Abstract
Colorectal cancer is the third most common cancer in men and the second most common in women worldwide, and the incidence is increasing among younger patients. 30% of these malignancies arise in the rectum. Patients with rectal cancer have historically been managed with preoperative radiation, followed by radical surgery, and adjuvant chemotherapy, with permanent colostomies in up to 20% of patients. Beginning in the early 2000s, non-operative management (NOM) of rectal cancer emerged as a viable alternative to radical surgery in select patients. Efforts have been ongoing to optimize neoadjuvant therapy for rectal cancer, thereby increasing the number of patients potentially eligible to forgo radical surgery. Magnetic resonance guided radiotherapy (MRgRT) has recently emerged as a treatment modality capable of intensifying preoperative radiation therapy for rectal cancer patients. This technology may also predict which patients will achieve a complete response to preoperative therapy, thereby allowing for more appropriate selection of patients for NOM. The present work seeks to illustrate the potential role MRgRT could play in personalizing rectal cancer treatment thus expanding the role of NOM in rectal cancer.
Keywords: rectal cancer, radiation therapy, quality of life, chemoradiation, adaptive radiation therapy
Introduction
Colorectal cancer is the third most common cancer in men and the second most common in women worldwide. 30% of these malignancies arise in the rectum and the incidence of rectal cancer is increasing among patients under the age of 50. The majority of patients with rectal cancer present with locoregional disease.1 Since the mid-2000s, management of patients with locally advanced rectal cancer has been preoperative radiation therapy (with or without chemotherapy), followed by radical surgery with total mesorectal excision, and post-operative systemic chemotherapy.2,3 This approach has resulted in excellent survival outcomes for most patients, at the expense of significant impairment in quality of life, primarily due to the consequences of rectal resection.2-4
Despite advances in surgical technique and perioperative care, surgery for rectal cancer is associated with significant morbidity, often permanently impairing quality of life.5,6 While the role of preoperative radiation therapy has historically been to improve rates of local control following surgery, 15-50% of patients are able to achieve a pathological complete response (pCR) to neoadjuvant therapy.7-10 Patients who achieve a pCR have been shown to have lower rates of local disease recurrence and improved disease-free and overall survival as compared to patients with residual disease in the surgical specimen.11-14 These favorable outcomes among patients achieving a pCR has led investigators to question the need for radical surgery in order to improve patients’ quality of life, primarily through rectal preservation.
Efforts have been ongoing to optimize neoadjuvant treatment approaches in order to increase response rates to preoperative therapy, thereby allowing more patients to pursue non-operative management (NOM). Researchers have investigated strategies to intensify both systemic and local preoperative therapies. Magnetic resonance guided radiotherapy (MRgRT) has recently emerged as a radiation treatment modality which may allow for more optimal delivery of preoperative radiation therapy for rectal cancer patients than what is currently achievable with computed-tomography based radiation techniques. The present work seeks to review the current literature regarding the intensification of preoperative therapies and to highlight the potential role MRgRT may play in the future. Taken together, these improvements in neoadjuvant therapy for rectal cancer may allow for a more individualized approach to treatment in which select patients can safely avoid the morbidity associated with radical surgery.
Rationale for NOM
While definitive surgery for rectal cancer is the standard of care, it is associated with significant morbidity. Studies have shown that surgical technique has a significant impact on patient-reported quality of life.15-18 Specifically, patients requiring low rectal anastomoses or a permanent stoma report the worst outcomes, indicating that surgery is the primary cause of rectal cancer morbidity in many patients.19,20 Unfortunately, a permanent stoma is required in up to 20% of patients and a significant number of temporary stomas are not reversed.21-24 These patients often experience delayed perineal wound healing and suffer numerous stoma related complications in the long-term, including leakage, skin toxicity, and parastomal hernia, in addition to the psychological stress of having a permanent colostomy.20 These long-term consequences of surgery are especially relevant considering that there is an increasing number of younger patients who are diagnosed with rectal cancer, many who will be cured.25
With the goal of reducing treatment-related morbidity and improving long-term quality of life, NOM has emerged as a promising treatment option for select patients who may potentially be spared the “over-treatment” of rectal resection, particularly when a stoma is required. NOM was initially proposed by Habr-Gama et al in 2004 as a viable alternative to radical surgery in patients who achieved a complete clinical response to neoadjuvant therapy.26 The authors reported that the 5-year overall survival for the 72 patients who underwent observation following a complete clinical response to neoadjuvant therapy was 100%. Since then, a number of single-institution series and meta-analyses have corroborated promising oncologic outcomes with NOM.27-38 The International Watch and Wait Database Study is the largest dataset of NOM rectal cancer patients treated at multiple institutions across Europe over the last 25 years.36 While the 2-year local tumor regrowth rate was 25%, the majority of these patients could be salvaged by surgery, resulting in a 5-year overall survival rate of 85%.36 The recently reported OPRA trial, randomizing patients to either induction (before chemo-radiation) or consolidative (after chemo-radiation) chemotherapy in patients with locally advanced rectal cancer, reported organ preservation rates of 43% and 58%, respectively.8 A summary of the data in support of NOM is shown in Table 1. While there are a number of ongoing trials evaluating NOM (Table 2), its utilization is likely to remain controversial until more data is able to confirm the oncologic safety of this compared to radical resection.
Table 1.
Existing Data in Support of Non-Operative Management of Rectal Cancer.
| Author (Reference) | Year Published | N | Median Follow-up (months) | LR (%) | DR (%) | DFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|
| Habr-Gama (26) | 2004 | 71 | 57.3 | 2.8 | 4.2 | 92# | 100# |
| Maas (28) | 2011 | 21 | 25 | 4.7 | 0 | 89* | 100* |
| Dalton (31) | 2012 | 6 | 25.5 | 0 | 0 | 100* | 100* |
| Smith (34) | 2012 | 32 | 28 | 18.7 | 9.4 | 88* | 96* |
| Li (33) | 2015 | 30 | 59 | 6.7 | 3.3 | 90# | 100# |
| Smith (32) | 2015 | 18 | 68.4 | 5.6 | 5.6 | NR | NR |
| Araujo (35) | 2015 | 42 | 47.7 | 11.9 | 9.5 | 60.9# | 71.6# |
| Lai (30) | 2016 | 18 | 49.9 | 11.1 | 0 | NR | 100# |
| van der Valk (36) | 2018 | 880 | 39 | 25.2 | 8 | NR | 84.7# |
| Smith (27) | 2019 | 113 | 43 | 19.5 | 8 | 75# | 73# |
| Garcia-Aguilar (8)^ | 2020 | 324 | 25 | NR | NR | NR | NR |
Abbreviations: N, number of patients; LR, local recurrence, DR, distant recurrence; DFS, disease-free survival, OS, overall survival; NR, not reported.
*: Reported at 2 years.
#: Reported at 5 years.
^: Final results of this trial are not available. Current data was reported for the cohort overall, not for patients undergoing organ preservation and was thus omitted from the above table.
Table 2.
Ongoing Trials for Non-Operative Management of Rectal Cancer.
| Trial Number (NCT) | Location | Study Arm 1 | Study Arm 2 | Primary Outcome(s) | Secondary Outcome(s) | Estimated Enrollment | Status |
|---|---|---|---|---|---|---|---|
| 02008656 | USA | 8c FOLFOX or 5c CapeOX followed by CRT | CRT followed by 6c CapeOX or 8c FOLFOX | 3-yr DFS | Adverse events | 325 | Recruiting |
| 02514278 | France | 4c Folfirinox then CRT | CRT alone | Rate of organ presevation and absence of stoma | Several, including, OS, DFS* | 218 | Recruiting |
| 02704520 | UK | Surgery followed by CapeOX or FOLFOX or single agent 5-Fu | CRT then chemotherapy followed by observation or surgery ^ | Patient recruitment rate | Several including, drug toxicity, surgical morbidity* | 90 | Recruiting |
| 01047969 | UK | CRT followed by chemo if cCR | CRT followed by surgery | Rate of NOM; 2-yr LF | Several including positive margin rate, OS | 99 | Unknown |
| 03426397 | The Nether-lands | Multicenter prospective observational cohort study including patients with rectal cancer who after a long course of CRT or a short course of radiation with a long waiting interval have a clinical complete response (ycT0N0). | 2-year non-regrowth rate and DFS | Several including LC, OS | 220 | Recruiting | |
Abbreviations: USA, United States of America; UK, United Kingdom; c, cyles; FOLFOX, folinic acid, 5-fluorouracil, and oxaliplatin; CapeOx, capecitabine and oxaliplatin; CRT, chemotherapy with concurrent radiotherapy; Folfirinox, 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; 5-Fu, 5-fluorouracil; DFS, disease-free survival; NOM, non-operative management; LF, local-failure.
^depending on response to neoadjuvant therapy
* For complete list of secondary outcome measures, please refer to http://clinicaltrials.gov.
Current Limitations to NOM
A major limitation to NOM is that it can only be offered to patients achieving a clinical response to neoadjuvant therapy. Thus, efforts are ongoing to optimize both neoadjuvant systemic and local treatments for patients with locally advanced rectal cancer.
Chemotherapy
Efforts are ongoing to increase pCR rates among patients with rectal cancer through the intensification of neoadjuvant treatments (Figure 1). A total neoadjuvant therapy (TNT) approach, delivering all therapies (systemic therapy and radiation therapy) prior to resection, has been explored and has yielded pCR rates which have reproducibly been higher than neoadjuvant radiation, with or without chemotherapy, alone. The TIMING trial showed that increasing the number of cycles of pre-operative consolidative FOLFOX chemotherapy after chemo-radiation resulted in an incremental increase in the rate of pCR (25% vs. 30% vs. 38% for 2, 4, and 6 cycles respectively).39 The recently presented PRODIGE 23 trial, comparing preoperative long-course chemo-radiation with or without neoadjuvant systemic chemotherapy consisting of 6 cycles of modified FOLFOX, found a more than doubling of the pCR rate with a TNT approach.10 Additional phase II studies and a number of meta-analyses have confirmed these findings, with pCR rates approaching 40%.40-44 Efforts are ongoing to further improve on these outcomes including the phase II NRG Oncology study (NRG GI-002; NCT02921256) evaluating the addition of veliparib or pembrolizumab with combination chemotherapy and radiation therapy in patients with high risk rectal cancer. The emerging data suggest that delivering all therapy upfront results in more patients achieving a complete response, challenging the accepted standard of reflexive rectal resection for all non-metastatic locally advanced rectal cancer patients.
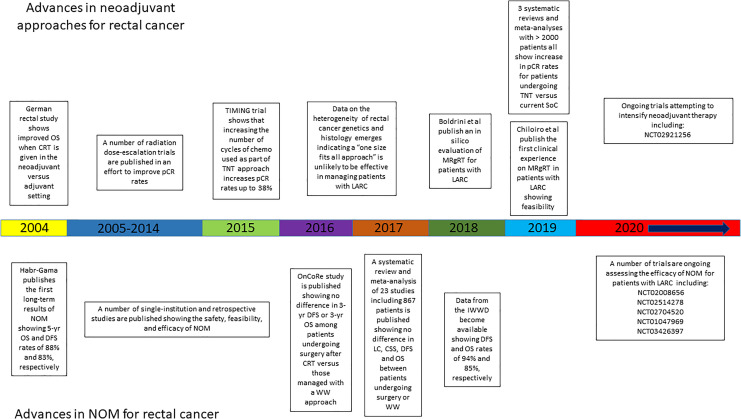
Figure 1.
Timeline depicting advances in neoadjuvant approaches in conjunction with advances in NOM for rectal cancer patients. Abbreviations: NOM, non-operative management; TNT, total neoadjuvant therapy; MRgRT, magnetic resonance guided radiation therapy.
Radiation Therapy
Efforts have also been made to intensify neoadjuvant radiation therapy for rectal cancer with the goal of improving tumor downstaging. Passoni et al showed that delivering a boost dose to residual disease (to 54 Gy total) following whole pelvic radiotherapy resulted in a pCR rate of 35%.45 A meta-analysis showed that the pCR rate after preoperative radiation to a dose greater than or equal to 60 Gy was 20%.46 A number of studies, including a randomized trial, have shown that higher radiation doses result in increased pathological down-staging.46-49 Of 51 eligible patients enrolled in a European prospective dose escalation study to 60 Gray in 30 fractions, 40 (78%) had a complete response and underwent observation with low rates of local regrowth.50 A large population based study evaluating 3298 patients from the National Oncology Data Alliance showed a dose-response relationship between radiation dose escalation and tumor regression.51 Patients treated to 54 Gy achieved greater tumor downstaging relative to patients receiving 50.4 Gy, who experienced greater tumor downstaging relative to patients receiving 45 Gy.51
Despite the promising results seen to date, radiation dose escalation is limited by the inability to safely deliver high doses of radiation therapy to the rectum, while sparing adjacent pelvic organs, using current treatment techniques. Organs of the pelvis, including the target and adjacent organs at risk, have high reproducibility uncertainty. The inter-fraction and intra-fraction variability in bladder and bowel filling cannot be adequately assessed and accounted for on standard computed tomography-based images acquired during radiation treatment. Moreover, rectal cancer is poorly visualized on computed tomography images. As a result, current protocols attempting to escalate dose to the tumor employ generous margins to account for uncertainties in tumor positioning, resulting in overlap with potentially large volumes of the small bowel, bladder, normal rectal mucosa, and anal sphincter muscles.50,52-54 Even through the use of more conformal radiation techniques, such as intensity modulated radiation therapy which has been shown to decrease toxicity rates from radiation therapy,55 dose escalation is limited by set-up uncertainties, organ motion, and poor-tumor tumor visualization.
Emerging genomic data has suggested distinct biological differences between rectal adenocarcinoma lesions,56-59 which may allow for a more tailored radiotherapy dose escalation. A genome-based model for tailoring radiotherapy dose has explored adaptive dosing to overcome individual rectal tumor intrinsic radiosensitivities.60,61 Despite this preliminary evidence, radiobiologic data is not currently available for clinical use to personalize the radiation dose in an attempt to increase tumor response. Current radiation dose prescriptions are mostly uniform and do not take into account the tumor’s genomic makeup or individual tumor biology. Given that patients with rectal cancer have significant heterogeneity at the genomic level, the ability to distinguish the individual intrinsic radiosensitivity is crucial when considering personalized RT in the precision medicine era.
Assessing Response to Neoadjuvant Therapy
In addition to the challenges faced in achieving a complete clinical response to therapy using current treatment techniques, the interpretation of what constitutes a complete clinical response limits the broader implementation of a NOM approach. Clinical complete responders are currently identified by a combination of clinical, endoscopic, and radiographic criteria, none of which have been validated on a large-scale. Thus, it is unknown if clinical complete response accurately predict pathological complete response.62 Efforts are therefore ongoing to optimize our ability to assess tumor response to neoadjuvant therapy through the use of imaging techniques including MRI and FDG-PET.63-66
Functional MRI using sequences such as diffusion-weighted imaging (DWI) and dynamic contrast enhanced (DCE) imaging, can provide biological data on tumor response to treatment. Studies have shown that changes in apparent diffusion coefficients (ADC) of rectal carcinoma obtained during CRT for rectal cancer correlate with tumor down-staging on pathology at the time of surgery.67-69 An ongoing European trial (TRIGGER trial) has incorporated MRI-based tumor regression grading at 4-6 weeks following completion of neoadjuvant chemo-radiation to determine response and subsequent management, including addition of chemotherapy or deferral of surgery.70 Thus, not only does pretreatment therapy need to be optimized in order to expand the proportion of rectal cancer patients eligible for organ preservation, but strategies to appropriately and reliably select patients for NOM are also required.
The Potential Role of Magnetic Resonance Guided Radiotherapy in NOM
On-board real-time Magnetic Resonance Imaging (MRI)-guided radiation therapy (MRgRT) has emerged as a promising technology that may facilitate safe tumor dose escalation in order to further optimize preoperative rectal cancer therapy. Unlike traditional image guidance which uses on-board cone-beam computed tomography, MRgRT couples an on-board MRI with a linear accelerator. MRgRT permits MRI acquisition immediately before, continuously during, and after a patient’s radiation treatment, in the treatment position.71 This temporal sequencing offers a number of advantages. First, MRI offers superior soft tissue delineation so that the rectal tumor can be more accurately visualized on daily pre-treatment imaging. Second, the increased soft tissue contrast provides dynamic anatomical information regarding rectal filling and subsequently tumor motion during treatment. Third, functional imaging can provide tumor response data over the course of treatment and is currently an active area of investigation. Fourth, on-board MRI can provide daily reassessment and adaptive re-planning to account for inter-fractional tumor and normal organ motion.72 Taken together, these features would allow for improved target accuracy, dose escalation, and enhanced tumor response assessment for rectal cancer patients undergoing preoperative radiotherapy using MRgRT.
MRI is the preferred imaging modality used for the initial local staging of rectal cancer.73 It follows, therefore, that MRI would also be the best imaging modality for on-board image guidance during radiation treatment delivery. MR-based imaging would provide the most accurate information on highly relevant tumor characteristics, such as the relationship to the mesorectal fascia, proximity to adjacent genitourinary structures, and the position of clinically suspicious lymph nodes, which would allow for a reduction in margins added to account for setup uncertainty. These margins could be further reduced through the use of adaptive re-planning during the course of treatment, made possible by the superior spatial resolution offered by MRgRT.74 Data on the use of MRgRT for pancreatic cancer has shown that adaptive re-planning results in superior target coverage and increased organ at risk sparing, allowing for the delivery of increasing doses of radiation to the target volume.75,76
MRgRT may also allow for the acquisition of functional imaging to not only guide treatment but also to assess response. MRgRT allows daily acquisition during treatment and may provide data which can be used to generate response prediction models during the course of radiotherapy.52,77 This innovative personalized approach to the treatment of rectal cancer might be used to better select patients who may safely avoid surgery. Furthermore, functional imaging may allow the radiation dose to be individualized if, for example, a particular ADC level were the goal of treatment rather than adhering to a pre-specified, empiric radiation dose.71 Similarly, MRgRT may provide radiomic biomarkers to guide radiation dose by identifying physiologically distinct regions within lesions where, for instance, a pCR may have been achieved. Although radiomics on diagnostic MRI is relatively well-established,78 it is just now beginning to be explored using MRgRT images.
Clinical data on the use of MRgRT for rectal cancer is limited. In 2018, Boldrini et al published their experience on an in silico evaluation of rectal cancer treatment using MRIdian, the first commercially available MRI-based linear accelerator system from Viewray.79 The authors compared Co-60 IMRT plans with and without the presence of the magnetic field in 10 consecutive patients receiving neoadjuvant radiation for rectal cancer to assess if the presence of the magnetic field would impact dosimetry. The authors found that there were no relevant differences between the plans with or without the magnetic field present. Their planning analysis represented the first proof of concept to verify the possibility of safely using MR-guided hybrid treatments in patients with rectal cancer. Their work served as the dosimetric benchmark for the newer MRIdian system using a 6MV linear accelerator in place of Co-60.79
Following the experience of Boldrini and colleagues, Chiloiro et al published on the first clinical experience with the MRIdian in rectal cancer treatment also using the earlier Co-60 model.80 The authors analyzed outcomes of 22 patients with LARC treated with the MRIdian Co-60 system. Most patients received a simultaneous integrated boost to 55 Gy in 25 fractions with concurrent chemotherapy. An MRI was acquired as an alignment image and used to define the radiation field. A cine-MRI gating protocol was used setting a 5% region of interest at a 3 mm boundary from the clinical treatment volume to ensure that the target was always in the appropriate position during beam-on time. The primary endpoint was complete response rate. Three patients (14%) achieved a complete clinical response and did not undergo surgery. They were alive without recurrence at the time of the analysis. Of those who underwent surgery, 16% achieved a complete pathological response. In total, 27% of patients achieved a complete clinical or pathological response to treatment.
Daily assessment of MRI tumor response to individualize the radiation dose is of significant clinical interest, particularly in advancing rectal organ preservation research efforts. MRI imaging parameters may also select out patients who are not candidates from a primary non-surgical approach and are best served with preoperative intent. Yet, what still remains unknown at this time are at what time point and how best to measure individual tumor response to therapy. Data from a 15 patients prospectively studied in Europe on a 3 T MRI measuring weekly response suggested that reduction in gross tumor volume burden occurs as early as week 1 with the main tumor regression occurring during the first half of a 5-6 week course of therapy.81 This preliminary research suggests that there may be an advantage to a sequential boost to the most radioresistant portion of the tumor can be targeted.
While there are many potential advantages of incorporating MRgRT into a personalized treatment approach, there are also some important limitations to be recognized. One of the most significant challenges associated with MRgRT is increased treatment time as compared to treatment on a conventional linear accelerator, which is inconvenient for both patient and treatment facility. Patients must lay motionless for up to 1.5 hours for treatment if adaptive re-planning is required, lessening the compliance of individual patients to tolerate the standard 5.5 week course of treatment. The MRgRT process entails delivery by step and shoot IMRT with static beams, while a much quicker Volumetric Modulated Arc Therapy plan may be delivered on a conventional linac.82 This mode of delivery lengthens treatment since more beams may be required to achieve a clinically acceptable plan, significantly increasing the time on the treatment table. For those patients requiring a real time adaptive re-plan on the MRI linac, additional time is needed to recontour the normal organs (10-15 minutes), re-optimize the dose, evaluate the new plan, perform real time quality assurance, and then treat. Re-contouring is especially time consuming because the current treatment planning system uses deformable image registration to deform the initial contours to the image of the day. These contours require significant manual edits that require excess physician time. Eventually, implementation of an artificial intelligence system may be used to accurately re-contour the normal tissues daily which would save considerable time in the future.83 For rectal cancer patients with rectal/urinary urgency, extended time on the treatment table may cause additional discomfort and distress.
In addition to increased treatment planning and delivery time, there are other limitations to MR-guided therapy which should be considered as well. One of these is machine down-time. While this is not an infrequent occurrence on a conventional treatment machine as well, down-time on an MR-linac may be significantly prolonged. Since the magnet is co-located within the linac, engineers will need additional time for repairs, translating to days, rather than hours, of patient delays. Finally, given the prolonged treatment time on the MR linac, fewer patients can be treated each day. Thus, patients’ start of therapy may be substantially delayed until there is availability on the machine for their treatment to be scheduled, which could lead to potentially worse outcomes.
These technical challenges with MRgRT require careful selection of those patients who will benefit the most from such a time intensive modality. Unanswered questions include whether MRgRT should be reserved for delivery of the boost only or in the setting of recurrent rectal cancer. Thus, while the scientific advantages are compelling, these must be balanced with the pragmatic issues relating to treatment using this technology. Future work is needed to explore how best to integrate MRgRT into the rectal cancer treatment paradigm.
Conclusions and Future Directions
The growing evidence in support of total neoadjuvant therapy, coupled with the implementation of strategies to enhance the delivery of local therapy, holds promise for increasing the rate of organ preservation in rectal cancer patients. Enhanced response to neoadjuvant therapy together with more appropriate patient selection, through the use of genomic data and functional imaging, may lead to more widespread implementation of NOM. This more personalized approach to therapy may permit an increasing number of patients to avoid the morbidity associated with radical surgery. Efforts are needed to appropriately define the optimal use of MRgRT in the management of these patients and will likely require multi-institutional collaborative efforts.
Footnotes
Author Contributions: LTT conceived the article, wrote and prepared the manuscript, and made the tables and figure. PBR contributed significantly to paper revisions, adding content and citations, and contributed to the editing of the manuscript and the tables. SF, SH, MDC, and SF added content, including data in their area of expertise to enhance the scope of the work, and edited the manuscript. MDC also contributed to the initial conception of the work.
Consent to Participate: Our study did not require an consent to participae because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PBR is a consultant for EMD Serono for work on radiation sensitizers and has received travel support from Elekta for work on the MR-LINAC Elekta Unity. MDC is on the speakers bureau for ViewRay, Accuray, and Sirtex; he has received research funding from ViewRay and AstraZeneca and is on the Medical Advisory Board for ViewRay. SHE has received research funding from Varian.
Ethics Approval: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Leila T. Tchelebi, MD  https://orcid.org/0000-0002-4789-449X
https://orcid.org/0000-0002-4789-449X
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi:10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 3. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi:10.1200/jco.2011.40.1836 [DOI] [PubMed] [Google Scholar]
- 4. Pucciarelli S, Gagliardi G, Maretto I, et al. Long-term oncologic results and complications after preoperative chemoradiotherapy for rectal cancer: a single-institution experience after a median follow-up of 95 months. Ann Surg Oncol. 2009;16(4):893–899. doi:10.1245/s10434-009-0335-6 [DOI] [PubMed] [Google Scholar]
- 5. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi:10.1056/NEJMoa060829 [DOI] [PubMed] [Google Scholar]
- 6. Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–687. doi:10.1016/s1470-2045(12)70187-0 [DOI] [PubMed] [Google Scholar]
- 7. Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi:10.1016/s1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38(15_suppl):4008–4008. doi:10.1200/JCO.2020.38.15_suppl.4008 [Google Scholar]
- 9. Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol. 2020;38(15_suppl):4006–4006. doi:10.1200/JCO.2020.38.15_suppl.4006 [Google Scholar]
- 10. Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38(15_suppl):4007–4007. doi:10.1200/JCO.2020.38.15_suppl.4007 [Google Scholar]
- 11. Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19(9):2822–2832. doi:10.1245/s10434-011-2209-y [DOI] [PubMed] [Google Scholar]
- 12. Lee YC, Hsieh CC, Chuang JP. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;56(9):1093–1101. doi:10.1097/DCR.0b013e318298e36b [DOI] [PubMed] [Google Scholar]
- 13. Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918–928. doi:10.1002/bjs.8702 [DOI] [PubMed] [Google Scholar]
- 14. Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72(1):99–107. doi:10.1016/j.ijrobp.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 15. Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;238(2):203–213. doi:10.1097/01.sla.0000080823.38569.b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lange MM, Marijnen CA, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer. 2009;45(9):1578–1588. doi:10.1016/j.ejca.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 17. Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48(7):1353–1365. doi:10.1007/s10350-004-0942-z [DOI] [PubMed] [Google Scholar]
- 18. Wilson TR, Alexander DJ. Clinical and non-clinical factors influencing postoperative health-related quality of life in patients with colorectal cancer. Br J Surg. 2008;95(11):1408–1415. doi:10.1002/bjs.6376 [DOI] [PubMed] [Google Scholar]
- 19. Nasvall P, Dahlstrand U, Lowenmark T, Rutegard J, Gunnarsson U, Strigard K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017;26(1):55–64. doi:10.1007/s11136-016-1367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007;14(7):2056–2068. doi:10.1245/s10434-007-9402-z [DOI] [PubMed] [Google Scholar]
- 21. Bokkerink GM, de Graaf EJ, Punt CJ, et al. The CARTS study: chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery. BMC Surg. 2011;11:34 doi:10.1186/1471-2482-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Homan J, Bökkerink GM, Aarts MJ, et al. Variation in circumferential resection margin: Reporting and involvement in the South-Netherlands. Eur J Surg Oncol. 2015;41(11):1485–1492. doi:10.1016/j.ejso.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 23. Anderin K, Gustafsson UO, Thorell A, Nygren J. The effect of diverting stoma on long-term morbidity and risk for permanent stoma after low anterior resection for rectal cancer. Eur J Surg Oncol. 2016;42(6):788–793. doi:10.1016/j.ejso.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 24. Kim MJ, Kim YS, Park SC, et al. Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery. 2016;159(3):721–727. doi:10.1016/j.surg.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 25. Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg. 2011;213(3):352–361. doi:10.1016/j.jamcollsurg.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 26. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–717; discussion 717-718 doi:10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5(4):e185896 doi:10.1001/jamaoncol.2018.5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–4640. doi:10.1200/jco.2011.37.7176 [DOI] [PubMed] [Google Scholar]
- 29. Li J, Liu H, Yin J, et al. Wait-and-see or radical surgery for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy: a cohort study. Oncotarget. 2015;6(39):42354–42361. doi:10.18632/oncotarget.6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int J Colorectal Dis. 2016;31(2):413–419. doi:10.1007/s00384-015-2460-y [DOI] [PubMed] [Google Scholar]
- 31. Dalton RS, Velineni R, Osborne ME, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14(5):567–571. doi:10.1111/j.1463-1318.2011.02752.x [DOI] [PubMed] [Google Scholar]
- 32. Smith RK, Fry RD, Mahmoud NN, Paulson EC. Surveillance after neoadjuvant therapy in advanced rectal cancer with complete clinical response can have comparable outcomes to total mesorectal excision. Int J Colorectal Dis. 2015;30(6):769–774. doi:10.1007/s00384-015-2165-2 [DOI] [PubMed] [Google Scholar]
- 33. Li J, Li L, Yang L, et al. Wait-and-see treatment strategies for rectal cancer patients with clinical complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Oncotarget. 2016;7(28):44857–44870. doi:10.18632/oncotarget.8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965–972. doi:10.1097/SLA.0b013e3182759f1c [DOI] [PubMed] [Google Scholar]
- 35. Araujo RO, Valadao M, Borges D, et al. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol. 2015;41(11):1456–1463. doi:10.1016/j.ejso.2015.08.156 [DOI] [PubMed] [Google Scholar]
- 36. van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi:10.1016/s0140-6736(18)31078-x [DOI] [PubMed] [Google Scholar]
- 37. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501–513. doi:10.1016/s2468-1253(17)30074-2 [DOI] [PubMed] [Google Scholar]
- 38. Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, et al. Pathologic complete response in rectal cancer: can we detect it? Lessons learned from a proposed randomized trial of watch-and-wait treatment of rectal cancer. Dis Colon Rectum. 2016;59(4):255–263. doi:10.1097/dcr.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–966. doi:10.1016/s1470-2045(15)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manthravadi S, Sun W, Saeed A, Baranda JC, Kasi A. Total neoadjuvant therapy compared with standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis. J Clin Oncol. 2019;37(4_suppl):709 doi:10.1200/JCO.2019.37.4_suppl.709 [Google Scholar]
- 41. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–448. doi:10.1097/sla.0000000000003471 [DOI] [PubMed] [Google Scholar]
- 42. Yu X, Wang QX, Xiao WW, et al. Neoadjuvant oxaliplatin and capecitabine combined with bevacizumab plus radiotherapy for locally advanced rectal cancer: results of a single-institute phase II study. Cancer Commun (Lond). 2018;38(1):24 doi:10.1186/s40880-018-0294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaborowski A, Stakelum A, Winter DC. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Br J Surg. 2019;106(8):979–987. doi:10.1002/bjs.11171 [DOI] [PubMed] [Google Scholar]
- 44. Fokas E, Allgauer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212–3222. doi:10.1200/jco.19.00308 [DOI] [PubMed] [Google Scholar]
- 45. Passoni P, Fiorino C, Slim N, et al. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. 2013;87(1):67–72. doi:10.1016/j.ijrobp.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 46. Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1–9. doi:10.1016/j.radonc.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 47. Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):74–80. doi:10.1016/j.ijrobp.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84(4):949–954. doi:10.1016/j.ijrobp.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 49. Gunther JR, Chadha AS, Shin US, et al. Preoperative radiation dose escalation for rectal cancer using a concomitant boost strategy improves tumor downstaging without increasing toxicity: a matched-pair analysis. Adv Radiat Oncol. 2017;2(3):455–464. doi:10.1016/j.adro.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Appelt AL, Ploen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. doi:10.1016/s1470-2045(15)00120-5 [DOI] [PubMed] [Google Scholar]
- 51. Hall MD, Schultheiss TE, Smith DD, Fakih MG, Wong JY, Chen YJ. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol. 2016;55(12):1392–1399. doi:10.1080/0284186x.2016.1235797 [DOI] [PubMed] [Google Scholar]
- 52. Gani C, Boldrini L, Valentini V. Online MR guided radiotherapy for rectal cancer. New opportunities. Clin Transl Radiat Oncol. 2019;18:66–67. doi:10.1016/j.ctro.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Radu C, Norrlid O, Braendengen M, Hansson K, Isacsson U, Glimelius B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013;52(3):528–537. doi:10.3109/0284186x.2012.737022 [DOI] [PubMed] [Google Scholar]
- 54. Kleijnen JJE, van Asselen B, Van den Begin R, et al. MRI-based tumor inter-fraction motion statistics for rectal cancer boost radiotherapy. Acta Oncol. 2019;58(2):232–236. doi:10.1080/0284186x.2018.1532598 [DOI] [PubMed] [Google Scholar]
- 55. Wee CW, Kang H-C, Wu H-G, et al. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta-analysis and pooled-analysis of acute toxicity. Jpn J Clin Oncol. 2018;48(5):458–466. doi:10.1093/jjco/hyy029 [DOI] [PubMed] [Google Scholar]
- 56. Bettoni F, Masotti C, Habr-Gama A, et al. Intratumoral genetic heterogeneity in rectal cancer: are single biopsies representative of the entirety of the tumor? Ann Surg. 2017;265(1):e4–e6. doi:10.1097/sla.0000000000001937 [DOI] [PubMed] [Google Scholar]
- 57. McCawley N, Clancy C, O’Neill BD, Deasy J, McNamara DA, Burke JP. Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Dis Colon Rectum. 2016;59(12):1200–1208. doi:10.1097/dcr.0000000000000635 [DOI] [PubMed] [Google Scholar]
- 58. Sagaert X, Vanstapel A, Verbeek S. Tumor heterogeneity in colorectal cancer: what do we know so far? Pathobiol. 2018;85(1-2):72–84. doi:10.1159/000486721 [DOI] [PubMed] [Google Scholar]
- 59. Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. 2017;14(4):235–246. doi:10.1038/nrclinonc.2016.171 [DOI] [PubMed] [Google Scholar]
- 60. Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi:10.1016/s1470-2045(16)30648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Torres-Roca JF, Eschrich S, Zhao H, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res. 2005;65(16):7169–7176. doi:10.1158/0008-5472.Can-05-0656 [DOI] [PubMed] [Google Scholar]
- 62. Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. No competing interests declared. J Am Coll Surg. 2002;194(2):131–135. doi:10.1016/S1072-7515(01)01159-0 [DOI] [PubMed] [Google Scholar]
- 63. Meng Y, Wan L, Zhang C, et al. The predictive value of pre-/postneoadjuvant chemoradiotherapy mri characteristics for patient outcomes in locally advanced rectal cancer. Acad Radiol. 2019;27(9):e233–e243. doi:10.1016/j.acra.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 64. Prezzi D, Goh V. Rectal cancer magnetic resonance imaging: imaging beyond morphology. Clin Oncol. 2016;28(2):83–92. doi:10.1016/j.clon.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 65. Pecori B, Lastoria S, Caracò C, et al. Sequential PET/CT with [18F]-FDG predicts pathological tumor response to preoperative short course radiotherapy with delayed surgery in patients with locally advanced rectal cancer using logistic regression analysis. PLOS One. 2017;12(1):e0169462 doi:10.1371/journal.pone.0169462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sorenson E, Lambreton F, Yu JQ, et al. Impact of PET/CT for restaging patients with locally advanced rectal cancer after neoadjuvant chemoradiation. J Surg Res. 2019;243:242–248. doi:10.1016/j.jss.2019.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun YS, Zhang XP, Tang L, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254(1):170–178. doi:10.1148/radiol.2541082230 [DOI] [PubMed] [Google Scholar]
- 68. Shaverdian N, Yang Y, Hu P, et al. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol. 2017;90(1071):20160 739-20160739. doi:10.1259/bjr.20160739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nougaret S, Vargas HA, Lakhman Y, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology. 2016;280(2):446–454. doi:10.1148/radiol.2016150702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Battersby NJ, Dattani M, Rao S, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials. 2017;18(1):394–394. doi:10.1186/s13063-017-2085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hall WA, Paulson ES, van der Heide UA, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: a review. Eur J Cancer. 2019;122:42–52. doi:10.1016/j.ejca.2019.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Winkel D, Bol GH, Kroon PS, et al. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi:10.1016/j.ctro.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019;39(2):367–387. doi:10.1148/rg.2019180114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Noel CE, Parikh PJ, Spencer CR, et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54(9):1474–1482. doi:10.3109/0284186x.2015.1062541 [DOI] [PubMed] [Google Scholar]
- 75. Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123–2132. doi:10.1002/cam4.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. El-Bared N, Portelance L, Spieler BO, et al. Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol. 2019;9(1):e46–e54. doi:10.1016/j.prro.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 77. Boldrini L, Cusumano D, Chiloiro G, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med. 2019;124(2):145–153. doi:10.1007/s11547-018-0951-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi:10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boldrini L, Placidi E, Dinapoli N, et al. Hybrid Tri-Co-60 MRI radiotherapy for locally advanced rectal cancer: an in silico evaluation. Tech Innov Patient Support Radiat Oncol. 2018;6:5–10. doi:10.1016/j.tipsro.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chiloiro G, Boldrini L, Meldolesi E, et al. MR-guided radiotherapy in rectal cancer: First clinical experience of an innovative technology. Clin Transl Radiat Oncol. 2019;18:80–86. doi:10.1016/j.ctro.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kleijnen JP. Towards MRI-guided rectal cancer boost radiotherapy PhD Thesis, Utrecht University, The Netherlands Elsevier; 2019. [Google Scholar]
- 82. Sahin B, Zoto Mustafayev T, Gungor G, et al. First 500 fractions delivered with a magnetic resonance-guided radiotherapy system: initial experience. Cureus. 2019;11(12):e6457 doi:10.7759/cureus.6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huynh E, Hosny A, Guthier C, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020. doi:10.1038/s41571-020-0417-8 [DOI] [PubMed] [Google Scholar]