Abstract
The aim of the experiment was to evaluate the process of neutrophil extracellular traps (NETs) formation in patients with oral squamous cell carcinoma (OSCC) in response to direct or indirect contact with SCC cells in comparison to results obtained in the cells of healthy subjects. To fulfill study objectives CAL 27 cell line and blood were obtained from cancer patients and control subjects. Parameters related to NETs formation were analyzed utilizing flow cytometry, fluorescence microscopy, and ELISA-type tests. The expression of selected phosphorylated proteins of the PI3K/Akt/PBK pathway in neutrophils was evaluated using the Western blot method. An increase in NETs formation was observed in a coculture of neutrophils with SCC cells, with the largest amount of NETs formed after stimulation with a supernatant obtained from the SCC culture. The enhanced process of NETs formation was accompanied by changes in the expression of proteins from the PI3K/Akt/PBK pathway. The obtained results prove the existence of interactions between neutrophils and cancer cells resulting in NETosis with the participation of the PI3K/Akt/PBK pathway in patients with OSCC.
Keywords: neutrophils, oral cancer, SCC, NETs, PI3K/Akt/PBK
Introduction
Discoverers of neutrophil extracellular traps (NETs) were the first to carry out studies explaining the hypothesis of neutrophils/traps participation in the cancer process.1 Their work demonstrated the presence of tumor-associated neutrophils (TANs) and NETs in diagnostic biopsies of pediatric patients with Ewing’s sarcoma. Moreover, NETs correlated with early relapse of the disease despite total remission after intensive chemotherapy.2
Studies conducted by other authors showed increased susceptibility of neutrophils to form and release NETs in the mouse model of breast cancer and chronic myeloid leukemia. Within this research it was observed that NETs formed spontaneously in the blood with the progression of the disease.3 Similar results were also obtained by Yang et al. in respect to patients with gastric cancer where neutrophils showed an increased capacity to release NETs and the number of traps correlated with the clinical stage of cancer.4 Yet other studies demonstrated an increase in the percentage of NETs-forming neutrophils in patients with SCC of the oral cavity mucosa at stage I/II of disease progression.5
The results of the initial studies dealing with the participation of NETs in cancer optimistically indicated the anticancer role of NETs through local high concentrations of active proteins, stimulation of the immune system and limiting the spread of cancer cells.6-8 A different concept assumes that NETs constitute a physical barrier between cancer and immunocompetent cells thus limiting the immune response.2 What is more, the pleiotropic function of NETs building blocks may affect various stages of cancer development, including tumor growth, angiogenesis, and metastasis.8 The presence of NETs was demonstrated in colorectal cancer metastases.9 Moreover, uncontrolled NETosis results in the development of chronic inflammation and the destruction of surrounding tissues conducive to the development of cancer.10
Literary data seems to confirm the existence of interactions between neutrophils and cancer cells but this process cannot be fully explained without a NETs analysis. For this reason, the current experiment aims to evaluate the formation of NETs by neutrophils in response to direct or indirect contact with SCC cells of patients with OSCC in comparison with results obtained from cells of healthy individuals.
The generation of NETs is a complex and ambiguous process whose molecular mechanisms are yet to be fully discovered. The main reason for the complexity of this issue is probably the fact that NETosis is induced by many signaling pathways. One such is the PI3K/Akt/PBK pathway.11,12 A study performed by Tatsiy and McDonald has proven that the use of PI3K/Akt/PBK pathway inhibitors blocked the formation of NETs despite activation with known stimulators of their formation clearly confirming the role of the pathway in NETosis.13 Available scientific data demonstrates pathway involvement in the course of infection or inflammation, however, no cancer-related information is available.
The present study aims to analyze parameters associated with the formation of NETs after neutrophil coculture with SCC cells and after stimulation with a supernatant—SCC cell culture products. Moreover, to explain mechanisms underlying the relationship between neutrophils and cancer cells, an attempt was made to analyze the processes influencing NETs generation by neutrophils through the PI3K/Akt/PBK pathway.
Materials and Methods
The research has been approved by the Bioethics Committee of the Medical University of Bialystok (No. R-I-002/348/2016 and R-I-002/360/2017).
The study was conducted on 5 OSCC patients whose disease was in its first stage of progression and who were hospitalized at the Department of Maxillofacial and Plastic Surgery of the University Clinical Hospital in Bialystok. Research in the early stages of OSCC is critical to understanding the basics of neoplastic transformation in the context of the primary immune response. Effective attempts to modify the immune response are made only in the early stages of cancer development, therefore the project included patients with OSCC in the early stage of the disease. Exluded late stage patients was a limitation for the study. The study was performed prior to any patient treatment. The control group consisted of 5 healthy subjects who were voluntary blood donors. Following donors’ consent, 2 blood samples were collected: one from the ectopic vein to isolate neutrophils—9 mL in a K3EDTA anticoagulant tube (Greiner Bio-One GmbH) and the other to obtain serum—6 mL in a Serum Clot Activator tube (Greiner Bio-One GmbH).
The presented test results are the product of preliminary studies hence the small study group. Additionally, patients with OSCC often report to the doctor at a late stage of disease progression when the lesions become inoperable and are referred to different facilities than the hospital at which the study was conducted. The low number of patients has also been dictated by the selection of participants without any chronic diseases, especially inflammation. The age difference between the study group and the control group is due to the selection to the latter of healthy people without any accompanying diseases.
Cells of the SCC cell line (CAL 27, ATCC® CRL-2095™) were used in the study. Due to the low number of neutrophils obtained from patients, only one SCC line was used.
White Blood Cells
The total number of leukocytes in whole blood was assessed for each subject through the use of the ventricular method utilizing a Bürker hemocytometer (Marienfeld Superior), Türk’s fluid (AQUA-MED) and a light microscope (Olympus).
Leucogram
A smear of each obtained whole blood sample was made on a basal slide. After fixation, the preparations were stained employing the May-Grünwald-Giemsa method (AQUA-MED) and evaluated using a light microscope (Olympus).
Isolation of Neutrophils
Leukocytes from blood samples with the anticoagulant were isolated in 2 stages. After preliminary isolation with gradient centrifugation using a Polymorphprep™ reagent (Axis-Shield) neutrophils were sorted during positive selection using a MACS® Magnetic Separator and a CD16 MicroBeads (Miltenyi Biotec) magnetic separator. This method does not require the removal of the MicroBeads because they biodegrade during polymorphonuclear neutrophils (PMNs) incubation and, according to the manufacturer’s assurances, do not affect cell function or cell viability.
SCC Cell Culture
SCC cells were cultured in a TC Dish 100, Cell+ (Sarstedt) plates in DMEM (1×) (GIBCO) medium with 10% FBS Good (PAN Biotech) and 1% Penicillin–Streptomycin (Sigma Life Sciences) antibiotic in a 5% CO2 environment in an incubator (NUAIRE) at 37°C. The supernatant and cancer cells (suspended in RPMI-1640 (GIBCO) with <5% FBS Good and 1% Penicillin-Streptomycin antibiotic) collected after 48 h from an SCC cell culture were used to stimulate the neutrophils.
Cell Incubation
The isolated neutrophils were suspended in RPMI-1640 medium enriched with <5% FBS Good with 1% Penicillin-Streptomycin antibiotic. Two 96-well plates (Microtest III-Falcon, BD Biosciences) containing a coculture of neutrophils with SCC cells (5 × 105 PMNs vs. 2.5 × 105 SCC in the well) or PMNs stimulated with a supernatant obtained from an SCC cell culture (100 µL), were prepared concurrently. The final volume of each well was 200 µL.
One plate was placed in an incubator. After 60 minutes of coculture, the supernatants were collected and the cells were separated by sorting neutrophils in a MACS® magnetic separator and a CD16 MicroBeads separator to produce a pure PMNs fraction.
PMNs Viability
Neutrophil viability was assessed in vitro under a light microscope, with basal slides treated with trypan blue (Lachema) which stains only dead cells. The preparations were made directly after isolation from whole blood, after coculture with SCC cells, and after stimulation with the supernatant obtained from the SCC cell culture.
PMNs Purity
The purity of neutrophils was assessed under a light microscope after isolation from whole blood in so-called “thick drop” preparations on basal slides stained using the May-Grünwald-Giemsa method (AQUA-MED). The purity of neutrophils sorted after coculture with SCC cells was also evaluated.
NETs Visualization
The second plate with coculture of neutrophils and SCC cells (105 PMNs vs. 0.5 × 105 SCC in the well) or PMNs stimulated with the supernatant obtained from SCC cell culture (100 µL) was incubated in the BD Pathway 855 system. Cells were suspended in an RPMI-1640, FBS Good (<5%) and Penicillin-Streptomycin (1%) medium and incubated at 37°C with 5% CO2 flow in a 96-well Black/Clear Tissue Culture Treated Imaging Plate with a Flat Bottom and a Lid (BD Falcon). Cell growth was recorded live using a microscopic system. A Hoechst 33342 solution (Invitrogen) was used for DNA staining while myeloperoxidase was visualized through the use of fluorescein-labeled monoclonal antibodies (Clone 8E6, Molecular Probes).
Determination of the percentage of NETs-forming cells occurred on the basis of same-time photos taken by a microscopic system. NETs-forming neutrophils and normal cells were counted in the field of view/photos (9 in each well). On this basis, the average percentage of NETs forming neutrophils was determined.
Myeloperoxidase-Positive Neutrophils
The number of myeloperoxidase (MPO)-positive PMNs was evaluated in neutrophils collected after incubation in BD FACSCalibur™ flow cytometer (BD Biosciences). Neutrophils, after permeabilization with FACS Permeabilizing Solution 2 (BD Biosciences), were marked with monoclonal antibodies conjugated with fluorescein (Clone 8E6, Molecular Probes). Forward scatter channel and side scatter channel parameters were considered when gating the neutrophils with the resulting data analyzed using the FlowJo software (TreeStar, Inc.).
Circulating Free DNA (cfDNA)
The concentration of cfDNA in cell supernatants obtained after neutrophil incubation was evaluated using an Abcam’s Circulating DNA Quantification Kit according to the manufacturer’s instructions. The intensity of fluorescence was directly proportional to DNA content (Supplementary 1).
MPO
Concentrations of MPO in supernatants obtained after neutrophil incubation and in blood serum were determined through the application of a Human Myeloperoxidase Quantikine ELISA Kit (R&D Systems). Color intensity was directly proportional to the amount of MPO (Supplementary 2).
Expression of p-Akt1/2/3 (B-5), p-Akt1/2/3 (Ser 473), and p-PI 3-Kinase p85α (Tyr 508) Proteins
The expression of phosphorylated p-Akt1/2/3 (B-5), p-Akt1/2/3 (Ser 473), and p-PI 3-kinase p85α (Tyr 508) proteins in non-stimulated neutrophils, neutrophils stimulated with a supernatant obtained from the SCC cell culture, and neutrophils after coculture with cancer cells was evaluated through the utilization of the Western blot method.
Neutrophils obtained after incubation were subjected to lysis by sonication (SONICS Vibra Cell) in the presence of a Protease Inhibitor Cocktail (Sigma-Aldrich, MERCK). The obtained cell lysate was suspended in a Laemmli Sample Buffer with βME (Bio-Rad). Electrophoresis (Bio-Rad Laboratories Mini-PROTEAN® Tetra Cell) was carried out using SDS-PAGE (Bio-Rad). The separated proteins were moved from gel to 0.45-µm nitrocellulose (Bio-Rad) during the transfer (Bio-Rad Laboratories Mini-PROTEAN® Tetra Cell) and then the nitrocellulose was trapped in a Millipore SNAP i.d.™ Protein Detection System and incubated with primary antibodies: mouse monoclonal p-Akt/1/2/3 (B-5) (Santa Cruz Biotechnology, 1:100), rabbit polyclonal p-Akt1/2/3 (Ser 473) (Santa Cruz Biotechnology, 1:200), and goat polyclonal p-PI 3-kinase p85α (Tyr 508) (Santa Cruz Biotechnology, 1:200). In the next stage, a membrane with secondary goat anti-mouse IgG (Jackson Immunoresearch, 1:5000), goat anti-rabbit IgG (Santa Cruz Biotechnology, 1:5000), and mouse anti-goat IgG (Jackson Immunoresearch, 1:5000) was incubated. Immunoreactive striations of proteins being analyzed were obtained by adding BCIP®/NBT Liquid Substrate (Sigma-Aldrich, MERCK).
The expression of β-Actin (9) (Santa Cruz Biotechnology, 1:200) was determined using the 3-Colour Prestained Protein Marker (BLIRT S.A.).
The color intensity of the bands was evaluated densitometrically using ImageJ software (NIH, Bethesda, MD) and presented in conventional units (Arbitrary Units, AU).
Statistics
Statistical analysis was done using the Statsoft Statistica, version 13.1, software. All data is reported as the mean ± standard deviation (SD). Student t-test, Shapiro-Wilk test, Mann-Whitney U-test, and Chi-square test were used for statistical analysis. Correlation coefficients were determined through the use of Pearson’s correlation with P-values based on 2-tailed tests. Differences of p < 0.05 were considered significant.
Results
Clinical data of cancer patients and healthy subjects has been presented in Table 1.
Table 1.
Data of Participants in the Study.
| Study groups | Number | Gender | Age | Cancer stage | Tumor location | Smokes |
|---|---|---|---|---|---|---|
| Healthy people | n = 2 | women | 38-41 | not applicable | not applicable | no |
| n = 3 | men | 29-44 | not applicable | not applicable | no | |
| Patients with OSCC | n = 2 | women | 68-75 | I stage | cheek floor of mouth |
no |
| n = 3 | men | 59-63 | I stage | floor of mouth 2x lower gingiva | no (2x) yes (1x) |
Results of the WBC, leucogram, PMNs viability, and purity are presented in Table 2.
Table 2.
List of Average Values of Basic Parameters.
| Study groups | WBC [cells/µL], median SD | Leucogram [%], median SD | PMNs viability [%], median SD |
PMNs purity [%], median SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophils | Eosinophils | Lymphocytes | Monocytes | After isolation | After coculture | After stimulation | After isolation | After coculture | ||
| Healthy people |
6100 954.33 | 51 10.78 |
2 1.14 |
37 8.03 |
5 2 |
99 0.84 |
98 0.71 |
99 0.84 |
100 0 |
100 0.45 |
| Patients with OSCC |
6700 1439.18 |
58 8.67 |
2 1.52 |
33 8.56 |
7 2.49 |
97 1.1 |
97 0.84 |
98 0.71 |
99 0.55 |
98 0.84 |
Microscopic Assessment of the Amount of NETs-Releasing Cells
Analysis of microscopic images obtained during the study showed a statistically significant higher percentage of NETs in neutrophils of patients with OSCC than in those of healthy subjects (Table 3).
Table 3.
Summary of Test Results.
| Study groups | TEST | PMNs | PMNs+SCC | PMNs+Supernatant |
|---|---|---|---|---|
|
Healthy
people mean SD |
NETs
[%] |
1.6 0.89 |
4.8a
1.92 |
8bc
2.92 |
|
MPO+ PMNs
[%] |
25.3 2.91 |
27.2 6.58 |
12.4bc
3.93 |
|
|
cfDNA
[ng/µL] |
135.2 29.06 |
162.4 29.15 |
187.5b
31.54 |
|
|
MPO
[ng/mL] |
63.3 3.16 |
69.6 7.15 |
82.4bc
8.99 |
|
|
p-Akt1/2/3 (B-5)
[AU] |
33821.4 2679.45 |
151434.8a
26631 |
41285.2bc
4180,91 |
|
|
p-Akt1/2/3 (Ser473)
[AU] |
51131.2 5818.89 |
142119.8a
22383.83 |
60040.4bc
3512.2 |
|
|
p-PI 3-kinase p85α
[AU] |
72494.8 13991.46 |
46140.4a
11912.71 |
60916.2 16761.75 |
|
|
Patients
with OSCC mean SD |
NETs
[%] |
5.8* 1.92 |
8.2* 2.59 |
16*bc
2.92 |
|
MPO+ PMNs
[%] |
19.3* 3.82 |
33.84*a
4.43 |
11.3bc
1.95 |
|
|
cfDNA
[ng/µL] |
174.8* 12.56 |
145.2a
30.04 |
189.5bc
6.44 |
|
|
MPO
[ng/mL] |
87* 12.28 |
79.3 17.56 |
97.8 21.85 |
|
|
p-Akt1/2/3 (B-5)
[AU] |
81366.4* 24338.02 |
214935.4*a
42405.4 |
95799.4*c
20417.36 |
|
|
p-Akt1/2/3 (Ser473)
[AU] |
99452.2* 23631.69 |
137623.6a
18433.95 |
88347.8*c
25434.17 |
|
|
p-PI 3-kinase p85α
[AU] |
142829.4* 29469.58 |
82966.4*a
9107.42 |
120566.6*c
24119.62 |
*Statistically significant difference with control.
a Statistically significant difference between PMNs and PMNs+SCC cells.
b Statistically significant difference between PMNs and PMNs+Supernatant.
c Statistically significant difference between PMNs+SCC cells and PMNs+Supernatant.
It was observed that after coculture with cancer cells (SCC), neutrophils of healthy subjects produced significantly higher amounts of NETs than non-stimulated PMNs. Similar results were obtained in samples from patients suffering from cancer but no statistical significance was noted (Table 3).
In both cancer patients (Figure 1) and in healthy individuals the highest percentage of trap-forming neutrophils was found after the PMNs were stimulated with a supernatant obtained from the SCC cell culture. Moreover, the amount of NETs was significantly higher than the non-stimulated or cocultured neutrophils (Table 3).
Figure 1.
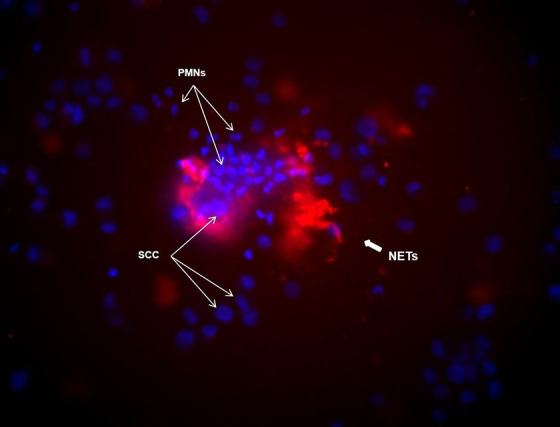
NETs under the microscope. Example of NETs image of coculture of neutrophils from a patient with cancer in response to SCC cells: in blue—DNA (Hoechst 33342), in red—MPO monoclonal antibodies labeled with fluorescein (Clone 8E6).
Cytometric Evaluation of the Number of MPO-Positive Neutrophils
The results of cytometric tests showed a significantly lower percentage of MPO-positive neutrophils in the non-stimulated cell samples from patients with cancer in relation to the number observed in the samples of healthy subjects (Figure 2, Table 3).
Figure 2.
Representative results for MPO-positive neutrophils determined by flow cytometry.
In respect to patients with cancer, the PMNs coculture with SCC cells resulted in a significant increase in the number of MPO-positive neutrophils compared to non-stimulated cells as well as those of healthy subjects. In the control group, the percentage of MPO-positive PMNs after coculture was the same as that of non-stimulated cells (Figure 2, Table 3).
Furthermore, a lower percentage of MPO-positive PMNs was observed in samples stimulated with supernatant from SCC cell culture compared to non-stimulated neutrophils or after coculture in both of the examined groups (Figure 2, Table 3).
cfDNA Concentration in Neutrophil Supernatants Evaluated According to the Manufacturer’s Instructions (Supplementary 1)
Comparison of cfDNA concentrations in supernatants of non-stimulated neutrophils in cancer patients and healthy subjects showed significantly higher levels of this marker in the former (Table 3).
When it comes to patients with cancer, lower cfDNA concentrations were observed after PMNs coculture with SCC cells with respect to non-stimulated cells or those stimulated with the supernatant from SCC cell cultures. In healthy subjects, however, the PMNs coculture with SCC cells was the cause of a slight increase in the level of cfDNA (Table 3).
The highest cfDNA concentrations were obtained after neutrophil stimulation with supernatant from SCC cell culture and were significantly higher compared to non-stimulated cells in patients with cancer and healthy subjects (Table 3).
Instruction Compliant Assessment of MPO Concentrations in Neutrophil Supernatants (Supplementary 2)
An evaluation of MPO concentrations in supernatants of non-stimulated neutrophils showed significantly higher values of this protein in patients with cancer than in healthy subjects (Table 3).
No significant changes were found in the amount of MPO in samples from PMNs and SCC coculture in both groups (Table 3).
Significantly higher MPO concentrations were observed after the stimulation of neutrophils of healthy subjects with an SCC cell culture supernatant compared to the other 2 groups of cells. A tendency toward a change in the amount of MPO in a similar sample obtained from cancer patients was found (Table 3).
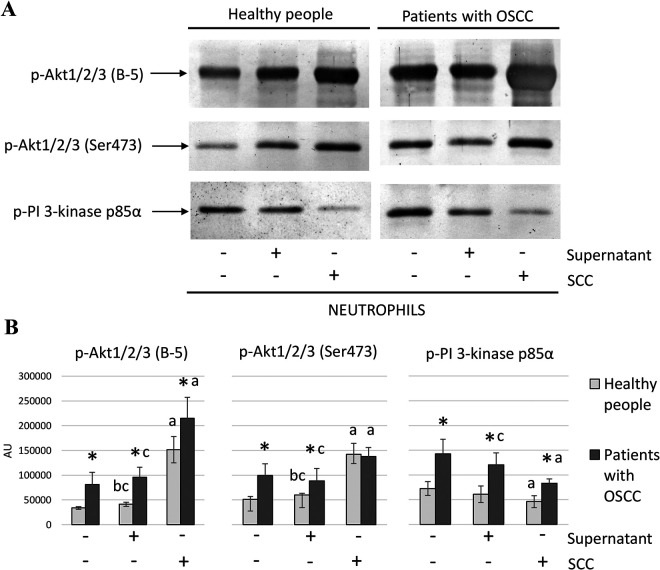
Analysis of p-Akt1/2/3 (B-5), p-Akt1/2/3 (Ser 473) and p-PI 3-Kinase Protein Expression in Neutrophil Lysates Obtained Using the Western Blot Method
Western blot results showed higher expression of p-Akt1/2/3 (B-5), p-Akt1/2/3 (Ser 473) (without any significance in the sample of PMNs coculture with SCC cells), and p-PI 3-kinase in neutrophil lysates in samples of patients with OSCC than in those of healthy subjects (Figure 3, Table 3).
Figure 3.
Western blot results. The figure shows the results obtained by the Western blot method: A—sample scans of membrane samples from healthy people and patients with OSCC; B—column charts of average expression values of tested proteins together with SD and statistically significant differences: *—with control; a—between PMNs and PMNs+SCC cells; b—between PMNs and PMNs+Supernatant; c—between PMNs+SCC cells and PMNs+Supernatant.
In both groups, the expression of p-Akt1/2/3 (B-5) and p-Akt1/2/3 (Ser 473) after PMNs coculture with SCC cells was significantly higher in relation to non-stimulated neutrophils or ones stimulated with supernatant from SCC cells. However, the expression of p-PI 3-kinase was lower after PMNs coculture with SCC cells compared to non-stimulated neutrophils in samples of patients with cancer and of healthy subjects (Figure 3, Table 3).
In the control group neutrophil stimulation with an SCC supernatant caused a significant increase in the expression of p-Akt1/2/3 (B-5) and p-Akt1/2/3 (Ser 473) compared to non-stimulated PMNs. Similar results were obtained for these proteins in patients with cancer but with no statistical significance. In respect to cancer patients, a significantly higher expression of p-PI 3-kinase was observed in lysates of neutrophils stimulated with supernatant from an SCC cell culture than that obtained after coculture. A similar trend was observed in the expression of p-PI 3-kinase in relation to the control group but without statistical significance (Figure 3, Table 3).
Correlations
Table 4 presents correlations noted between test parameters.
Table 4.
Correlations Between the Examined Parameters.
| Study groups | Correlations | Value | p | |
|---|---|---|---|---|
|
Healthy
people |
MPO PMNs |
MPO Serum |
0.906 | P ≤ 0.05 |
|
Patients
with OSCC |
NETs
PMNs+Supernatant |
p-Akt1/2/3 (Ser473)
PMNs+Supernatant |
0.965 | P < 0.05 |
|
PMNs MPO+
PMNs |
p-Akt1/2/3 (B-5)
PMNs |
0.989 | P < 0.05 | |
|
PMNs MPO+
PMNs |
p-Akt1/2/3 (Ser473)
PMNs |
0.893 | P < 0.05 | |
|
p-Akt1/2/3 (B-5)
PMNs |
p-Akt1/2/3 (Ser473)
PMNs |
0.926 | P < 0.05 | |
|
p-Akt1/2/3 (B-5)
PMNs+Supernatant |
p-Akt1/2/3 (Ser473)
PMNs+Supernatant |
0.933 | P < 0.05 | |
Discussion
High cfDNA concentrations and the percentage of spontaneously neutrophil-forming NETs, accompanied by a reduced number of MPO-positive PMNs in patients with cancer confirm the results of previous analyses and indicate a contribution of neutrophil traps to the course of oral mucosa SCC.5 The low percentage of MPO-positive neutrophils probably results from the release of neutrophil traps outside the cell evidenced by the observed increase in MPO concentrations in neutrophil supernatants and a strong correlation between the concentrations of this protein in the PMNs supernatant and blood serum. Increased NETs release in non-stimulated neutrophils was accompanied by a high expression of p-Akt (T308), p-Akt (S473), and p-PI 3 k proteins which indicates that, in the studied groups of patients, the PI3K/Akt/PKB pathway was involved in NETosis. This hypothesis is confirmed by correlations observed between the expression of signaling proteins of this pathway and the assessed NETs formation markers. Liu et al. have proven that the activation of Akt requires threonine phosphorylation in position 479 (T479) and serine phosphorylation in position 477 (S477), which affects S473 phosphorylation, and have related the results of their study to cancer formation.14 Feng et al. showed a 10-fold higher Akt/PKB kinase activity as the result of S473 phosphorylation with DNA-PK (DNA-dependent protein kinase) participation. The authors of the present article consider the existence of another kinase having S473 phosphorylation ability that is activated by growth factors or DNA damage.15
The intensity of NETs formation in response to direct contact with the SCC cell line not only confirms the participation of NETs in the cancer process but also indicates the existence of interactions between cells. To date, it has been believed that peripheral blood neutrophils do not provide information about the course of the immune response to cancer in situ and are not capable of directly reacting with cancer cells. Only TANs have been reported to have such ability. The results of the present study are similar to those obtained by Jung et al., which have shown that pancreatic cancer cells induced NETs formation after coculture with neutrophils.16 Similar evidence can also be seen in the work of other authors who found the presence of extensive neutrophil traps after neutrophil incubation with breast adenocarcinoma cells.17
An increased formation of NETs was accompanied by changes in the expression of p-Akt after coculture with SCC cells which suggests that neutrophils react to the appearance of cancer cells by activating the Akt/PKB pathway. Surprising is the simultaneous low expression of p-PI 3 k, possibly caused by the presence of PI3 K inhibitors and which, at the same time, can activate Akt. There is also a hypothesis assuming Akt kinase activation excluding PI3 K kinase after neutrophils’ direct contact with SCC cells. Kozak et al. described mutations of PIK3CA genes in tumor cells of patients with oral mucosa SCC. An increase in the expression of Akt was observed during PIK3CA gene mutations18 which may explain an increase in p-Akt expression by the mutant gene product with a simultaneous decrease in p-PI 3 k expression. Additionally, the regulation of Akt activity is dependent on PTEN (phosphatase and tensin homolog) and PDK1 (phosphoinositide-dependent kinase 1) or other proteins independent of PI3 K kinase. Maira et al. described the role of casein kinase 2 (CK2) in the process of Akt phosphorylation. The CK2 protein physiologically plays a role in apoptosis and cell survival control.19-21 According to literary data levels of CK2 are elevated in patients with cancer which may be indicative of its role in the over-activation of Akt, a result that can be also seen in our studies.22,23 Using PTEN-free cells, Maira et al. observed that CK2 phosphorylates serine in position 129 and correlates with increased activity of Akt.20 It can, therefore, be concluded that CK2 contributes to the regulation of Akt activity without affecting levels of the PI3 K protein. Furthermore, elevated CK2 levels were found in patients with head and neck SCC and an increase in CK2 activity correlated with less differentiated types of cancer and the presence of metastases.24
Results of recent studies show that the interaction and modulation of the blood microenvironment by circulating cancer cells (CTCs) is essential for the development of metastases.25 Data seen in literature shows that tumors induce changes in distant organs preparing their tissues for CTC colonization by releasing proinflammatory cytokines. It has been demonstrated that neutrophils mobilize and accumulate at sites of future metastases, release NETs binding CTCs, take part in the formation of a “premetastatic niche,” and promote the formation of tumors having aggressive phenotypes.26-28 The results of our study confirm that factors released by cancer cells cause an increase in NETs formation. However, the formation of larger amounts of NETs after stimulation with a supernatant obtained from an SCC cell culture than that seen after direct contact with SCC cells is surprising and may indicate a dominant role of the primary tumor in the formation of a metastasis microenvironment. Our results confirm observations of other authors who were able to activate NETosis in neutrophils of healthy subjects with plasma or platelets obtained from patients with gastric cancer.4
It is worth noting that MPO serum concentrations increased fourfold (MPO = 1931.96 ng/µL) in patients with cancer as compared to those of healthy subjects (MPO = 483.08 ng/µL). According to Odajima et al., MPO plays an important anticancer role in NETs because of its cytotoxic effect on cancer cells and these authors, using a mouse model, have shown that MPO kills melanoma cells and inhibits their growth after implantation.29 Rymaszewski et al. on the other hand, have expressed a different opinion and in their belief, the phase of initiation and promotion of cancer requires the activity of MPO and during its progression protects cancer cells against caspase-3 activity.30 The high percentage of MPO-positive neutrophils after coculture with SCC cells observed in our study in samples of patients with cancer indicates a strong activation of PMNs during NETs formation. Roncucci et al. also noted an increase in the number of MPO-positive neutrophils during cancer transformation in the colon mucosa.31 Lack of changes in MPO concentrations and the small amount of cfDNA in PMNs supernatants in these patients suggest a delayed reaction of NETs release by neutrophils after contact with SCC cells. However, an increase in cfDNA and MPO concentrations in patients with cancer after neutrophil stimulation with a supernatant obtained from an SCC cell culture confirms the results of the microscopic examination performed within this study and indicates a rapid reaction of cells to products released by cancer cells resulting in NETs release. Lower expression of p-Akt after stimulation with supernatant from SCC cell cultures in relation to PMNs incubated in the presence of SCC cells suggests that the direct contact of cancer cells with neutrophils activates kinase more strongly than the presence of substances secreted by tumor cells or is the result of neutrophil breakdown in favor of NETs. Currently available literature does not contain data with a similar research profile warranting further studies to obtain reliable conclusions.
Conclusions
Neutrophils are increasingly scrutinized as an important element of cancer progression. New aspects of neutrophil biology have been clarified by the discovery of NETs and their participation in the cancer process is considered important. The results obtained through the present study indicate a different mechanism of Akt kinase regulation, independent of PI3 K, which leads to the formation of NETs in patients with early stage of SCC of the oral mucosa. The lack of research in the group of patients with advanced OSCC is a limitation for the study. Therefore, more detailed studies considering a larger group of patients that will explain the type of factor activating Akt/PKB kinase, which may also influence the inhibition of PI3 K, are justified. These could become a point of reference for new therapies which could help increase OSCC patients’ chances for survival and improve their quality of life.
Supplemental Material
Supplemental Material, CCX-20-0250_Graphic_abstract for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control
Supplemental Material, CCX-20-0250_Supplementary_1 for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control
Supplemental Material, CCX-20-0250_Supplementary_2 for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control
Acknowledgments
This study was conducted with the use of equipment purchased by the Medical University of Bialystok as part of the RPOWP 2007-2013 funding, Priority I, Axis 1.1, contract No. UDA- RPPD.01.01.00-20-001/15-00 dated 26.06.2015.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marzena Garley  https://orcid.org/0000-0002-6727-3850
https://orcid.org/0000-0002-6727-3850
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 2. Berger-Achituv S, Brinkmann V, Abed UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad S. 2012;109(32):13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang C, Sun W, Cui W, et al. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol. 2015;8(11):14075–14086. [PMC free article] [PubMed] [Google Scholar]
- 5. Garley M, Dziemiańczyk-Pakieła D, Grubczak K, et al. Differences and similarities in the phenomenon of NETs formation in oral inflammation and in oral squamous cell carcinoma. J Cancer. 2018;9(11):1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demers M, Wagner DD. Neutrophil extracellular traps: a new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2(2):e22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci. 2014;71(21):4179–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arelaki S, Arampatzioglou A, Kambas K, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11(5):e0154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Haidari AA, Algethami N, Lepsenyi M, Rahman M, Syk I, Thorlacius H. Neutrophil extracellular traps promote peritoneal metastasis of colon cancer cells. Oncotarget. 2019;10(12):1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Garley M, Jabłońska E, Dąbrowska D. NETs in cancer. Tumour Biol. 2016;37(11):14355–14361. [DOI] [PubMed] [Google Scholar]
- 11. Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18(4):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dąbrowska D, Jabłońska E, Garley M, Ratajczak-Wrona W, Iwaniuk A. New aspects of the biology of neutrophil extracellular traps. Scand J Immunol. 2016;84(6):317–322. [DOI] [PubMed] [Google Scholar]
- 13. Tatsiy O, McDonald PP. Physiological stimuli induce PAD4-dependent, ROS-independent NETosis, with early and late events controlled by discrete signaling pathways. Front Immunol. 2018;9:2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu P, Begley M, Michowski W, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508(7497):541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279(39):41189–41196. [DOI] [PubMed] [Google Scholar]
- 16. Jung HS, Gu J, Kim JE, Nam Y, Song JW, Song JW. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One. 2019;14(4):e0216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo L, Chen G, Zhang W, et al. A high-risk luminal A dominant breast cancer subtype with increased mobility. Breast Cancer Res Treat. 2019;175(2):459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kozaki K, Imoto I, Pimkhaokham A, et al. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 2006;97(12):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakahata S, Ichikawa T, Maneesaay P, et al. Loss of NDRG2 expression activates PI3K-AKT signaling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5(1):3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Maira G, Salvi M, Arrigoni G, et al. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12(6):668–677. [DOI] [PubMed] [Google Scholar]
- 21. Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369(Pt 1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20(2):391–408. [DOI] [PubMed] [Google Scholar]
- 23. Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16(2):573–582. [DOI] [PubMed] [Google Scholar]
- 24. Gapany M, Faust RA, Tawfic S, et al. Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med. 1995;1(6):659–666. [PMC free article] [PubMed] [Google Scholar]
- 25. Heeke S, Mograbi B, Alix-Panabières C, Hofman P. Never travel alone: the crosstalk of circulating tumor cells and the blood microenvironment. Cells. 2019;8(7):E714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jablonska J, Lang S, Sionov RV, Granot Z. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget. 2017;8(67):112132–112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Homa-Mlak I, Majdan A, Mlak R, Małecka-Massalska T. Metastatic potential of NET in neoplastic disease. Postepy Hig Med Dosw (Online). 2016;70(0):887–895. [DOI] [PubMed] [Google Scholar]
- 28. Wu L, Saxena S, Awaji M, Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers (Basel). 2019;11(4):E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odajima T, Onishi M, Hayama E, Motoji N, Momose Y, Shigematsu A. Cytolysis of B-16 melanoma tumor cells mediated by the myeloperoxidase and lactoperoxidase systems. Biol Chem. 1996;377(11):689–693. [PubMed] [Google Scholar]
- 30. Rymaszewski AL, Tate E, Yimbesalu JP, et al. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers (Basel). 2014;6(2):1111–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roncucci L, Mora E, Mariani F, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2291–2297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, CCX-20-0250_Graphic_abstract for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control
Supplemental Material, CCX-20-0250_Supplementary_1 for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control
Supplemental Material, CCX-20-0250_Supplementary_2 for Cancers Cells in Traps? The Pathways of NETs Formation in Response to OSCC in Humans—A Pilot Study by Marzena Garley, Ewa Jabłońska, Wojciech Miltyk, Kamil Grubczak, Arkadiusz Surażyński, Wioletta Ratajczak-Wrona, Małgorzata Grudzińska, Kinga H. Nowacka, Marcin Moniuszko, Jerzy A. Pałka, Jan Borys and Dorota Dziemiańczyk-Pakieła in Cancer Control