Abstract
Hepatocellular carcinoma (HCC), one of the most common lethal diseases in the world, has a 5-year survival rate of only 7%. Hepatocellular carcinoma has no symptoms in the early stage but obvious symptoms in the late stage, leading to delayed diagnosis and reduced treatment efficacy. In recent years, as the scope of HCC research has increased in depth, the clinical development and application of molecular targeted drugs and immunotherapy drugs have brought new breakthroughs in HCC treatment. Targeted therapy drugs for HCC have high specificity, allowing them to selectively kill tumor cells and minimize damage to normal tissues. At present, these targeted drugs are mainly classified into 3 categories: small molecule targeted drugs, HCC antigen-specific targeted drugs, and immune checkpoint targeted drugs. This article reviews the latest research progress on the targeted drugs for HCC.
Keywords: HCC, targeted therapy, immune checkpoint, small molecule drugs, sorafenib
Liver cancer is one of the most common fatal diseases in the world.1 Early-stage liver cancer has no symptoms, whereas late-stage symptoms become obvious, leading to delays in diagnosis and reduced therapeutic efficacy.2 There are more than 850 000 new cases of liver cancer every year worldwide, 90% of which are hepatocellular carcinoma (HCC).3 In China in particular, due to the high incidence of hepatitis B, the number of HCC deaths in 2015 reached roughly 422 000, accounting for 57% of the global HCC deaths.4 At present, surgery is still an effective way to improve the survival rate of patients with HCC.5 However, incomplete resection, high recurrence rate, and high metastasis rate are the main problems affecting HCC’s clinical therapeutic efficacy.
In recent years, with advances in understanding of HCC’s molecular biology, the clinical development and application of molecular targeted drugs and immunotherapy drugs have brought new breakthroughs in HCC treatment, greatly improving its diagnosis and treatment efficacy, prolonging the survival of patients with HCC, and improving their quality of life.6 However, HCC’s morbidity and mortality have not decreased, and they are still increasing year by year. In this article, the latest research progress in targeted therapy for HCC from recent years is briefly reviewed, including small molecule targeted inhibitors, targeted drugs designed based on specific antigens for HCC, and immune checkpoint inhibitors.
Small Molecule Targeted Drugs
In recent years, molecular targeted therapy has emerged as a new cancer treatment method.7 Compared with traditional therapy, molecular targeted therapy has improved targeting, can specifically kill tumor cells and reduce damage to normal tissues, has low rates of drug resistance, and is safer and better tolerated by patients.8 In 1906, Enrlich first proposed the concept of targeted drug delivery, that is, to use a specific carrier to selectively deliver drugs or other antitumor active substances to the target site, therefore limiting the therapeutic effect or drug effect to specific target cells, tissues, or organs as far as possible, without affecting the function of their normal counterparts, so as to improve efficacy and reduce adverse reactions.9
Molecular targeted therapy targets overexpressed cell receptors, key genes, and some marker molecules of tumor cells by selecting specific blockers to inhibit tumor growth, progress, and metastasis.10 The principle of molecular targeted therapy is to target key genes and signaling pathways in the process of tumor development, or the proto oncogene, tumor suppressor gene, and suicide gene, among others, by designing small molecule inhibitors to reverse the biological behavior of tumor cells at the molecular level, so as to inhibit the proliferation and metastasis of tumor cells.11 At present, there are dozens of molecular targeted drugs both on the market and in the clinical research stage, including sorafenib, regorafenib, lenvatinib, cabozantinib, and other drugs, which have achieved significant results (Figure 1) (Table 1).
Figure 1.
Overview of various targeted drugs. Molecular targeted therapy selects specific blockers to effectively intervene in the regulation of cell receptors, key genes, and marker molecules, in order to achieve tumor-inhibiting effects.
Table 1.
Characteristics of Agents Approved for Second-Line Treatment of Patients With Advanced HCC.
| Regorafenib | Cabozantinib | Ramucirumab | Nivolumab | Pembrolizumab | Ipilimumab plus nivolumab | |
|---|---|---|---|---|---|---|
| Drug class | Multitarget kinase inhibitor | Multitarget kinase inhibitor | Monoclonal antibody | Monoclonal antibody | Monoclonal antibody | Monoclonal antibody |
| Molecular targets | VEGFR-1–3, TIE2, KIT, RET, RAF1, BRAF, BRAFV600E, PDGFR, FGFR | VEGFR-2, MET, RET, AXL, FLT3, c-KIT | VEGFR-2 | PD-1 | PD-1 | CTLA-4/PD-1 |
| Route of administration | Oral | Oral | Intravenous infusion | Intravenous infusion | Intravenous infusion | Intravenous infusion |
| Study | RESORCE (NCT01774344) | CELESTIAL (NCT01908426) | REACH-2 (NCT02435433) | CheckMate 040 (NCT01658878) | KEYNOTE-224 (NCT02702414) | CheckMate 040 (NCT01658878) |
| Design | Phase 3 | Phase 3 | Phase 3 | Phase 1-2 | Phase 2 | Phase 1-2 |
| Primary end point | OS | OS | OS | ORR | ORR | ORR |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte antigen 4; BRAF, B-Raf Proto-Oncogene; PDGFR, Platelet Derived Growth Factor Receptor Beta; ORR, Objective Response Rate; OS, overall survival; FGFR, fibroblast growth factor receptor; FLT3, fms-like tyrosine kinase-3; PD, programmed cell death; RET, ret proto-oncogene; VEGFR, vascular endothelial growth factor receptor.
Sorafenib
Sorafenib is a broad-spectrum, small molecule inhibitor that can inhibit the expansion, angiogenesis, and apoptosis of many tumor cells.12 Sorafenib primarily targets serine/threonine kinase, vascular endothelial growth factor receptor (VEGFR), Platelet-derived growth factor receptor beta (PGFRβ), Kit, fms-like tyrosine kinase-3 (FLT3), ret proto-oncogene (RET), and other receptor tyrosine kinases to inhibit tumor cell proliferation and angiogenesis, which subsequently inhibits tumor growth.13 In 602 patients with advanced HCC who had not received systematic treatment before, the median survival time of the sorafenib group was 2.8 months longer than that of placebo group (44%). There was a significant difference between the sorafenib group and the placebo group.13 The patients with advanced HCC had good tolerance to sorafenib, indicating that sorafenib can be used as a first-line drug to treat patients with advanced HCC.
Regorafenib
The molecular structures of regorafenib and sorafenib are very similar, as are their mechanisms of action.8 However, regorafenib has higher biological activity than sorafenib, capable of widely inhibiting the kinases related to angiogenesis and tumorigenesis, such as VEGFR 1-3, tyrosine protein kinase receptor Tie, RET, PDFGR, basic fibroblast growth factor receptor (FGFR), serine/threonine protein kinase RAF, mitogen-activated protein, and kinase p38, so as to play an antitumor role.14
The researchers tested regorafenib as a second-line drug in 573 patients with HCC who had been treated with sorafenib, 194 of whom received placebo.15 The final data showed that, compared with the placebo group, regorafenib significantly improved the overall patient survival time from 7.8 months in the placebo group to 10.6 months in the experimental group. In 2 of the patients treated with regorafenib, the tumor shrank to an undetectable state.16 These data indicate that regorafenib has a good therapeutic effect in patients with HCC who have been treated with sorafenib.
Lenvatinib
Lenvatinib is an inhibitor of VEGFR1-3, FGFR1-4, PDGFRα, and tyrosine protein kinase receptor RET and Kit.17 In June 2017, the annual meeting of the American Society of Clinical Oncology reported the phase III clinical research results of lenvatinib in the first-line treatment of unresectable liver cancer.18 It was found that the total survival time of the main clinical end point in the lenvatinib group was longer than that of the sorafenib group (13.6 vs 12.3 months), and the secondary clinical end point in the lenvatinib group was also significantly better than that of the sorafenib group, including progression-free survival time (7.4 vs 3.7 months), disease progression time (8.9 vs 3.7 months), and objective remission rate (24% vs 9%). Although the main clinical end point overall survival of the lenvatinib group and sorafenib group did not reach statistical difference, progression-free survival of the lenvatinib group was twice as long as that of the sorafenib group, and time to progress was nearly 3 times longer than that of sorafenib group (Figure 2).19
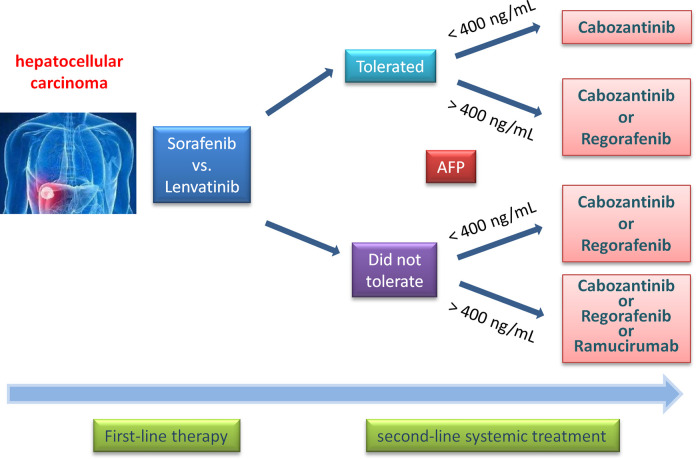
Figure 2.
Second-line systemic treatment options in patients with hepatocellular carcinoma. PD-1 inhibitors can be considered given objective response rates of 15% to 20%, although phase III studies have failed to demonstrate statistically significant survival benefit compared to other agents in phase III studies. PD-1 indicates programmed cell death 1.
In addition, 83% of the Asian patients (288 cases) in the REFLECT study had been infected with the hepatitis B virus (HBV). Among the patients with HBV-related HCC, the effective rate of the lenvatinib group was 21.5%, which was 2.6 times higher than that of the sorafenib group (8.3%). The median overall survival time of the lenvatinib group (15.0 months) was significantly longer than that of the sorafenib group (10.2 months), and the median progression-free survival time and median time to progression in the lenvatinib group were significantly better than those of the sorafenib group.18,20
It has been confirmed that lenvatinib is not inferior to sorafenib in the first-line treatment of advanced liver cancer, especially in patients with HBV-related HCC.8 This shows that lenvatinib has good application prospects in Asia, especially in China, and is expected to become the latest standard for the treatment of advanced HCC.
Cabozantinib
Cabozantinib is an effective multireceptor tyrosine kinase inhibitor, which can target VEGFR2, c-Met, Kit, Axl, and FLT3 with 0.035, 1.3, 4.6, 7, and 11.3 nM half-maximal inhibitory concentration, respectively. In the second-line phase III CELESTIAL trial, cabozantinib significantly improved overall survival in patients with liver cancer and was approved for use in patients with unresectable liver cancer.21,22
Inhibitors of VEGF
Vascular endothelial growth factor is the only known growth factor that specifically acts on vascular endothelial cells.23,24 Studies have shown that VEGF was expressed in both hepatocytes and HCC cells, and its expression intensity gradually increased with the development of HCC.25 Vascular endothelial growth factor and its receptor are powerful factors for inducing angiogenesis. When combined, they can strongly induce endothelial cell proliferation and tubular formation, which is an important part of angiogenesis.26 Furthermore, HCC is a vascular-rich cancer.27 Vascular endothelial growth factor and microvessel density in HCC are significantly increased,27,28 and the high expression of VEGF in HCC indicates poor prognosis.29 At each stage of HCC progression, the proliferation of vascular endothelial cells is active, and the expression of VEGFR molecules on the cell surface is significantly upregulated.30 Angiogenesis in cancer tissues has an important impact on the biological invasion abilities of the tumor.31 Therefore, blocking VEGF/VEGFR and reducing angiogenesis in the tissues are considered a novel idea for targeted therapy in HCC.
Bevacizumab is a recombinant human-mouse chimeric monoclonal antibody against VEGF.32 The humanization process prolongs the half-life of the drug and weakens its immunogenicity.33 Through competitive binding with VEGF in the circulation, bevacizumab can prevent the binding of VEGF to the corresponding receptor, thus blocking the formation of new blood vessels in HCC.24 At the same time, bevacizumab can normalize the distribution of blood vessels in HCC and its surrounding tissues, which improves the delivery of chemotherapy drugs by reducing the interstitial pressure.34
The efficacy of bevacizumab in advanced HCC treatment has been initially confirmed.35 Zhu et al reported a GE2MOX2B scheme for the treatment of advanced liver cancer in a phase II clinical study.36 All of the 33 patients were enrolled into the group, 30 of whom were evaluable, with an effective rate of 20% and a disease stability rate of 27%; the median survival time was 9.6 months, the median progression-free survival time was 5.3 months, and the disease-free survival times at 3 and 6 months were 70% and 48%, respectively. Therefore, the scheme has certain antitumor activity as well as a high disease-free survival rate in 6 months, which is worth further study. Siegel et al also used bevacizumab in a phase II clinical drug trial.37 The results showed that 65% of the 46 patients had a median progression-free survival time of 6 months. Adverse reactions included hypertension, thrombosis, and bleeding. Through dynamic contrast-enhanced nuclear magnetic resonance imaging of the tumor, it could be observed that the concentration of VEGF in patients’ plasma was decreased, and the vasoactivity was also decreased.
Ramucirumab is an anti-VEGF monoclonal immunoglobulin G (IgG) antibody. It was first evaluated in the REACH trial, a second-line phase III trial in patients with progression or intolerance leading to the failure of sorafenib.38-40 Ramucirumab significantly improved the overall survival rate to 8.5 months (hazard ratio: 0.71, 95% CI: 0.5-0.95), compared with the median survival rate of 7.3 months in the placebo group. Ramucirumab is the first approved systemic therapy for liver cancer in a biomarker selection population. Ramucirumab has demonstrated survival benefits in patients having unresectable HCC with α-fetoprotein (AFP) ≥ 400 ng/dL.41,42
Thalidomide can inhibit angiogenesis by interfering with the effect of VEGF and fibroblast growth factor.43 Several clinical trials discussed the therapeutic effect of thalidomide on patients with HCC whose cancer could not be resected or treated locally.44 It was found that the objective response rate of thalidomide was about 5%, and 10% to 30% of patients demonstrated disease stability for 2 months after thalidomide single drug treatment.45
Matrix Metalloproteinase Inhibitors
Metastasis is one of the characteristics of cancer.46-49 One of the important steps is the degradation of extracellular matrix (ECM), which is especially important for liver cancer.50-52 The invasion and metastasis that result after ECM degradation are largely related to integrin and matrix metalloproteinases (MMPs).53-55 Integrin not only mediates the adhesion of HCC cells to other HCC cells and HCC cells to the ECM but also participates in the chemotaxis, proliferation, and apoptosis of tumor cells, and thus, integrin involvement extends nearly throughout the entire process of HCC cell invasion and metastasis.56-60 The monoclonal antibodies LM609 and Vitaxin of integrin aVβ3 also showed good antiangiogenic effects in vitro and in vivo, and Vitaxin has subsequently entered phase II clinical trials.61
Matrix metalloproteinase is a type of proteolytic enzyme involved in the degradation of the ECM and basement membrane.62-65 Its structure, function, and regulatory level are closely related to the growth, invasion, and metastasis of liver cancer.66-69 A large number of studies have shown that MMPs, including MMP-7, MMP-9, and MMP-2, are highly expressed in liver cancer cells and tissues.70-73 Marimastat is a synthetic MMP inhibitor.74 Like tissue inhibitor of metalloproteinases-2, Marimastat can inhibit the invasion induced by hepatocyte growth factor (HGF).75 Clinically related drugs also include Bamalabaster, Novartis, BAY12-9566, AG-3340, 0PB-3206, KBR07785, and KBR-8301.76-78 At present, it is suggested that the response rate of these drugs is low, and they may be used as chemopreventive drugs for liver cirrhosis and other patients with a higher risk of developing liver cancer.
Liver Cancer-Specific Antigen Targeting Drugs
α-Fetoprotein Targeted Drugs
α-Fetoprotein is a sensitive serum marker of HCC.79-81 A clinical study of Japanese chronic hepatitis showed that the sensitivity, cutoff, and specificity of AFP were 79%, 78 ng/mL, and 78%, respectively.82 In small liver cancer, the sensitivity of AFP is relatively low (33%-65%),83 and the level of AFP in serum seems to be related to the size and differentiation of HCC.84 In a considerable number of patients with chronic liver disease, AFP (20-200 ng/mL) increased significantly.85 Therefore, AFP has long been used in the clinical diagnosis of HCC.
With the development of new research, tumor immunology researchers are increasingly interested in the antitumor immune response of AFP.86 In a phase I/II clinical trial, 4 kinds of AFP-activated dendritic cells were used to treat patients with HCC to test their immune response.87 The results of ELISPOT showed that interferon γ (IFN-γ) increased in 6 of 10 individuals after inoculation with at least 1 AFP polypeptide, resulting in AFP-specific T-cell response.88 In addition, studies of AFP-DNA vaccines and adenovirus-driven immunotherapy in 2 pretreated AFP-positive HCC patients reported expected safety and immunogenic T-cell responses.89
Glypican-3 Targeted Drugs
Glypican-3 is a heparin sulfate proteoglycan anchored to the cell surface by glycosylphosphatidylinositol.90 Glypican-3 is a carcinoembryonic antigen, and its abnormal overexpression in 81% of patients with HCC is related to poor prognosis.91 Therefore, it may be an ideal target for HCC targeting therapy.
So far, many research institutions have invented 4 high-affinity antibodies, GCC-3, HN3, HS20, and YP7, for GPC-3 using phage display and hybridoma technology.92 A phase I clinical study of GCC3 showed that patients with high GPC-3 expression had significantly higher progression-free survival than those with low GPC-3 expression.93 HN3 and YP7 can fuse with the fragment pe38 of Pseudomonas exotoxin A to produce immunotoxin.94 The heterotopic tumor of Hep3B and HepG2 can be eliminated using HN3-PE38 alone or combined with chemotherapy.95 The immunotoxin produced by YP7-PE38 has better antitumor activity than that produced by HN3-PE38.96 Therefore, GPC-3 is expected to be a new potential target for HCC treatment.
Hepatocyte Growth Factor and Its Receptor Inhibitors
Hepatocyte growth factor is an important liver regeneration factor.97 Its receptor is c-Met, which belongs to the tyrosine kinase receptor family.98 c-Met is highly expressed or mutated in many tumor cells, including HCC.99 Hepatocyte growth factor combined with c-Met, through a series of signal transduction pathways, is closely related to cell growth, differentiation, angiogenesis, and other processes, and promotes the infiltration and metastasis of HCC cells.100-103 This indicates that HGF may become an effective target for the treatment of HCC.
At present, the inhibitors and antagonists of HGF include the following: (1) NK4, an endogenous fragment truncated by HGF, which can bind to the c-Met receptor, but cannot activate it, thus antagonizing the interaction between HGF and c-Met, and in turn inhibiting HCC cell invasion and metastasis104; (2) small molecule c-Met selective inhibitors, such as SOMCL-863, PHA.665752, and SU11274105; (3) tivantinib (ARQ 197), which mainly binds to retinoic acid receptor and can downregulate the expression of c-Met protein, inhibit HGF-induced invasion, and inhibit the intrahepatic spread of HCC cells and liver metastasis of other types of cancer by inhibiting transcription factor subuni (AP1) activity.106 Clinical trials have confirmed that tivantinib is a drug with better efficacy and less adverse reactions, and thus, it may become a new anti-HCC drug.
Drugs Targeted at Immuno Checkpoint
Immuno checkpoint blocking therapy has recently become a promising treatment for many kinds of malignant tumors, including HCC.107 At present, cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death (PD) 1, T-cell immunoglobulin and mucin domain-containing protein 3, and B- and T-lymphocyte attenuator are the most studied immuno checkpoint receptors.108 Blocking the above negative regulatory immune regulatory targets can significantly improve the median overall survival and response rate of patients and improve the prognosis of patients with melanoma, renal cancer, and non-small cell lung cancer.109 The blocking monoclonal antibodies of CTLA-4 and PD-1/PD-L1 have been approved by the Food and Drug Administration (FDA) for melanoma treatment.110
Cytotoxic T-lymphocyte Antigen 4 Targeted Drugs
Cytotoxic T-lymphocyte antigen 4 is expressed in activated T cells and Treg cells and also in the initial T cells.111 Compared with CD28, CTLA-4 has higher affinity to CD80 and CD86.112 Therefore, CTLA-4 can inhibit the binding of CD28, CD80, and CD86, and thus inhibit T-cell activation.113 Cytotoxic T-lymphocyte antigen 4 signaling may also stimulate the expression of transforming growth factor β in CD4-positive T cells.114 In addition, inhibition of CD28 binding to CD80 and CD86 in Regulator Of WNT Signaling Pathway (APC) may result in a decrease in T-cell activation.115 Specific knockout or blocking of CTLA-4 can activate the autoimmune response and improve anticancer immunity.116 In 2011, the US FDA approved a randomized phase III clinical trial using the monoclonal antibody ipilimumab blocking CTLA-4 for the treatment of melanoma.117
The phase I clinical trial (NCT01008358) of human IgG2 monoclonal antibody tremelimumab against CTLA-4 in patients with HCC showed that 21 patients with advanced liver cirrhosis (liver function grade of Child-Pugh A or B) who were not suitable for percutaneous ablation or transarterial embolization had good tolerance to tremelimumab, and there were no treatment-related deaths.112 About 17.6% of the patients had partial reactions, and 45% of them were stable for more than 6 months. Interestingly, patients with stable IFN-γ during treatment had a better response than those with decreased IFN-γ, which indicated that patients with stable IFN-γ had active antitumor immunity. A phase I clinical trial of tremelimumab in patients undergoing radiofrequency ablation or arterial therapy is currently in progress (NCT01853618).118 Another phase II clinical trial (NCT01649024), in which tremelimumab was used to treat patients with advanced HCC, showed that among the 17 evaluable cases, 3 had partial remission, 10 had stable tumor control, and the disease control rate was as high as 76.4%.119
Programmed Cell Death 1 Targeted Drugs
As a member of the CD28 superfamily, PD-1 was first discovered by Professor Yoshio Benshu of Kyoto University in 1992.120 At present, PD-1, as a significant target of immunotherapy, has attracted wide attention in the field of tumor-targeted therapy.121 Programmed Cell Death 1 was mainly expressed in CD8-positive T cells but could also be detected in Treg cells and myeloid-derived suppressor cells (MDSCs).122 Programmed Cell Death 1 not only mediates the differentiation and proliferation of Treg but also regulates peripheral blood tolerance and autoimmunity.123 Long-term antigen exposure leads to overexpression of PD-1 in T cells, leading to T-cell depletion or nonresponse.124
Tumor cells can express PD-L1 or PD-L2 to activate the expression of PD-1 in tumor-infiltrating lymphoid tissue and escape from immune surveillance.8 The clinical study results showed that the PD-1/PDL-1 pathway may induce immune tolerance for HCC. At the same time, the expression levels of PD-1 and PD-L1 are closely related to the development stage, local recurrence rate, and poor HCC prognoses.125 Similarly, the level of PD-1 and CD8 positive T cells in the tumor is related to HCC progression and postoperative recurrence.126
In addition, in the HBV-positive HCC patients who received cryoablation, the prognosis of those who expressed PD-1 and PD-L1 on circulating tumor cells was poor.127 In vitro, Treg cells, MDSCs, and PD-1-positive T cells from the peripheral blood of patients with advanced HCC were consumed simultaneously to restore the activation of CD8-positive T cells.128
In 2017, El Khoueiry et al released the latest data of nivolumab, a PD-1 inhibitor, in the treatment of patients with advanced HCC.129 In the dose extension stage, 42 (20%) patients observed objective response, 96 (45%) patients observed stable disease, 138 (64%) patients observed disease control, 28 (67%) patients had sustained response, and the median response time was 9.9 months. Most of the diseases were stable for at least 6 months, and 79 (57%) of 138 patients demonstrated disease control. At the dose extension stage, the median progression time was 4.1 months, and the overall survival rate was 83% at 6 months and 74% at 9 months. The 6-month progression-free survival rate was 37%, and the 9-month progression-free survival rate was 28%.130,131
Outlook
Multiple combination regimens, including immunotherapy, multireceptor kinase inhibitors, and anti-VEGF agents likely portend the future of HCC treatment, in which combination therapies will hopefully increase objective responses and overall survival. In recent years, PD-1 antibody has made great achievements in further promoting the application and research of various drugs targeting immunoassay sites in HCC, and liver cancer treatments targeting immunoassay sites have entered development. Although the available treatment methods of advanced liver cancer are greatly expanding at present, single administration treatment methods do not yield satisfactory results. Therefore, it is necessary to increase the development of new targeted drugs and to simultaneously promote the research and application of targeted drugs combined with other treatment methods or multiple targeted drugs combined with each other. These combinations may include targeted therapy combined with surgical resection, radiotherapy, chemotherapy, interventional therapy, and immunotherapy, and even targeted therapy combined with cell therapy, gene therapy, and other conventional or emerging treatment methods for liver cancer. As research broadens and deepens in scope, targeted therapy will likely continue to demonstrate its advantages of high specificity, good therapeutic effects, long-lasting benefits, and less adverse reactions.
Acknowledgments
We would like to thank Prof Fei Yu for data analysis and critical discussion of the manuscript. We thank LetPub (https://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Authors’ Note: YSM and DF designed the study. YSM, JBL, and TMW contributed equally to this work. All authors performed the statistical analyses and interpreted the data. D.F. wrote the manuscript. All authors contributed to the final version of the manuscript and approved the final manuscript. Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported partly by grants from the National Natural Science Foundation of China (81972214, 81772932, 81472202, 81201535, 81302065, 81671716, 81301993, 81372175 and 81472209), The Fundamental Research Funds for the Central Universities (22120170212 and 22120170117), The Scientific Research Fund Project of Anhui Medical University (2018xkj058), Shanghai Natural Science Foundation (12ZR1436000), Shanghai Municipal Commission of Health and Family Planning (201540228), Special funding fund for clinical research of Wu Jieping Medical Foundation (320.6750.14326), Nantong science and technology project (YYZ15026), The Peak of Six personnel Foundation in Jiangsu Province (WSW-009), The Fifth Phase of 333 Talents Engineering Science and Technology Project of Jiangsu Province (2017205), and Jiangsu Province Science Foundation for Youths (BK2012101).
ORCID iD: Da Fu, PhD  https://orcid.org/0000-0002-0878-2575
https://orcid.org/0000-0002-0878-2575
References
- 1. Ma YS, Lv ZW, Yu F, et al. MiRNA-302a/d inhibits the self-renewal capability and cell cycle entry of liver cancer stem cells by targeting the E2F7/AKT axis. J Exp Clin Cancer Res. 2018;37(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Luo Q, Liu L, Song G. Low-level shear stress promotes migration of liver cancer stem cells via the FAK-ERK1/2 signalling pathway. Cancer Lett. 2018;427:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Abou-El-Enein M, Grainger DW, Kili S. Registry contributions to strengthen cell and gene therapeutic evidence. Mol Ther. 2018;26(5):1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu G, Ma Z, Cheng Y, et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer. 2018;17(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escobar G, Barbarossa L, Barbiera G, Norelli M, Genua M, Ranghetti A. Interferon gene therapy reprograms the leukemia microenvironment inducing protective immunity to multiple tumor antigens. Nat Commun. 2019;9(1):2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwon S, Velasquez FC, Rasmussen JC, et al. Nanotopography-based lymphatic delivery for improved anti-tumor responses to checkpoint blockade immunotherapy. Theranostics. 2019;9(26):8332–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Z, Lin Y, Zhang J, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oshima G, Guo N, He C, et al. In vivo delivery and therapeutic effects of a microrna on colorectal liver metastases. Mol Ther. 2018;25(7):1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Fu S, Zhao J, et al. Transbronchoscopic patient biopsy-derived xenografts as a preclinical model to explore chemorefractory-associated pathways and biomarkers for small-cell lung cancer. Cancer Lett. 2019:180–188. [DOI] [PubMed] [Google Scholar]
- 12. Zheng JF, He S, Zeng Z, Gu X, Cai L, Qi G. PMPCB silencing sensitizes HCC tumor cells to sorafenib therapy. Mol Ther. 2019;27(10):1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raoul JL, Kudo M, Finn RS, Edeline J, Reig M, Galle PR. Systemic therapy for intermediate and advanced hepatocellular carcinoma: sorafenib and beyond. Cancer Treat Rev. 2018;68:16–24. [DOI] [PubMed] [Google Scholar]
- 14. He L, Zhu W, Chen Q, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 16. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–358. [DOI] [PubMed] [Google Scholar]
- 17. Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. [DOI] [PubMed] [Google Scholar]
- 18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. [DOI] [PubMed] [Google Scholar]
- 19. Suyama K, Iwase H. Lenvatinib: a promising molecular targeted agent for multiple cancers. Cancer Control. 2018;25(1):1073274818789361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garbuzenko OB, Kuzmov A, Taratula O, Pine SR, Minko T. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019;9(26):8362–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Kasim V, Yan X, Li L, et al. Yin Yang 1 facilitates hepatocellular carcinoma cell lipid metabolism and tumor progression by inhibiting PGC-1β-induced fatty acid oxidation. Theranostics. 2019;9(25):7599–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maeda O, Ando Y. Cabozantinib in hepatocellular carcinoma. N Engl J Med. 2018;379(14):1384. [DOI] [PubMed] [Google Scholar]
- 23. Hu F, Li H, Liu L, et al. Correction to: histone demethylase KDM4D promotes gastrointestinal stromal tumor progression through HIF1β/VEGFA signalling. Mol Cancer. 2018;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tzchori I, Falah M, Shteynberg D, et al. Improved patency of ePTFE grafts as a hemodialysis access site by seeding autologous endothelial cells expressing fibulin-5 and VEGF. Mol Ther. 2018;26(7):1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukherjee S, Sonanini D, Maurer A, Daldrup-Link HE. The yin and yang of imaging tumor associated macrophages with PET and MRI. Theranostics. 2019;9(25):7730–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Havunen R, Santos JM, Sorsa S, et al. Abscopal effect in non-injected tumors achieved with cytokine-armed oncolytic adenovirus. Mol Ther Oncolytics. 2018;11:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xin X, Wu M, Meng Q, et al. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol Cancer. 2018;17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mauseth B, Camilio KA, Shi J, et al. The novel oncolytic compound LTX-401 induces antitumor immune responses in experimental hepatocellular carcinoma. Mol Ther Oncolytics. 2019;14:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim Y, Jo M, Schmidt J, et al. Enhanced potency of GalNAc-conjugated antisense oligonucleotides in hepatocellular cancer models. Mol Ther. 2019;27(9):1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang JL, Cao SW, Ou QS, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer. 2018;17(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nuti M, Zizzari IG, Botticelli A, Rughetti A, Marchetti P. The ambitious role of anti angiogenesis molecules: turning a cold tumor into a hot one. Cancer Treat Rev. 2018;70:41–46. [DOI] [PubMed] [Google Scholar]
- 32. Rodell CB, Koch PD, Weissleder R. Screening for new macrophage therapeutics. Theranostics. 2019;9(25):7714–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajaraman S, Canjuga D, Ghosh M, et al. Measles virus-based treatments trigger a pro-inflammatory cascade and a distinctive immunopeptidome in glioblastoma. Mol Ther Oncolytics. 2018;12:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grépin R, Guyot M, Dumond A, et al. The combination of bevacizumab/Avastin and erlotinib/Tarceva is relevant for the treatment of metastatic renal cell carcinoma: the role of a synonymous mutation of the EGFR receptor. Theranostics. 2020;10(3):1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett. 2016;370(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(12):1898–1903. [DOI] [PubMed] [Google Scholar]
- 37. Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. [DOI] [PubMed] [Google Scholar]
- 39. Gilabert M, Raoul JL. Potential of ramucirumab in treating hepatocellular carcinoma patients with elevated baseline alpha-fetoprotein. J Hepatocell Carcinoma. 2018;5:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alphafetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. [DOI] [PubMed] [Google Scholar]
- 41. Kudo M, Okusaka T, Motomura K, et al. Ramucirumab as secondline treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: pooled efficacy and safety in Japanese patients across two global randomized phase III studies (REACH-2 and REACH). J Clin Oncol. 2019;37:320. [Google Scholar]
- 42. Zhu AX, Baron AD, Malfertheiner P, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH trial results by child-pugh score. JAMA Oncol. 2017;3(2):235–243. [DOI] [PubMed] [Google Scholar]
- 43. Abdallah MA, Singal AK. Mitochondrial dysfunction and alcohol-associated liver disease: a novel pathway and therapeutic target. Signal Transduct Target Ther. 2020;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patt YZ, Hassan MM, Lozano RD, et al. Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial. Cancer. 2005;103(4):749–755. [DOI] [PubMed] [Google Scholar]
- 45. Lin AY, Brophy N, Fisher GA, et al. Phase II study of thalidomide in patients with unresectable hepatocellular carcinoma. Cancer. 2005;103(1):119–125. [DOI] [PubMed] [Google Scholar]
- 46. Ma YS, Huang T, Zhong XM, et al. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol Cancer. 2018;17(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Di L, Liu LJ, Yan YM, et al. Discovery of a natural small-molecule compound that suppresses tumor EMT, stemness and metastasis by inhibiting TGFβ/BMP signaling in triple-negative breast cancer. J Exp Clin Cancer Res. 2019;38(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin Z, Sun L, Xie S, et al. Chemotherapy-induced long non-coding RNA 1 promotes metastasis and chemo-resistance of TSCC via the Wnt/β-catenin signaling pathway. Mol Ther. 2018;26(6):1494–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ji Q, Xu X, Song Q, et al. MiR-223-3p inhibits human osteosarcoma metastasis and progression by directly targeting CDH6. Mol Ther. 2018;26(5):1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177–187. [DOI] [PubMed] [Google Scholar]
- 51. Huang YN, Qian TT, Dang F, Yin YG, Li M, Zhou DM. Significant contribution of metastable particulate organic matter to natural formation of silver nanoparticles in soils. Nat Commun. 2019;10(1):3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yue B, Liu C, Sun H, et al. A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther. 2018;26(5):1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shen M, Zhao X, Zhao L, et al. Met is involved in TIGAR-regulated metastasis of non-small-cell lung cancer. Mol Cancer. 2018;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang CF, Shi ZM, Li DM, et al. Estrogen-induced miR-196a elevation promotes tumor growth and metastasis via targeting SPRED1 in breast cancer. Mol Cancer. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mooney R, Majid AA, Batalla-Covello J, et al. Enhanced delivery of oncolytic adenovirus by neural stem cells for treatment of metastatic ovarian cancer. Mol Ther Oncolytics. 2018;12:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Whilding LM, Parente-Pereira AC, Zabinski T, et al. Targeting of aberrant αvβ6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol Ther. 2017;25(10):2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Sun J, Tan M, et al. Species-specific involvement of integrin αiibβ3 in a monoclonal antibody CH12 triggers off-target thrombocytopenia in cynomolgus monkeys. Mol Ther. 2018;26(6):1457–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sarathy A, Wuebbles RD, Fontelonga TM, et al. SU9516 increases α7β1 integrin and ameliorates disease progression in the mdx mouse model of duchenne muscular dystrophy. Mol Ther. 2017;25(6):1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang MH, Zhao L, Wang L, et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin β3 transcriptional activating and MAPK/AKT signalling. Mol Cancer. 2019;18(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duhachek-Muggy S, Qi Y, Wise R, et al. Metalloprotease-disintegrin ADAM12 actively promotes the stem cell-like phenotype in claudin-low breast cancer. Mol Cancer. 2017;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu H, Beuerlein G, Nie Y, et al. Stepwise in vitro affinity maturation of Vitaxin, an alphav beta3-specific humanized mAb. Proc Natl Acad Sci U S A. 1998;95(11):6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang R, Zhang S, Chen X, et al. EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer. 2018;17(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lwin ST, Fowler JA, Drake MT, Edwards JR, Lynch CC, Edwards CM. A loss of host-derived MMP-7 promotes myeloma growth and osteolytic bone disease in vivo. Mol Cancer. 2017;16(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu KP, Ma XL, Zhang CL. LncRNA ODRUL contributes to osteosarcoma progression through the miR-3182/MMP2 Axis. Mol Ther. 2017, 25(10):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Cheng J, Guo J, North BJ, et al. Functional analysis of deubiquitylating enzymes in tumorigenesis and development. Biochim Biophys Acta Rev Cancer. 2019, 1872(2):188312. [DOI] [PubMed] [Google Scholar]
- 66. Hadi T, Boytard L, Silvestro M, et al. Macrophage-derived netrin-1 promotes abdominal aortic aneurysm formation by activating MMP3 in vascular smooth muscle cells. Nat Comm. 2018;9(1):5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luo F, Tran AP, Xin L, et al. Modulation of proteoglycan receptor PTPσ enhances MMP-2 activity to promote recovery from multiple sclerosis. Nat Comm. 2018;9(1):4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beug ST, Pichette SJ, St-Jean M, et al. Combination of IAP antagonists and TNF-α-armed oncolytic viruses induce tumor vascular shutdown and tumor regression. Mol Ther Oncolytics. 2018;10:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Santiago CP, Keuthan CJ, Boye SL, Boye SE, Imam AA, Ash JD. A drug-tunable gene therapy for broad-spectrum protection against retinal degeneration. Mol Ther. 2018;26(10):2407–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma Y, Cao G, Hu J, Liu X, Liu J. Silence of lncRNA HEIH suppressed liver cancer cell growth and metastasis through miR-199a-3p/mTOR axis. J Cell Biochem. 2019;120(10):17757–17766. [DOI] [PubMed] [Google Scholar]
- 71. Gasch C, Ffrench B, O’Leary JJ, Gallagher MF. Catching moving targets: cancer stem cell hierarchies, therapy-resistance & considerations for clinical intervention. Mol Cancer. 2017;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang Y, Wang J, Lian Y, et al. Linking long non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancer. Mol Cancer. 2017;16(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang HL, Thiyagarajan V, Shen PC, et al. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J Exp Clin Cancer Res. 2019;38(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lyu Y, Xiao Q, Yin L, Yang L, He W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduct Target Ther. 2019;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ulasov I, Borovjagin AV, Kaverina N, et al. MT1-MMP silencing by an shRNA-armed glioma-targeted conditionally replicative adenovirus (CRAd) improves its anti-glioma efficacy in vitro and in vivo. Cancer Lett. 2015;365(2):240–250. [DOI] [PubMed] [Google Scholar]
- 76. Lv Y, Zhao X, Zhu L, et al. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018, 8(10):2830–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Heath EI, O’Reilly S, Humphrey R, et al. Phase I trial of the matrix metalloproteinase inhibitor BAY12-9566 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2001;48(4):269–274. [DOI] [PubMed] [Google Scholar]
- 78. Wojtowicz-Praga S. Clinical potential of matrix metalloprotease inhibitors. Drugs R D. 1999;1(2):117–129. [DOI] [PubMed] [Google Scholar]
- 79. Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pursell N, Gierut J, Zhou W, et al. Inhibition of glycogen synthase II with RNAi prevents liver injury in mouse models of glycogen storage diseases. Mol Ther. 2018;26(7):1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nwosu ZC, Battello N, Rothley M, et al. Correction to: liver cancer cell lines distinctly mimic the metabolic gene expression pattern of the corresponding human tumours. J Exp Clin Cancer Res. 2018;37(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol Ther Oncolytics. 2019;13:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tahmasebi-Birgani M, Ansari H, Carloni V. Defective mitosis-linked DNA damage response and chromosomal instability in liver cancer. Biochim Biophys Acta Rev Cancer. 2019;1872(1):60–65. [DOI] [PubMed] [Google Scholar]
- 84. Guo Y, Wu Z, Shen S, et al. Nanomedicines reveal how PBOV1 promotes hepatocellular carcinoma for effective gene therapy. Nat Comm. 2018;9(1):3430. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85. Bian Z, Hann HW, Ye Z, et al. Ferritin level prospectively predicts hepatocarcinogenesis in patients with chronic hepatitis B virus infection. Oncol Lett. 2018;16(3):3499–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xiong W, Chen Y, Kang X, et al. Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol Ther. 2018;26(4):963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li W, Turaga RC, Li X, et al. Overexpression of smac by an armed vesicular stomatitis virus overcomes tumor resistance. Mol Ther Oncolytics. 2019;14:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nakagawa H, Mizukoshi E, Kobayashi E, et al. Association between high-avidity t-cell receptors, induced by α-fetoprotein-derived peptides, and anti-tumor effects in patients with hepatocellular carcinoma. Gastroenterology. 2017;152(6):1395–1406. [DOI] [PubMed] [Google Scholar]
- 89. Duperret EK, Wise MC, Trautz A, et al. Synergy of immune checkpoint blockade with a novel synthetic consensus DNA Vaccine Targeting TERT. Mol Ther. 2018, 26(2): 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu X, Luo H, Shi B, et al. Combined antitumor effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma. Mol Ther. 2019;27(8):1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yu M, Luo H, Fan M, et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018;26(2):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang Y, Zhang H, Wei M, et al. Recombinant adenovirus expressing a soluble fusion protein PD-1/CD137 L subverts the suppression of CD8(+) T Cells in HCC. Mol Ther. 2019;27(11):1906–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun Q, Hu Y, Gu Y, et al. Deciphering the regulatory and catalytic mechanisms of an unusual SAM-dependent enzyme. Signal Trans Target Ther. 2019;4(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gao W, Tang Z, Zhang YF, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of WNT signalling and protein synthesis. Nat Comm. 2015;6:6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hassan R, Alewine C, Pastan I. New life for immunotoxin cancer therapy. Clin Cancer Res. 2016;22(5):1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mazor R, Zhang J, Xiang L, et al. Recombinant immunotoxin with t-cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin-expressing tumors. Mol Cancer Ther. 2015;14(12):2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Demkova L, Kucerova L. Role of the HGF/c-MET tyrosine kinase inhibitors in metastasic melanoma. Mol Cancer. 2018;17(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Capsomidis A, Benthall G, Van Acker HH, et al. Chimeric antigen receptor-engineered human gamma delta t cells: enhanced cytotoxicity with retention of cross presentation. Mol The. 2018, 26(2):354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Phelps MP, Yang H, Patel S, Rahman MM, McFadden G, Chen E. Oncolytic virus-mediated RAS targeting in rhabdomyosarcoma. Mol Ther Oncolytics. 2018;11:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin Y, Fang ZP, Liu HJ, et al. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5(+) liver stem cells. Nat Comm. 2017;8(1):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Huang X. The potential role of HGF-MET signaling and autophagy in the war of alectinib versus crizotinib against ALK-positive NSCLC. J Exp Clin Cancer Res. 2018;37(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gao X, Jiang P, Zhang Q, et al. Peglated-H1/pHGFK1 nanoparticles enhance anti-tumor effects of sorafenib by inhibition of drug-induced autophagy and stemness in renal cell carcinoma. J Exp Clin Cancer Res. 2019;38(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang D, Saga Y, Sato N, et al. The hepatocyte growth factor antagonist NK4 inhibits indoleamine-2,3-dioxygenase expression via the c-Met-phosphatidylinositol 3-kinase-AKT signaling pathway. Int J Oncol. 2016;48(6):2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang L, Ai J, Shen Y, et al. SOMCL-863, a novel, selective and orally bioavailable small-molecule c-Met inhibitor, exhibits antitumor activity both in vitro and in vivo. Cancer Lett. 2014;351(1):143–150. [DOI] [PubMed] [Google Scholar]
- 106. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–693. [DOI] [PubMed] [Google Scholar]
- 107. Zheng Z, Sun R, Zhao HJ, et al. MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+T cells. Mol Cancer. 2019;18(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Redmer T. Deciphering mechanisms of brain metastasis in melanoma - the gist of the matter. Mol Cancer. 2018;17(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu L, Wang Y, Miao L, et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Soldevilla MM, Villanueva H, Meraviglia-Crivelli D, et al. ICOS costimulation at the tumor site in combination with CTLA-4 blockade therapy elicits strong tumor immunity. Mol The. 2019;S1525-0016(19): 30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu L, Wang Y, Miao L, et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ock CY, Hwang JE, Keam B, et al. Genomic landscape associated with potential response to anti-CTLA-4 treatment in cancers. Nat Comm. 2017;8(1):1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. [DOI] [PubMed] [Google Scholar]
- 116. Goswami S, Apostolou I, Zhang J, et al. Modulation of EZH2 expression in T cells improves efficacy of anti-CTLA-4 therapy. J Clin Invest. 2018;128(9):3813–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wolchok JD, Rollin L, Larkin J. Nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(25):2503–2504. [DOI] [PubMed] [Google Scholar]
- 118. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14(11):1104–1111. [DOI] [PubMed] [Google Scholar]
- 120. von Knethen A, Brüne B. PD-L1 in the palm of your hand: palmitoylation as a target for immuno-oncology. Signal Trans Target Ther. 2019, 4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Andrieu GP, Shafran JS, Smith CL, et al. BET protein targeting suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer and elicits anti-tumor immune response. Cancer Lett. 2019;465:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Veldman J, Visser L, Berg AVD, Diepstra A. Primary and acquired resistance mechanisms to immune checkpoint inhibition in Hodgkin lymphoma. Cancer Treat Rev. 2019;82:101931. [DOI] [PubMed] [Google Scholar]
- 123. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cai J, Wang D, Zhang G, Guo X. The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther. 2019;12:8437–8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. [DOI] [PubMed] [Google Scholar]
- 126. Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019;18(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (check mate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. [DOI] [PubMed] [Google Scholar]