a diagnostic guideline to facilitate personalized treatment in the future.
In the present study, the analysis of Bd in gastric cancer was based on the 2016 ITBCC criteria. The results demonstrated a relationship of pathological characteristics such as histological type, Lauren classification, differentiated grade, lymph node metastasis, LVI, and perineural infiltration with recurrence and survival. A high Bd grade was significantly associated with relapse and short survival. Nevertheless, the findings did not retain its significant prognosis for association between the Bd grade and pTNM stage, in this mode. At present, only few studies have been performed on Bd in stomach cancer, with each study applying different scoring systems.4,6,20,29 Despite the methodical variation, early cohorts displayed that high Bd is related to various adverse clinicopathological characteristics, including an advanced T stage, positive lymph node, distant metastasis, advanced UICC stage, poor differentiated grade, LVI, perineural infiltration, and poor prognosis.4,5,17,20,26,44 Additionally, 2 cohorts demonstrated that Bd was an independent hallmark of prognosis in stomach cancer.4,20 The findings of the present study are consistent with some of the findings in the above studies.
Regarding the relationship between Bd and early-stage GC, although patients with early-stage disease may show favorable outcomes after radical gastrectomy, many of them still die due to cancer and exhibit high recurrent rates, even after potentially curative operation.45,46 Bd provides valuable information regarding the decision of adjuvant therapy, especially in patients with Bd3 grade having early-stage cancer, to help oncologists identify high-risk patients who may benefit from systemic therapy. Bd assessment might help identify patients at risk for worse outcome in the following ways: (1) identifying patients with pT1 stage or cancer-associated polyps endoscopically resected with increased risk for the metastatic lymph node and (2) identifying patients with stage II cancer who may benefit from adjuvant treatment.30 In the present study, 2% patients with early-stage gastric cancers exhibited high Bd grade. The findings of this study may support the previously described role of Bd in early stomach cancer.17,29 The above mentioned result illustrates that assessing Bd in early-stage gastric cancer is essential to help make suitable decisions on adjuvant therapy after surgery for these cancers.
Regarding the Lauren classification, Kemi et al. indicated that a significant relationship was not found between Bd and OS in the diffuse-type gastric cancer.20 The relationship between different types (Lauren classification) of gastric cancer and Bd grades may vary. The diffuse-type gastric cancer is usually considered to be an aggressive tumor with poor prognosis. The present study included the intestinal -and diffuse-type gastric cancers. Bd assessment in both intestinal- and diffuse-type gastric cancers showed its prognostic significance for OS and DFS in both univariate and multivariate analyses in the present study. In the future, the relationship between Bd and different types of gastric cancer according to the Lauren classification should be evaluated in separate studies to investigate better the impact of Bd on the prognosis of stomach cancer.
Regarding the present study results on relapse and survival in patients with gastric cancer, the high Bd group exhibited higher recurrence and shorter survival, both OS and DFS, than the low Bd group in univariate and multivariate analyses as well. However, Ulase et al. did not find Bd to be an independent prognostic parameter upon multivariate analysis.29 High Bd was demonstrated to show a correlation with low 3- and 5-year OS. Nevertheless, patients with intestinal-type gastric cancer still showed a significant correlation between Bd and survival.44 Conversely, both Kemi et al. and Che et al. showed that Bd is valuable as an independent prognostic indicator in univariate and multivariate analyses.4,20 The present study also demonstrated that Bd is an independent prognostic factor in gastric cancer.
Bd was defined as an independent prognostic marker in colorectal cancer by the ITBCC and recognized as an additional hallmark of prognosis by the UICC and numerous treatment guidelines.1,8 However, Bd assessment and scoring methods vary, and hitherto only Ulase et al. have conducted a study on Bd evaluation in patients with gastric cancer using the ITBCC 2016 standards.29 The present study also used this guideline to evaluate Bd in gastric cancer. To improve the survival of patients with gastric cancer, making more suitable decisions on the adjuvant treatment after surgery for gastric cancer is very important, especially in patients with early-stage disease. This necessitates the validation of treatment guidelines that are used in Vietnam for managing gastric cancer patients. Vietnam is a developing country, and most of this patient population cannot spend a lot of money on the expensive molecular tests; hereby, choosing tools of risk stratification that are suitable in terms of expenses and value in selecting the exact adjuvant treatment is crucial. The Bd categories may be a good candidate, and this classification has not yet been applied in Vietnam to identify risk groups. To the best of our knowledge, the present study is the first in Vietnam to use the 2016 ITBCC criteria for Bd classification and to assess the utility of Bd for predicting gastric cancer patient survival. The patients with Bd1 and Bd2 grades were combined into the low Bd group, and those with the Bd3 grade comprised the high Bd group. Therefore, the cutoff value for budding is 10 buds per standardized high-power field; low Bd grade was assigned to cases with <10 buds and high Bd grade to those with ≥ 10 buds. The present studyfindings showed that the 3 Bd grades (Bd1, Bd2, and Bd3) and 2 Bd groups (low and high Bd groups) have a prognostic significance for OS and DFS in univariate and multivariate analyses. Hence, making the exact decision on adjuvant treatment is convenient when Bd is classified in 2 main Bd groups.
Bd was assessed only on HE-stained slides, and immunohistochemical staining was not necessary. According to the recommendations of the ITBCC 2016, this method is informative, available, inexpensive, and readily applicable to routine practice and does not seem to demonstrate a different prognostic power from immunohistochemistry.1 Occasionally, tumors with small signet-ring–like cancer cells at the infiltrated margin were observed, leading to difficulties in Bd assessment. Because signet-ring malignant cells invade deeply into the gastric wall, they usually become smaller and release less secretion and start mimicking plasmocytes. In that case, observing the slide more carefully is necessary, and it may even be stained immunohistochemically for identifying Bd.
There are some limitations to the present study. Because of the small sample size and the relative heterogeneity of the subjects, evaluating the median survival of the Bd groups exactly according to pTNM staging was impossible. In future cohorts, the further investigation would increase the precision of these evaluations. Especially, assessing Bd in early-stage gastric cancer for calculating survival is crucial. Larger studies regarding Bd in early-stage stomach carcinoma should be designed to help stratify patients into the different Bd groups and to make correct decisions on adjuvant treatment.
Conclusions
The Bd grades of patients with gastric cancer exhibited distinct OS and DFS. These findings suggest that the 2016 ITBCC criteria can be used to stratify risk categories of gastric cancer in Vietnam, and Bd status should be mentioned in the histopathological reports of patients with stomach cancer, as tumor budding provides precious information for the treatment and prognosis of Vietnamese patients with gastric cancer. Bd may be a unique predictive hallmark, and the method of Bd assessment used in the present study is simple, reproducible, and inexpensive.
Acknowledgments
The authors want to say thank Prof. Tran Van Thuan, MD, PhD, who is the Vice-Minister of Vietnam MOH, Director of the National Cancer Hospital and National Cancer Institute, Vietnam; and we would like to thank the Cancer Research and Clinical Trials Center, NCH, for your assistance and support with this study. The authors would like to thank ENAGO for their assistance of language edit, too.
Authors’ Note: Tu Van Dao, MD, PhD, and Chu Van Nguyen, MD, PhD, equally major contributed to this work. The Scientific and Ethical Committee of Hanoi Medical University approved this study as number: 2866/QD-DHYHN. All of the patients provided written informed consent before enrolling them to the study. Participants could withdraw from the study at any time without any threats or disadvantages, and for no stated reasons. Regarding the Vietnamese condition, we should apply the ITBCC 2016 for identifying the Bd to stratify risk groups for gastric cancer. Thuan Van Tran is now affiliated with Ministry of Health, Vietnam.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Tu Van Dao, MD, PhD  https://orcid.org/0000-0001-6858-8347
https://orcid.org/0000-0001-6858-8347
Chu Van Nguyen, MD, PhD  https://orcid.org/0000-0001-8928-5089
https://orcid.org/0000-0001-8928-5089
Oanh Thi Bui, Pharm, MPH  https://orcid.org/0000-0002-0850-759X
https://orcid.org/0000-0002-0850-759X
Dung Khac Nguyen, Pharm, PhD  https://orcid.org/0000-0002-6916-6135
https://orcid.org/0000-0002-6916-6135
Thuan Van Tran, MD, PhD  https://orcid.org/0000-0002-5839-0895
https://orcid.org/0000-0002-5839-0895
References
- 1. Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the international tumor budding consensus conference (ITBCC) 2016. Modern Pathology. 2017;30(9):1299–1311. [DOI] [PubMed] [Google Scholar]
- 2. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour budding as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40(2):127–132. [DOI] [PubMed] [Google Scholar]
- 3. Lugli A, Vlajnic T, Giger O, et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol. 2011;42(12):1833–1840. [DOI] [PubMed] [Google Scholar]
- 4. Che K, Zhao Y, Qu X, et al. Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma. Onco Targets Ther. 2017;10:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanaka K, Shimura T, Kitajima T, et al. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br J Cancer. 2014;110(12):2923–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koelzer VH, Langer R, Zlobec I, Lugli A. Tumor budding in upper gastrointestinal carcinomas. Front Oncol. 2014;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karsa LV, Patnick J, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st ed 2010; European Commission. [DOI] [PubMed] [Google Scholar]
- 8. Schmoll HJ, Van Cutsem E, Stein AE, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479–2516. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17(1):1–29. [DOI] [PubMed] [Google Scholar]
- 10. van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50(1):1.e1-1.e34. [DOI] [PubMed] [Google Scholar]
- 11. Greene FL, Balch CM, Fleming ID, et al. AJCC Cancer Staging Manual. 7th ed: Springer-Verlag; 2010. [Google Scholar]
- 12. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumors of the Digestive System. 4th ed IARC; 2010. [Google Scholar]
- 13. Tomita S, Yamauchi M, Ichikawa K, Mitomi H, Fujimori T. The brand new trend of colorectal carcinoma pathology. Nihon Rinsho. 2014;72(1):63–70. [PubMed] [Google Scholar]
- 14. Choudhury KR, Yagle KJ, Swanson PE, Krohn KA, Rajendran JG. A robust automated measure of average antibody staining in immunohistochemistry images. J Histochem Cytochem. 2010;58(2):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoo CH NS, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. [DOI] [PubMed] [Google Scholar]
- 17. Gulluoglu M, Yegen G, Ozluk Y, et al. Tumor budding is independently predictive for lymph node involvement in early gastric cancer. Int J Surg Pathol. 2015;23(5):349–358. [DOI] [PubMed] [Google Scholar]
- 18. Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum. 1993;36(7):627–635. [DOI] [PubMed] [Google Scholar]
- 19. Karamitopoulou E, Zlobec I, Born D, et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer. 2013;49(5):2458–2459. [DOI] [PubMed] [Google Scholar]
- 20. Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH. Tumor budding and prognosis in gastric adenocarcinoma. Am. J Surg Pathol. 2019;43(2):229–234. [DOI] [PubMed] [Google Scholar]
- 21. Koike M, Kodera Y, Itoh Y, et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann Surg Oncol. 2008;15(7):1977–1982. [DOI] [PubMed] [Google Scholar]
- 22. Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z. The prognostic value of tumor budding in invasive breast cancer. Pathol Res Pract. 2013;209(5):269–275. [DOI] [PubMed] [Google Scholar]
- 23. Manjula BV, Augustine S, Selvam S, Mohan AM. Prognostic and predictive factors in gingivo buccal complex squamous cell carcinoma: role of tumor budding and pattern of invasion. Indian J Otolaryngol Head Neck Surg. 2015;67(suppl 1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masuda R, Kijima H, Imamura N, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep. 2012;6(5):937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Connor K, Li-Chang HH, Kalloger SE, et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2015;39(4):472–478. [DOI] [PubMed] [Google Scholar]
- 26. Olsen S Jin L, Fields RC, Yan Y, Nalbantoglu I. Tumor budding in intestinal-type gastric adenocarcinoma is associated with nodal metastasis and recurrence. Hum Pathol. 2017;68:26–33. [DOI] [PubMed] [Google Scholar]
- 27. Sarioglu S, Acara C, Akman FC, et al. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract. 2010;206(2):88–92. [DOI] [PubMed] [Google Scholar]
- 28. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127(2):385–394. [DOI] [PubMed] [Google Scholar]
- 29. Ulase D, Heckl S, Behrens HM, Krüger S, Röcken C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the international tumour budding consensus conference. Histopathology. 2020;76(3):433–446. [DOI] [PubMed] [Google Scholar]
- 30. Koelzer VH, Zlobec I, Berger MD, et al. Tumor budding in colorectal cancer revisited: results of a multicenter interobserver study. Virchows Archiv. 2015;466(5):485–493. [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi Y, Ishii G, Kojima M, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol. 2010;5(9):1361–1368. [DOI] [PubMed] [Google Scholar]
- 32. Zhao Y, Shen H, Qiu C, et al. Invasion types are associated with poor prognosis in lung squamous carcinoma patients. Medicine (Baltimore). 2015;94(43):e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang C, Huang H, Huang Z, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40(7):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. IMAI T. Growth patterns in human carcinoma. Their classification and relation to prognosis. Obstet Gynecol. 1960;16(3):296–308. [PubMed] [Google Scholar]
- 35. Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor budding: the name is EMT. Partial EMT. J Clin Med. 2016;5(5):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. [DOI] [PubMed] [Google Scholar]
- 37. Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget. 2010;1(7):651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Regulated genes in mesenchymal stem cells and gastric cancer. World J Stem Cells. 2015;7(1):208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. [DOI] [PubMed] [Google Scholar]
- 42. Bronsert P, Ammour KE, Bader M, et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol. 2014;234(3):410–422. [DOI] [PubMed] [Google Scholar]
- 43. Vieth M, Quirke P, Lambert R, von Karsa L, Risio M. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—annotations of colorectal lesions. Endoscopy. 2012;44(Suppl 3):SE131–S139. [DOI] [PubMed] [Google Scholar]
- 44. Gabbert HE, Meier S, Gerharz CD, Hommel G. Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer. 1992;50(2):202–207. [DOI] [PubMed] [Google Scholar]
- 45. Ito H, Inoue H, Ikeda H, et al. Clinicopathological characteristics and treatment strategies in early gastric cancer: a retrospective cohort study. J Exp Clin Cancer Res. 2011;30(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. [DOI] [PubMed] [Google Scholar]