Abstract
The surgical stress and inflammatory response and volatile anesthetic agents have been shown to promote tumor metastasis in animal and in-vitro studies. Regional neuraxial anesthesia protects against these effects by decreasing the surgical stress and inflammatory response and associated changes in immune function in animals. However, evidence of a similar effect in humans remains equivocal due to the high variability and retrospective nature of clinical studies and difficulty in directly comparing regional versus general anesthesia in humans. We propose a theoretical framework to address the question of regional anesthesia as protective against metastasis.
This theoretical construct views the immune system, circulating tumor cells, micrometastases, and inflammatory mediators as distinct populations in a highly connected system. In ecological theory, highly connected populations demonstrate more resilience to local perturbations but are prone to system-wide shifts compared with their poorly connected counterparts. Neuraxial anesthesia transforms the otherwise system-wide perturbations of the surgical stress and inflammatory response and volatile anesthesia into a comparatively local perturbation to which the system is more resilient. We propose this framework for experimental and mathematical models to help determine the impact of anesthetic choice on recurrence and metastasis and create therapeutic strategies to improve cancer outcomes after surgery.
Keywords: onco-anesthesia, regional neuraxial anesthesia, cancer outcomes, immunosurveillance, immunoediting
Background and Significance
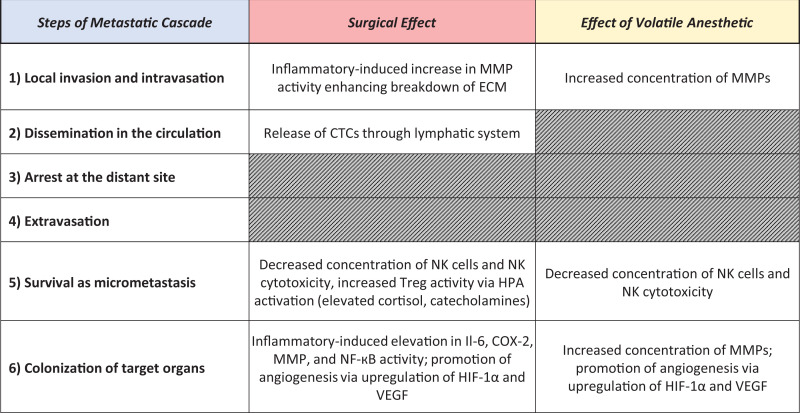
Growing evidence suggests that the pathophysiological disturbances of surgery and anesthetic medications administered during the perioperative period impact cancer recurrence and metastasis.1,2 These system-wide disturbances impart their influence at several key points in the metastatic cascade as illustrated in Figure 1.3-6 The perioperative timeframe, while small in comparison to the full life cycle of a cancer, is a critical time period of pathophysiological disturbance.7 Regional neuraxial anesthesia has a potentially protective effect that dampens this overall disturbance via reduction of the surgical stress response, reduction of surgical inflammation, and a decrease in volatile anesthetic requirement during surgery. The sum of these 3 effects would theoretically reduce the probability of metastasis and recurrence by decreasing the survival of micrometastases, colonization of target organs, and local invasion and intravasation. While shedding of circulating tumor cells (CTCs) during surgery is likely not preventable, the ability of those CTCs to survive and create metastases appears to be decreased by regional neuraxial anesthesia based on these known mechanisms. However, human clinical data has not provided clear evidence of a benefit of regional anesthesia in improving cancer survival and outcomes.1,2
Animal studies demonstrate protective effects of regional versus general anesthesia and of certain medications discussed below.8,9 While selected human studies also suggest protective effects of regional anesthesia, meta-analyses in humans remain equivocal.1,2,10-14 This is likely because most human studies are retrospective, with uncontrolled variability in administered anesthesia medications, recovery protocols, pain control modalities, and postoperative medication administration. Variability in the length of patient follow-up among these studies may also contribute to the ambiguity of their findings. In those studies that demonstrate effects of anesthetic choice on cancer recurrence and metastases, regional anesthesia inhibits, whereas general anesthesia with inhaled agents promotes metastases and recurrence.1,12,14,15 A recent nationwide retrospective study of 196,303 eligible patients found no difference in overall or recurrence-free survival when comparing inhaled volatile anesthesia versus propofol-based total intravenous anesthesia for digestive cancer surgery.16 And a randomized controlled trial by Hovaguimian and colleagues found no difference in the amount of circulating tumor cell counts over 72 hours after primary breast cancer surgery under inhaled volatile (sevoflurane) versus propofol-based total intravenous anesthesia.17 Previous retrospective human studies have shown differing results with some indicating a benefit of propofol-based total intravenous anesthesia versus inhaled volatile anesthesia in cancer surgery, versus others which found no such benefit.18-29 Independent of anesthetic choice, the stress response and inflammation secondary to surgical stimulation appears to increase the likelihood of metastasis and recurrence, which may explain the protective effect of regional anesthesia7,30,31 (Figure 1). Opioids appear to be detrimental based on in-vitro and animal studies as a result of decreased NK-cell activity and promotion of angiogenesis by VEGF upregulation.1 This effect may be partially negated at very large doses that decrease the stress response to pain.1,32 COX inhibitors demonstrate a protective effect in both animal and human studies as COX-2 is upregulated in many cancer types and PGE2 promotes cancer progression.1 Amide local anesthetics, which are commonly used in regional anesthesia, also appear to have a protective effect via the Src-tyrosine kinase signaling pathway, reversal of DNA hypermethylation, and direct effects on voltage gated Na+ channels in cancer cells.1 While several preclinical studies showed evidence of protection against recurrence and metastasis, overall evidence on perioperative beta blockade remains equivocal.33
Figure 1.
Surgery, volatile anesthesia, and the metastatic cascade. Published literature suggests that surgery and volatile anesthesia contribute to various stages of the metastatic cascade in similar and mostly overlapping ways. Effects of volatile anesthesia may amplify those of surgery itself, leading to increased rates of metastasis and recurrence. MMP, matrix metalloproteinase; ECM, extracellular matrix; CTC, circulating tumor cell; NK, natural killer; Treg, regulatory T cells; HPA, hypothalamic pituitary adrenal axis; Il-6, interleukin 6; COX-2, cyclooxygenase-2; NF-кB, nuclear factor kappa B; HIF, hypoxia inducible factor; VEGF, vascular endothelial growth factor.
A typical adult surgical patient undergoing colon resection demonstrates the complexity of this research in humans. This patient will likely have either general anesthesia alone or general anesthesia coupled with regional anesthesia such as an epidural. These options necessarily confound a comparison of the effects of general versus regional anesthesia on recurrence or metastasis, which requires a juxtaposition of general anesthesia versus regional anesthesia alone. Further complications are introduced because the degree of surgical stimulation and stress response will vary with the surgeon, the surgical approach (open versus laparoscopic or robotic), and the size and type of incision. There is also evidence that surgical skill level and overall case volume can impact oncological outcomes in cancer surgery.34-36 The amount and type of opioids, COX inhibitors, local anesthetics, and beta blockade required, if these agents are used at all, will also vary among patients. If used, the amount of inhaled volatile agent required will also differ by patient and is impacted by the use of regional anesthesia, opioids, neuromuscular blockade, neoadjuvant non-opioid pain medications, and patient-specific factors. Furthermore, there is heterogeneity in patient tumor characteristics and co-morbidities. In contrast, experimental parameters like surgical incision, type of anesthesia, surgical population, tumor type, and elimination of confounding medications are highly controlled in animal models. Therefore, while animal and in-vitro studies provide clear results regarding effects of general and regional anesthesia on cancer recurrence and metastasis, data for humans are difficult to interpret.
Several large, randomized controlled studies underway for humans are of great interest and relevance to the intersection of anesthesia and cancer progression. A recently completed study by Sessler and colleagues reported no difference in recurrence of breast cancer in patients undergoing regional anesthesia (paravertebral block with propofol) versus general anesthesia (inhaled anesthesia with opioids) for primary breast cancer resection.37 The authors noted that additional studies of patients undergoing surgeries that stimulate more pain and a larger stress response than is typical of breast surgery are needed.
The Theoretical Framework
Based on available data, we hypothesize that regional anesthesia protects against cancer recurrence and metastasis when surgical stimulation is large enough to exceed an as-yet undefined threshold. We propose a theoretical framework to explain this phenomenon by crossing disciplines with ecology, evolutionary biology, and mathematical modeling. Evolutionary therapy for treatment of cancer is a newly evolving field.38,39 Through collaboration with evolutionary biologists, ecologists, and mathematicians, new theoretical frameworks view cancers cells as distinct, evolving populations in a shared ecosystem.40 From initial theoretical work combined with mathematical modeling, cancer researchers developed adaptive therapy, which has increased survival in metastatic prostate cancer.16,17
Using a similar interdisciplinary approach, we develop a conceptual framework to explain the hypothesized effect of regional anesthesia on cancer recurrence and metastasis. We hypothesize that surgical resection of a tumor triggers cancer dissemination by shedding of tumor cells, enhancement of motility, invasion, and proliferation from proinflammatory factors, disruption of immunosurveillance, and alteration of the equilibrium that exists between the immune system and CTCs.41 This disruption results from either induction or disinhibition of seeded CTCs, effectively “releasing” them by enabling their metastatic potential. The immune system is compromised by general volatile anesthesia in addition to the surgical stress response (Figure 1). We propose that regional anesthesia prevents immune dampening by decreasing the surgical stress response and the general volatile anesthetic requirement, and CTCs are therefore less likely to metastasize owing to a functional immune response. Further, regional anesthesia diminishes the proinflammatory response reducing the probability of successful colonization of micrometastases.
A relevant and analogous ecological theoretical construct that may explain this process is that of local and system-wide adaptation as influenced by population connectivity.42 The core of this theory is the idea that highly connected systems respond to perturbation differently from poorly connected or fragmented systems. Highly connected systems tend to be more resilient to local perturbation in comparison to poorly connected ones. Poorly connected systems, on the other hand, are less prone to sudden, system-wide shifts but more susceptible to local perturbation. Thus, there is a tradeoff between local and system-wide resilience. In biology, this theory explains why poorly connected, fragmented populations can be devastated by local environmental changes, while more highly connected populations are more resilient (e.g. birds that can fly between islands compared with a terrestrial mammal that cannot easily disperse across water).
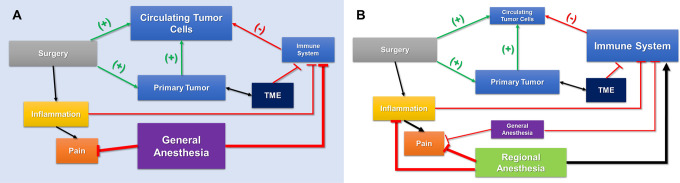
We suggest that within the human body CTCs, micrometastases, inflammatory mediators, and the immune system represent a dynamic and highly connected system. Most patients with solid tumors have circulating tumor cells and, as highlighted previously, surgical resection of primary tumor releases CTCs, inhibits proper immune function, and enhances proinflammatory factors. The complex interactions between the immune system and cancer are well-documented as intricately connected, involving innate and adaptive immunity, in a process known as “immunoediting.”37,43 General anesthesia and the proinflammatory and stress responses to surgery represent sudden, system-wide perturbations. The addition of regional anesthesia dampens these stress and proinflammatory responses, which makes the surgical incision and manipulation a comparatively local perturbation. This results in more resilience in this highly connected system in the form of increased immune protection and decreased inflammation leading to overall decreased metastatic potential of CTCs and micrometastases and a lower likelihood of tumor recurrence and metastasis (Figure 2). Additionally, regional anesthesia decreases and, in some instances, obviates the need for general anesthesia which effectively dampens the system-wide perturbation and associated effects of inhaled general anesthesia with volatile agents (Figure 2).
Figure 2.
Anesthetic techniques and tumor immunosurveillance. A. Homeostasis exists between a primary tumor and circulating tumor cells. The systemic inflammatory response by surgical resection of the tumor, and concurrent general anesthesia, dampens the immune system’s surveillance of these cells. B. When regional anesthesia modalities are employed and decreased general anesthesia is required, immune function is more well-preserved and the circulating tumor cells are less likely to metastasize. TME = tumor microenvironment.
In addition to population connectivity dynamics, our theory will also incorporate the predator-prey dynamic that exists in which key immune effector cells are “predators” and CTCs, disseminated tumor cells, and micrometastases are “prey.”44 Further, the Allee effect will be considered which is an evolutionary phenomenon in which mean individual fitness decreases as population sizes dwindle to low enough thresholds, which eventually contributes to background extinctions.45 In our case, the populations are cancer cells within the primary tumor, CTCs, and micrometastases and the immune-conserving effect of regional anesthesia may serve to promote the Allee effect and associated background extinctions.45
Application of Mathematical Modeling Within the Theoretical Framework
Mathematical models of biological systems can be designed to create useful frameworks for investigating system perturbations. These models can be used to determine the impact of a specific insult or pathway on the overall functioning of the system. Furthermore, the functioning of small subsystems can be investigated to determine their relevance within a larger system. These unique features of mathematical modeling make it an ideal modality to study the effect of regional anesthesia on the body’s proinflammatory and stress response to surgery. Mathematical modeling is particularly well-suited for the study of regional anesthesia and cancer recurrence and metastasis, because of its ability to model many small components of a complex system and estimate an overall cumulative effect of various interventions, effectively filtering through the heterogeneity seen so far in clinical human studies.46
When modeling these responses to surgery and their impact on metastatic potential, the dynamic processes of cellular behavior need to be considered. This time dependent behavior is best captured with differential equations. There is precedent for mathematical models which demonstrate the body’s response to stress and the metastatic potential after surgery.47 There have been recent breakthroughs in mathematically modeling the interconnectivity between the immune system, primary tumors, and distant metastases during treatment.48 Not only has this modeling been applied to and validated in an animal model, it has been used retrospectively to evaluate clinical data in humans and suggests that altering the sequence of radiotherapy with respect to surgical cancer resection can improve outcomes by harnessing the power of antitumor immunity.49 We suggest that it is possible to create models similarly to help determine the impact of regional anesthesia on metastatic potential via its effects on the immune system. We also aim to use such models to estimate a threshold of surgical stimulation at which the addition of regional anesthesia will result in a significant decrease in metastatic potential. As Sessler and colleagues suggested, there may be a requisite level of surgical stimulation necessary before the addition of regional anesthesia results in a statistically and clinically significant reduction in metastatic potential.37 Further, if the threshold is found to be too high to achieve significance with regional anesthesia alone, then other perioperative interventions may be necessary additions for a beneficial cumulative effect. There is currently data lacking on potential synergies of perioperative interventions implemented with the intent of harnessing immunologic protection for improved cancer outcomes.
The body’s inflammatory response to infection and trauma has been modeled repeatedly using both agent-based and differential equation-based mathematical models.50 Translational systems biology applies the use of dynamic mathematical models to study the effect of inflammatory mediators on tissue remodeling and immunosuppression.51 Iwata and colleagues proposed an initial formalism which described tumor colony growth and metastasis using the Gompertz function.52 Benzekry and colleagues went further, using the mathematical principles of dissemination and proliferation, and derived a nonlinear, quantitative model to relate presurgical primary tumor growth to postsurgical metastatic potential.47
We envision building upon these earlier works to create mathematical models that are mindful of the evolutionary and ecological framework of evolving tumor populations and metastases and the known impact of anesthetic agents and regional anesthesia on immune function as it relates to cancer immunosurveillance. These models can be tested by applying them to animal models of metastases, which will pave the way for subsequent application in humans in a similar manner as was done by López Alfonso and colleagues.49 Initially, modeling will focus on areas that can be measured quantitatively but may later incorporate human factors, such as surgeon and anesthesiologist skill level, which may affect outcomes. An iterative process of measurement, calibration, and validation will be employed before any applications in the human clinical setting. We plan to create these models to evaluate prospective, therapeutic anesthetic and surgical strategies intended to decrease the likelihood of recurrence and metastases and investigate the potential role for immune stimulating therapies for patients undergoing cancer surgery.
Footnotes
Authors’ Note: Our study did not require an ethical board approval because it did not contain human or animal trials. AM: Overall conceptual design and structure of manuscript, drafting of manuscript, figures, and revisions, coordination of co-authors. SP: Significant contributions to concept, drafting of manuscript, and revisions. CW: Significant contributions to concept, drafting of manuscript, and revisions. RA: Substantial contributions to concept, drafting of figures and associated manuscript content, and revisions. RG: Significant contributions to concept and revising manuscript for intellectual content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aaron R. Muncey, MD  https://orcid.org/0000-0002-6401-5370
https://orcid.org/0000-0002-6401-5370
References
- 1. Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115(Suppl 2):ii34–45. [DOI] [PubMed] [Google Scholar]
- 2. Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–277. [DOI] [PubMed] [Google Scholar]
- 4. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. [DOI] [PubMed] [Google Scholar]
- 5. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1(1):76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sessler DI, Riedel B. Anesthesia and cancer recurrence: context for divergent study outcomes. Anesthesiology. 2019;130(1):3–5. [DOI] [PubMed] [Google Scholar]
- 8. Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94(6):1066–1073. [DOI] [PubMed] [Google Scholar]
- 9. Wada H, Seki S, Takahashi T, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106(3):499–506. [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, Li T, Gan TJ. The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: a systematic review and meta-analysis. Reg Anesth Pain Med. 2015;40(5):589–598. [DOI] [PubMed] [Google Scholar]
- 11. Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One. 2013;8(2):e56540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pei L, Tan G, Wang L, et al. Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: a meta-analysis of retrospective and prospective studies. PLoS One. 2014;9(12):e114667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5(5):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016;7(12):15262–15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lusty AJ, Hosier GW, Koti M, et al. Anesthetic technique and oncological outcomes in urology: a clinical practice review. Urol Oncol. 2019;37(12):845–852. [DOI] [PubMed] [Google Scholar]
- 16. Makito K, Matsui H, Fushimi K, Yasunaga H. Volatile versus total intravenous anesthesia for cancer prognosis in patients having digestive cancer surgery: a nationwide retrospective cohort study. Anesthesiology. 2020;133(4):764–773. [DOI] [PubMed] [Google Scholar]
- 17. Hovaguimian F, Braun J, Z’Graggen BR, et al. Anesthesia and circulating tumor cells in primary breast cancer patients: a randomized controlled trial. Anesthesiology. 2020;133(3):548–558. [DOI] [PubMed] [Google Scholar]
- 18. Yoo S, Lee HB, Han W, et al. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology. 2019;130(1):31–40. [DOI] [PubMed] [Google Scholar]
- 19. Oh TK, Kim K, Jheon S, et al. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control. 2018; 25(1):1073274818775360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim MH, Kim DW, Kim JH, Lee KY, Park S, Yoo YC. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget. 2017;8(52):90477–90487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119(3):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong B, Lee S, Kim Y, et al. Anesthetics and long-term survival after cancer surgery-total intravenous versus volatile anesthesia: a retrospective study. BMC Anesthesiol. 2019;19(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng X, Wang Y, Dong L, et al. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther. 2018;11:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu ZF, Lee MS, Wong CS, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129(5):932–941. [DOI] [PubMed] [Google Scholar]
- 25. Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7(1):14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. [DOI] [PubMed] [Google Scholar]
- 27. Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69(2):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B. Global Onco-Anesthesia Research Collaboration Group. Correction to: anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(8):1007–1008. [DOI] [PubMed] [Google Scholar]
- 29. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B; Global Onco-Anesthesia Research Collaboration Group. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–561. [DOI] [PubMed] [Google Scholar]
- 30. Cata JP, Bauer M, Sokari T, et al. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25(4):255–262. [DOI] [PubMed] [Google Scholar]
- 31. Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133(5):547–555. [DOI] [PubMed] [Google Scholar]
- 32. Kim R. Anesthetic technique for cancer surgery: harm or benefit for cancer recurrence? Eur J Surg Oncol. 2018;44(5):557–558. [DOI] [PubMed] [Google Scholar]
- 33. Musselman RP, Bennett S, Li W, et al. Association between perioperative beta blocker use and cancer survival following surgical resection. Eur J Surg Oncol. 2018;44(8):1164–1169. [DOI] [PubMed] [Google Scholar]
- 34. Pohle M, Magheli A, Fischer T, Ralla B, Miller K, Hinz S. Influences of surgical volume on perioperative and oncological outcomes following radical prostatectomy. Urol Int. 2018;101(3):256–262. [DOI] [PubMed] [Google Scholar]
- 35. Barzi A, Lara PN, Jr, Tsao-Wei D, et al. Influence of the facility caseload on the subsequent survival of men with localized prostate cancer undergoing radical prostatectomy. Cancer. 2019;125(21):3853–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grande P, Campi R, Roupret M. Relationship of surgeon/hospital volume with outcomes in uro-oncology surgery. Curr Opin Urol. 2018;28(3):251–259. [DOI] [PubMed] [Google Scholar]
- 37. Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394(10211):1807–1815. [DOI] [PubMed] [Google Scholar]
- 38. Gatenby RA, Vincent TL. An evolutionary model of carcinogenesis. Cancer Res. 2003;63(19):6212–6220. [PubMed] [Google Scholar]
- 39. Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459(7246):508–509. [DOI] [PubMed] [Google Scholar]
- 40. Maley CC, Aktipis A, Graham TA, et al. Classifying the evolutionary and ecological features of neoplasms. Nature Reviews Cancer. 2017;17(10):605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–1828. [DOI] [PubMed] [Google Scholar]
- 42. Nosil P, Soria-Carrasco V, Feder JL, Flaxman SM, Gompert Z. Local and system-wide adaptation is influenced by population connectivity. Conserv Genet. 2019;20(1):45–57. [Google Scholar]
- 43. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. [DOI] [PubMed] [Google Scholar]
- 44. Wilkie KP. A review of mathematical models of cancer-immune interactions in the context of tumor dormancy. Adv Exp Med Biol. 2013;734:201–234. [DOI] [PubMed] [Google Scholar]
- 45. Johnson KE, Howard G, Mo W, et al. Cancer cell population growth kinetics at low densities deviate from the exponential growth model and suggest an Allee effect. PLoS Biol. 2019;17(8):e3000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sekandarzad MW, van Zundert AAJ, Doornebal CW, Hollmann MW. Regional anesthesia and analgesia in cancer care: is it time to break the bad news? Curr Opin Anaesthesiol. 2017;30(5):606–612. [DOI] [PubMed] [Google Scholar]
- 47. Benzekry S, Tracz A, Mastri M, Corbelli R, Barbolosi D, Ebos JM. Modeling spontaneous metastasis following surgery: an in vivo-in silico approach. Cancer Res. 2016;76(3):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker R, Poleszczuk J, Pilon-Thomas S, et al. Immune interconnectivity of anatomically distant tumors as a potential mediator of systemic responses to local therapy. Sci Rep. 2018;8(1):9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez Alfonso JC, Poleszczuk J, Walker R, et al. Immunologic consequences of sequencing cancer radiotherapy and surgery. JCO Clin Cancer Inform. 2019;3:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vodovotz Y, Clermont G, Chow C, An G. Mathematical models of the acute inflammatory response. Curr Opin Crit Care. 2004;10(5):383–390. [DOI] [PubMed] [Google Scholar]
- 51. Day JD, Metes DM, Vodovotz Y. Mathematical modeling of early cellular innate and adaptive immune responses to ischemia/reperfusion injury and solid organ allotransplantation. Front Immunol. 2015;6:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iwata K, Kawasaki K, Shigesada N. A dynamical model for the growth and size distribution of multiple metastatic tumors. J Theor Biol. 2000;203(2):177–186. [DOI] [PubMed] [Google Scholar]