Abstract
There are few studies on the cause of death in patients with stage I non-small cell lung cancer after surgery. Our aim is to study the trend of cause of death and risk factors affecting prognosis in the patients. We retrospectively reviewed patients in Surveillance, Epidemiology and End results database from 2004 to 2015. The change trend between cause of death and follow-up time was studied by calculating the proportion of cause of death at different periods and analyzing the cumulative risk. COX risk regression model was performed by univariate and multivariate analyses for survival analysis. Finally, 23,652 patients were enrolled. In the whole cohort, lung cancer accounted for 18.68% of deaths, followed by other causes (9.57%), heart disease (5.12%) and COPD (3.89%). With the increasing of follow-up time, the cumulative incidence of lung cancer was always the highest, but the growth rate in the late follow-up period was slower than that caused by heart disease and COPD. The proportion of death due to lung cancer decreased from 53.1%-73.1% in 0-30 months after follow-up to 7.8%-41.4% in 90 months after follow-up, while the proportion of deaths due to heart disease and COPD increased. Age was an independent risk factor for lung cancer-, heart disease- and COPD-specific survival, while lobectomy resection was a protective factor, even in patients older than 70 years old. In conclusion, during the follow-up period, lung cancer was still the main cause of death, but the proportion of patients died of heart disease and COPD increased gradually, especially in elderly. Furthermore, age was an important independent factor affecting prognosis, particularly for heart disease- and COPD-related mortality. The application of wedge resection in elderly patients needs further exploration.
Keywords: cause of death, NSCLC, lobectomy, elderly, SEER
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, accounting for 18.4% of all deaths in 2018.1 Non-small cell lung cancer (NSCLC) is the main pathological type of lung cancer.2 Lobectomy is the most important treatment for early operable patients with NSCLC, and has been widely accepted as the standard treatment since 1995.3 However, the 5-year survival rate of patients with stage I NSCLC after lobectomy is only 45%-65%.4 For early stage patients, the prognosis of lobectomy and wedge resection is still controversial,5,6 and a study comparing the prognosis of the 2 groups is in progress.7 Thus, it is very important to further improve the overall survival (OS) rate of patients with early stage NSCLC after surgery.
Understanding the cause of death (COD) in patients with early stage NSCLC after surgery is significant for developing individual follow-up strategies, designing clinical trials and implementing secondary prevention, and ultimately improving the OS rate in these patients. However, little research had been done on the COD in patients with stage I NSCLC after surgery. The previous studies found that the risk of dying from lung cancer continue to increase within 5 years after surgery in stage I-II NSCLC patients and the impact from non-cancer-specific diseases in patients who had undergone resection for stage I NSCLC increased with age.8,9 The limitations of previous studies were that their data came from a single center, the sample size was small, and the changing trend of the COD in patients of different ages was not well described. Research based on multi-center often has a larger sample size and better applicability and reliability. Therefore, it is necessary to study the COD in patients with stage I NSCLC after surgery through large population-based sample size.
We retrospectively studied the distribution of COD at different age groups in patients with stage I NSCLC after surgery in Surveillance, Epidemiology and End results (SEER) database, compared the prognosis between lobectomy resection and wedge resection in elder group and determined the prognostic factors affecting different COD. This study is critical for the customized individualized long-term surveillance for patients with stage I NSCLC after surgery and for the prevention of potential underlying diseases that may lead to death.
Materials & Methods
Selection of Patients
In this study, we obtained the permission to use the SEER database by signing the corresponding agreement on the official website of the SEER database (user name: 10067-Nov2018). The SEER database is one of the world’s largest public cancer databases, established by the National Cancer Institute of the United States, and accounts for about 30% of the U.S. population. The data in our study came from Incidence-SEER 18 Registries Custom Data (with additional treatment fields), released April 2019, based on the November 2018 submission (http://www.seer.cancer.gov). Patients with stage I based on 8th AJCC stage NSCLC after lobectomy (or wedge) resection from January 1, 2004 to December 31, 2015 were included in this study. In view of the controversial role of adjuvant chemotherapy in patients with early postoperative NSCLC and the adverse effects of adjuvant radiotherapy on prognosis,10-13 we selected patients who underwent lobectomy (or wedge) resection without radiotherapy and chemotherapy as subjects for the study. Patients with unknown marital status, race, degree of tumor differentiation and survival time were excluded, as were patients with previous history of malignant tumors and non-stage I NSCLC. The flow chart of patient selection was shown in Figure 1.
Figure 1.
Flow chart of stage I NSCLC patients after surgery diagnosed at 2004-2015 from SEER database.
Variable Classification
Age at diagnosis, gender, race, laterality, degree of tumor differentiation, pathological subtypes, marital status and cause of death were included in our work as variables. The age groups were divided into age <60 years, between 60–69 years and 70–79 years, and >79 years. Race was classified into whites, backs and others. Gender was divided into male and female. The laterality was divided into the left and right. Marital status was evaluated into 4 categories: married, single (never married), windowed and separated/divorced. According to the International Classification of Diseases for Oncology histology codes, pathological subtypes were divided into adenocarcinoma (8140, 8250–8253, 8255, 8260, 8323, 8480–8481, 8550, 8560, 8570 and 8574), squamous cell carcinoma (8070–8073 and 8083) and Others NSCLC.14 The degree of tumor differentiation was classified by adopting the standard SEER grade recode (I- well differentiated, II- moderately differentiated, III- poorly differentiated and IV- undifferentiated).15 The COD were classified into 4 categories: lung cancer, heart disease, COPD and other causes. The 4 periods after diagnosis were classified as 0-30 months, 31-60 months, 61-90 months and months over 90 after surgery.
Statistical Methods
The distribution of characteristics (age at diagnosis, gender, race, laterality, degree of tumor differentiation, pathological subtypes, marital status and cause of death) of stage I NSCLC after surgery was summarized by counting and percentage. The associations between age and the risk of each cause of death were evaluated with competing risks analysis. The distribution of COD and the death rates were assessed according to age groups and follow-up periods. For example, in the < 60-year-old group, the number of deaths from lung cancer during the 0-30 months after surgery was 288, while the total number of deaths during that period was 469, so lung cancer accounted for 61.4% of the deaths. A Cox proportional hazard regression was used to assess the association between various specific survival and potential prognostic factors. Statistical significance was considered at 2-sided P value < 0.05. All data were obtained using SEER*Stat Software version 8.3.5. Competing risks analysis was performed using R version 3.6.0 (http://www.r-project.org), the distribution of different COD according to the follow-up time of diagnosis was calculated by Microsoft Excel 2016 and other analyses were performed using SPSS Statistics 25 (IBM, NY).
Results
Patients Characteristics
Finally, a total of 23,652 patients with stage I NSCLC after surgery diagnosed from 2004 to 2015 were screened from the SEER database. Table 1 summarized the clinical characteristics of the patients. Most of the people in the cohort were 60-79 years old (68.87%), whites (84.6%) and married (57.75%). The proportion of male was less than that of female. Most of the tumors were on the right (60.11%). Adenocarcinoma was the most common pathological type (68.35%). The degree of tumor differentiation was mainly moderate differentiation (47.62%). Most of patients received lobectomy resection (83.02%), but the proportion of wedge resection increased with the age. Lung cancer (18.68%) was the leading cause of death, followed by other causes (9.57%), heart disease (5.12%) and COPD (3.89%).
Table 1.
Characteristics of Patients With Stage I NSCLC After Surgery Diagnosed at 2004-2015 in SEER Database.
| Age | |||||
|---|---|---|---|---|---|
| Variables | All patients | <60 | 60-69 | 70-79 | >79 |
| 23,652(100) | 4,625(19.55) | 8,042(34.00) | 8,247(34.87) | 2,738(11.58) | |
| Race | |||||
| White | 20,009(84.60) | 3,658(79.09) | 6,765(84.12) | 7,153(86.73) | 2,433(88.86) |
| Black | 1,867(7.89) | 575(12.43) | 701(8.72) | 483(5.86) | 108(3.94) |
| Others | 1,776(7.51) | 392(8.48) | 576(7.16) | 611(7.41) | 197(7.20) |
| Gender | 0 | ||||
| Male | 10,456(44.21) | 1,890(40.86) | 3,669(45.62) | 3,713(45.02) | 1,184(43.24) |
| Female | 13,196(55.79) | 2,735(59.14) | 4,373(54.38) | 4,534(54.98) | 1,554(56.76) |
| Laterality | 0 | ||||
| Left side | 9,434(39.89) | 1,757(37.99) | 3,238(40.26) | 3,329(40.37) | 1,110(40.54) |
| Right side | 14,218(60.11) | 2,868(62.01) | 4,804(59.74) | 4,918(59.63) | 1,628(59.46) |
| Tumor grade | 0 | ||||
| I | 5,099(21.56) | 1,104(23.87) | 1,688(20.99) | 1,709(20.72) | 598(21.84) |
| II | 11,280(47.69) | 2,134(46.14) | 3,843(47.79) | 4,009(48.61) | 1,294(47.26) |
| III | 6,907(29.20) | 1,316(28.45) | 2,386(29.67) | 2,405(29.16) | 800(29.22) |
| IV | 366(1.55) | 71(1.54) | 125(1.55) | 124(1.5) | 46(1.68) |
| Pathological subtypes | 0 | ||||
| Adenocarcinoma | 16,165(68.35) | 3,495(75.57) | 5,531(68.78) | 5,330(64.63) | 1,809(66.07) |
| Squamous cell carcinoma | 5,604(23.69) | 619(13.38) | 1,886(23.45) | 2,362(28.64) | 737(26.92) |
| Others NSCLC | 1,883(7.96) | 511(11.05) | 625(7.77) | 555(6.73) | 192(7.01) |
| Marital status | 0 | ||||
| Married | 13,658(57.75) | 2,668(57.69) | 4,863(60.47) | 4,799(58.19) | 1,328(48.50) |
| Single (never married) | 2,700(11.42) | 946(20.45) | 978(12.16) | 634(7.69) | 142(5.19) |
| Windowed | 4,050(17.12) | 170(3.68) | 914(11.37) | 1,902(23.06) | 1,064(38.86) |
| Separated/Divorced | 3,244(13.72) | 841(18.18) | 1,287(16.00) | 912(11.06) | 204(7.45) |
| Surgery | 0 | ||||
| Wedge resection | 4,015(16.98) | 577(12.48) | 1,173(14.59) | 1,526(18.50) | 739(26.99) |
| Lobectomy resection | 19,637(83.02) | 4,048(87.52) | 6,869(85.41) | 6,721(81.50) | 1,999(73.01) |
| Vital status | 0 | ||||
| Alive | 14,844(62.76) | 3,578(77.36) | 5,513(68.55) | 4,625(56.08) | 1,128(41.20) |
| Dead | 8,808(37.24) | 1,047(22.64) | 2,529(31.45) | 3,622(43.92) | 1,610(58.80) |
| Lung cancer | 4,418(18.68) | 619(13.38) | 1,364(16.96) | 1,758(21.32) | 677(24.73) |
| Heart disease | 1,210(5.12) | 108(2.34) | 303(3.77) | 530(6.43) | 269(9.82) |
| COPD | 916(3.89) | 80(1.73) | 271(3.37) | 395(4.79) | 170(6.21) |
| Other causes | 2,264(9.57) | 240(5.19) | 591(7.35) | 939(11.39) | 494(18.04) |
Cumulative Incidence of Death in Patients
Figure 2 shown the cumulative incidence of COD in patients with stage I NSCLC after surgery. In most age groups, the cumulative incidence of death (CID) of lung cancer increased significantly in the early follow-up period, but then slowed down after about 90 months after surgery. The CID of heart disease was similar to that of COPD death in patients aged younger than 60 years old with lobectomy or wedge resection, patients aged between 60 and 69 with lobectomy resection and patients aged over 79 years old with wedge resection. The CID of heart disease was higher than that of COPD death in patients aged over 70 years old with lobectomy resection, while in patients aged between 60 and 79 with wedge resection, it was the opposite.
Figure 2.
Cumulative incidence of causes of death in patients with stage I NSCLC after surgery. Patients with wedge resection aged <60 years old (a), aged 60-69 years old (c), aged 70-79 years old (e), aged >79 years old (g); Patients with lobectomy resection aged <60 years old (b), aged 60-69 years old (d), aged 70-79 years old (f), aged >79 years old (h).
The detailed cumulative incidence of different causes of death at 30, 60, 90, and 120 months after surgery was presented in Table 2. In patients with wedge resection, the CID of lung cancer doubled from 30 months to 90 months after follow-up, but the maximum growth rate was only 11.9% (age 60-69 years old) between 90 months and 120 months. However, the CID of heart disease and COPD not only more than doubled from 30 months to 90 months after follow-up, but also increased by 21.5% (age >79 years old) and 44.4% (age <60 years old) respectively from 90 months to 120 months in patients with wedge resection. In patients with lobectomy resection, the CID of lung cancer at 90 months after followed up in different age was 2.6, 2.5, 2.1 and 1.9 times higher than that at 30 months after followed up, and the corresponding values were 3, 2.4, 2.7 and 2.9 in CID of heart disease and 4.3, 4.1, 3.7 and 3.1 in CID of COPD, respectively. Similarly, in patients with lobectomy resection, the maximum growth rate in CID of lung cancer was only 18.4% (age 60-69 years old) between 90 months and 120 months, while the corresponding value was 44.1% (age >79 years old) in CID of heart disease and 52.9% (age <60 years old).
Table 2.
Cumulative Incidence of Different Causes of Death at 30, 60, 90, and 120 Months After Surgery.
| Variables | Wedge resection | Lobectomy resection | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 | |
| <60 | ||||||||
| Lung cancer | 11.0 | 17.5 | 22.5 | 24.7 | 6.2 | 12.4 | 16.4 | 18.8 |
| Heart disease | 2.2 | 3.9 | 6.3 | 6.9 | 0.9 | 1.6 | 2.7 | 3.2 |
| COPD | 1.8 | 2.9 | 3.6 | 5.2 | 0.4 | 1.1 | 1.7 | 2.6 |
| Other disease | 3.6 | 5.0 | 7.3 | 7.3 | 2.3 | 4.3 | 6.3 | 8.2 |
| 60-69 | ||||||||
| Lung cancer | 12.7 | 21.3 | 26.1 | 29.2 | 8.4 | 15.8 | 20.6 | 24.4 |
| Heart disease | 2.5 | 5.1 | 6.1 | 6.7 | 1.8 | 3.0 | 4.4 | 5.8 |
| COPD | 2.5 | 4.6 | 8.7 | 11.9 | 0.9 | 2.1 | 3.7 | 5.0 |
| Other disease | 4.7 | 8.8 | 10.7 | 13.4 | 3.1 | 6.2 | 8.6 | 11.1 |
| 70-79 | ||||||||
| Lung cancer | 14.2 | 24.2 | 31.0 | 33.2 | 12.2 | 20.0 | 25.5 | 28.5 |
| Heart disease | 3.5 | 5.6 | 8.5 | 9.4 | 2.8 | 5.1 | 7.5 | 10.5 |
| COPD | 2.9 | 5.6 | 11.4 | 14.9 | 1.3 | 3.0 | 4.8 | 7.2 |
| Other disease | 5.5 | 12.2 | 15.6 | 18.8 | 5.1 | 9.0 | 13.3 | 17.5 |
| >79 | ||||||||
| Lung cancer | 20.5 | 30.2 | 35.2 | 38.2 | 14.7 | 22.7 | 27.5 | 29.2 |
| Heart disease | 3.4 | 9.1 | 12.1 | 14.7 | 3.8 | 7.8 | 11.1 | 16.0 |
| COPD | 4.0 | 7.7 | 10.0 | 13.1 | 2.1 | 4.3 | 6.6 | 8.3 |
| Other disease | 6.4 | 14.8 | 21.9 | 24.0 | 7.1 | 14.3 | 21.0 | 28.5 |
Trends Between COD in Different Periods and Follow-Up Time After Surgery
In patients with wedge resection, the proportion of death due to lung cancer continued to decline during the follow-up time in all age groups. The proportion of death due to heart disease generally increased in the early follow-up period, although there were some differences in different age groups, then the proportion declined from period of 61-90 months to over 90 months in most patients (excluding age old than 79 years old). The proportion of death due to COPD continued to increase during the follow-up time in all age groups, and even in patients younger than 60 years old, it reached to 50%. The detailed change trend of different COD was presented in Figure 3.
Figure 3.
Proportion of causes of death at different periods in patients with stage I NSCLC after wedge resection. a, Patients aged <60 years old; b, Patients aged 60-69 years old; c, Patients aged 70-79 years old; d, Patients aged >79 years old.
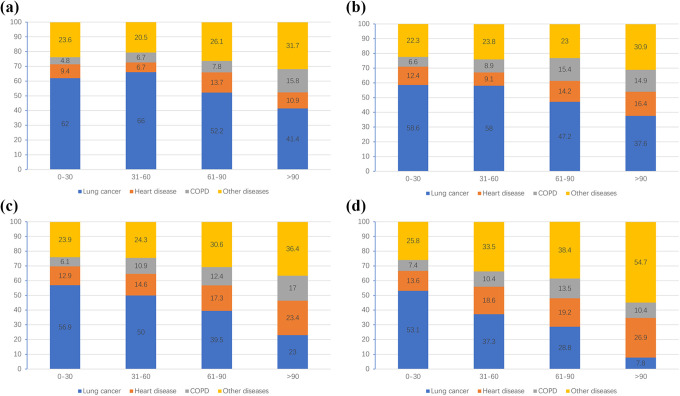
As shown in Figure 4, in patients with lobectomy resection, the proportion of death due to other causes continued to increase during the follow-up time in all age groups, and even reached 54.7% in patients aged over 79 years old after 90 months of followed up. In contrast, the proportion of death due to lung cancer generally decreased during the follow-up time in all age groups, and even in patients aged over 79 years old after 90 months of followed up, it was only 7.8%. The proportion of death due to heart disease risen steadily during the follow-up time in patients age over 70 years old. The proportion of death due to COPD increased in patients aged younger than 79 years old during the follow-up time, and the proportion diseased from period of 61-90 months (13.5%) to over 90 months (10.4%) in patients older than 79 years old.
Figure 4.
Proportion of causes of death at different periods in patients with stage I NSCLC after lobectomy resection. a, Patients aged <60 years old; b, Patients aged 60-69 years old; c, Patients aged 70-79 years old; d, Patients aged >79 years old.
Survival Analysis
According to the results of univariate analysis, we conducted multivariate analysis and found that age, gender, degree of tumor differentiation and marital status were independent factors affecting lung cancer-specific survival (Table 3). In details, patients undergoing lobectomy resection (HR:0.72 (0.67-0.78), p < 0.001) and female had a better lung cancer-specific survival than male (HR:0.74 (0.69-0.78), p < 0.001), and a worse prognosis was found in older patients (>79 years old: HR:2.26 (2.02-2.54), p < 0.001), low differentiated tumor (IV: HR:2.64 (2.09-3.33), p < 0.001) and single (HR:1.12 (1.02 -1.24), p = 0.025), windowed (HR:1.19 (1.09 -1.29), p < 0.001) or separated/divorced (HR:1.20 (1.09 -1.31), p < 0.001).
Table 3.
Multivariable COX Analysis* of Factors Associated With Death From Different Cause of Death in Whole Cohort.
| Variables | Lung cancer | Heart disease | COPD | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age | <0.001 | <0.001 | <0.001 | |||
| <60 | Ref. | Ref. | Ref. | |||
| 60-69 | 1.31(1.93 -1.45) | <0.001 | 1.62(1.30-2.03) | <0.001 | 1.90(1.48-2.44) | <0.001 |
| 70-79 | 1.81(1.65 -1.99) | <0.001 | 3.13(2.53-3.87) | <0.001 | 2.87(2.24-3.68) | <0.001 |
| >79 | 2.26(2.02-2.54) | <0.001 | 5.28(4.18-6.68) | <0.001 | 3.92(2.96-5.18) | <0.001 |
| Surgery | <0.001 | <0.001 | <0.001 | |||
| Wedge resection | Ref. | Ref. | Ref. | |||
| Lobectomy resection | 0.72(0.67-0.78) | <0.001 | 0.78(0.67-0.90) | <0.001 | 0.42(0.37-0.49) | <0.001 |
| Race | 0.086 | <0.001 | <0.001 | |||
| White | Ref. | Ref. | Ref. | |||
| Black | 1.04(0.92 -1.16) | 0.556 | 1.09 (0.88 -1.36) | 0.442 | 0.66(0.49-0.90) | 0.008 |
| Others | 0.88(0.77-0.99) | 0.037 | 0.45(0.33-0.62) | <0.001 | 0.36(0.59-0.78) | <0.001 |
| Gender | <0.001 | <0.001 | <0.001 | |||
| Male | Ref. | Ref. | Ref. | |||
| Female | 0.74(0.69-0.78) | <0.001 | 0.50(0.44-0.56) | <0.001 | 0.68(0.59-0.78) | <0.001 |
| Tumor grade | <0.001 | <0.001 | <0.001 | |||
| I | Ref. | Ref. | Ref. | |||
| II | 2.19(1.98-2.42) | <0.001 | 1.25(1.06 -1.48) | 0.009 | 1.98(1.58-2.49) | <0.001 |
| III | 2.90(2.61-3.22) | <0.001 | 1.43(1.19 -1.72) | <0.001 | 2.51(1.98-3.18) | <0.001 |
| IV | 2.64(2.09-3.33) | <0.001 | 2.36(1.60-3.48) | <0.001 | 2.51(1.53-4.13) | <0.001 |
| Pathological subtypes | 0.077 | <0.001 | <0.001 | |||
| Adenocarcinoma | Ref. | Ref. | Ref. | |||
| Squamous cell carcinoma | 1.04(0.97 -1.12) | 0.276 | 1.51(1.32 -1.71) | <0.001 | 1.80(1.56-2.08) | <0.001 |
| Others NSCLC | 1.13(1.01 -1.26) | 0.029 | 0.96(0.76 -1.22) | 0.753 | 1.17(0.91 -1.51) | 0.213 |
| Marital status | <0.001 | <0.001 | <0.001 | |||
| Married | Ref. | Ref. | Ref. | |||
| Single (never married) | 1.12(1.02 -1.24) | 0.025 | 1.17(0.96 -1.44) | 0.128 | 1.28(1.02 -1.62) | 0.036 |
| Windowed | 1.19(1.09 -1.29) | <0.001 | 1.49(1.28 -1.74) | <0.001 | 1.69(1.42-2.01) | <0.001 |
| Separated/Divorced | 1.20(1.09 -1.31) | <0.001 | 1.48(1.25 -1.76) | <0.001 | 1.60(1.32 -1.95) | <0.001 |
*: based on all variables excluding laterality.
We also performed univariate and multivariate analyses of heart disease- and COPD-specific survival. The detailed results were shown in Table 3. Age, surgery, race, gender, degree of tumor differentiation, pathological subtypes and marital status were independent factors affecting cardiac specific survival. Advanced age (>79 years old: HR:5.28 (4.18-6.68), p < 0.001), low differentiated tumors (IV: HR:2.36 (1.60-3.48), p < 0.001), squamous cell carcinoma (HR:1.51 (1.32 -1.71), p < 0.001) and windowed (HR:1.49 (1.28 -1.74), p < 0.001) or separated/divorced (HR:1.48 (1.25 -1.76), p < 0.001) were independent risk factors affecting cardiac specific survival, while patients undergoing lobectomy resection (HR:0.78 (0.67-0.90), p < 0.001) and female(HR: 0.50 (0.44-0.56), p < 0.001) was independent protective factors affecting cardiac specific survival. Similarly, age, surgery, race, gender, degree of tumor differentiation, pathological subtypes and marital status were also independent factors affecting COPD-specific survival, in which lobectomy resection (HR0.42 (0.37-0.49), p < 0.001), blacks (HR:0.66 (0.49-0.90), p = 0.008) and female (HR:0.68 (0.59-0.78), p < 0.001) were independent protective factors for COPD-specific survival, while advanced age (>79 years old: HR:3.92 (2.96-5.18), p < 0.001), low differentiated tumors (IV: HR:2.51 (1.53-4.13), p < 0.001), squamous cell carcinoma (HR:1.80 (1.56-2.08), p < 0.001) and single (HR:1.28(1.02 -1.62), p = 0.036), windowed (HR:1.69 (1.42-2.01), p < 0.001) or separated/divorced (HR:1.60 (1.32 -1.95), p < 0.001) were independent risk factors for COPD-specific survival.
In addition, we further compared the effects of different surgical methods on the prognosis of elderly patients (Table 4). The multivariate analyses revealed age was still an important factor affecting prognosis of different COD-specific survival. Compared with wedge resection, patients with lobectomy resection had a better lung cancer-specific survival (HR:0.74 (0.68-0.82), p < 0.001) and COPD-specific survival (HR:0.45 (0.38-0.54), p < 0.001), while the mode of operation was not an independent factor affecting cardiac specific survival (p = 0.065).
Table 4.
Multivariable COX Analysis of Factors Associated With Death From Different Cause of Death in Elderly Cohort.
| Variables | Lung cancer* | Heart disease** | COPD*** | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age | <0.001 | <0.001 | 0.001 | |||
| 70-79 | ||||||
| >79 | 1.28(1.17 -1.40) | <0.001 | 1.81(1.56-2.09) | <0.001 | 1.38(1.15 -1.66) | 0.001 |
| Surgery | <0.001 | 0.065 | <0.001 | |||
| Wedge resection | Ref. | Ref. | Ref. | |||
| Lobectomy resection | 0.75(0.68-0.82) | <0.001 | 0.85(0.72 -1.01) | 0.065 | 0.45(0.38-0.54) | <0.001 |
| Race | 0.001 | 0.001 | ||||
| White | Ref. | Ref. | ||||
| Black | 0.89(0.63 -1.24) | 0.61 | 0.61(0.39-0.95) | 0.030 | ||
| Others | 0.50(0.35-0.72) | <0.001 | 0.46(0.29-0.74) | 0.001 | ||
| Gender | <0.001 | <0.001 | <0.001 | |||
| Male | Ref. | Ref. | Ref. | |||
| Female | 0.75(0.69-0.81) | <0.001 | 0.54(0.46-0.62) | <0.001 | 0.63(0.53-0.76) | <0.001 |
| Tumor grade | <0.001 | 0.004 | <0.001 | |||
| I | Ref. | Ref. | Ref. | |||
| II | 2.05(1.80-2.35) | <0.001 | 1.19(0.97 -1.46) | 0.092 | 1.66(1.27-2.19) | <0.001 |
| III | 2.72(2.36-3.14) | <0.001 | 1.40(1.12 -1.75) | 0.003 | 2.08(1.56-2.78) | <0.001 |
| IV | 2.85(2.12-3.85) | <0.001 | 2.08(1.27-3.40) | 0.004 | 2.09(1.12-3.93) | 0.022 |
| Pathological subtypes | 0.003 | <0.001 | <0.001 | |||
| Adenocarcinoma | Ref. | Ref. | Ref. | |||
| Squamous cell carcinoma | 1.09(1.00 -1.20) | 0.061 | 1.60(1.37 -1.88) | <0.001 | 1.76(1.46-2.12) | <0.001 |
| Others NSCLC | 1.28(1.10 -1.49) | 0.001 | 1.14(0.85 -1.53) | 0.369 | 1.27(0.91 -1.77) | 0.156 |
| Marital status | <0.001 | |||||
| Married | Ref. | |||||
| Single (never married) | 1.21(0.86 -1.71) | 0.280 | ||||
| Windowed | 1.64(1.34-2.01) | <0.001 | ||||
| Separated/Divorced | 1.47(1.11 -1.94) | 0.008 | ||||
*: based on variable including gender, grade, age, surgery and pathological subtypes.
**: based on variable including gender, race, grade, age, surgery and pathological subtypes.
***: based on variable including gender, race, grade, age, surgery, pathological subtypes and marital status.
Discussion
Based on the analysis of data from patients with stage I NSCLC after surgery diagnosed from 2004 to 2015 in the SEER database, we highlighted 3 important findings. First, lung cancer remained the leading cause of death, while the contribution of heart disease, COPD and other COD to death gradually increased after longer follow-up. Second, patients in different age groups had different composition of COD during different periods of follow-up, such as patients aged 70-79 years old with wedge resection should pay more attention to COPD than heart disease after 61 months of follow-up, while patients over 79 years old with lobectomy resection should pay more attention to heart disease than COPD during the period of follow-up time. Third, multivariate analysis results showed age was a very important factor, especially for COPD-specific survival and heart disease-specific survival, and lobectomy resection might be more appropriate for elderly patients.
As far as we know, this was the first analysis that a multi-center data to study the COD in long-term survivors with stage I NSCLC after surgery. Bugge et al8 studied the cause of death of their 756 patients with stage I/II NSCLC who underwent surgical resection and found that the risk of having died of lung cancer was 36.1% at the end of the follow-up period (9.3 years), which was close to 38% as we reported. Another study published9 pointed out that the 5-year lung cancer-specific CID in patients with stage I NSCLC after lobectomy was 9.3% (95%CI = 7.7%-10.8%), but it exceeded 20% in our study. This difference may be due to the variance in clinical characteristics of the cohorts, especially gender and pathological subtypes. In our cohort, difference in the ratio of male to female was small, while in their cohort it was 2:3. In addition, the proportion of adenocarcinomas in their cohort was as high as 80%, while in our cohort it was 68%. Besides lung cancer, noncancer-specific COD, including heart disease, COPD and other COD, are also important competing factors affecting the OS rate of patients with stage I NSCLC after surgery. One previous study showed that if a patient survived to 7 years after lobectomy, the conditional probability of dying from cancer was equal to the conditional probability of dying from cardiovascular disease and the equilibrium became earlier with increasing age.16 This phenomenon was not observed in our study. This may be explained by the fact that their subjects were early stage (including stage I and II) NSCLC patients who underwent lobectomy, bilobectomy, wedge resection and pneumonectomy, while we screened patients with stage I NSCLC who underwent lobectomy or wedge resection. In Eguchi et al’s study, for patients with stage I NSCLC after resection (including lobectomy and sublobar), the non-cancer specific CID was higher than that of lung cancer-specificity within 1.5 years after surgery, but after 1.5 years, the lung cancer-specific CID exceeded that of noncancer-specificity.9 Similarly, Bugge et al reported that at day 129 after resection, lung cancer surpassed all other COD.8 Although the results of these 2 studies were somewhat different, both of them pointed out that noncancer-specific COD had an increasing impact on mortality during the follow-up period, which was consistent with our findings.
The proportion of COD in NSCLC patients during different periods of long-term follow-up was not constant.17,18 As Janssen-Heijnen et al reported, in 3 groups of I-II NSCLC patients of different ages, the proportion of death due to lung cancer continued to decline at different periods after diagnosis (such as age 45-59 years old: 83% <1 year, 46% 4-6 years, 41% 12-16 years), while other COD, such as COPD (such as age 45-59 years old: 1% <1 year, 3% 4-6 years, 11% 12-16 years) and cardiovascular diseases (such as age 45-59 years old: 4% <1 year, 8% 4-6 years, 18% 12-16 years) continued to rise. This was the same trend that we found in patients with stage I NSCLC after surgery. In the early stage of follow-up, lung cancer was the main cause of death, but after 60 months of follow-up, the percentage of lung cancer in the cause of death decreased, and the degree of decline was more significant in elderly patients. In addition, what deserves the attention of clinicians and researchers is that after 90 months of follow-up, patients under 79 years old with wedge resection were more likely to die from COPD (age <60 years old: 50%; age 60-69 years old: 35.1%; age 70-79 years old: 35.4%) than from heart disease (age <60 years old: 12.5%; age 60-69 years old: 5.4%; age 70-79 years old: 10.4%), whereas in patients over 70 years old with lobectomy resection, the risk of death from heart disease (age 70-79 years old: 23.4%; age >79 years old: 26.9%) was higher than that of COPD (age 70-79 years old: 17%; age >79 years old: 10.4%). This may be due to the difference in the incidence of COPD and heart disease at diagnosis. The proportion of COPD in NSCLC patients under 70 years old is higher than that in patients with heart disease, while the proportion of heart disease is higher in patients over 70 years old.19 In addition, studies have shown that comorbidity has significant impact on the prognosis of patients with stage I NSCLC after surgery, and the prognosis of patients with comorbidity is worse.20-22 Therefore, besides paying attention to the influence of comorbidity itself on prognosis, the age and follow-up time of the patients are also closely related to the impact of the disease on the prognosis.
To further improve the prognosis of patients with stage I NSCLC after surgery, we conducted a multivariate analysis of different COD. Among the variables we enrolled, age, surgery, gender, degree of tumor differentiation and marital status were independent factors affecting specific COD. Our findings were consistent with previous studies.8,23-25 It is worth pointing out that the risk of death from heart disease and COPD in elderly patients is significantly higher than that in young patients. As Abdel-Rahman et al17 reported, HR of patients over 70 years old died of heart disease and lung disease were 33.367 and 16.890 respectively in long-term follow-up patients with NSCLC compared with those under 40 years old. This is also consistent with the significant upward trend in the risk of non-lung cancer specific COD as we show in this study. Patients with squamous cell carcinoma have a higher risk of death from heart disease and COPD than adenocarcinoma. As previously reported, smoking may be more closely related to squamous cell cancer than adenocarcinoma, and smoking has been proven to be an important risk factor for heart disease and COPD.26-28 It is worth noting that race has different effects on the 3 COD in this study. Compared with whites, blacks with stage I NSCLC after surgery had a lower risk of death from COPD. This phenomenon may be related to the incidence of the disease, studies have shown that among lung cancer patients undergoing surgery, blacks have a lower prevalence of respiratory diseases.29 In this study, we also compared the prognosis of patients with lobectomy or wedge resection in whole cohort and elderly group. We found that with the increase of age, the proportion of patients who received wedge resection increased gradually, but patients with lobectomy resection had a better survival, even in elderly group. Lobectomy resection could improve the specific survival rate of different causes of death, especially for COPD-specific survival, which was constant with previous studies.30 Recent study revealed that there was no significant difference in survival between IA1 and IA2 based 8th AJCC stage patients with lobectomy and wedge resection.31 However, in theory, wedge resection was less invasive surgical procedure than lobectomy, which might have benefit of preserving lung function and preventing cardiovascular complications. Previous studies reported lobectomy destroyed more pulmonary vascular beds than wedge resection, which had an impact on hemodynamics and eventually led to greater pressure on the cardiovascular system, as well as to respiratory system.32 Past systematic review suggested that wedge resection was especially suitable for elderly patients and patients with high comorbidity index or reduced respiratory functional reserve.33 In our opinion, the prognosis of patients with wedge resection was often poor (including different cause of death) might because of their poor physical condition, such as elderly, poor lung function. In patients undergoing lobectomy, their overall condition was often better and the resection range was wider, which might be the reason for better overall survival rate and fewer complications after surgery, even if more pulmonary vascular beds were damaged. Previous studies comparing the prognosis of lobectomy and wedge resection were mostly based on retrospective studies. Prospective studies may provide more accurate answers. In addition, it is more important to select the people who could benefit from wedge resection among patients with early stage NSCLC. The role of wedge resection in patients with early stage operable NSCLC needs to be further explored.
This study has several limitations. First, we screened patients in the SEER database whose radiotherapy records were “refused, no / unknown” and chemotherapy records were “no / unknown,” because we did not know the proportion of patients with unknown treatment, this could have an impact on our results. Second, other risk factors, such as smoking, drinking and co-morbidity, are not available from the database, so more detailed data are needed to improve our research. Finally, our study may only apply to patients in the United States.
Conclusions
In conclusion, we studied the COD of long-term survivors of stage I NSCLC after surgery in SEER database, and found that lung cancer was the predominant cause of death, but the COD caused by respiratory diseases and cardiovascular diseases could not be ignored, especially in elderly patients. Furthermore, age is a key factor affecting cardiac-specific mortality and COPD-specific mortality. The indication of wedge resection in patients with early stage NSCLC needs to be further clarified. Therefore, it is still important to strengthen the management of related diseases in elderly patients.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Footnotes
Author Contribution: Wan-da Peng and Jun Xie are authors contributed equally to this work and are co-first authors
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Wan-da Peng  https://orcid.org/0000-0001-9877-6226
https://orcid.org/0000-0001-9877-6226
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11(10):1653–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–622; discussion 622-613. [DOI] [PubMed] [Google Scholar]
- 4. Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110(7):1532–1541. [DOI] [PubMed] [Google Scholar]
- 5. Taioli E, Yip R, Olkin I, et al. Survival after Sublobar resection for early-stage lung cancer: methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol. 2016;11(3):400–406. [DOI] [PubMed] [Google Scholar]
- 6. Cao C, Chandrakumar D, Gupta S, Yan TD, Tian DH. Could less be more? A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89(2):121–132. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka K, Tsutani Y, Wakabayashi M, et al. Sublobar resection versus lobectomy for patients with resectable stage I non-small cell lung cancer with idiopathic pulmonary fibrosis: a phase III study evaluating survival (JCOG1708, SURPRISE). Jpn J Clin Oncol. 2020;50(9):1076–1079. [DOI] [PubMed] [Google Scholar]
- 8. Bugge AS, Lund MB, Valberg M, Brustugun OT, Solberg S, Kongerud J. Cause-specific death after surgical resection for early-stage non-small-cell lung cancer. Eur J Cardiothorac Surg. 2018;53(1):221–227. [DOI] [PubMed] [Google Scholar]
- 9. Eguchi T, Bains S, Lee MC, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35(3):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998;352(9124):257–263. [PubMed] [Google Scholar]
- 11. Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev. 2015;(3):CD011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burdett S, Rydzewska L, Tierney J, et al. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2016;9(9):CD002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, Wozniak AJ. Risk of second lung cancer in patients with previously treated lung cancer: analysis of surveillance, epidemiology, and end results (SEER) data. J Thorac Oncol. 2018;13(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milano AF. 20-Year comparative survival and mortality of cancer of the stomach by age, sex, race, stage, grade, cohort entry time-period, disease duration & selected ICD-O-3 oncologic phenotypes: a systematic review of 157,258 cases for diagnosis years 1973-2014: (SEER*Stat 8.3.4). J Insur Med. 2019;48(1):5–23. [DOI] [PubMed] [Google Scholar]
- 16. Groth SS, Rueth NM, Hodges JS, et al. Conditional cancer-specific versus cardiovascular-specific survival after lobectomy for stage I non-small cell lung cancer. Ann Thorac Surg. 2010;90(2):375–382. [DOI] [PubMed] [Google Scholar]
- 17. Abdel-Rahman O. Causes of death in long-term lung cancer survivors: a SEER database analysis. Curr Med Res Opin. 2017;33(7):1343–1348. [DOI] [PubMed] [Google Scholar]
- 18. Janssen-Heijnen ML, van Erning FN, De Ruysscher DK, Coebergh JW, Groen HJ. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol. 2015;26(5):902–907. [DOI] [PubMed] [Google Scholar]
- 19. Janssen-Heijnen ML, Smulders S, Lemmens VE, Smeenk FW, van Geffen HJ, Coebergh JW. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax. 2004;59(7):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ambrogi V, Pompeo E, Elia S, Pistolese GR, Mineo TC. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur J Cardiothorac Surg. 2003;23(5):811–817. [DOI] [PubMed] [Google Scholar]
- 21. Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123(2):280–287. [DOI] [PubMed] [Google Scholar]
- 22. Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005;26(3):480–486. [DOI] [PubMed] [Google Scholar]
- 23. Nelson DB, Cata JP, Niu J, et al. Persistent opioid use is associated with worse survival after lobectomy for stage I non-small cell lung cancer. Pain. 2019;160(10):2365–2373. [DOI] [PubMed] [Google Scholar]
- 24. Ost D, Goldberg J, Rolnitzky L, Rom WN. Survival after surgery in stage IA and IB non-small cell lung cancer. Am J Respir Crit Care Med. 2008;177(5):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shoji F, Haratake N, Akamine T, et al. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37(2):741–747. [DOI] [PubMed] [Google Scholar]
- 26. U. S. Department of Health and Human Services, Zimmerhoff J. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Create Space Independent Publishing Platform; 2014. [Google Scholar]
- 27. Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. 2004;59(8):679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. [DOI] [PubMed] [Google Scholar]
- 29. Williams CD, Stechuchak KM, Zullig LL, Provenzale D, Kelley MJ. Influence of comorbidity on racial differences in receipt of surgery among US veterans with early-stage non-small-cell lung cancer. J Clin Oncol. 2013;31(4):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao J, Yuan P, Wang Y, et al. Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg. 2018;105(5):1483–1491. [DOI] [PubMed] [Google Scholar]
- 31. Moon Y, Park JK, Lee KY, Kim ES. Prognosis after wedge resection in patients with 8(th) edition TNM stage IA1 and IA2 non-small cell lung cancer. J Thorac Dis. 2019;11(6):2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seok Y, Cho S, Lee JY, Yang HC, Kim K, Jheon S. The effect of postoperative change in bronchial angle on postoperative pulmonary function after upper lobectomy in lung cancer patients. Interact Cardiovasc Thorac Surg. 2014;18(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Divisi D, De Vico A, Zaccagna G, Crisci R. Lobectomy versus sublobar resection in patients with non-small cell lung cancer: a systematic review. J Thorac Dis. 2020;12(6):3357–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]