Abstract
Objective:
The aim is to explore the prediction effect of 5 machine learning algorithms on peritoneal metastasis of gastric cancer.
Methods:
1080 patients with postoperative gastric cancer were divided into a training group and test group according to the ratio of 7:3. The model of peritoneal metastasis was established by using 5 machine learning (gbm(Light Gradient Boosting Machine), GradientBoosting, forest, Logistic and DecisionTree). Python pair was used to analyze the machine learning algorithm. Gbm algorithm is used to show the weight proportion of each variable to the result.
Result:
Correlation analysis showed that tumor size and depth of invasion were positively correlated with the recurrence of patients after gastric cancer surgery. The results of the gbm algorithm showed that the top 5 important factors were albumin, platelet count, depth of infiltration, preoperative hemoglobin and weight, respectively. In training group: Among the 5 algorithm models, the accuracy of GradientBoosting and gbm was the highest (0.909); the AUC values of the 5 algorithms are gbm (0.938), GradientBoosting (0.861), forest (0.796), Logistic(0.741) and DecisionTree(0.712) from high to low. In the test group: among the 5 algorithm models, the accuracy of forest, DecisionTree and gbm was the highest (0.907); AUC values ranged from high to low to gbm (0.745), GradientBoosting (0.725), forest (0.696), Logistic (0.680) and DecisionTree (0.657).
Conclusion:
Machine learning can predict the peritoneal metastasis in patients with gastric cancer.
Keywords: machine learning, peritoneal metastasis, gastric cancer, predictive modeling
Introduction
Gastric cancer (GC) is a highly malignant and heterogeneous tumor. Among the malignant tumors worldwide, the incidence rate is the fourth and the mortality rate is the second.1 Peritoneal membrane is the common metastatic site of gastric cancer. The studies2,3 showed that 8.0% ∼ 13.5% of patients with newly diagnosed gastric cancer were complicated with malignant ascites, while the incidence of peritoneal metastasis was higher than 39.0% ∼ 43.0% in patients with advanced gastric cancer. Patients with advanced gastric cancer, especially those with malignant ascites due to peritoneal metastases, have a poorer prognosis. The median survival of patients with gastric cancer and peritoneal metastasis is only4-6 months.2 At the same time, malignant ascites can lead to complications such as intestinal obstruction, infection, malnutrition and renal insufficiency, which seriously affect the patients’ quality of life. The early stage of peritoneal metastasis of gastric cancer is mainly micro-metastasis, the small size and low density of peritoneal tumor nodules, so how to correctly diagnose peritoneal metastasis in early-stage has been the subject of clinical researchers’ attention. Laparoscopic or laparotomy pathology is the “gold standard” for the diagnosis of peritoneal metastases, but both are invasive and are not suitable for routine performance. CT and PET/ CT have commonly used imaging examinations for the diagnosis of recurrent metastasis after radical gastrostomy for gastric cancer, but there is some radiation and high cost.
At present, there are some predictive models for peritoneal metastasis of advanced gastric cancer. Studies4 have shown that the clinical features combined with CT can predict the peritoneal metastasis of advanced gastric cancer. Similarly, some studies5 have shown that texture features obtained from preoperative CT images of patients with advanced gastric cancer can be used to predict peritoneal metastasis. Other studies6 have shown that venous CT radiological analysis based on primary tumors provides valuable information for predicting peritoneal metastasis of advanced gastric cancer. However, there is no research on the peritoneal metastasis of gastric cancer related to artificial intelligence.
Currently, based on large data sets and in-depth learning, researchers can use medical data and machine learning to predict disease risk better. Machine learning can translate measurements into relevant prediction models. Through machine learning, the diagnostic and drug genetics experts can find out the complexity of the disease, perform treatments, and customize medical options for individual patients. This study has reported that the combination of anti-cancer antigen 125 level and machine learning can predict the recurrence of abdominopelvic cancer.7 Machine learning combined with MRI can predict the prognosis of breast tumor patients early8; Machine-based learning and radiomics can help improve the diagnostic performance of prostate cancer.9 Convolutional neural network classifier can effectively distinguish bone metastasis of prostate cancer patients.10 Other studies11 have shown that machine learning can predict the effect of immune tumor-related gene expression on immune checkpoint inhibition in gastrointestinal cancer. Moreover, machine learning analysis can help clinicians determine the scope of lymph node dissection in gastric cancer before surgical resection.12
However, studies on peritoneal metastasis of gastric cancer related to machine learning have not been reported. Therefore, this study intends to investigate the effect of 5 machine learning algorithms on predicting peritoneal recurrence in gastric cancer.
Materials and Methods
Patients
In this retrospective analysis, we reviewed the data of 1199 GC patients who underwent GC surgery. Data is available at BioStudies database(https://www.ebi.ac.uk/biostudies/studies?query=S-EPMC5383064), accession numbers: S-EPMC5383064. All patients underwent a preoperative CT scan and were CT negative for peritoneal metastasis. The following information was collected and recorded: the patient’s personal information (i.e., age, sex, body mass index, family history), tumor characteristics (i.e., location, size, type of pathology, histopathological differentiation, lymphatic invasion), and blood routine indices (i.e., neutrophils, lymphocytes, platelets, monocytes, NLR, and PLR). Pathological types were divided into ulcerative group and non-ulcerative group. The diagnosis was confirmed by histological examination in all patients. Exclusion criteria included (1) history of gastrectomy (8 patients), (2) liver disease such as cirrhosis (14 patients), (3) history of other malignancies (15 patients), (4) severe bleeding and autoimmune disease (22 patients), (5) preoperative chemoradiotherapy (2 patients), (6) severe inflammatory or hematological disorders (34 patients), and (7) distant metastasis (except abdominal metastases) (6 patients). Finally, the study enrolled in 1080 patients.
Diagnosis of Peritoneal Metastases
According to the “Japanese Guidelines for the Treatment of Gastric Cancer” (fifth edition), the diagnostic criteria for peritoneal metastasis are as follows: metastasis is limited to the greater omentum, lesser omentum, anterior lobe of transverse colon, pancreatic capsule and spleen; Metastases and metastases to upper abdominal peritoneum (visceral peritoneum above transverse position and parietal peritoneum above umbilical cord). These patients with peritoneal metastases were diagnosed by intraoperative cryosections and postoperative pathological diagnosis.
Machine Learning
Logistic regression is essentially a surveillance classification algorithm. Logistic regression establishes a regression model to predict the probability that a given data entry falls into a category numbered “1.”
Decision tree learning is a decision model established by tree structure based on the attributes of data, which can be used to solve the classification and regression problems.
A random forest is a proprietary noun that represents the overall decision tree. In order to classify it according to the attributes of a new object, each decision tree has a classification called this decision tree “vote” to the classification.
GBDT (Gradient Boosting Decision Tree) is a decision tree algorithm based on iteration. It is a Boosting method, and its main idea is that each establishment of the model is the gradient descent direction in which the model loss function was established before.
LightGBM (Light Gradient Boosting Machine) adopts the Histogram algorithm. The idea is to divide the continuous floating-point characteristics into k discrete values and construct the Histogram with k width.
Data Processing
The data were processed by R language, and the measurement data were expressed by the “mean, standard deviation” and tested by T-test. Counting data are expressed by the number of examples (n) and percentage (%) and x2 test is adopted. The difference was statistically significant with p < 0.05. Multiple interpolations are used for missing variables. Python pair was used to analyze the machine learning algorithm. The total population was randomly divided into a training group and test group according to the ratio of 7:3. Meanwhile, the data were normalized and the prognostic weight was constructed.
Correlation analysis is used to observe the relationship between variables and show the correlation values. In particular, 5 different classification techniques have been evaluated: gbm, GradientBoosting, forest, Logistic and DecisionTree. Gbm algorithm is used to show the weight proportion of each variable to the result. Regularization is used to correct the over-fitting of the model. The prediction performance of the model is adjusted by manual and net style parameters.The following indicators are used to evaluate the prediction ability of the model: Accuracy, MSE and AUC.
Accuracy = (number of samples correctly classified)/(number of all samples classified). MSE (Mean Squared Error) is called mean squared error, the smaller, the better. ROC curve: receiver operating characteristic curve is a comprehensive index reflecting sensitivity and continuous specificity variables. AUC value is between 0 -1, the greater, the better. The parameters and data packets used to build the machine learning model are shown in Appendix Table 1. Codes related to this research can be downloaded from GitHub website(https://github.com/qazq124/-Peritoneal-Metastasis-of-Gastric-Cancer/blob/master/ML%20code12.pdf).
Result
Of the 1080 patients, 839 were men and 241 were women. Patients with GC and peritoneal metastases had a significantly higher PLR than patients without peritoneal metastases (P < 0.001). Similarly, GC patients with peritoneal metastases had higher NLR (P = 0.011). Age and height were not statistically different between the 2 groups.(See in Table 1)The basic information features of the training and test group are shown in Appendix Table 2.
Table 1.
Baseline Data.
| Peritoneal metastasis | NO | YES | P-value |
|---|---|---|---|
| N | 979 | 101 | |
| Age (years) | 63.8 ± 11.2 | 63.6 ± 12.1 | 0.958 |
| Height(cm) | 165.2 ± 8.0 | 164.4 ± 9.1 | 0.584 |
| Weight(kg) | 59.4 ± 10.5 | 57.0 ± 11.0 | 0.035 |
| BMI (kg/m2) | 21.7 ± 3.0 | 21.1 ± 3.2 | 0.037 |
| Tumor size (cm) | 3.9 ± 2.1 | 5.0 ± 2.2 | <0.001 |
| PLR | 148.8 ± 73.7 | 170.3 ± 77.7 | <0.001 |
| NLR | 2.6 ± 1.6 | 2.9 ± 1.6 | 0.011 |
| PREOPERATIVE. HEMOGLOBIN | 118.4 ± 24.0 | 112.4 ± 22.6 | 0.003 |
| Platelet count | 225.3 ± 69.6 | 241.9 ± 72.4 | 0.035 |
| Albumin (g/L) | 39.7 ± 5.2 | 38.6 ± 5.1 | 0.016 |
| Neutrophil count | 4.1 ± 3.9 | 4.0 ± 1.3 | 0.151 |
| Lymphocyte count | 1.8 ± 2.0 | 1.6 ± 0.5 | 0.034 |
| Monocyte count | 0.5 ± 0.4 | 0.4 ± 0.2 | 0.144 |
| WBC count | 6.1 ± 1.6 | 6.2 ± 1.5 | 0.735 |
| Sex | 0.893 | ||
| Male | 760 (77.6%) | 79 (78.2%) | |
| Female | 219 (22.4%) | 22 (21.8%) | |
| ASA | 0.905 | ||
| 1 | 61 (6.2%) | 5 (5.0%) | |
| 2 | 828 (84.6%) | 86 (85.1%) | |
| 3 | 90 (9.2%) | 10 (9.9%) | |
| TNM | 0.958 | ||
| I | 236 (24.1%) | 23 (22.8%) | |
| II | 67 (6.8%) | 8 (7.9%) | |
| III | 513 (52.4%) | 52 (51.5%) | |
| IV | 163 (16.6%) | 18 (17.8%) | |
| Borrmann types | 0.041 | ||
| 1 | 55 (5.6%) | 8 (7.9%) | |
| 2 | 859 (87.7%) | 80 (79.2%) | |
| 3 | 65 (6.6%) | 13 (12.9%) | |
| Pathological type [n, (%)] | 0.015 | ||
| Ulcerative | 120 (12.3%) | 21 (20.8%) | |
| Nonulcerative | 859 (87.7%) | 80 (79.2%) | |
| Depth of invasion [n, (%)] | <0.001 | ||
| T1/T2 | 341 (34.8%) | 6 (5.9%) | |
| T3/T4 | 638 (65.2%) | 95 (94.1%) |
Note: NLR, neutrophil-tolymphocyte ratio; PLR, platelet-to-lymphocyte ratio; WBC, white blood cell.
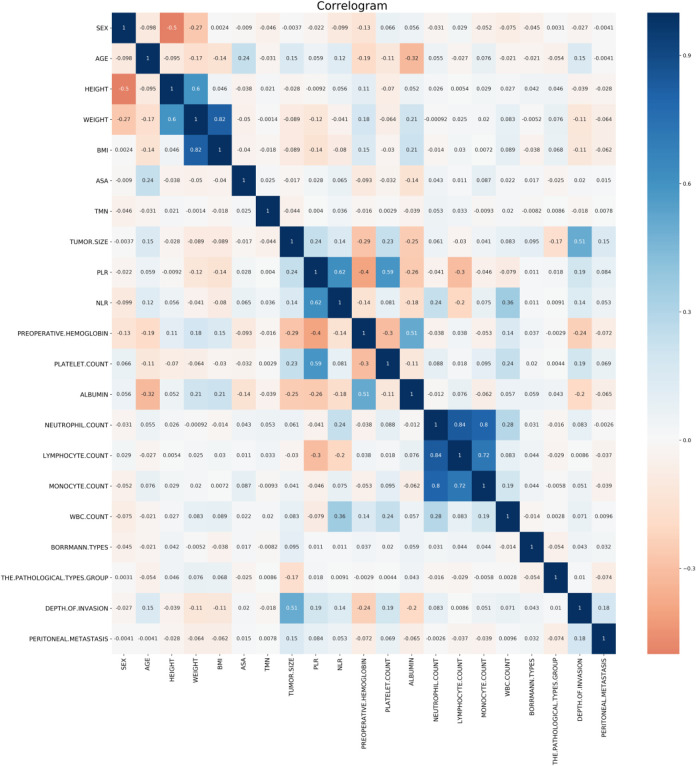
Correlation analysis showed that tumor size and depth of invasion were positively correlated with the recurrence of patients after gastric cancer surgery. Among them, tumor size and invasion depth were positively correlated with peritoneal metastasis, while body weight and BMI index were weakly correlated with peritoneal metastasis.(Figure 1) In addition, the results of the gbm(Light Gradient Boosting Machine) algorithm showed that the top 5 important factors were albumin, platelet count, depth of infiltration, preoperative hemoglobin and weight, respectively.(Figure 2)
Figure 1.
Correlation between variables.
Figure 2.
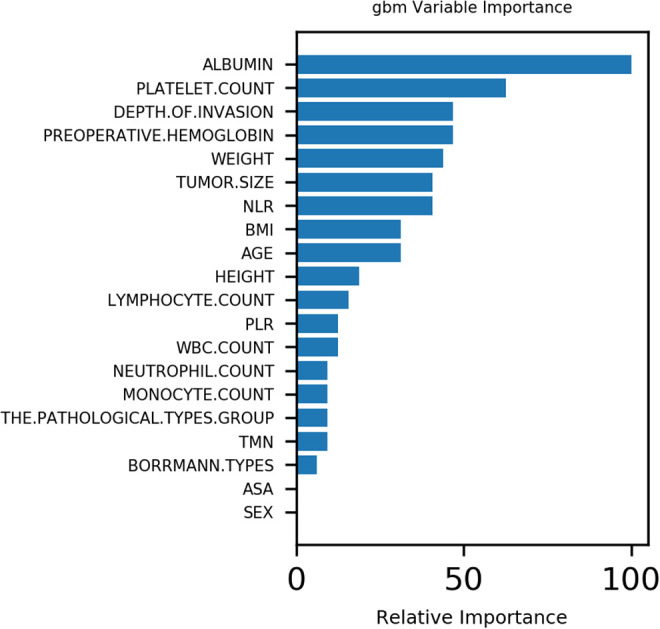
Variable importance of features included in machine learning algorithm for prediction of peritoneal metastasis.
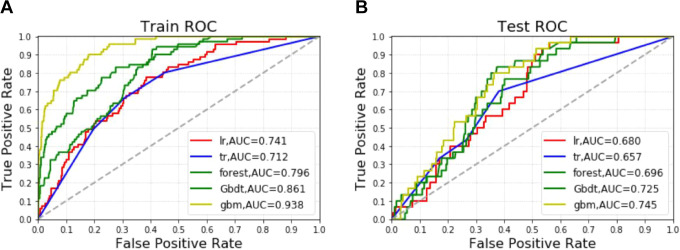
Effect of postoperative recurrence model of gastric cancer patients in a training group: Among the 5 algorithm models, the accuracy of GradientBoosting and gbm was the highest (0.909), and the accuracy of the other 3 algorithm models was (0.906). The AUC values of the 5 algorithms are gbm(0.938), GradientBoosting(0.861), forest(0.796), Logistic(0.741) and DecisionTree(0.712) from high to low. Of the 5 algorithms, both GradientBoosting and gbm have the lowest MSE value of 0.091.(Figure 3A and Table 2)
Figure 3.
Different machine learning algorithms predict the peritoneal metastasis in the training group(A) and test group(B).
Note: gbm: Light Gradient Boosting Machine.
Table 2.
Forecast Results for Training and Testing Group.
| Training | Testing | |||||
|---|---|---|---|---|---|---|
| Accuracy | AUC | MSE | Accuracy | AUC | MSE | |
| Logistic | 0.906 | 0.741 | 0.094 | 0.904 | 0.680 | 0.096 |
| DecisionTree | 0.906 | 0.712 | 0.094 | 0.907 | 0.657 | 0.093 |
| forest | 0.906 | 0.796 | 0.094 | 0.907 | 0.696 | 0.093 |
| GradientBoosting | 0.909 | 0.861 | 0.091 | 0.904 | 0.725 | 0.096 |
| gbm | 0.909 | 0.938 | 0.091 | 0.907 | 0.745 | 0.093 |
Note: gbm: Light Gradient Boosting Machine.
The effect of postoperative recurrence model of gastric cancer patients in the test group: among the 5 algorithm models, the accuracy of the forest, DecisionTree and gbm was the highest 0.907, and the accuracy of the other 2 algorithm models was 0.904; AUC values ranged from high to low to gbm(0.745), GradientBoosting(0.725), forest(0.696), Logistic(0.680) and DecisionTree(0.657). Among the 5 algorithms, DecisionTree, gbm and forest had the lowest MSE value of 0.093.(Figure 3B and Table 2)
Discussion
The peritoneum is a membrane-like structure covering the inner surface of the abdominopelvic wall and the surface of the viscera, which is mainly composed of mesothelial cells and a small amount of underlying connective tissue.13 It is a predilection site for malignant tumor metastasis of the abdominopelvic cavity. The incidence of peritoneal metastasis in patients after radical gastrectomy for gastric cancer is 40% ∼ 50%, which is the main type of recurrent metastasis after radical gastrectomy for gastric cancer, and also the main cause of death. There was no obvious symptom in the early stage of peritoneal metastasis, but further development could lead to ascites, gastrointestinal and ureteral obstruction, hydronephrosis, etc. which seriously affected the quality of life and prognosis of patients.14 Peritoneal metastasis of gastric cancer is often associated with a poor prognosis.15 The results of this study show that the machine learning algorithm can better predict peritoneal metastasis in patients with gastric cancer, and the accuracy of the 5 algorithms is as high as 90%.In addition, the results of the gbm algorithm showed that the top 5 important factors were albumin, platelet count, depth of infiltration, preoperative hemoglobin and weight, respectively.
The high-risk factors for peritoneal metastasis of gastric cancer include TNM staging, extranodal infiltration, Borrmann type Ⅲ ∼ Ⅳ, Lauren type diffused and abdominal free cancer cells positive. The incidence of peritoneal metastasis was 25% in T3, T4, and N positive patients, and postoperative pathology showed that the incidence of peritoneal metastasis was 3.84 times higher in lymph node-positive patients than in negative patients, and the risk of peritoneal metastasis was higher in patients with extranodal metastases. Albumin improves prognosis in patients with peritoneal metastases from gastric cancer.16 Low pretreatment hemoglobin levels may reflect poor prognosis in patients with endometrial cancer.17 This is similar to our findings, and albumin and preoperative hemoglobin are one of the top 5 important factors in peritoneal metastases in patients with gastric cancer.
In the report by Pawlik et al,18 regarding BMI and gastric cancer, patients with underweight BMI <18.5 kg/ m2 had worse overall survival after gastric cancer resection than patients with BMI above 18.5. Low body mass index has been reported to be associated with more serious postoperative complications and poor prognosis compared with patients with normal body mass index.19 Body mass index (BMI) may be a prognostic factor for diffuse gastric cancer in the peritoneum.20 This is supported by our findings.
Recently, NLR has been reported as an important independent predictor of peritoneal metastasis in patients with advanced GC.21 The predictive value of NLR for peritoneal metastasis during SL has been studied in early gastric cancer or lower esophageal cancer but has not been reported in advanced GC.22 Studies23 have also shown that high NLR is an important independent predictor of P/ CY-positive outcomes during SL in patients with advanced GC. Similarly, a meta-analysis24 of 26 studies, including 13964 patients, showed that PLR was a poor prognostic factor for OS in patients with gastric cancer and colorectal cancer, hepatocellular carcinoma, ovarian cancer, and non-small cell lung cancer, and was not a poor prognostic factor for pancreatic cancer. Gunaldi et al.25 studied the relationship between PLR and prognosis of gastric cancer by taking 160 as the cut-off value of PLR; The results showed that PLR was related to depth of invasion and stage of disease, and was not related to OS. Lian et al.26 followed up 162 patients with gastric cancer surgery, and selected PLR = 208 as demarcation value. The tumor invasion degree was deep in patients with high PLR, the lymph node metastasis was large, the clinical stage was late, and the OS and DFS of patients with high PLR were short. Our results also suggest that PLR and NLR are one of the important factors in peritoneal metastasis of gastric cancer.
There are still some limitations of this study that cannot be overlooked. This was a retrospective study. Therefore, tumor characteristics such as depth of invasion, lymphangitic invasion, and type of pathology were obtained after surgery. These parameters can be obtained pre-operatively by endoscopy. However, the results may not be accurate. In addition, we did not add prognostic analysis to the study due to incomplete data and partial loss of follow-up data. In addition, all these patients were from the same hospital. And in this study, T1, T2, T3 and T4 stages are not subdivided, which fails to compare the prediction performance of different stages. As this is a second retrospective analysis, we can only re-study the specific classification of T when we carry out relevant prospective research in the future.Therefore, our findings still need to be validated through a large prospective multicenter study. Besides, the clinical-pathological factors analyzed were few, and Ki-67, C-reactive protein, carcinoembryonic antigen and CA199 could be combined to select more significant independent prognostic indicators in the future.
Conclusion
This is the first attempt to study the use of machine learning to predict peritoneal metastases in patients with gastric cancer. We found that machine learning was a good predictor of peritoneal metastases in gastric cancer patients with an accuracy of up to 90%.
Acknowledgment
We are also very grateful to the BioStudies database (public database) for including and providing Professor Shen’s original data.27
Appendix A
Table 1.
Functions, Packages, and Tuning Parameters in the Anaconda Software Used for Each Machine Learning Algorithm.
| Algorithm | Classifier | Package | Tuning Parameters |
|---|---|---|---|
| Logistic regression | LogisticRegression | from sklearn.linear_model import LogisticRegression | Penalty = “l2,” tol = 0.0001, C = 1, intercept_scaling = 1, max_iter = 100 |
| DecisionTree | DecisionTreeClassifier | from sklearn.tree import DecisionTreeClassifier | splitter = “best,” max_depth = 2, min_samples_split = 20, min_samples_leaf = 5, min_weight_fraction_leaf = 0.1 |
| forest | RandomForestClassifier | from sklearn.ensemble import RandomForestClassifier | n_estimators = 10, max_depth = 3, min_samples_split = 70, min_samples_leaf = 6, random_state = 41 |
| GradientBoosting | GradientBoostinglassifier | from sklearn.ensemble import GradientBoostinglassifier | learning_rate = 0.06, n_estimators = 50, max_depth = 2, random_state = 41 |
| gbm | lgb.LGBMClassifier | lightgbm 2.2.0 | learning_rate = 0.1, n_estimators = 30, max_depth = 3 |
Note: gbm:(Light Gradient Boosting Machine).
Table 2.
Basic Characteristics of Training Group and Test Group.
| Training | Test | P-value | |
|---|---|---|---|
| Number | 756 | 324 | |
| Age (years) | 63.9 ± 11.1 | 63.3 ± 11.6 | 0.409 |
| Height(cm) | 165.0 ± 8.2 | 165.5 ± 7.7 | 0.359 |
| Weight(kg) | 58.7 ± 10.4 | 60.1 ± 10.7 | 0.051 |
| BMI (kg/m2) | 21.6 ± 3.0 | 21.9 ± 3.1 | 0.061 |
| Tumor size (cm) | 4.1 ± 2.2 | 3.8 ± 2.0 | 0.048 |
| PLR | 151.0 ± 71.5 | 150.4 ± 80.6 | 0.890 |
| NLR | 2.6 ± 1.5 | 2.6 ± 1.7 | 0.936 |
| PREOPERATIVE. HEMOGLOBIN | 117.5 ± 23.7 | 118.5 ± 24.4 | 0.539 |
| Platelet count | 229.0 ± 70.2 | 221.9 ± 69.4 | 0.128 |
| Albumin (g/L) | 39.5 ± 5.2 | 40.0 ± 5.2 | 0.136 |
| Neutrophil count | 4.0 ± 3.0 | 4.2 ± 5.1 | 0.627 |
| Lymphocyte count | 1.8 ± 1.9 | 1.8 ± 1.9 | 0.865 |
| Monocyte count | 0.4 ± 0.3 | 0.4 ± 0.5 | 0.838 |
| WBC count | 6.2 ± 1.6 | 6.0 ± 1.5 | 0.022 |
| Sex | 0.034 | ||
| Male | 574 (75.9%) | 265 (81.8%) | |
| Female | 182 (24.1%) | 59 (18.2%) | |
| ASA | 0.885 | ||
| 1 | 47 (6.2%) | 19 (5.9%) | |
| 2 | 641 (84.8%) | 273 (84.3%) | |
| 3 | 68 (9.0%) | 32 (9.9%) | |
| TNM | 0.314 | ||
| I | 183 (24.2%) | 76 (23.5%) | |
| II | 56 (7.4%) | 19 (5.9%) | |
| III | 383 (50.7%) | 182 (56.2%) | |
| IV | 134 (17.7%) | 47 (14.5%) | |
| Borrmann types | 0.382 | ||
| 1 | 44 (5.8%) | 19 (5.9%) | |
| 2 | 652 (86.2%) | 287 (88.6%) | |
| 3 | 60 (7.9%) | 18 (5.6%) | |
| Pathological type [n, (%)] | 0.650 | ||
| Ulcerative | 101 (13.4%) | 40 (12.3%) | |
| Nonulcerative | 655 (86.6%) | 284 (87.7%) | |
| Depth of invasion [n, (%)] | 0.401 | ||
| T1/T2 | 237 (31.3%) | 110 (34.0%) | |
| T3/T4 | 519 (68.7%) | 214 (66.0%) |
Footnotes
Authors’ Contributions: All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Availability of Data and Material: Data is available at BioStudies database(https://www.ebi.ac.uk/biostudies/studies?query=S-EPMC5383064), accession numbers: S-EPMC5383064.
Consent for Publication: All consent of the personal data were obtained from corresponding person.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to participate: Data is available at BioStudies database (https://www.ebi.ac.uk/biostudies/studies?query=S-EPMC5383064), accession numbers: S-EPMC5383064. Our study did not require the approval of an ethics committee as it is a secondary analysis of BioStudies database of a public domain and of free access[28] [29]. This article does not contain any studies with human participants or animals performed by any of the authors.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX19_0113) and the Recruitment Program of Overseas High-Level Young Talents, “Innovative and Entrepreneurial Team” (No.(2018)2015) of Jiangsu Province.
ORCID iD: Chengmao Zhou, PhD  https://orcid.org/0000-0001-5680-791X
https://orcid.org/0000-0001-5680-791X
References
- 1. Ong HS, Smithers BM. Epidemiology of gastric cancer. Cancer Rev: Asia-Pacific. 2004;2(01):1–7. [Google Scholar]
- 2. Thomassen I, Van Gestel YR, Van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–628. [DOI] [PubMed] [Google Scholar]
- 3. Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol. 2015;21(39):10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong D, Tang L, Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. 2019;30(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HY, Kim YH, Yun G, et al. Simpson, could texture features from preoperative CT image be used for predicting occult peritoneal carcinomatosis in patients with advanced gastric cancer? PLoS One. 2018;13(3):e0194755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, He J, Liu S, et al. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur Radiol. 2020;30(1) 239–246. [DOI] [PubMed] [Google Scholar]
- 7. Shinagare AB, Balthazar P, Ip IK, et al. High-grade serous ovarian cancer: use of machine learning to predict abdominopelvic recurrence on CT on the basis of serial cancer antigen 125 levels. J Am Coll Radiol. 2018;15(8):1133–1138. [DOI] [PubMed] [Google Scholar]
- 8. Tahmassebi A, Wengert GJ, Helbich TH, et al. Impact of machine learning with multiparametric magnetic resonance imaging of the breast for early prediction of response to neoadjuvant chemotherapy and survival outcomes in breast cancer patients. Invest Radiol. 2019;54(2):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Wu CJ, Bao ML, Zhang J, Wang XN, Zhang YD. Machine learning-based analysis of MR radiomics can help to improve the diagnostic performance of PI-RADS v2 in clinically relevant prostate cancer. Eur Radiol. 2017;27(10):4082–4090. [DOI] [PubMed] [Google Scholar]
- 10. Papandrianos N, Papageorgiou E, Anagnostis A, Papageorgiou K. Bone metastasis classification using whole body images from prostate cancer patients based on convolutional neural networks application. PloS One. 2020;15:e0237213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu Z, Chen H, Jiao X, et al. Prediction of immune checkpoint inhibition with immune oncology-related gene expression in gastrointestinal cancer using a machine learning classifier. J Immunother Cancer. 2020;8(2):e000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu C, Qi L, Feng Q, Sun S, Zhang Y, Liu X. Performance of a machine learning-based decision model to help clinicians decide the extent of lymphadenectomy (D1 vs. D2) in gastric cancer before surgical resection. Abdom Radiol (NY). 2019;44(9):3019–3029. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Yan R, Lei R, Jiang C. Comparison of PET with PET/CT in detecting peritoneal carcinomatosis: a meta-analysis. Abdom Imaging. 2015;40(7):2660–2666. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20(1):111–121. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed A, Ukwenya AY, Makama JG, Mohammad I. Management and outcome of gastric carcinoma in Zaria, Nigeria. Afr Health Sci. 2011;11(3):353–361. [PMC free article] [PubMed] [Google Scholar]
- 16. Ishikawa M, Iwasa S, Nagashima K, et al. Retrospective comparison of nab-paclitaxel plus ramucirumab and paclitaxel plus ramucirumab as second-line treatment for advanced gastric cancer focusing on peritoneal metastasis. Invest New Drugs. 2020;38(2):533–540. [DOI] [PubMed] [Google Scholar]
- 17. Metindir J, Dilek GB. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol. 2009;135(1):125–129. [DOI] [PubMed] [Google Scholar]
- 18. Ejaz A, Spolverato G, Kim Y, et al. Impact of body mass index on perioperative outcomes and survival after resection for gastric cancer. J Surg Res. 2015;195(1):74–82. [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Chen XZ, Zhang WH, et al. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10-Year, Single-Institution Cohort Study. Medicine (Baltimore). 2015;94(42):e1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Nie R, OuYang L, et al. Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakayama Y, Gotohda N, Shibasaki H, Nomura S, Kinoshita T, Hayashi R. Usefulness of the neutrophil/lymphocyte ratio measured preoperatively as a predictor of peritoneal metastasis in patients with advanced gastric cancer. Surg Today. 2014;44(11):2146–2152. [DOI] [PubMed] [Google Scholar]
- 22. Grenader T, Plotkin Y, Mohammadi B, et al. Predictive value of the neutrophil/lymphocyte ratio in peritoneal and/or metastatic disease at staging laparoscopy for gastric and esophageal adenocarcinoma. J Gastrointest Cancer. 2015;46(3):267–271. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura N, Kinami S, Fujii Y, et al. The neutrophil/lymphocyte ratio as a predictor of peritoneal metastasis during staging laparoscopy for advanced gastric cancer: a retrospective cohort analysis. World J Surg Oncol. 2019;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PloS One. 2014;9(6):e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunaldi M, Goksu S, Erdem D, et al. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. 2015;8(4):5937–5942. [PMC free article] [PubMed] [Google Scholar]
- 26. Lian L, Xia Y, Zhou C, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15(6):899–907. [DOI] [PubMed] [Google Scholar]
- 27. Chen XD, Mao CC, Wu RS, et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PloS One. 2017;12(4):e0175074. [DOI] [PMC free article] [PubMed] [Google Scholar]