Abstract
Background:
Cervical cancer is a public health problem and one of the leading causes of death in women worldwide. In Ethiopia, the government expands cervical cancer screening centers and recommends services to age-eligible and high-risk groups of women. However, evidence indicates that the utilization of services among eligible and high-risk women in the country has remained very low, and data are scarce in Dire Dawa. Therefore, this study aimed to assess cervical cancer screening service utilization and associated factors among women aged 30 to 49 years in Dire Dawa, eastern Ethiopia.
Methods:
A facility-based cross-sectional study was undertaken in Dire Dawa from February 01 to March 01, 2017. Only two facilities provided the screening service in Dire Dawa Administration. Six- hundred and one women aged 30 to 49 years were selected using a systematic sampling method. Data were collected using a pretested face-to-face interview administered questionnaire. Data were entered using EpiData 3.1, and analyzed using the Statistical Package for Social Science Version 21. Multivariable logistic regression was used to examine the factors associated with cervical cancer screening utilization. An adjusted odds ratio (AOR) with a 95% confidence interval (CI) was used, and a p-value <0.05 was considered statistically significant.
Results:
In this study, the magnitude of cervical cancer screening service utilization was 4.0% (95% CI: 2.5-5.7). The factors associated with cervical cancer screening service utilization were older age (AOR = 4.2; 95% CI:1.3-13.8), attending private health facilities (AOR = 8.9; 95% CI: 2.8-28.0), being employed (AOR = 3.3; 95% CI: 1.3-8.8), visiting the gynecology departments (AOR = 3.8; 95% CI: 1.5-9.8), being knowledgeable (AOR = 4.8; 95% CI: 1.5-15.5), being counseled by health professionals (AOR = 4.1; 95% CI: 1.5-11.3), and user’s of family planning (AOR = 4.9; 95% CI: 1.2-20.0).
Conclusion:
The magnitude of cervical cancer screening utilization was very low. Hence, to improve the screening service utilization of cervical cancer, a campaign on community awareness, strengthening service linkage among departments, expansion of the centers for cervical cancer screening, and promotion of family planning method utilization are recommended.
Keywords: cervical cancer, screening utilization, Dire Dawa, Ethiopia
Introduction
Cervical cancer is a serious public health problem worldwide. It is one of the leading causes of death in women and the second leading cause of female cancer-related deaths.1-4 Globally, more than 2.7 million women, of whom approximately 85% in low- and middle-income countries are at risk of acquiring cervical cancer.2,5 Of these, an estimated one million new cases of cervical cancer are diagnosed each year, half of them die, and over 85% of deaths each year as a result of cervical cancer occur in low- and middle-income countries.6-10
Cervical cancer screening utilization (CCSU) is low in low-income countries, particularly in sub-Saharan Africa. As a result, it is the leading cause of morbidity and mortality in the region.9 In Ethiopia, there are an insignificant number of facilities providing cervical cancer diagnostic modalities that aim to diagnose and treat based on World Health Organization (WHO) recommendations. They are meant for women aged 30 to 49 years and high-risk groups of women, such as those who have multiple sexual partners, smoke cigarettes, practice sex early, human immunodeficiency virus (HIV) positive, and have other sexually transmitted diseases.10,11
The burden is increasing because of late detection of most of the cases, unfavorable attitudes toward the screening services, low socioeconomic conditions, and lack of service, awareness, enough resources, and trained human power.12-15 The incidence and mortality of cervical cancer are 26.4 and 18.4 per 100,000 women per year, respectively.5,16 Women aged 30-49 years have a higher chance of developing precancerous lesions so that screening at this point has an opportunity to detect lesions earlier, which can be effectively treated with simple and low-cost interventions.17-20
Cervical cancer screening is testing for precancerous lesions and cancer among women who have no symptoms. Early screening is the most effective measure for early detection, treatment, and prevention of precancerous lesions and cancer.3,4,20,21 WHO recommends the use of screening and treatment approaches for women using visual inspection with acetic acid (VIA) for screening and cryotherapy for treatment.22 It is the most efficient and cost-effective screening technique for low-income countries.3,23 This method also increases coverage in all countries and ultimately decreases cervical cancer incidence and mortality.22,24 Moreover, early detection and treatment of precancer lesions at an early stage can reduce the incidence and mortality by 65%. If these interventions are universally available for women (i.e., 40% to 90% coverage), almost three thousand women can be saved each year.3,9,25 The burden of cervical cancer could also be prevented by increasing women’s awareness of its key prevention methods and creating and organizing opportunities for screening at local community levels.4,6,24
The Federal Minister of Health (FMOH) of Ethiopia has organized a national cancer control plan to be implemented from 2015 to 2020, which is a scale-up of the screening and treatment for cervical precancer into 800 health facilities (one health facility per district). The plan primarily targeted 30 to 49 years-old and high-risk groups of women.16 It aims to promote cervical cancer prevention and early detection, improving diagnosis and treatment to palliative care and maximize screening coverage to more than 80%. However, poor infrastructure, limited awareness, late-stage arrival for screening, and lack of trained human power are the main obstacles to achieving the plan across the nation, including our study setting.10,26,27
Despite efforts from governmental and non-governmental organizations to improve access to the screening service in Ethiopia, utilization has not raised.16,28 Different studies have documented factors associated with cervical cancer screening utilization worldwide, including the age of the women, risk perception, financial constraints, marital status, and parity, although there are inconsistencies among the studies.29,30 However, there is a paucity of data on CCSU in Ethiopia, particularly in Dire Dawa. Therefore, this study aimed to assess cervical cancer screening utilization and associated factors among women aged 30-49 years in Dire Dawa, eastern Ethiopia.
Materials and Methods
Study Setting, Design, and Population
A facility-based cross-sectional study was conducted in Dire Dawa from February 01 to March 01, 2017. Dire Dawa is found in eastern Ethiopia and located 515 km away from Addis Ababa, the capital of Ethiopia. According to the Central Statistical Agency population projections of 2017, its total estimated population was 359,000. Of these, 175,782 were women. Of the total number of women, approximately 55% were in the reproductive age group. Of these, nearly 5% were 30 to 49 years old.20,31 In Dire Dawa, there are two public hospitals and 15 public health centers, as well as 3 private hospitals and more than ten private higher clinics. However, only two of the health facilities; Dilchora Hospital and the Family Guidance Association Higher Clinic provide a cervical screening service. All women aged 30 to 49 years and attending the two facilities were included in the study, but those with severe mental illness and critically ill women were excluded. The facilities are integrated to link any eligible and high-risk women to screening service units from the antenatal care (ANC) unit, family planning unit, gynecology care unit, and adult outpatient departments.
Sample Size and Sampling Procedure
The sample size was determined using a single proportion formula considering the proportion of women who underwent cervical cancer screening in Mekelle, Ethiopia (19.8%),32 4% level of significance (α = 0.04), 95% CI, a design effect of 1.5 and 5% a non-response rate. The final sample size was 601. The two health facilities were selected based on service availability in the catchment area. We allocated the final sample size to the selected health facilities proportional to their monthly client flow from the previous year’s quarterly report of the same periods. The study subjects were selected from the ANC unit, family planning unit, gynecology outpatient unit, and adult outpatient unit attendants using a systematic sampling technique. A sampling interval (k) of 2 was used for each unit/department to select women aged 30-49 and high-risk women, where the first eligible woman was selected randomly.
Data Collection and Procedure
Data were collected using a pretested structured questionnaire adapted from different studies14, 16,22,32-34 and modified to suit the local context. The questionnaire was translated from English into local languages. Then, it was translated back into English to maintain consistency. Through face-to-face interviews, eight diploma nurses and midwives collected the data, and two supervisors checked the collection. Reproductive health-related questions were incorporated. The utilization of cervical cancer screening services was defined as women who ever used cervical cancer screening services within the past three to five years. Good knowledge was defined as those who scored > the mean score value of the knowledge measuring questions, while those who scored less than the mean value were labeled as having poor knowledge. We defined favorable attitudes as women who scored > the mean score value of the attitude measuring questions and unfavorable attitude for a score less than the mean value of the attitude measuring questions.33
Data Quality Control
To ensure the quality of the data, one-day training was given to all the data collectors and supervisors. Before the data collection, a pretest was carried out on 5% of the sample size (in Harar family guidance clinic), who were not included in the study. Based on the findings of the pretest, modifications to the questionnaire were made. The data collection process was closely supervised, and the completeness of each questionnaire was checked by the investigators and the supervisors on a daily basis. Finally, double data entry was performed to check the consistency of the data.
Data Processing and Analysis
The data were coded and entered EPI Data Version 3.1, and then exported to SPSS Version 21 statistical software for analysis. Data were summarized and presented using descriptive statistics. The outcome variables were coded as “1” for women who used the screening service whereas “0” for others. The associations between the outcome variables (i.e., received the screening service) and the independent variables were analyzed using a binary logistic regression model. Covariates with a p-value <0.2 were retained and entered into the multivariable logistic regression analysis. Hosmer and Lemeshow goodness-of-fit tests were used to assess whether the necessary assumptions were fulfilled. The results were presented as adjusted odds ratio (AOR) with 95% confidence interval (CI). A p-value <0.05 was considered for declaring statistical significance.
Ethical Consideration
This study was approved by the Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University (approval no.IHRERC 84/2017). All patients provided informed written consent before enrollment in the study. Following approval, a written official letter of cooperation was given to the Dire Dawa Regional Health Bureau and the respective facility heads. Then, a permission letter was obtained from the respective officials. The purpose and importance of the study were explained to the study participants and facility heads. To ensure confidentiality, names and other identifiers of the women were not recorded on the data collection tools.
Results
Sociodemographic Characteristics
In this study, 595 participants were interviewed, yielding a response rate of 99%. Their mean age was 37.4 (SD ±4.1) years. Of all the respondents, 397 (66.7%) were from a public health facility; 425 (70.3%) were urban dwellers; 345 (58.0%) were married; 426 (71.5%) attended formal education; and 426 (70.5%) were employed (Table 1).
Table 1.
Socio-Demographic Characteristics Among Women Who Attended Health Facilities in Dire Dawa, Eastern Ethiopia, February 01 to March 01, 2017 [N = 595].
| Variables | Frequency(N) | Percent (%) | |
|---|---|---|---|
| Age women in years | 30-39 | 444 | 74.6 |
| 40-49 | 151 | 25.4 | |
| Educational status | No Formal Education | 169 | 28.4 |
| Primary Level | 75 | 12.6 | |
| Secondary Level | 122 | 20.5 | |
| College and Above | 229 | 38.5 | |
| Marital Status | Married | 345 | 58.0 |
| Single | 104 | 17.5 | |
| Divorced | 109 | 18.3 | |
| Widowed | 37 | 6.2 | |
| Occupational status | Gov’t employee | 180 | 30.1 |
| Private worker | 230 | 38.7 | |
| House Wife | 160 | 27.0 | |
| Unemployment | 25 | 4.2 | |
| Residence | Urban | 418 | 70.3 |
| Rural | 177 | 29.7 | |
| Monthly Income in USD | <22.2$ | 163 | 27.4 |
| 22.3$ -61.1$ | 197 | 33.1 | |
| 61.14$-118.5$ | 202 | 34.0 | |
| >118.6$ | 33 | 5.5 | |
Reproductive Health Characteristics
Two hundred and forty-three women (40.8%) had a history of chronic lower pelvic pain. The majority of the women 507 (85.2%) had ever taken the HIV test and counseling. More than half 378 (63.5%) of the participants had sexual intercourse before the age of sixteen years. Three hundred eighty-three (64.4%) used modern family planning methods. Of these, 198 (51.7%) used injections, and 84 (21.9%) used the implant. Three hundred twenty-five (54.6%) participants said that they had a history of sexually transmitted infections (STIs) during the last one year, and 37 (6.2%) participants had a history of smoking. Regarding parity, 513 (86.2%) women were multiparous and 82 (13.8%) of them were nulliparous.
Knowledge Related to Cervical Cancer Screening Services
Among the total women, three hundred ten (52.1%) heard about cervical cancer. Of them, two hundred (64.5%) heard from mass media, 87 (28.0%) from health care providers, and 82 (26.5%) from the Community Cervical Cancer Screening campaign. Regarding the main risk factors for cervical cancer development, 98 (31.6%) of the 310 respondents believed it was having multiple sexual partners; for 63 (20.3%), it was early sexual practice; for 45 (14.5%), it was related to having a weak immune system; and for 58 (18.7%), it was smoking cigarette. Moreover, one hundred and one (42.3%) of the 310 women knew the availability of screening services in the facilities, they were attending; 45 (14.5%) knew bleeding or pain after coitus is a sign of cervical cancer. The majority of 132 (42.6%) women mentioned abnormal vaginal discharge, followed by abnormal vaginal bleeding 65 (21.0%). Regarding the prevention methods, only 133 (43.0%) knew at least one prevention method, 85 (27.4%) mentioned cervical screening, 74 (23.9%) mentioned avoiding risk factors such as premarital sex, STI, multiple sexual partners used to prevent cervical cancer and only 3 (1.0%) human papillomavirus vaccine. The overall mean score for knowledge measurement questions was 15, and 173 (29.1%) participants had good knowledge about CCSSU (Table 2).
Table 2.
Knowledge related to cervical cancer risks, signs, prevention, consequence and service availability among women who attended Health facilities in Dire Dawa, eastern Ethiopia, February 01-March 01, 2017 [n=310].
| Variables | Yes | Percent (%) | No | Percent (%) |
|---|---|---|---|---|
| Human Papilloma Virus infection risk for cervical cancer | 28 | 9.1 | 282 | 90.9 |
| Having Multiple Sexual Partners risk for cervical cancer | 98 | 31.6 | 212 | 68.4 |
| Performed early sexual practice <20 years risk for cervical cancer | 63 | 20.3 | 247 | 79.7 |
| Having a weak immune system risk for cervical cancer | 45 | 14.5 | 265 | 85.5 |
| Use of contraceptive for more than 6 years risk for | 17 | 5.5 | 293 | 94.5 |
| Having Cigarette smoking exposed to cervical cancer | 58 | 18.7 | 252 | 81.3 |
| Cervical cancer symptoms gradually appear | 181 | 58.4 | 129 | 41.6 |
| Abnormal vaginal bleeding is a sign of cervical cancer | 65 | 21.0 | 245 | 79.0 |
| Abnormal Vaginal discharge is a sign of cervical cancer | 132 | 42.6 | 178 | 57.4 |
| Bleeding or pain after coitus is a sign of cervical cancer | 45 | 14.5 | 265 | 85.5 |
| Bleeding after menopause is a sign of cervical cancer | 21 | 6.8 | 289 | 93.2 |
| Cervical screening used to prevent cervical cancer | 85 | 27.4 | 225 | 72.6 |
| Avoid risk factors like Premarital sex, STI, multiple sexual partners used to prevent cervical cancer | 74 | 23.9 | 236 | 76.1 |
| HPV vaccine is used for prevent cervical cancer | 3 | 1.0 | 307 | 99.0 |
| Women with untreated cervical cancer can die | 215 | 69.4 | 95 | 30.6 |
| Advanced stage of cervical cancer causes infertility | 105 | 33.9 | 205 | 66.1 |
| Cervical cancer causes reproductive organ disability | 91 | 29.4 | 219 | 70.6 |
| The advanced stage of cervical cancer causes psychosocial problems in women’s life. | 65 | 21.0 | 245 | 79.0 |
| Cervical cancer is easily cured if detected at an early stage | 198 | 63.9 | 112 | 36.1 |
| Women know cervical screening service is available in this facility. | 131 | 42.3 | 179 | 57.7 |
| Women’s aged 30-49 is recommended to take the cervical screening | 73 | 23.6 | 237 | 76.4 |
| Any high-risk groups of women are advised to take cervical screening at the health facility. | 57 | 18.4 | 253 | 81.6 |
Attitude and Perception of Respondents Toward Cervical Cancer Screening
Two hundred (64.6%) participants agreed that cervical cancer can be prevented. Two hundred fifty-one (81.0%) strongly support recommending screening services for friends. The mean attitude score was 3, and 137 (23.0%) respondents had a favorable attitude towards cervical cancer and its screening services. (Table 3).
Table 3.
Attitude of Respondents Toward Cervical Cancer Screening Utilization in Dire Dawa, eastern Ethiopia, February-March 2017 [n = 310].
| Variable | Respondents attitude toward screening service utilization (n = 310) | ||||
|---|---|---|---|---|---|
| Strongly disagree (1) | Disagree (2) | Neutral (3) | Agree (4) | Strongly agree (5) | |
| Cervical cancer is easily cured | 4 (1.2%) | 13 (4.2%) | 93 (30.0%) | 103 (33.2%) | 98 (31.6%) |
| Cervical cancer affects any women | 4 (1.2%) | 33 (10.6%) | 135 (43.5%) | 88 (28.4%) | 49 (15.8%) |
| Cervical cancer can prevented | 2 (0.6%) | 14 (4.5%) | 93 (30.0%) | 122 (39.4%) | 78 (25.2%) |
| Being Screen no Harm | 3 (1.0%) | 29 (9.4%) | 140 (45.2%) | 90 (29.0%) | 47 (15.1%) |
| Free cost screening | 5 (1.6%) | 35 (11.3%) | 133 (42.9%) | 94 (30.0%) | 42 (13.5%) |
| Recommend to friends | 2 (0.7%) | 5 (1.6%) | 51 (16.5%) | 144 (46.5%) | 107 (34.5%) |
Cervical Cancer Screening Utilization
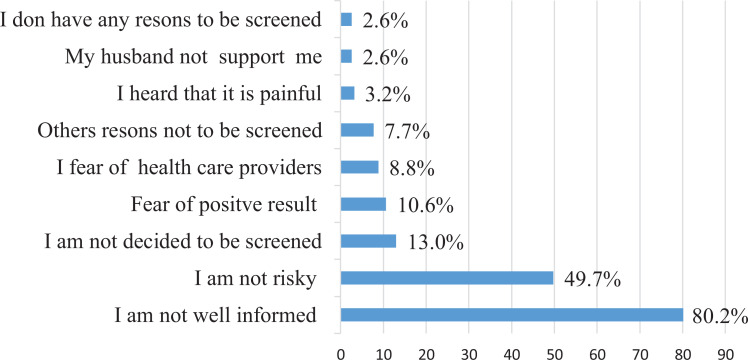
The magnitude of cervical cancer screening utilization was 24 (4.0%; 95% CI: 2.5%- 5.7%). Of these, 8 were in the early stages of the disease, and 16 were screened in private health facilities. Among the women who were not screened, the main reason for 458 (80.2%) was lack of awareness about cervical cancer and screening services, and 284 (49.7%) were believed not risky for the disease (Figure 1).
Figure 1.
The reasons for not utilizing cervical cancer screening among women who attended health facilities in Dire Dawa, eastern Ethiopia, February- March 2017 [N = 571].
Factors Associated With Cervical Cancer Screening Service Utilization
In the multivariable logistic regression analysis, women aged 40-49 years, attending private health facilities, being government employed, attending care in the gynecology unit, being knowledgeable, being counseled by health care providers, and users of family planing were significantly associated with CCSSU. Older women (aged 40-49 years) were almost four times (AOR = 4.2; 95% CI: 1.3-13.8) more likely to use the screening service compared with women aged less than 40 years. The number of women who attended private health facilities were approximately nine times (AOR = 8.9; 95% CI: 2.8- 28.0) more likely to use screening services compared with those who attended public health facilities. Furthermore, government employee women were three times (AOR = 3.3; 95% CI: 1.3- 8.8) more likely to utilize the service compared with others. The women who visited the gynecology unit were almost four times (AOR = 3.8; 95% CI: 1.5- 9.8) more likely to undergo cervical cancer screening. Moreover, knowledgeable women were five times (AOR = 4.8; 95% CI: 1.5- 15.5) more likely to use the services than their counterparts. Additionally, those who were counseled by healthcare providers about cervical cancer and those who used family planning were four and five times (AOR = 4.1; 95% CI: 1.5-11.3), (AOR = 4.9; 95% CI: 1.2- 20.0) more likely to utilize cervical cancer screening, respectively (Table 4).
Table 4.
Factors Associated With Cervical Cancer Screening Utilization Among Women Who Attended Health Facilities in Dire Dawa, eastern Ethiopia, February-March 2017 [N = 595].
| Variables | Screening service utilization | COR (95% CI) | AOR (95% CI) | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Age in years | 30-39 | 430 (92.8%) | 13 (7.2%) | 1.00 | 1.00 |
| 40-49 | 141 (97.1%) | 11 (2.9%) | 2.10 (1.1, 5.9)* | 4.2 (1.3, 13.8)** | |
| Type of health facility | Private | 182 (91.9%) | 16 (8.1%) | 4.28 (1.8, 10.2)* | 8.9 (2.8, 28.0)** |
| Public | 389 (98.0%) | 8 (2.0%) | 1.00 | 1.00 | |
| Occupation | Government employee | 170 (91.4%) | 16 (8.6%) | 2.99 (1.2-7.5)* | 3.3 (1.3, 8.8)** |
| Non-governmental | 401 (98.0%) | 8 (2.0%) | 1.00 | 1.00 | |
| Income in USD | ≥129.6$ | 151 (92.6%) | 12 (7.4%) | 2.7 (1.2, 6.3)* | 4.7 (0.9, 23.8) |
| ≤129.5$ | 420 (97.2%) | 12 (2.8%) | 1.00 | 1.00 | |
| Attending unit | Women visits gynecology OPD | 124 (90.5%) | 13 (9.5%) | 4.26 (1.9, 9.7)* | 3.8 (1.5, 9.8)** |
| Other unit | 447 (97.5%) | 11 (2.4%) | 1.00 | 1.00 | |
| Knowledge | Good knowledge | 155 (89.6%) | 18 (10.4%) | 8.05 (3.1, 20.6)* | 4.8 (1.5,15.5)** |
| Poor knowledge | 416 (98.6%) | 6 (1.4%) | 1.00 | 1.00 | |
| Attitude | Unfavorable Attitude | 289 (94.1%) | 18 (5.9%) | 2.9 (1.16-7.5)* | 1.4 (0.3, 5.2) |
| Favorable Attitude | 282 (97.9%) | 6 (2.1%) | 1.00 | 1.00 | |
| Counseled by HCW | Yes | 139 (89.7%) | 16 (10.3%) | 6.2 (2.6, 14.8)* | 4.1 (1.5, 11.3)** |
| No | 432 (98.2%) | 8 (1.8%) | 1.00 | 1.00 | |
| Know the availability of screening service | Yes | 182 (91.9%) | 16 (8.1%) | 4.0 (1.7, 9.2)* | 1.6 (0.1, 17.6) |
| No | 389 (98.0%) | 8 (2.0%) | 1.00 | 1.00 | |
| Invitation by HCP from clients | Yes | 389 (98.0%) | 16 (8.1%) | 4.3 (1.8,10.8)* | 2.2 (0.6, 8.2) |
| No | 362 (94.8%) | 8 (2.0%) | 1.00 | 1.00 | |
| Family planning | Used | 209 (98.1%) | 20 (5.2%) | 2.9 (1, 8.8)* | 4.9 (1.2,20.0)** |
| Not used | 227 (93.4%) | 4 (1.9%) | 1.00 | 1.00 | |
| Chronic pelvic pain | Had treated | 344 (97.7%) | 16 (6.6%) | 3.03 (1.3-7.19)* | 2.3 (0.7, 7.5) |
| Not treated | 227 (94.5%) | 8 (2.3%) | 1.00 | 1.00 | |
| STIs | Had Exposure | 264 (97.8%) | 18 (5.5%) | 2.57 (1.0, 6.6)* | 1.7 (0.5, 5.8) |
| Had No exposure | 309 (94.6%) | 6 (2.2%) | 1.00 | 1.00 | |
| SRH service | Attending | 352 (98.2%) | 20 (5.4%) | 3.1 (1, 9.1)* | 1.7 (0.5, 6.6) |
| Not attend | 219 (89.7%) | 4 (1.8%) | 1.00 | 1.00 | |
* significant COR, P ≤ 0.05, **significant association at AOR P ≤ 0.05, SRH; Sexual and Reproductive health service and STIs; sexually transmitted infections
Discussion
The Ethiopian FMOH recommends cervical cancer screening every three-five years irrespective of HIV status. In resource-poor settings, including Ethiopia, 30-49 years old women are the target population for screening because cervical cancer is rare in women under 30 years old, except for high-risk groups of women. It is an interventional period to minimize the devastating effects on women’s lives.16 Therefore, we conducted this study to determine cervical cancer screening service uptake and the associated factors among women aged 30-49 years in Dire Dawa, eastern, Ethiopia. Thus, the findings will help health planners to improve service-related barriers and plan an effective intervention applicable in the local context.
The findings of the present study indicated that the magnitude of CCSU was 4.0% (95% CI: 2.5%, 5.7%). This finding is extremely lower than those found in low- and middle-income countries; for example, 39% in Botswana,35 25% in Tanzania,36 and 25% in Kenya.37 It is also lower than reported in Ethiopia: 10.7% to 19.8% in Mekelle,32,33 8.3% in Dessie,34 and 21.8% in Addis Ababa and southern Ethiopia.38,39 The possible explanation for this low cervical cancer screening utilization could be due to the low level of awareness, limited access to screening services, socio-cultural, sociodemographic, economic disparities, and lack of specificity of national cancer prevention and control strategies in Ethiopia. Moreover, Ethiopia has invested little in the infrastructure, training, and laboratory capacity required for successful screening services. However, this study is in agreement with study findings in some African countries: for example, 6% in Morocco,40 5% in Nigeria,41, 4.8% in Uganda,42 and 2.9% in a nationwide study in Ethiopia.43
In the multivariable logistic regression analysis, older age, attendants of private health facilities, being employed, visiting the gynecology unit, being knowledgeable, receiving information from health professionals, and user of family planning were independently associated with CCSU. The women aged 40-49 years were four times more likely to be screened compared to the women aged 30-39 years. This is in line with study results in Ethiopia,34 Malaysia,44 and Kenya.23,45 The explanation for this could be that individuals would consider being at risk and seeking care after recognizing symptoms and perceiving susceptibility. Most of the women said that they would have made gynecological examinations and screenings if they had obtained the information and felt the symptoms of the disease. Furthermore, the women who were knowledgeable about cervical cancer and screening were five times more likely to use screening services than their counterparts. This finding is also supported by studies in Kenya,45 Ethiopia,46 Nigeria,47 Malaysia,21 Jamaica,48 and Tanzania,36 which revealed that knowledge of cervical cancer and its prevention increases the odds of screening utilization.
Furthermore, employed women were three times more likely to practice screening services compared to unemployed women. This is consistent with reports from Kenya49 and Korea,50 where the odds of screening utilization increased by two times more among employed women. This might be due to an invitation by coworkers in the workplace. In addition, employed women are educated and thus can solve the constraints of money to attend their health condition. Moreover, the women who attended private health facilities were approximately nine times more likely to use screening services than those who attended public health facilities. This might be due to the need for safety and privacy issues and promotion and intervention activities to eligible women disseminate information about cervical cancer and its screening by private health facilities. In addition, there might be adequate counseling to the individual client about cervical cancer and its screening service by healthcare providers in these facilities.
The respondents’ perception of potential susceptibility to cervical cancer was another critical factor in predicting the chance of screening service utilization. The participants who attended the gynecology unit were about four times more likely to undergo screening, which is consistent with study findings in Ethiopia.14,30,32,33 This might be due to the strong relationship between gynecological problems with cervical cancer and most reproductive problems referred to this unit. When women become more risk full and become symptomatic for cervical cancer, this increases the chance of utilizing screening services.
As per this study, the women who were counseled by healthcare providers about cervical cancer and their prevention methods were four times more likely to utilize screening services than their counterparts. This finding is in agreement with the studies in France,30 Thailand,51 Uganda,42 Korea,50 and Kenya.49,52 This might be due to the trust of healthcare providers, which increases the probability of screening services utilization. Similarly, those users of family planning were five times more likely to accept CCSU compared to those who did not use family planning. This finding is in line with those in Malaysia44 and Kenya.23 This could be due to an increased chance of communicating with health care providers to obtain information about cervical screening and the availability of service screening services.
This study was not without limitations. First, as a cross-sectional study, it is difficult to establish a cause-and-effect relationship between dependent and independent variables. Second, it used self-reporting (interview response), which might have a social desirability bias. Some questions also required the participants to recall, which could have affected the results, as most of them could have forgotten. Third, this finding is from a facility-based study, which is not representative of the whole population. Its strength was based on WHO recommendations and aged eligible and high-risk groups of women in inclusive data in the facility, which can infer for facilities attendants.
Conclusion
In conclusion, the prevalence of CCSU was low in Dire Dawa. Women’s age, attending private health facilities, being employed, visiting the gynecology department, being knowledgeable, being counseled by healthcare providers, and using family planning were independently associated with CCSU in Dire Dawa, eastern Ethiopia. Hence, to improve screening service utilization, campaigns on community awareness, working on women’s education, strengthening service linkage among departments, expansion of the centers for cervical cancer screening, and promotion of family planning method utilization are recommended. In addition, cervical cancer screening can be increased through updating the healthcare providers’ knowledge and counseling skills on cervical cancer and health workers to discuss the disease when women visit the health facilities.
Acknowledgments
We would like to thank Haramaya University, Dire Dawa University, and the Ministry of Education for unreserved technical support. Then, we also thank the Health Bureau, hospital heads, and the study participants for collaborations.
Abbreviations
- ANC
Antenatal Care
- AOR
Adjusted Odds Ratio
- CI
Confidence Interval
- CSA
Central Statistical Agency
- CCSU
Cervical Cancer Screening Utilization
- FMOH
Federal Minsters of Health
- HIV
Human Immunodeficiency Virus
- HPV
Human Papillomavirus
- SPSS
Statistical Package for Social Science
- STD
Sexually Transmitted Disease
- STIs
Sexually Transmitted Infections
- WHO
World Health Organization.
Authors’ Note: All the data of this study are available from the corresponding author upon request. All authors contributed equally to this work. YB, AS, MD, AD and NA contributed to study concept, design, acquisition of data, contributed to analysis and interpretation of data. AD and YB contributed to draft of the manuscript. All authors read and approved the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yalelet Belay  https://orcid.org/0000-0003-1249-600X
https://orcid.org/0000-0003-1249-600X
Assefa Desalew  https://orcid.org/0000-0001-6065-0708
https://orcid.org/0000-0001-6065-0708
References
- 1. Nega AD, Woldetsadik MA, Gelagay AA. Low utilization of cervical cancer screening among HIV positive women in Gondar University referral hospital, Northwest Ethiopia: cross-sectional study design. BMC Womens Health. 2018;18(1):1–7. doi:10.1186/s12905-018-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions. Published 2019. https://www.who.int/reproductivehealth/publications/thermal-ablation-for-cervicalpre-cancer-lesions/en/ [PubMed]
- 3. WHO. Comprehensive Cervical Cancer Control. WHO Publications; 2014:366–378. [Google Scholar]
- 4. Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World; Summary Report 2015 2016;04–08. www.hpvcentre.com. [Google Scholar]
- 5. Bruni L, Albero G, Serrano B, et al. Human Papillomavirus and Related Diseases Report. ICO/IARC Inf Cent HPV and Cancer (HPV Inf Centre) 2019:307 Accessed July 10, 2019 https://hpvcentre.net/statistics/reports/XWX.pdf.
- 6. O’Donovan J, O’Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: a systematic scoping review of the literature. BMJ Glob Heal. 2019;4(3):1–8. doi:10.1136/bmjgh-2019-001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO. International Agency for Research on Cancer World cancer factsheet. World Heal Organ. 2014;2012(2012):2012–2015. Accessed September 20, 2016 www.iarc.fr. [Google Scholar]
- 8. WHO. Technical Guidance and Specifications of Medical Devices for Screening and Treatment of Precancerous Lesions in the Prevention of Cervical Cancer. World Health Organization; 2020. Accessed May 20, 2020 http://apps.who.int/iris. [Google Scholar]
- 9. Ntekim A. Cervical cancer in sub Sahara Africa In: Rajamanickam R, ed. Topics on Cervical Cancer With an Advocacy for Prevention [Internet]. InTech; Published 2012. Accessed July 6, 2016 http://www.intechopen.com/books/topics-on-cervical-cancer-with-an-advocacy-for-prevention/cervical-cancer-in-sub-sahara-africa. [Google Scholar]
- 10. ICO (Information Centre on HPV and Cancer (HPV Information. Human Papillomavirus and Related Diseases Report(ICO (Information Centre on HPV and Cancer (HPV Information). 2016. Accessed May 10, 2017. [Google Scholar]
- 11. Gebre Y, Zemene A, Fantahun A, Aga F. Assessment of treatment compliance and associated factors among cervical cancer patients in Tikur Anbessa specialized hospital, Oncology Unit, Ethiopia 2012. Int J Cancer Stud Res. 2015;4(2):67–74. doi:10.19070/2167-9118-1500010 [Google Scholar]
- 12. Path. Cervical cancer screening and treatment in low-resource settings practical experience from path |2013 Cervical Cancer Prevention: Practical Experience Series acknowledgments. 2013:1–32. Accessed September 20, 2016 https://path.azureedge.net/media/documents/RH_ccp_screening_treatment.pdf.
- 13. PSI Kenya. Evidence series: cervical cancer screening and prevention, and barriers to utilization. Popul Serv Int. 2016;16(1):1–11. [Google Scholar]
- 14. Bante SA, Getie SA, Getu AA, Mulatu K, Fenta SL. Utilization of pre-cervical cancer screening and associated factors among reproductive-age women in Debre Markos town, Northwest Ethiopia, 2017. BMC Public Health. 2019;19(1):1–9. doi:10.1186/s12889-019-7398-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ethiopian Public Health Institute (EPHI).Ethiopian public health Institute Ethiopia Service Availability and Readiness Assessment (SARA) 2018 Final Report. 2018:119 Accessed May 10, 2019 https://www.ephi.gov.et/index.php/2014-04-10-07-22-18/download.
- 16. Federal Ministry of Health. National Cancer Control Plan 2016-2020 of Ethiopia. Dis Prev Control Dir. 2015:83 Accessed July 22, 2019. [Google Scholar]
- 17. Addis T. Combating cervical cancer in ethiopia addressing the screening and treatment gap: the single-visit approach. Pathfind Int. 2010:1–2. Accessed October 26, 2016 http://www2.pathfinder.org/site/DocServer/Ethiopia_CC_launch_brief.pdf
- 18. WHO. Latest world cancer statistics global cancer burden rises to 14.1 million new cases in 2012: marked increase in breast cancers must be addressed. 2013:2012–2014.
- 19. Federal Ministry of Health. . National Cancer Control Plan 2016-2020: disease prevention and control directorate. Published 2016. Accessed July 3, 2016 http://www.fmohe.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hf
- 20. ICF and (CSA) [Ethiopia]. Ethiopia Demographic and Health Survey 2016: Key Indicators Report . CSA and ICF; 2016. [Google Scholar]
- 21. Nwabichie CC, Manaf RA, Ismail SB. Factors affecting the utilization of cervical cancer screening among African women in Klang Valley, Malaysia. Asian Pac J Cancer Prev. 2018;19(3):825–831. doi:10.22034/APJCP.2018.19.3.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. New guidelines for the screening and treatment of cervical cancer. Published 2014. http://www.who.int/reproductivehealth/topics/cancers/guidelines/en/
- 23. Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132 doi:10.1371/journal.pmed.0050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. HPV Information Centre. Human papillomavirus and related cancers, fact sheet 2018; key data on HPV and HPV-related cancers. Econ Outlook. 2006;2013:2013–2014. Accessed September 20, 2016 www.hpvcentre.net [Google Scholar]
- 25. Kahesa C, Mwaiselage J, Wabinga HR, Ngoma T, Kalyango JN, Karamagi CAS. Association between invasive cancer of the cervix and HIV-1 infection in Tanzania: the need for dual screening. BMC Public Health. 2008;8(1):1–8. doi:10.1186/1471-2458-8-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Central Statistical Agency (CSA). 2016 Ethiopia demographic and health survey (EDHS) Introduction and Methodology. Published 2016. Accessed September 20, 2017 http://www.ethiodemographyandhealth.org/Measure_DHS_Ethiopia2016.pdf.
- 27. Kolo HT. Increasing breast and cervical cancer screening utilization in women of child-bearing age in Niger state, Nigeria. J Glob Oncol. 2018;(4_suppl_2):213s–213s. doi:10.1200/jgo.18.85900 [Google Scholar]
- 28. Waktola EA, Mihret W, Bekele L. HPV and burden of cervical cancer in East Africa. Gynecol Oncol. 2005;99(3):201–202. doi:10.1016/j.ygyno.2005.07.083 [DOI] [PubMed] [Google Scholar]
- 29. Islam RM, Billah B, Hossain MN, Oldroyd J. Barriers to cervical cancer and breast cancer screening utilization in low-income and middle-income countries: a systematic review. Asian Pacific J Cancer Prev. 2017;18(7):1751–1763. doi:10.22034/APJCP.2017.18.7.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tron L, Lert F, Spire B, et al. Levels and determinants of breast and cervical cancer screening utilization in HIV-infected women compared with the general population in France. HIV Med. 2017;18(3):181–195. doi:10.1111/hiv.12412 [DOI] [PubMed] [Google Scholar]
- 31. CSA. 2007 Population and housing census of Ethiopia administrative report. 2007:125 catalog.ihsn.org›Home›CentralDataCatalog%0A
- 32. Bayu H, Berhe Y, Mulat A, Alemu A. Cervical cancer screening service utilization and associated factors among age-eligible women in Mekelle zone, Northern Ethiopia, 2015: a community-based study using the health belief model. PLoS One. 2016;11(3):1–13. doi:10.1371/journal.pone.0149908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gebreegziabher M, Asefa NG, Berhe S. Factors affecting the practices of cervical cancer screening among female nurses at public health institutions in Mekelle town, Northern Ethiopia, 2014: a cross-sectional study. J Cancer Res. 2016;2016:1–7. doi:10.1155/2016/4743075 [Google Scholar]
- 34. Assefa Andargie S, Reddy. Knowledge, attitude, practice, and associated factors of cervical cancer screening among women in Dessie Referral Hospital and Dessie Health Center, Northeast Ethiopia. Glob J Res Anal. 2015;4(12):248–251. [Google Scholar]
- 35. Ibekwe CM. Factors Influencing Cervical Cancer Screening Utilization Among Women Attending Mahalapye District Hospital in Botswana—Use of the Health Belief Model [Dissertation]. 2009;12(1). Accessed September 20, 2016 http://hdl.handle.net/10386/227 [Google Scholar]
- 36. Kileo NM, Michael D, Neke NM, Moshiro C. Utilization of cervical cancer screening services and its associated factors among primary school teachers in Ilala Municipality, Dar es Salaam, Tanzania. BMC Health Serv Res. 2015;15(1):1–9. doi:10.1186/s12913-015-1206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murugi NA. Cervical Cancer Knowledge and Screening Utilization Among Women in Embu County, Kenya [Dissertation]. Published 2014. Accessed September 20, 2016 10.1111/ajco.12332 [DOI]
- 38. Gebrie MH. Knowledge, preventive practice, and associated factors of female nurses? Towards cervical cancer in the selected government hospitals in Addis Ababa, Ethiopia. J Diabetes Metab. 2015;6(7):569 doi:10.4172/2155-6156.1000569 [Google Scholar]
- 39. Gedefaw A, Astatkie A, Tessema GA. The prevalence of precancerous cervical cancer lesion among HIV-infected women in Southern Ethiopia: a cross-sectional study. PLoS One. 2013;8(12):1–8. doi:10.1371/journal.pone.0084519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Selmouni F, Zidouh A, Alvarez-Plaza C, El Rhazi K. Perception and satisfaction of cervical cancer screening by Visual Inspection with Acetic acid (VIA) at Meknes-Tafilalet Region, Morocco: a population-based cross-sectional study. BMC Womens Health. 2015;15(1):1–6. doi:10.1186/s12905-015-0268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adeoye OO, Fawole O, Ajayi I, Nguku P. Cervical cancer knowledge, screening service utilization, and predictors of precancerous cervical changes: a population-based survey of sexually active women in Lagos, South-Western Nigeria. Online J Public Health Inform. 2014;6(1):2579 doi:10.5210/ojphi.v6i1.5054 [Google Scholar]
- 42. Ndejjo R, Mukama T, Musabyimana A, Musoke D. Utilization of cervical cancer screening and associated factors among women in rural Uganda: a cross-sectional study. PLoS One. 2016;11(2):1–13. doi:10.1371/journal.pone.0149696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terefe G, Lizeth R, Theodros G, Abebe B. Coverage and factors associated with cervical cancer screening: results from a population-based WHO steps study in Ethiopia. J Oncol Res Treat. 2017;2(1):115. [Google Scholar]
- 44. Gan DE, Dahlui M. Cervical screening utilization and its predictors among rural women in Malaysia. Singapore Med J. 2013; 54(3):163-168. doi:10.11622/smedj.2013047 [DOI] [PubMed] [Google Scholar]
- 45. Mbaka P, Waihenya R, Oisebe C, Lihana R. Factors affecting the utilization of cervical cancer screening in mama Lucy Kibaki Hospital, Nairobi, Kenya. Cancer Res J. 2018;6(3):106 doi:10.11648/j.crj.20180603.16 [Google Scholar]
- 46. Geremew AB, Gelagay AA, Azale T. Utilization of pre cervical cancer screening service and associated factors among women aged 30-49 years in Finote Selam town Northwest Ethiopia. Int J Collab Res Intern Med Public Heal. 2018;10(2):829–842. [Google Scholar]
- 47. Getahun F, Mazengia F, Abuhay M, Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in Northwest Ethiopia. BMC Cancer. 2013;13(1):2 doi:10.1186/1471-2407-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ncube B, Bey A, Knight J, Bessler P, Jolly PE. Factors associated with the utilization of cervical cancer screening among women in Portland, Jamaica. N Am J Med Sci. 2015;7(3):104–113. doi:10.4103/1947-2714.153922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mbatia S, Ngure K, Muniu E, Musenjeri S. Factors associated with cervical cancer screening utilization in Kenya. J Heal Med Nurs. 2016;24:43–51. [Google Scholar]
- 50. Chang HK, Myong JP, Byun SW, et al. Factors associated with participation in cervical cancer screening among young Koreans: a nationwide cross-sectional study. BMJ Open. 2017;7(4):e013868 doi:10.1136/bmjopen-2016-013868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wongwatcharanukul L, Promthet Supannee, Bradshaw Peter, Chananya Jirapornkul NT. Factors affecting cervical cancer screening utilization by Hmong Hilltribe women in Thailand. Asian Pac J Cancer Prev. 2014;15(8):3753–3756. doi:10.7314/APJCP.2014.15.8.3753 [DOI] [PubMed] [Google Scholar]
- 52. Njuguna E, Ilovi S, Muiruri P, Mutai K, Kinuthia J, Njoroge P. Factors influencing cervical cancer screening in a Kenyan Health Facility: a mixed qualitative and quantitative study. Int J Reprod Contraception Obstet Gynecol. 2017;6(4):1180 doi:10.18203/2320-1770.ijrcog20171381 [Google Scholar]