Abstract
At present, concurrent chemoradiotherapy (CRT) is considered the standard treatment of limited-stage small cell lung cancer (LS-SCLC). However, LS-SCLC is highly heterogeneous in the T stage, N stage, and prognosis. Increasing evidence has shown that individual treatment should be considered when treating LS-SCLC patients. The aim of the present study was to explore the optimal combination model of thoracic radiotherapy (TRT) and chemotherapy in N3 LS-SCLC. We retrospectively analyzed 93 N3 LS-SCLC patients treated in the Department of Oncology of Binzhou Medical University Hospital (Shandong, China) between March 2010 and October 2015. A total of 52 (52/93; 55.9%) patients received sequential CRT, and 41 (41/93; 44.1%) patients received concurrent CRT. All patients received 4-6 cycles of chemotherapy and TRT (50-60 Gy). The median follow-up time was 25.4 months (range was 6-65 months).The overall response rate was 88.5% in the sequential CRT group (9.6% complete response rate and 78.8% partial response rate) and 90.2% in the concurrent CRT group (14.6% complete response rate and 75.6% partial response rate). The PFS and OS were 15.4 months and 19.1 months in sequential CRT group, and 16.9 months and 20.5 months in concurrent CRT group. There was no significant difference in treatment response rate, PFS, and OS between sequential and concurrent CRT patients. The most common treatment-related toxicities were nausea/vomiting, neutropenia, and esophagitis. In conclusion, when concurrent CRT is performed in N3 LS-SCLC patients, tolerance to treatment should be fully considered. In our study, sequential CRT and concurrent CRT showed the same efficacy, and sequential CRT demonstrated better tolerance. However, these results require confirmation in future follow-up studies.
Keywords: small cell lung cancer, radiotherapy, chemotherapy, chemoradiotherapy sequence, prognosis
Introduction
Small cell lung cancer (SCLC) accounts for approximately 20-25% of all lung cancer, which is the leading cause of cancer-related deaths worldwide.1 According to the Veterans Administration’s classification system, limited stage small cell lung cancer (LS-SCLC) is confined to the ipsilateral hemithorax and can be safely encompassed within a single radiation portal. Based on the TNM classification system (AJCC 8th Edition), LS-SCLC is generally considered to include T1-4N1-3M0 cases except for T3-4 with multiple lung nodules, which is a group of diseases that display a wide range of involvement. Since SCLC is prone to metastasis and sensitive to cytotoxic agents, chemotherapy is the basis for LS-SCLC treatment. Several studies have shown that thoracic radiotherapy (TRT) improves the outcome of LS-SCLC and concurrent chemoradiotherapy (CRT) is superior to sequential CRT, which produced a modest, but significant, long-term benefit (3.9% 3-year OS benefit).2-4 However, these findings are based on clinical trials involving selected LS-SCLC, some excluding patients with contralateral hilar and supraclavicular lymph node metastasis.5,6 Patients with N3 LS-SCLC would require large radiation target volumes of radiotherapy. Large radiation target volumes increase acute and chronic toxicities and sometimes lead to delay of concurrent chemotherapy. A recent study showed that N3 LS-SCLC treated with concurrent CRT still had a lower 3-year survival than heterogeneous LS-SCLC patients.7 In addition, our previous work found that patients with high-dose TRT showed a lower 2-year survival than those with low-dose TRT in SCLC patients with superior vena cava syndrome (SVCS, most of them are N3).8 Therefore, the prognosis of N3 LS-SCLC remains poor and the best treatment model is unclear. Due to more lymph node regions involvement and the tolerability of treatment, many patients did not receive concurrent CRT and received sequential CRT. The aim of this retrospective study was to explore the optimal combination model of TRT and chemotherapy in N3 LS-SCLC.
Patients and Methods
Patient and Tumor Characteristics
N3 LS-SCLC was defined as LS-SCLC with contralateral mediastinal node metastasis, contralateral hilar node metastasis, and/or supraclavicular (both ipsi- and contralateral) node metastasis. We enrolled 93 N3 LS-SCLC patients in our retrospective study. The patients were treated in the Department of Oncology of Binzhou Medical University Hospital (Shandong, China) between March 2010 and October 2015. All patients were diagnosed by tissue pathology (bronchoscopic biopsy and supraclavicular lymph node biopsy). In all patients, initial staging included computed tomography (CT) examination of the neck/thorax/abdomen, bone scintigraphy, and contrast-enhanced cranial CT (or MRI). PET-CT scan was performed in 17 of the 93 patients. Contralateral mediastinal, contralateral hilar, and supraclavicular lymph node metastasis occurred in 90.4%, 21.2%, 19.2% and 85.4%, 12.2%, 17.1% in sequential group and concurrent group, respectively. A total of 22 (22/93; 23.7%) patients showed 2 or more N3 lymph node region involvement; 52/93 (55.9%) patients received sequential CRT, and 41/93 (44.1%) patients received concurrent CRT. All patients received 4-6 cycles of chemotherapy. The average number of chemotherapy cycles was 5.0(range 4-6 cycles) in sequential CRT and 4.5(range 4-6 cycles) in concurrent CRT. TRT consisted of 2 Gy daily in 30 fractions up to 60 Gy. The minimum dose in this study was 50 Gy. The average dose of TRT was 59.6 Gy (range 52-60 Gy) in sequential CRT and 58.6 Gy (range 50-60 Gy) in concurrent CRT. The median patient age was 57.6 years old (range 40-71 years old). The characteristics of the enrolled N3 LS-SCLC patients were shown in Table 1. Age, gender, performance status, involvement of lymph node regions, cycles of chemotherapy, and prophylactic cranial irradiation (PCI) were similar in patients treated concurrently or sequentially. Our study was approved by the Ethics Committee of Binzhou Medical University Hospital (Shandong, China). Due to the retrospective nature of the study, informed consent was waived. The median follow-up time was 25.4 months (range 6-62 months).
Table 1.
Characteristics of N3 LS-SCLC Patients.
| Characteristics | Sequential patients (n = 52) | Concurrent patients (n = 41) | p-valuea | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| <60 | 30 | 57.7 | 25 | 61.0 | 0.749 |
| ≥60 | 22 | 42.3 | 16 | 39.0 | |
| Gender | |||||
| Male | 45 | 86.5 | 36 | 87.8 | 0.856 |
| Female | 7 | 13.5 | 5 | 12.2 | |
| Performance status | |||||
| 0-1 | 46 | 88.5 | 39 | 95.1 | 0.784 |
| 2 | 6 | 11.5 | 2 | 4.9 | |
| N3 lymph node regions | |||||
| Contralateral mediastinum | 47 | 90.4 | 35 | 85.4 | 0.696 |
| Contralateral hilum | 11 | 21.2 | 5 | 12.2 | |
| Supraclavicular | 10 | 19.2 | 7 | 17.1 | |
| Cycles of chemotherapy | |||||
| 4 | 25 | 48.1 | 26 | 63.4 | 0.140 |
| 5-6 | 27 | 51.9 | 15 | 36.6 | |
| Prophylactic brain radiation | |||||
| Yes | 28 | 53.8 | 19 | 46.3 | 0.420 |
| No | 24 | 46.2 | 22 | 53.7 | |
| PTVb volume (cm3) | |||||
| ≤310 | 34 | 65.4 | 21 | 51.2 | 0.168 |
| >310 | 18 | 34.6 | 20 | 48.8 | |
| Radiation dose (Gy) | |||||
| 60 | 49 | 94.2 | 35 | 85.4 | 0.279 |
| 50-60 | 3 | 5.8 | 6 | 14.6 | |
a Chi-square test; P < 0.05 was considered statistically significant; b PTV: Planning target volume.
Treatments
Chemotherapy consisted of cisplatin and etoposide. Chemotherapy was administered in a 3-week cycle using sequential chemotherapy (100 mg/m2 of etoposide was given intravenously on days 1-3 and 25 mg/m2 of cisplatin was given intravenously on days 1-3) and was administered in a 4-week cycle using concurrent chemotherapy (80mg/m2 of etoposide was given intravenously on days 1-3 and 25 mg/m2 of cisplatin was given intravenously on days 1-3). After completing radiotherapy, patients with good performance status continued to receive no more than 2 cycles of chemotherapy. All patients received at least 4 cycles of chemotherapy. Dose adjustment and delay were based on adverse effects. We administered granulocyte colony stimulating factor subcutaneously if the absolute granulocyte count was <0.5 × 109/L.
In patients treated with concurrent CRT, TRT was administered at the beginning of the second or third cycles of chemotherapy. In patients that received sequential CRT, TRT was performed after 4 cycles of chemotherapy. During radiation delivery, patients were immobilized with a thermoplastic mask in the supine position and then scanned with a CT slice thickness of 5 mm. All patients received intensity modulated radiotherapy (IMRT). The gross tumor volume (GTV) was based on the planning CT images. Initially involved nodal regions were covered. Clinical tumor volume (CTV) included the GTV with a 7 mm margin. An expansion of 5 mm of the CTV created the planning target volume (PTV). The prescription dose applied to 95% PTV was 60 Gy given in 30 fractions using a fraction dose of 2.0 Gy. Dose constrains of normal tissues included V20 ≤30% for bilateral lungs, V5≤70% for bilateral lungs, mean lung dose ≤18 Gy for bilateral lungs, One-third of the heart volume ≤40 Gy, maximum dose for esophagus≤64 Gy, and maximum dose for spinal cord ≤45 Gy.
After TRT and chemotherapy, PCI was administered to patients with a complete or partial response. Forty-seven patients received PCI (25 Gy in 10 fractions or 30 Gy in 10 fractions).
Similar percentages of patients received subsequent therapy after disease progression (59.1% in sequential group and 64.7% in concurrent group), including whole brain radiotherapy (WBRT) for brain metastasis and subsequent chemotherapy.
Assessment and Follow-Up
After completion of treatments, patients were evaluated every 3 months during the first 2 years and then every 6 months from the third year. History, physical examination, and CT of the chest and upper abdomen (or ultrasonography) were obtained at every evaluation. Brain magnetic resonance imaging or enhanced CT was performed if there were central nervous system symptoms. Response to treatment was categorized according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1). Toxicities were evaluated by Common Terminology Criteria Adverse Events (CTCAE) version 4.0.
Statistical Analyses
We used SPSS 17.0 (SPSS Inc., Chicago, IL, USA) to perform statistical analyses. Progression-free survival (PFS) and overall survival (OS) were analyzed according to the Kaplan–Meier method and compared by log-rank test. PFS was measured from the date of treatment to the date of the first observation of disease progression or mortality. OS was measured from the date of treatment to the date of death or last follow-up. The response rates of patients were based on the initial follow-up CT. Chi-square test was used for comparisons of categorical data. All P values are based on 2-sided tests. A P-value of <0.05 indicated a statistically significant difference.
Results
Treatment Response
Treatment response is described in Table 2. The overall response rate was 88.5% in the sequential CRT group (9.6% complete response rate and 78.8% partial response rate) and 90.2% in the concurrent CRT group (14.6% complete response rate and 75.6% partial response rate). There was no significant difference in treatment response rates between the sequential and concurrent CRT patients.
Table 2.
Response According to Treatment Regimen.
| Characteristics | Sequential patients (n = 52) | Concurrent patients (n = 41) | p-valuea | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Objective Response | 46 | 88.5 | 37 | 90.2 | 0.783 |
| Complete Response | 5 | 9.6 | 6 | 14.6 | 0.475 |
| Partial Response | 41 | 78.8 | 31 | 75.6 | |
| Stable Disease | 3 | 5.8 | 1 | 2.4 | – |
| Progressive Disease | 3 | 5.8 | 3 | 7.3 | – |
a Chi-square test; P < 0.05 was considered statistically significant.
Progression-Free Survival and Overall Survival
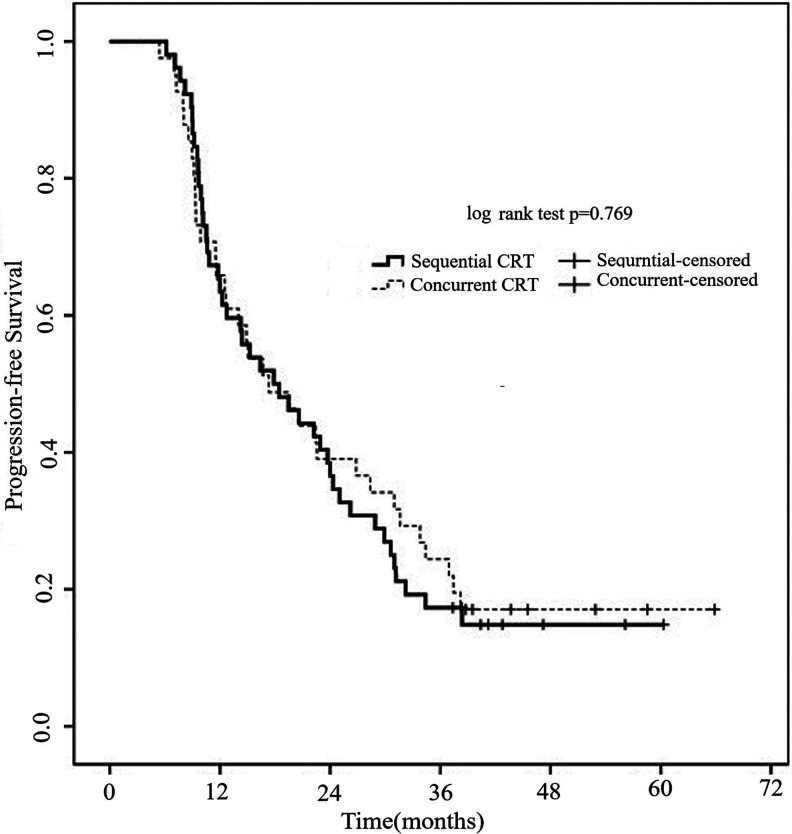
Figure 1 shows the PFS according to treatment model. The median PFS was 16.2 months (95% CI 12.4-19.2 months) for the entire cohort. There was no significant difference in the median PFS between sequential group and concurrent groups (15.4 vs. 16.9 months; p = 0.769).
Figure 1.
Progression-free survival of patients with N3 LS-SCLC according to treatment regimen. There was no significant difference in the median PFS between sequential group and concurrent group (15.4 vs. 16.9 months; p = 0.769).
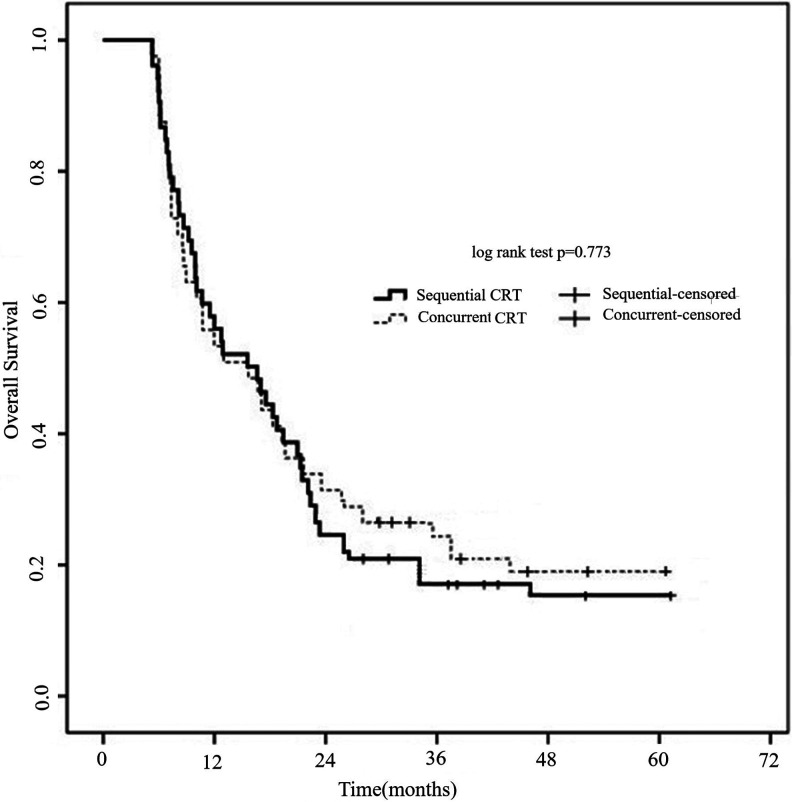
Figure 2 shows the OS according to treatment model. The median survival time was 19.1 months for the patients that received sequential CRT (95% CI 14.5-22.3 months) and 20.5 months (95% CI 16.3-25.1 months) for patients with concurrent CRT. The 2- and 3-year survival rates were 28.9%, 17.5% for sequential CRT patients and 33.1%, 23.8% for concurrent CRT patients, respectively (p = 0.773).
Figure 2.
Overall survival of patients with N3 LS-SCLC according to treatment regimen. The 2- and 3-year survival rates were 28.9%, 17.5% for sequential CRT patients and 33.1%, 23.8% for concurrent CRT patients, respectively (p = 0.773).
Patterns of Failure
The patients in sequential group and concurrent group had similar pattern of failure (Table 3). Of the 93 patients in this study, 12 patients in sequential group and 8 patients in concurrent group had the local failure as the first site of failure (27.3% vs. 23.5%; p = 0.707). Twenty-four in sequential group and 21 in concurrent group had distant metastasis as the first site of failure (50.0% vs. 55.9%; p = 0.606). Twenty-nine patients in this study (32/93, 34.4%) developed brain metastasis (Table 4). Brain metastasis as the first site of failure occurred in 12 patients (7 in sequential group and 5 in concurrent group). Brian metastasis was more common in patients without PCI (43.5% vs. 25.5%; p = 0.203). However, there was no significant difference in brain metastasis in sequential and concurrent groups (p > 0.05).
Table 3.
Site of First Progression According to Treatment Regimen.
| Site | Sequential patients (n = 44) | Concurrent patients (n = 34) | P-valuea | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Within the thorax | 12 | 27.3 | 8 | 23.5 | 0.707 |
| Outside the thorax | 22 | 50.0 | 19 | 55.9 | 0.606 |
| Within and outside the thorax | 10 | 22.7 | 7 | 20.6 | 0.820 |
| Brain | 7 | 15.9 | 5 | 14.7 | 0.884 |
a Chi-square test; P < 0.05 was considered statistically significant.
Table 4.
Brain Metastasis of Patients with or Without PCIa.
| PCI | All patients (n = 93) | P-valueb | Sequential patients (n = 52) | Concurrent patients (n = 41) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| n | Brain metastasis | n | Brain metastasis | n | Brain metastasis | |||
| Yes | 47 | 13 | 0.166 | 28 | 7 | 19 | 6 | 0.621 |
| No | 46 | 19 | 24 | 11 | 22 | 8 | 0.515 | |
a PCI: prophylactic cranial irradiation; b Chi-square test; P < 0.05 was considered statistically significant.
Toxicity
Hematologic and nonhematologic toxicities are summarized in Table 5. The most common treatment-related toxicities were nausea/vomiting, neutropenia, and esophagitis. Grade 3/4 neutropenia occurred in 25/52 patients (48.1%) received sequential CRT and 30/41 patients (73.2%) received concurrent CRT (p = 0.015). Grade 3/4 esophagitis was more frequent in the concurrent group, occurring in 9/41 patients (22.0%) compared with 6/52 patients (11.5%) in sequential group. Two patients in each group developed ≥grade3 radiation pneumonia. In addition, we had a treatment-related death in the concurrent group (radiation pneumonitis combined with pulmonary infection).
Table 5.
Toxic Effects According to Treatment Regimen.
| Toxic effects | Sequential patients (n = 52) | Concurrent patients (n = 41) | P-valueb | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hematologic toxicities | |||||
| Neutropenia | |||||
| Grade 1/2 | 27 | 51.9 | 11 | 26.8 | 0.015 |
| Grade 3/4 | 25 | 48.1 | 30 | 73.2 | |
| Thrombocytopenia | |||||
| Grade 1/2 | 35 | 67.3 | 22 | 53.7 | 0.142 |
| Grade 3/4 | 15 | 28.8 | 18 | 43.9 | |
| Anemia | |||||
| Grade1/2 | 34 | 65.4 | 23 | 56.1 | 0.335 |
| Grade3/4 | 11 | 21.2 | 12 | 29.3 | |
| Nonhematologic toxicities | |||||
| Nausea/vomiting | |||||
| Grade1/2 | 37 | 71.2 | 33 | 80.5 | 0.423 |
| Grade3/4 | 12 | 23.1 | 7 | 17.1 | |
| Esophagitis | |||||
| Grade1/2 | 46 | 88.5 | 32 | 78.0 | 0.021 |
| Grade3/4 | 6 | 11.5 | 9 | 22.0 | |
| Fever | |||||
| Yes | 1 | 1.9 | 2 | 4.9 | 0.581 |
| No | 51 | 98.1 | 39 | 95.1 | |
a Chi-square test; P < 0.05 was considered statistically significant.
Discussion
The PFS and OS were comparable in N3 LS-SCLC patients who received sequential and concurrent CRT treatments. Regardless of the combination model of chemotherapy and radiotherapy, the median survival time of N3 LS-SCLC patients in this cohort was approximately 20 months. However, it was shorter than that of previous studies on LS-SCLC patients with different TNM stages.5 Our results were consistent with a recent study of N3 LS-SCLC.7 According to previously published results, the prognosis of LS-SCLC is better than ES-SCLC.9 Although some studies have found that patients with broader lymph node metastasis showed decreased survival, there are few trials that focus on survival of LS-SCLC with different N staging.8,10 It has been gradually recognized that LS-SCLC patients with different T stages and N stages have great heterogeneity in tumor burden, radiation tolerance, and prognosis. Therefore, it has been recommended that TNM staging should be reported in clinical trials.11 A recent study reported that patients with stage I to II LS-SCLC treated with concurrent CRT had much better outcomes compared with patients that had stage III disease (median OS: 50 vs. 25 months, respectively).9 In addition, some evidence has shown that surgical resection improves prognosis in patients with T1-2N0M0 SCLC.12,13 We suggest that LS-SCLC patients need more individual treatments regarding the radiation target of TRT, timing of TRT, and intensity of concurrent chemotherapy.
Concurrent CRT is an effective treatment model for malignancies because of spatial cooperation, cytotoxic enhancement, biological cooperation, temporary modulation, and normal tissue protection.14 Concurrent CRT is widely used in different cancers.3,15,16 However, while concurrent CRT improves efficacy, severe toxicities occur more frequently than sequential CRT. Toxicities as a result of concurrent CRT are related to many factors, such as chemotherapeutic drugs, radiation target areas, and radiation dosage. Hence, when concurrent CRT is used to improve therapeutic effect, the influence of patient selection and systemic treatment should be taken into account. SCLC is characterized by rapid growth, early dissemination, and sensitivity to chemotherapy and radiotherapy. Chemotherapy is the fundamental treatment for all patients with SCLC. TRT yields 25-30% reduction in local recurrence and a corresponding 5-7% improvement in 2-year survival for LS-SCLC compared with chemotherapy alone.17,18 At present, the standard care for LS-SCLC consists of combined chemotherapy and thoracic radiotherapy, followed by prophylactic cranial irradiation (PCI) for patients who have complete or partial treatment response. Early concurrent TRT results in significant improvement in overall survival and has led to more severe toxicities compared to late concurrent or sequential radiotherapy.2,3,19 However, the timing of TRT is unclear. One study showed that late TRT appeared to be superior to early TRT in terms of complete response and OS in patients with LS-SCLC.20 In our study, concurrent CRT showed a trend toward improving the complete response rate, PFS and OS, but there was no significant difference between sequential group and concurrent group. For N3 LS-SCLC patients, systemic therapy plays a more important role compared with N0-2 patients. The early-starting radiation target volume in N3 LS-SCLC patients is large and treatment-related toxicities are more common and severe, especially with concurrent chemotherapy. Because of intolerance, the intensity of chemotherapy and radiotherapy has to be reduced in some patients. Therefore, the efficacy is sometimes impaired.21 Although there was no statistical difference, patients were more likely to receive a substantially reduced radiation therapy volume after 4 cycles of induction chemotherapy in sequential group. In the present study, patients receiving concurrent CRT had fewer chemotherapy cycles than those receiving sequential CRT. Reduced intensity of chemotherapy might offset the advantages of concurrent CRT. In the present study, G3/4 neutropenia and esophagitis were more common in concurrent group than in sequential group.
The patterns of relapse in sequential and concurrent groups were similar, including local progression, extracranial distant metastasis, and brain metastasis. JCOG 9104 showed concurrent CRT had OS superiority on sequential CRT, but local control was the same in both arms.22 Early TRT might attribute survival benefit to early local control that prevents distant spread of the primary tumor. Consistent with a previous study, the sites of first relapse in this study were also similar in the concurrent and sequential groups.23 Extrathoracic metastasis was the main cause of treatment failure in both groups. Patients without PCI were more likely to develop brain metastasis in the present study. PCI reduced brain metastasis from 43.8% to 25.5%. The difference was not statistically significant, which might be due to the small sample size. PCI is considered as an important component of the treatment of the patients with LS-SCLC.24 Studies have confirmed that WBRT led to neurocognitive dysfunction and worsening quality of life, and even a recent retrospective report indicated that PCI had detrimental effect on the OS of patients with LS-SCLC.25-27 With the prolongation of the survival time of the patients with SCLC, both the risk of brain metastasis and neurocognitive toxicity need to be paid more attention to. Hippocampus-sparing WBRT has been proved to reduce the neurological damage caused by PCI. However, sparing the hippocampus during WBRT poses important technical challenges with respect to contouring and treatment planning. So in practice, not all radiotherapy institutes use hippocampus-sparing technology, and some patients refuse PCI because of their fear of neurocognitive toxicity. Nearly half of the patients in this study did not undergo PCI.
There are limitations to this study, including its nature of a retrospective study and its associated biases. A small percentage of patients underwent PET/CT, which might lead to inaccurate staging. Another limitation is the sample size, especially in patients with contralateral hilar and supraclavicular lymph node metastasis. Therefore, we did not compare the prognosis of N3 LS-SCLC with different lymph node regions. Furthermore, the rate of patients received PCI were relatively low. Although a recent study from Japan showed PCI played a detrimental role in the outcome of patients with LS-SCLC,28 PCI is generally considered as a component of the treatment of patients with LS-SCLC. PFS and OS might be impaired by low PCI rate.
In summary, LS-SCLC is a group of highly heterogeneous tumors, and N3 LS-SCLC is a subcategory with a wider range of involvement. When choosing concurrent CRT, the patient’s tolerance should be fully evaluated. In our study, sequential CRT and concurrent CRT demonstrated the same efficacy and sequential CRT had better tolerance in patients with N3 LS-SCLC. This finding should be confirmed in large-cohort, prospective studies.
Acknowledgments
We thank Wei Qu for her assistance in the data collection and statistical analysis.
Abbreviations
- CRT
chemoradiotherapy
- LS-SCLC
limited-stage small cell lung cancer
- TRT
thoracic radiotherapy
- SVCS
superior vena cava syndrome
- PCI
prophylactic cranial irradiation
- IMRT
received intensity modulated radiotherapy
- GTV
gross tumor volume
- CTV
clinical tumor volume
- PTV
planning target volume
- WBRT
whole brain radiotherapy
- PFS
progression-free survival
- OS
overall survival
- ES-SCLC
extensive-stage small cell lung cancer
Authors’ Note: All persons listed as authors have read the manuscript and given their approval for the submission. Zhenbo Wang and Jinliang Wan participated in the study design and performed the statistical analysis. Haitao Geng, Lei Li, and Changmin Liu collected data and wrote the manuscript. Xinjun Dong collected data, participated in the revision of manuscript. Our study was approved by the Ethics Committee of Binzhou Medical University Hospital (Shandong, China). 2019-LW-018.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zhenbo Wang, MD  https://orcid.org/0000-0002-0523-8697
https://orcid.org/0000-0002-0523-8697
References
- 1. Herrmann MK, Bloch E, Overbeck T, et al. Mediastinal radiotherapy after multidrug chemotherapy and prophylactic cranial irradiation in patients with SCLC-treatment results after long-term follow-up and literature overview. Cancer Radiother. 2011;15(2):81–88. [DOI] [PubMed] [Google Scholar]
- 2. Pijls-Johannesma MCG, De Ruysscher D, Lambin P, Rutten I, Vansteenkiste JF. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev. 2005;25(1):CD004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22(23):4837–4845. [DOI] [PubMed] [Google Scholar]
- 4. Gridelli C, Casaluce F, Sgambato A, Monaco F, Guida C. Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that’s the question. Transl Lung Cancer Res. 2016;5(2):150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park J, Kang MK. Impact of radiation dose on concurrent chemoradiotherapy for limited-stage small-cell lung cancer. Radiat Oncol J. 2018;36(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59(4):943–951. [DOI] [PubMed] [Google Scholar]
- 7. Valan CD, Slagsvold JE, Halvorsen TO, et al. Survival in limited disease small cell lung cancer according to N3 lymph node involvement. Anticancer Res. 2018;38(2):871–876. [DOI] [PubMed] [Google Scholar]
- 8. Wang ZB, Ning FL, Wang XL, et al. Radiation dose is associated with prognosis of small cell lung cancer with superior vena cava syndrome. Int J Clin Exp Med. 2015;8(3):4263–4268. [PMC free article] [PubMed] [Google Scholar]
- 9. Bremnes RM, Sundstrom S, Aasebø U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5-year follow-up. Lung Cancer. 2003;39(3):303–313. [DOI] [PubMed] [Google Scholar]
- 10. Salem A, Mistry H, Hatton M, et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: secondary analysis of a randomized clinical trial. JAMA Oncol. 2019;5(3):e185335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2(12): 1067–1077. [DOI] [PubMed] [Google Scholar]
- 12. Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: its time has come. J Thorac Oncol. 2008;3(11):1267–1271.18978561 [Google Scholar]
- 13. Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Cardiovasc Surg. 2005; 129(1):64–72. [DOI] [PubMed] [Google Scholar]
- 14. Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Clin Pract Oncol. 2007;4(3):172–180. [DOI] [PubMed] [Google Scholar]
- 15. Lu H, Peng L, Yuan X, et al. Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev. 2009;35(4):345–353. [DOI] [PubMed] [Google Scholar]
- 16. Hiroshima Y, Fukumitsu N, Saito T, et al. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother Oncol. 2019;136:37–43. [DOI] [PubMed] [Google Scholar]
- 17. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10(6):890–895. [DOI] [PubMed] [Google Scholar]
- 18. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327(23):1618–1624. [DOI] [PubMed] [Google Scholar]
- 19. Spiro SG, James LE, Rudd RM, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24(24):3823–3830. [DOI] [PubMed] [Google Scholar]
- 20. Bayman E, Etiz D, Akcay M, Ak G. Timing of thoracic radiotherapy in limited stage small cell lung cancer: results of early versus late irradiation from a single institution in Turkey. Asian Pac J Cancer Prev. 2014;15(15):6263–6267. [DOI] [PubMed] [Google Scholar]
- 21. Wong PW, Enriquez A, Barrera R. Nutritional support in critically ill patients with cancer. Crit Care Clin. 2001;17(3):743–767. [DOI] [PubMed] [Google Scholar]
- 22. Takada M, Fukuoka1 M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20(14):3054–3060. [DOI] [PubMed] [Google Scholar]
- 23. Ohara S, Kanda S, Okuma H, et al. Effect of sequential chemoradiotherapy in patients with limited-disease small-cell lung cancer who were ineligible for concurrent therapy: a retrospective study at two institutions. Jpn J Clin Oncol. 2018;48(1):82–88. [DOI] [PubMed] [Google Scholar]
- 24. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med. 1999;341(7):476–484. [DOI] [PubMed] [Google Scholar]
- 25. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 26. Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008; 72(5):1311–1318. [DOI] [PubMed] [Google Scholar]
- 27. Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71(1):64–70. [DOI] [PubMed] [Google Scholar]
- 28. Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Saito T. Treatment outcomes of patients with small cell lung cancer without prophylactic cranial irradiation. J Thorac Dis. 2016;8(9):2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]