Abstract
In this retrospective study we analyze and compare clinical characteristics and outcomes of patients with and without cancer history who were infected with novel coronavirus disease 19 (COVID-19). Medical records were reviewed and a comparative analysis of 53 cancer and 135 non-cancer patients with COVID-19 were summarized. Results: The median age for COVID-19 patients with and without cancer was 71.5 and 61.6 years, respectively. Patients aged 60 years and above were 86.8% and 60.7% in cancer and non-cancer groups, respectively. A high proportion of cases were seen in African Americans 73.6% (with cancer) and 75.6% (without cancer) followed by Hispanic patients. Male and female patients had a high percentage of prostate (39.3%) and breast (32%) cancer respectively. Prostate cancer (18.9%) and myeloma (11.3%) were common among solid and hematological cancers respectively. Hypertension and smoking were prevalent among cancer (83% and 41.5%) compared to non-cancer (67.4% and 9.6%) patients. The common symptoms in cancer patients were dyspnea (64.2%) followed by fever and cough (50.9%) compared to fever (68.1%) and cough (66.7%) in non-cancer patients. Cancer patients had higher levels of lactic acidosis, C-reactive protein, lactate dehydrogenase, and alkaline phosphatase than non-cancer patients (p < 0.05). Conclusions: Rapid clinical deterioration was seen in cancer patients who were aged 60 years and above. Higher mortality was seen in this subgroup, especially when they had associated hypertension and elevated levels of CRP and LDH.
Keywords: cancer, COVID-19, mortality, SARS-CoV-2, retrospective, risk factors
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) arising from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection become a global health crisis unlike any in recent history. There have been 5,304,772 cases of SARS-CoV-2 virus cases as of May 25th, 2020 globally with 270,404 deaths of which 28,978 have been in New York state, the epicenter of coronavirus outbreak in the USA. The WHO declared this a public health emergency of international concern and the disease was named Coronavirus disease 2019 (COVID-19). COVID-19 has a high infectious rate and not much information is known on how this disease targets patients who have cancer and on immunosuppressants, information is especially scarce for local NY state patients. Few studies have shown the rate of spread of COVID-19 and its propensity to cause severe damage to a subpopulation.1-3 We decided to analyze cancer patients admitted to our teaching hospital in Brooklyn, New York to estimate disease risk in cancer patients. Cancer patients, by the nature of the underlying disease and being on immunosuppressive therapies are often immunocompromised and are susceptible to infection.1 A study from China of 28 patients with cancer and COVID-19 disease showed them to be at risk of severe disease and poor outcomes.2 Similarly, a report investigating 72,314 cases from the Chinese center for disease control and prevention showed an overall case-fatality rate (CFR) of 2.3% among COVID-19 confirmed cases in comparison to CFR of 5.6% in patients with preexisting cancer, showing a high vulnerability in cancer patients.4 The current literature review shows a few case studies and reports on COVID-19 patients with cancer from NY with limited information on their clinical course, prognostic indicators, and their progress during the illness. We aim to analyze the clinical characteristics of cancer patients with COVID-19 and compare the differences with the non-cancer group to identify risk factors that will help stratify patients and potentially open doors for early and effective interventions for better outcomes in cancer patients.
Methods
Setting
Retrospective data from 725 consecutive admitted patients who were COVID-19 positive was collected from Brookdale University Hospital and Medical Center, New York, a regional tertiary care center covering the underserved population of East Brooklyn with the predominantly African American population. Data was collected from March 18 to April 30, 2020. All adult patients more than 18 years old, admitted to Brookdale Hospital with COVID-19 infection as evidenced by laboratory confirmation by RT-PCR were included in the study. We identified 53 patients with a history of cancer (n = 53) and included the first 135 non-cancer patients admitted with COVID-19 as a comparison group to investigate the clinical characteristics between cancer and non-cancer groups. We collected epidemiological, demographics, clinical presentation, laboratory values, and outcome data in addition to their cancer history. The study was approved by the IRB Committee of Brookdale University Hospital and Medical Center.
Statistical Methods
Statistical analysis was performed using IBM SPSS software version 26 (SPSS Inc, Armonk, NY). Descriptive summary statistics are presented as means with standard deviation (SD) for normally distributed continuous variables, median with interquartile range (IQR) for non-normally distributed continuous variables, and frequencies with percentages for categorical variables. Categorical and continuous variables were tested for statistical significance using chi-square tests and t-tests or non-parametric tests, respectively. Multivariate analysis by Cox regression was performed to identify factors independently associated with inpatient mortality. The predictors were considered statistically significant in the multivariate model at P < 0.05.
Results
Demographic and Clinical Characteristics
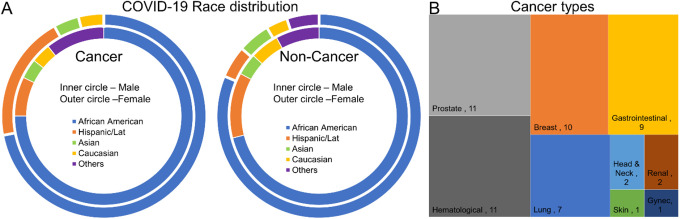
The average age for COVID-19 patients with and without cancer was 71.5 and 61.6, respectively with a significant difference in the age group (p ≤ 0.001). COVID-19 patients with and without cancer who were 60 years and above were 86.8% and 60.7%, respectively with a significant difference (p = 0.001). Irrespective of patients with or without cancer, the COVID-19 was seen more frequently in the population above 60 years. There is no significant difference in gender across COVID-19 patients irrespective of cancer status. In the inpatient cohort, high frequency was seen in African Americans 73.6% (with cancer) and 75.6% (without cancer) followed by the Hispanic population 13.2% (with cancer) and 8.9% (without cancer), respectively. The frequency was less than 5% in the Asian and Caucasians (Figure 1A and Table 1).
Figure 1.
Race distribution in cancer and non-cancer patients (A) different types of cancer (B).
Table 1.
Demographic and Baseline Clinical Characteristics of COVID-19 Patients With and Without Cancer.
| Characteristic | COVID-19 patients without Cancer n = 135 | COVID-19 patients with Cancer n = 53 | P value | ||
|---|---|---|---|---|---|
| Age, mean (SD) | 61.6 | (15.5) | 71.5 | (10.0) | <0.001 |
| Age, median (IQR) | 63 | (52, 73) | 74 | (63.5,77) | <0.001 |
| Age >60 years | 82 | (60.7) | 46 | (86.8) | <0.001 |
| Female gender | 59 | (43.7) | 25 | (47.2) | 0.75 |
| BMI, median (IQR) | 29 | (25.8, 34.9) | 27.4 | (24.2, 34.3) | 0.46 |
| Race | n | % | n | % | |
| White | 6 | 4.4 | 2 | 3.8 | |
| African American | 102 | 75.6 | 39 | 73.6 | |
| Hispanic | 12 | 8.9 | 7 | 13.2 | |
| Asian | 6 | 4.4 | 2 | 1.1 | |
| Unknown | 9 | 6.7 | 3 | 5.7 | |
| Comorbidities | |||||
| Hypertension | 91 | 67.4 | 44 | 83.0 | 0.047 |
| Dyslipidemia | 48 | 35.6 | 11 | 20.8 | 0.056 |
| CAD | 26 | 19.3 | 10 | 18.9 | 1 |
| DM | 58 | 43.0 | 19 | 35.8 | 0.41 |
| COPD | 12 | 8.9 | 5 | 9.4 | 1 |
| Smoker | 13 | 9.6 | 22 | 41.5 | <0.001 |
| Medications | |||||
| ACEI/ARB | 41 | 30.4 | 23 | 43.4 | 0.12 |
| NSAID | 32 | 23.7 | 5 | 9.4 | 0.03 |
| Aspirin | 45 | 33.3 | 19 | 35.8 | 0.74 |
| Statin | 63 | 46.7 | 18 | 34.0 | 0.14 |
| Symptoms | |||||
| Cough | 90 | 66.7 | 27 | 50.9 | 0.07 |
| Fever | 92 | 68.1 | 27 | 50.9 | 0.03 |
| Dyspnea | 89 | 65.9 | 34 | 64.2 | 0.87 |
| Fatigue | 72 | 53.3 | 18 | 34.0 | 0.02 |
| Myalgia | 54 | 40.0 | 14 | 26.4 | 0.09 |
| GI symptom | 27 | 20.0 | 6 | 11.3 | 0.2 |
SD = Standard deviation; IQR = interquartile range; BMI = Body mass index; CAD = Coronary artery disease; DM = Diabetes mellitus; COPD = Chronic obstructive pulmonary disease; ACEI = Angiotensin converting enzyme inhibitor; ARB = Angiotensin receptor blocking.
The most common COVID-19 presenting symptoms in cancer patients were dyspnea (64.2%) followed by fever and cough (50.9%), fatigue (34%), and myalgia (26.4%), whereas in patients without cancer symptoms were fever (68.1%), cough (66.7%), dyspnea (65.9%), fatigue (53.3%) and myalgia (40%). More COVID-19 patients without cancer showed symptoms of fever and fatigue compared to patients with cancer. Cough is the second most common symptom in both cancer and non-cancer patients, indicative of a clinical symptom that is the involved organ as well as in the mode of transmission.
Hypertension (HT) was the most frequently reported comorbidity in COVID-19 patients, documented in 83% and 67.4% in patients with and without cancer, respectively. Though HT was commonly seen in both groups, COVID-19 patients with cancer showed a significantly higher association with HT in comparison to patients without cancer (p = 0.047). Smoking history was frequent in COVID-19 patients with cancer (41.5%) in comparison to patients without cancer (9.6%). In terms of medication, NSAIDs was used significantly higher in COVID-19 patients without cancer (23.7%) in comparison with cancer patients (9.4%) (p = 0.03).
Cancer Characteristics
The percentage of COVID-19 infection in cancer patients above and below 60 years was 86.6% (n = 46) and 13.2% (n = 7), respectively. Gender distribution showed a male preponderance (52.8%, n = 28). Blood group data from COVID-19 patients revealed a 26.4% of type A+ blood group with a mortality of 17% followed by O+ (22.6%) with mortality of 18.9%. Table 2. The percentage of solid organ and hematological cancers were 77% and 20%, respectively. Among those who had solid cancers, prostate cancer was the most common (n = 10, 18.9%) followed by breast cancer (n = 8, 15.1%). Myeloma was the most common type of malignancy (n = 6, 11.3%) among hematological cancer. The mortality rate in cancer patients were 60.4% with solid tumor patients contributing to 49% (n = 26) followed by hematological cancers (n = 6, 11.3%). The current and the past cancer treatment for each tumor type were summarized in Table 3. The mortality rate in cancer patients who have aged above and below 60 years were (n = 28, 52.8%) and (n = 4, 7.5%), respectively. Similarly, cancer remission (n = 14, 26.4%) is seen more in patients aged above 60 years whereas cancer non-remission (n = 3, 5.6%) was seen more in patients aged below 60 years. Older age was an important poor prognostic factor irrespective of cancer status, whereas active cancer status was a poor prognostic predictor in patients younger than 60 years old. Figure 1B and Table 3.
Table 2.
Blood Group Types and Mortality in COVID-19 Cancer Patients.
| Blood group | COVID-19 patients with cancer n = 53 | Mortality (overall) | ||
|---|---|---|---|---|
| N | % | n | % | |
| A+ | 14 | 26.4 | 9 | 17.0 |
| 0+ | 12 | 22.6 | 10 | 18.9 |
| B+ | 5 | 9.4 | 2 | 3.8 |
| AB+ | 2 | 3.8 | 1 | 1.9 |
| A- | 1 | 1.9 | 0 | 0.0 |
| AB- | 1 | 1.9 | 1 | 1.9 |
| B- | 1 | 1.9 | 0 | 0.0 |
| Not known | 17 | 32.1 | 9 | 17.0 |
Table 3.
Cancer Types, Treatment, and Outcome in COVID-19 Cancer Patients.
| Characteristic | Gender | Staging | Cancer treatment (past) | Cancer treatment (current) | Cancer in remission | Mortality | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Diagnosis | n | (%) | M | F | L | A | NK | C | S | R | C/R | S/R | S/C/R | S/C/R/H | NK | None | C | H | I | T | S | C/I | C/R | NK | Yes | No | NK | n | (%) |
| Prostate | 10 | 18.9 | 10 | 5 | 3 | 2 | 2 | 3 | 2 | 1 | 5 | 3 | 5 | 2 | 7 | 13.2 | |||||||||||||
| Breast | 8 | 15.1 | 2 | 6 | 8 | 0 | 0 | 1 | 5 | 1 | 1 | 6 | 2 | 5 | 9.4 | ||||||||||||||
| Gastrointestinal | 8 | 15.1 | 5 | 3 | 5 | 3 | 0 | 1 | 6 | 2 | 1 | 6 | 2 | 6 | 11.3 | ||||||||||||||
| Lung | 5 | 9.4 | 2 | 3 | 1 | 4 | 0 | 3 | 1 | 1 | 4 | 1 | 1 | 1.9 | |||||||||||||||
| Head & Neck | 2 | 3.8 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 2 | 1 | 1.9 | |||||||||||||||||
| Renal | 2 | 3.8 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 3.8 | |||||||||||||||||
| Skin | 1 | 1.9 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| Gynecological (ovarian) | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| Brain | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| Combined | |||||||||||||||||||||||||||||
| Prostate & hematological | 1 | 1.9 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| Lung & renal | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| Lung & breast | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||
| Uterine & breast | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| Hematological | |||||||||||||||||||||||||||||
| Myeloma | 6 | 11.3 | 3 | 3 | 6 | 1 | 5 | 6 | 3 | 5.7 | |||||||||||||||||||
| CLL | 1 | 1.9 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| MDS | 1 | 1.9 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| CML | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| CMML | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
| Hodgkin Lymphoma | 1 | 1.9 | 1 | 1 | 1 | 1 | 1 | 1.9 | |||||||||||||||||||||
M = Male; F = Female; L = Local; A = Advanced; NK = Not Known; C = Chemotherapy; S = Surgery; R = Radiation; H = Hormone; I = Immunotherapy; T = Target therapy; CLL = Chronic lymphocytic leukemia; MDS = Myelodysplastic syndrome; CML = Chronic myeloid leukemia; CMML = Chronic myelomonocytic leukemia.
Laboratory Data
The median value for hemoglobin was 11.8 and 13.2 gm/dl in COVID-19 patients with and without cancer, respectively. The level of hemoglobin was significantly lower in cancer patients compared to patients without cancer (p = 0.04).
On comparing patients with cancer and COVID 19 and those without cancer, the other laboratory parameters which showed statistically significant differences were lactic acidosis [M 1.9 mmol/L (IQR 1.6-3.2) vs M 1.6 mmol/L (IQR 1.2-2); p < 0.05], elevated C-reactive protein(CRP) [M 20 mg/dL (IQR 7.3-26) vs M 7.4 mg/dL (IQR 4.3-18),; p < 0.05] and elevated lactate dehydrogenase (LD) [M 1168 IU/L (IQR 888-1782) vs M 972 IU/L (IQR 734-1359); p < 0.05].
On analyzing liver enzyme levels, the difference in alkaline phosphatase levels (ALP) and alanine aminotransferase (ALT) levels between the 2 groups were noted to be statistically significant [cancer vs non-cancer: (ALP) M 82.5 U/L (IQR 68-129) vs M 73 U/L (58.5-88); p < 0.05 and (ALT) M 32.5 U/L (IQR 23.3-47.8) vs M 40 U/L (IQR 28-58); p < 0.05]. Significant ALP elevation in cancer patients and ALT elevation in non-cancer patients may be due to the metastatic propensity of cancer in addition to the liver damage caused by COVID-19 disease. Other lab parameters were comparable between the 2 groups and details are listed in Table 4.
Table 4.
Laboratory Values and Outcome in COVID-19 Patients With and Without Cancer.
| Characteristic | COVID-19 patients without Cancer n = 135 | COVID-19 patients with Cancer n = 53 | P value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Hemoglobin | 13.2 | 11.7, 14.3 | 11.8 | 10.3, 13.6 | 0.04 |
| Ferritin | 490 | 159, 1120 | 498 | 229, 1190 | 0.91 |
| D-dimer | 706 | 229, 3289 | 1680 | 339, 10367 | 0.23 |
| WBC | 6.4 | 5.1, 8.3 | 8.5 | 5.3, 12.4 | 0.11 |
| Lymphocyte count | 1 | 0.7, 1.3 | 0.9 | 0.6, 1.3 | 0.52 |
| Creatinine | 1.2 | 0.8, 1.8 | 1.2 | 0.8, 2.6 | 0.95 |
| CPK | 207 | 110.5, 464 | 188 | 53, 255 | 1 |
| Lactate | 1.6 | 1.2, 2 | 1.9 | 1.6, 3.2 | 0.003 |
| LDH | 972 | 734, 1359 | 1168 | 888, 1782 | 0.03 |
| CRP | 7.4 | 4.3, 18 | 20 | 7.3, 26 | 0.009 |
| Bilirubin | 0.6 | 0.4, 0.9 | 0.7 | 0.5, 1.1 | 0.42 |
| Alkaline Phosphatase | 73 | 58.5, 88 | 82.5 | 68, 129 | 0.02 |
| AST | 49 | 36, 77 | 43 | 34, 68 | 0.19 |
| ALT | 40 | 28, 58 | 32.5 | 23.3, 47.8 | 0.047 |
| Platelets | 193 | 155, 243 | 212 | 142.5, 271 | 0.87 |
| Length of stay | Median | IQR | Median | IQR | |
| 7 | 5, 10 | 5 | 3, 7 | 0.001 | |
| n | % | n | % | ||
| Died | 49 | 36.3 | 32 | 60.4 | 0.02 |
| Mechanical Ventilation | 36 | 26.7 | 5 | 9.4 | 0.01 |
| Shock | 39 | 28.9 | 12 | 22.6 | 0.47 |
IQR = Interquartile range; WBC = White Blood cells; CPK = Creatine phosphokinase; LDH = Lactate dehydrogenase; CRP = C-reactive protein; AST = Aspartate aminotransferase; ALT = Alanine aminotransferase.
Outcome Data
COVID-19 cancer patients had a higher mortality rate (60.4%) in comparison to patients without cancer (36.3%) with statistical significance reaching a p = 0.02 Table 4. Multivariable cox regression analysis showed that the presence of cancer was significantly associated with increased risk of mortality when accounting for other variables including age (Hazard Ratio 2.3, 95% CI 1.2-4.5) Table 5. The median length of stay in cancer patients (5days) is significantly lower in comparison to patients without cancer (7days) (p ≤ 0.001). Similarly, the use of mechanical ventilation is significantly lower in cancer patients (9.4%) in comparison to non-cancer patients (26.7%) in Table 4.
Table 5.
Association of Cancer and Other Variables With Mortality in COVID-19 Patients by Using Cox Regression Analysis.
| Variable | Hazard Ratio | 95% Confidence Interval | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Cancer | 2.28 | 1.15 | 4.54 | 0.018 |
| Age >60 years | 1.18 | 0.51 | 2.73 | 0.705 |
| Hypertension | 0.90 | 0.44 | 1.82 | 0.766 |
| Smoking | 1.28 | 0.64 | 2.54 | 0.487 |
| Hemoglobin (admission) | 1.01 | 0.86 | 1.19 | 0.902 |
| Lactate (admission) | 0.97 | 0.84 | 1.13 | 0.731 |
| C-reactive protein (admission) | 1.02 | 0.97 | 1.06 | 0.456 |
| Alkaline Phosphatase (admission) | 1.00 | 1.00 | 1.01 | 0.666 |
Discussion
Cancer patients face unique challenges in terms of being susceptible to infections given their immunocompromised state attributed both due to cancer itself and from cancer therapies.5,6 Our study describes the clinical characteristics and outcomes of 53 cancer patients with COVID-19 and compared the differences with 135 non-cancer patients with COVID-19 infection.
Both cancer and non-cancer groups of patients with COVID-19 had a wide range of symptoms at presentation, including dyspnea, fever, cough, fatigue, myalgia, and gastrointestinal symptoms (nausea, vomiting, diarrhea). Based on data available so far, the most common presenting symptoms in patients likely to present with COVID-19 were fever and cough.7 Our data showed symptoms of fever and fatigue are significantly higher in non-cancer patients in comparison to cancer patients (p < 0.05) whereas cough and dyspnea showed no statistical difference in their presentation between the same group. This shows the clinical symptoms in cancer patients might be different compared to non-cancer patients, indicative of different requirements in the screening procedures. Also, effective screening methods may decrease viral transmission, particularly from asymptomatic COVID-19 patients.8 The mechanism of SARS-CoV-2 infection involves the virus binding with the cell membrane-bound (Angiotensin Converting Enzyme-2) ACE-2 receptor followed by internalization of the complex.9 The ACE-2 receptor is widely distributed in the lungs, heart, kidneys, brain, and gut, providing a rationale for the wide variety of symptomatology noted in our study population.
Analysis of comorbidities showed a striking prevalence of hypertension in patients with cancer and SARS-CoV-2 infection compared to the non-cancer patients [44 (83%) vs 91 (67.4%); p < 0.05]. Studies have shown that among patients with COVID-19, patients with comorbidities had poorer clinical outcomes than patients without comorbidities.3,10-14 On analyzing specific comorbidities, research has shown that hypertension is most associated with the occurrence of severe disease in patients with COVID-19.15 The correlation between hypertension and ACE-2 receptor expression has been studied16 and provides clinical insight for patients with hypertension developing more severe disease. The difference in the prevalence of smokers in the cancer group was higher compared to the non-cancer group [41.5% vs 9.6%; p < 0.001]. It is known that smoking upregulates the expression of ACE-2 receptors and novel methods of nicotine consumption including electronic cigarettes may do the same.17 The clinical significance of smoking status to outcomes in COVID 19 disease is poorly understood and requires further research.
There is a dearth of research data comparing laboratory parameters between COVID-19 patients with and without cancer. Our study was able to identify several key differences between the 2 groups. As described in Table 4, higher blood levels of lactic acid, LDH, CRP, and ALP were seen in patients with cancer compared to those without cancer. We also noted lower hemoglobin and ALT levels in cancer patients compared to their counterparts. The lower hemoglobin may be due to the myelosuppressive effects of cancer therapy and the significantly lower hemoglobin may be the reason why cancer patients showed dyspnea as the number one complaint, due to poor oxygenation. Multiple studies have shown that COVID 19 patients present with higher levels of LDH, CRP, AST, ALT, and lymphopenia.7,18,19 Higher LDH and CRP levels are associated with more severe forms of SARS-COV-2 infections and are harbingers of poor outcomes in terms of mortality and morbidity.15,20,21
Given that our study showed patients with cancer and COVID-19 have 2.1 times the risk of mortality than patients without cancer [HR 2.09; p < 0.05], we emphasize the necessity to trend levels of LDH and CRP on a daily basis to identify patients who require pre-emptive escalation of care to avoid negative outcomes. We echo the sentiment expressed by Liang et al that cancer patients with COVID-19 warrant more intensive care as they are at risk of rapid deterioration.1 Notably, our study found that in our cancer patients, mortality was higher in those older than 60 years of age compared to those less than 60 [52.8% vs 7.5%]. The higher mortality in cancer patients might be due to several reasons but the laboratory data showed elevated levels of inflammatory and enzyme markers of tissue damage leading to poor oxygenation and outcome. Interestingly, 14 (26.4%) older patients had their cancer in remission compared to just 3 (5.6%) patients below 60 years of age. In older cancer patients, age appears to be a stronger indicator of mortality while active cancer and treatment status seems to play a bigger role in deciding outcomes for younger patients. These associations require further exploration in future studies. Although studies have shown patients with cancer have a higher risk of mechanical ventilation.1 Our study noted a significantly lower incidence of mechanical ventilation [9.4% vs 26.7%; p < 0.05] and shorter hospital stay [Mn 5 days, IQR 3-7 vs Mn 7 days, IQR 5-10; p < 0.05] in COVID-19 cancer patients compared to non-cancer patients. We attribute this difference to the fact that a large proportion of cancer patients admitted during the study period decompensated rapidly (as indicated by the higher levels of LDH and CRP and a higher risk of mortality compared to their non-cancer counterparts) and several patients had advance directives reflecting no-intubation given the nature of their comorbidities.
There are many limitations to our study in that it was based on small sample size, retrospective in nature, the comparison group was arbitrarily selected and the study sample was non-randomized. Further studies with larger sample size, analysis stratified by types of malignancy, prospective studies with larger sample sizes will be needed to shed further light on the matter.
In summary, we recommend closer observation of patients with cancer who have COVID-19, particularly older patients with hypertension and higher levels of CRP and LDH as they are at risk of rapid deterioration and have increased risk of mortality. More extensive surveillance with broad community testing focused on cancer patients would help identify asymptomatic and mild cases who would benefit from regular follow up as outpatients and promote timely intervention prior to progression to more severe disease. We agree with Liang et al1 and Zhang et al2 that immunosuppressive treatment strategies should either be postponed for stable cancer patients or done under vigorous screening strategies for SARS-CoV-2, especially in younger patients in whom immunosuppression from active cancer or active antitumor treatment seems to play a major role in deciding outcomes. Pre-testing all patients with cancer, prior to elective surgeries and antitumor therapy should be explored. Special care should be taken to minimize community exposure to COVID-19 in patients with cancer, which could be accomplished by more stringent guidelines for personal protective gear for these patients and people in close contact with them. In the early stages of a pandemic, where minimizing community spread is essential, it is imperative we take precautions to prevent our most vulnerable populations from being infected. These measures should result in a significant reduction in overall mortality from SARS-CoV-2 and in patients with cancer.
Footnotes
Authors’ Note: IRB approved: Yes.
Author Contributions: Conceptualizations and methodology: PR, JCW. Statistical and data analysis: MG, UZ. Data collection &literature review: SG, IO, JTN, AC. Manuscript Writing, reviewing, and editing PR, BK, JCW, FA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval and Consent: IRB of Brookdale University Hospital and Medical Center, NY approved this study. Reference Number: Protocol 20-12.IRB granted a waiver of informed consent due to the retrospective nature of the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Preethi Ramachandran, MD, MRCP, FRCPath  https://orcid.org/0000-0003-3032-8886
https://orcid.org/0000-0003-3032-8886
Faiz Anwer, MD, FACP  https://orcid.org/0000-0001-6914-7439
https://orcid.org/0000-0001-6914-7439
References
- 1. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in china. Lancet Oncol. 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 5. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10(6):589–597. [DOI] [PubMed] [Google Scholar]
- 6. Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Hum Immunol. 2016;77(8):631–636. [DOI] [PubMed] [Google Scholar]
- 7. Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020;71(15):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee LYW, Hill T, Topping O, et al. Utility of COVID-19 screening in cancer patients. Cancer Cell. 2020;38(3):306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5): H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in china: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. [DOI] [PubMed] [Google Scholar]
- 17. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin Chem Lab Med. 2020;58(7):1095–1099. [DOI] [PubMed] [Google Scholar]
- 19. Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng Y, Xu H, Yang M, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020;127:104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]