Abstract
The sex-determining region Y-box 12 (SOX12) is implicated in several oncogenic signaling pathways of multiple types of cancer; however, the biological effects of SOX12 on breast cancer has yet to be elucidated. Here, we assessed SOX12 expression using real-time quantitative PCR in 142 pairs of breast cancer and adjacent normal tissues (ANTs) and immunohistochemistry in 524 breast cancer and 147 ANTs. The effects of SOX12 on breast cancer progression, clinicopathological variables, and prognostic value were then investigated. SOX12 expression was markedly elevated in breast cancer tissues relative to that in ANTs at both mRNA and protein levels. Positive SOX12 expression was correlated to tumor size (P = 0.005), estrogen receptor (ER) (P = 0.018) and human epidermal growth factor receptor (HER2) (P = 0.004) status, lymph node metastasis (P < 0.001), and the tumor-node-metastasis (TNM) stage (P < 0.001). Notably, the positive rate of SOX12 expression gradually increased with breast cancer progression. Multivariate analysis indicated that SOX12 was an independent prognostic factor for overall survival (OS, P = 0.023) and distant metastasis-free survival (DMFS, P = 0.012). Subgroup analysis revealed that luminal and HER2 patients with positive SOX12 expression had a shorter OS period than those with negative SOX12 expression. Moreover, SOX12 expression was associated with a high risk of distant metastasis in invasive carcinoma with the lymph node metastasis subgroup. In summary, SOX12 correlates with progression and poor prognosis in human breast cancer, suggesting that SOX12 is a potential target for breast cancer treatment and warrants further functional research.
Keywords: Breast cancer, SOX12, progression, prognostic factor, metastasis
Introduction
Breast cancer is one of the most frequently occurring malignant tumors, with a rising incidence rate and the highest cancer mortality in females worldwide [1]. An estimated 2.1 million new cases and 626,679 deaths were reported in 2018 [2,3]. Various methods have been developed for early diagnosis and multidisciplinary treatment to lower mortality rates [4]; regardless, 25%-50% of patients with breast cancer were predicted to ultimately develop distant metastasis and then succumbed after decades of diagnosis and primary tumor resection [5,6]. The identification of cancer progression-related genes that exhibit potential therapeutic value remains as a major objective of breast cancer research.
The sex-determining region Y-box (SOX) transcriptional factors include more than 20 members in vertebrates. These SOX transcriptional factors are characterized by the conserved DNA-binding high-mobility group box and subdivided into 8 groups named A to H [7-9]. SOX genes present a specific or more complex expression pattern and play an essential role during embryonic development, cell differentiation, tumorigenesis, and cancer [8,10,11]. The SOXC group consists of SOX4, SOX11, and SOX12, which have a critical role in neuron and retina formation, skeletogenesis, and notably in multiple types of malignancies [12,13]. SOX4 mediates epithelial-to-mesenchymal transition (EMT) in normal and breast cancer cells and is related to tumorigenesis and metastasis in vivo as well as cell viability in vitro [14]. Song et al. reported that high SOX4 expression is an independent unfavorable prognostic factor in breast cancer patients [15]. SOX11 is regarded as an essential regulatory factor for cell growth, migration, and invasion in triple-negative breast cancer [16,17]. High levels of SOX11 expression are associated with poor overall survival and increased formation of metastasis in breast cancer patients [16,18]. SOX12 is markedly elevated in colorectal carcinoma tissues and facilitates cancer cell proliferation and metastasis by inducing asparagine synthesis [19]. Moreover, SOX12 enhances the metastatic capacity of cancer cells by regulating matrix metalloproteinase 7 (MMP7) and insulin-like growth factor 1 (IGF1) in gastric cancer [20]. Intriguingly, a recent study demonstrated that low SOX12 expression correlated with poor survival in malignant gastric tumors [21]. Ding et al. reported an increase in the SOX12 mRNA level in samples from patients with breast cancer and confirmed its function in facilitating the proliferation, migration, and metastasis of breast cancer cells [22]; however, their study lacked the detection of SOX12 expression at the protein level and associated survival analysis. Indeed, there has been no evidence concerning the specific effect of SOX12 expression on breast cancer outcomes. Verification is clearly needed to explore the function and prognostic value of SOX12 expression in breast cancer.
SOX12 mRNA expression was determined by real-time quantitative polymerase chain reaction (RT-qPCR). Immunohistochemistry (IHC) was conducted using 524 formalin-fixed paraffin-embedded breast cancer tissues with complete clinical information and outcome data. In this study, we examined the SOX12 expression in breast cancer samples and its association with progression as well as clinicopathologic characteristics, particularly prognosis.
Materials and methods
Patients and clinicopathological information
The research protocol was reviewed and approved by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from each participant. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. This article does not contain any animal studies conducted by any of the authors.
A series of 524 patients with pathologically diagnosed breast cancer were retrospectively enrolled in our study from 2005 to 2009 at the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, consisting of 45 patients with ductal carcinoma in situ (DCIS) and 479 invasive carcinoma (IC) cases; 147 pairs of adjacent normal tissues (ANTs) were enrolled as well. In this retrospective cohort, 203 patients had lymph node metastasis. Moreover, 30 normal breast epithelial tissues and 142 pairs of frozen breast cancer and ANTs were collected and then snap-frozen with liquid nitrogen after surgical resection. The inclusion and exclusion criteria are presented in detail in Table 1.
Table 1.
Inclusion and exclusion criteria of the present study
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Written informed consent | • Declined to participate |
| • Female patients with unilateral, stage I-III breast cancer who had undergone standard surgical treatment and confirmed histologically | • Receipt of neoadjuvant radiotherapy, chemotherapy, or hormonal therapy for breast cancer |
| • Available primary tumor samples; Adjacent normal breast tissues selected from an area more than 5 cm from the edge of the tumor | • Patients had distant metastasis at the time of diagnosis |
| • Complete clinicopathological and follow-up information | • Other concurrent malignant diseases or previous diagnosis of carcinoma |
| • Severe cardiac or cardiovascular disease | |
| • Severe cerebrovascular disease | |
| • Severe renal failure | |
| • Severe respiratory insufficiency | |
| • Prior organ transplantation | |
| • Pregnancy or lactation |
Estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 expression status were available from standardized histopathology reports. ER and PR were defined as positive with more than 10% positively staining nucleus [23]. Fluorescence in situ hybridization was performed to determine HER2 status in the equivocal judgment of HER2 expression by immunohistochemistry (IHC) [24]. Ki-67 index was dichotomized to high- and low-expression groups, with 20% as the cut-off point [25]. The patients were staged using the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system for breast cancer staging [26]. All specimens were evaluated by two independent pathologists. Data on patient age, menstrual status, tumor size, histological grade, and lymph node status were obtained from medical records and pathological reports.
Intrinsic molecular subtypes
Patients were subdivided into 4 groups in accordance with the St. Gallen Consensus 2013 [27]: luminal A: ER-positive and/or PR-positive, HER2-negative, and Ki-67 ≤ 20%; luminal B (HER2-negative): ER-positive and/or PR-positive, HER2 negative, and Ki-67 > 20%; luminal B (HER2 positive): ER-positive and/or PR-positive and HER2 positive; HER2 type: ER-negative, PR-negative, and HER2-positive; Triple-negative breast cancer (TNBC): ER-negative, PR-negative, and HER2-negative.
Immunohistochemistry
Whole-tissue blocks from 524 breast cancer tissues and 147 ANTs were sliced into 4 µm sections and heated in a hot chamber for 1 h at 60°C, followed by dewaxing and epitope retrieval. Slides were immunoreacted for 1 h with the anti-SOX12 primary antibody (diluted at 1:100; Sigma-Aldrich) and then incubated with the secondary antibody for 30 min. Hematoxylin was used for counterstaining. Two indexes-staining intensity and percentage of the staining cells-were evaluated by two senior pathologists blinded to patient medical information. The staining intensities were assigned as follows: 0 (no stain), 1 (weak), 2 (moderate), and 3 (strong). The percentages of staining cancer cells were graded as follows: 0 (< 5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (> 75%). SOX12 expression was determined based on the semi-quantitative immunoreactive score (IRS) [28], which was determined by multiplying the two indexes mentioned above. Slices with a score from 0 to 5 were regarded as negative expression, whereas those with a score from 6 to 12 were considered as positive expression.
Real-time quantitative polymerase chain reaction
Total RNA from snap-frozen tissues was extracted with TRIzol Reagent (Invitrogen), followed by reverse transcription using the PrimeScript™ RT Reagent Kit (TaKaRa). For RT-qPCR, cDNA was amplified using the SYBR Green Real-time PCR Kit (QIAGEN). The primers of SOX12 were designed as follows: forward, 5’-AAGAGGCCGATGAACGCATT-3’; reverse, 5’-TAGTCCGGGTAATCCGCCAT-3’. GAPDH was utilized as the internal control, and primers were the following: forward, 5’-AATGGACAACTGGTCGTGGAC-3’; reverse, 5’-CCCTCCAGGGGATCTGTTTG-3’. The cycling parameters were set up as follows: 45 cycles of 95°C for 15 s, 55°C-60°C for 15 s, and 72°C for 15 s. The results of RT-qPCR were calculated using the 2-ΔΔCt method.
Statistical analysis
Overall survival (OS) was defined as the period from breast cancer surgery to death from any causes. Distant metastasis-free survival (DMFS) was defined as the time after surgery until the diagnosis of distant metastatic lesions derived from breast cancer.
Data were statistically analyzed using IBM SPSS 22.0 and GraphPad Prism 8.0. Relationships between SOX12 expression and clinicopathologic factors were evaluated using the chi-squared test. Differences in the immunoreactive score (IRS) between the two groups were evaluated using the Mann-Whitney U test. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used to compare the survival of the different groups. Univariate and multivariate Cox proportional hazard regression analyses were performed to evaluate the effect of SOX12 expression and other clinicopathological factors on OS and DMFS. P < 0.05 was considered statistically significant.
Results
SOX12 is highly expressed in breast cancer tumors
A total of 524 patients with pathologically diagnosed primary breast cancer from a retrospective cohort were included in this study. All patients were female with a median age of 51 y (range 22-84 y); 16.4% of the patients were younger than 35 y when diagnosed, and 38.7% had regional lymph node involvement during the postoperative pathological examination.
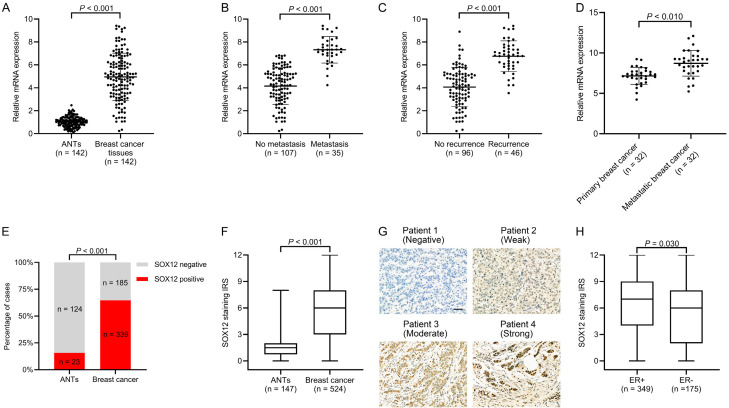
We first analyzed the SOX12 mRNA levels in 142 paired breast cancer specimens and ANTs by RT-qPCR. The SOX12 levels were markedly increased in breast cancer tissues relative to that in ANTs (n = 142) (Figure 1A). As shown in Figure 1B and 1C, breast cancer cases with metastasis (n = 35) or recurrence (n = 46) showed markedly higher SOX12 levels than those without metastasis (n = 107) or without recurrence (n = 96). SOX12 mRNA levels were elevated in the paired metastatic lesions (n = 32) relative to those in primary breast cancer specimens (Figure 1D).
Figure 1.
Markedly elevated SOX12 in human breast cancer tissues. A. Real-time PCR results of SOX12 mRNA levels in paired breast cancer tissues and adjacent normal tissues (ANTs) (n = 142). B. Relative expression of SOX12 mRNA in breast cancer cases with metastasis (n = 35) and without metastasis (n = 107). C. Relative expression of SOX12 mRNA in breast cancer cases with recurrence (n = 46) and without recurrence (n = 96). D. Relative expression of SOX12 mRNA expression in paired tissues from primary breast cancer and metastatic lesions (n = 32). E. Proportion of breast cancer tissues with positive SOX12 expression being higher than that of ANTs (P < 0.001). F. Higher median immunoreactive score (IRS) of breast cancer tissues than that of ANTs (P < 0.001). G. Representative immunohistochemistry (IHC) images of different SOX12 staining intensities in breast cancer tissues (Scale bar, 50 µm). H. Higher median IRS in estrogen receptor (ER)-positive breast cancer tissues than in ER-negative cases (P = 0.030).
SOX12 expression was determined by IHC using a previously reported antibody specific for SOX12 [19]. SOX12 was mainly expressed in the nucleus of the cancer cells, which was consistent with previous reports [19,20,29]. SOX12 expression was positive in 339 (64.7%) of the 524 evaluable primary breast cancer tissues; meanwhile, only 15.6% (23/147) of ANTs showed positive immunoreactivity for SOX12. A significant difference in SOX12 expression was found between ANTs and breast cancer tissues (Figure 1E). Compared with that in ANTs, the median IRS of SOX12 expression in breast cancer tissues was markedly higher (Figure 1F). Representative images of SOX12 staining in breast cancer tissues are presented in Figure 1G. Of all 524 patients, 63.9% (349) were ER-positive, consisting of 6.6% (23) DCIS and 93.4% (326) IC. Compared with the ER-negative group in breast cancer, the median SOX12 staining IRS of the ER-positive group was higher (Figure 1H), indicating that SOX12 is correlated to ER expression in breast cancer.
Overall, SOX12 expression is elevated in breast cancer relative to that in ANTs, particularly in the ER-positive group.
SOX12 expression is related to clinicopathological characteristics
The association between SOX12 expression and clinicopathological parameters in breast cancer was analyzed (Table 2). Positive SOX12 expression was markedly related to pathological large tumor size (χ2 = 10.462, P = 0.005), lymph node metastasis (χ2 = 12.344, P < 0.001), and advanced TNM staging (χ2 = 7.622, P = 0.022). SOX12 expression was also positively associated with ER (χ2 = 5.605, P = 0.018) and PR (χ2 = 8.453, P = 0.004) expression. For molecular subtypes, 68.8% (97/141) of breast cancer tissues showed positive SOX12 expression in the luminal A subtype, 66.1% (160/242) in the luminal B subtype, 58.4% (52/89) in the HER2 subtype, and 57.7% (30/52) in TNBC. However, no statistical difference in SOX12 expression was detected in several molecular subtypes of breast cancer (χ2 = 3.899, P = 0.273). SOX12 expression showed no significant association with other clinicopathologic characteristics, including age at diagnosis, menopausal status, histologic classification, HER2, Ki-67, and P53 expression. To summarize, positive SOX12 expression was significantly related to tumor size, ER and PR status, lymph node metastasis, and TNM staging.
Table 2.
Association between SOX12 expression and clinicopathologic characteristics
| Clinicopathological parameters | Expression of SOX12 | χ2 | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Positive (n = 339) | Negative (n = 185) | Total (n = 524) | |||
| Age at diagnosis (years) | |||||
| ≤ 35 | 43 | 16 | 59 | 1.951 | 0.163 |
| > 35 | 296 | 169 | 465 | ||
| Pathologic tumor size (cm) | |||||
| T1 | 130 | 98 | 228 | 10.462 | 0.005 |
| T2 | 183 | 77 | 260 | ||
| T3 | 26 | 10 | 36 | ||
| Menopausal status | |||||
| Premenopausal | 189 | 94 | 283 | 1.177 | 0.278 |
| Postmenopausal | 150 | 91 | 241 | ||
| Histological grade | |||||
| 1 | 30 | 16 | 46 | 7.498 | 0.058 |
| 2 | 153 | 97 | 250 | ||
| 3 | 116 | 63 | 179 | ||
| Unknown | 40 | 9 | 49 | ||
| ER | |||||
| Positive | 238 | 111 | 349 | 5.605 | 0.018 |
| Negative | 101 | 74 | 175 | ||
| PR | |||||
| Positive | 245 | 125 | 370 | 1.276 | 0.259 |
| Negative | 94 | 60 | 154 | ||
| HER2 | |||||
| Negative | 252 | 115 | 367 | 8.453 | 0.004 |
| Positive | 87 | 70 | 157 | ||
| Lymph node metastasis | |||||
| Negative | 181 | 128 | 309 | 12.344 | < 0.001 |
| Positive | 158 | 57 | 215 | ||
| TNM | |||||
| I | 52 | 58 | 110 | 22.958 | < 0.001 |
| II | 206 | 104 | 310 | ||
| III | 81 | 23 | 104 | ||
| Ki-67 status | |||||
| < 20% | 98 | 59 | 165 | 4.235 | 0.120 |
| ≥ 20% | 241 | 126 | 316 | ||
| P53 status | |||||
| Positive | 227 | 125 | 328 | 2.456 | 0.293 |
| Negative | 75 | 47 | 132 | ||
| Unknown | 37 | 13 | 21 | ||
| Molecular subtype | |||||
| Luminal A | 97 | 44 | 141 | 3.899 | 0.273 |
| Luminal B | 160 | 82 | 242 | ||
| HER2 type | 52 | 37 | 89 | ||
| TNBC | 30 | 22 | 52 | ||
SOX12, sex-determining region Y-box 12; TNM, tumor-node-metastasis; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer; P-values that reach significance are in bold.
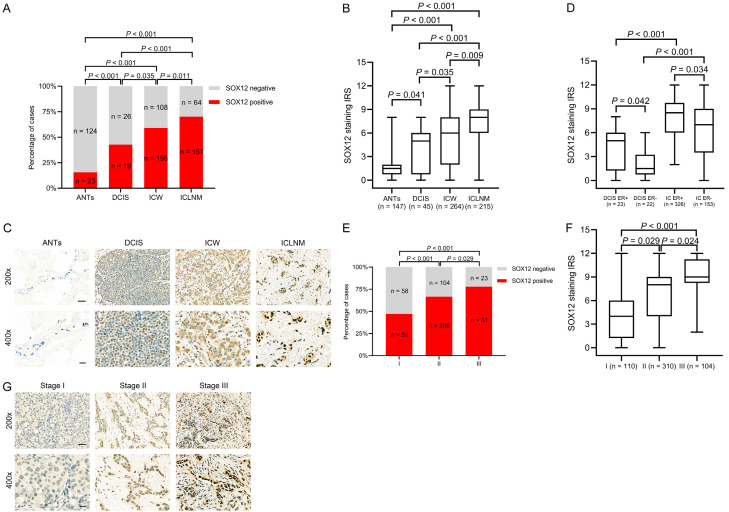
SOX12 expression correlated with breast cancer progression
We then determined the SOX12 expression levels in ANTs (n = 147), DCIS (n = 45), invasive cancer with no lymph node metastasis (ICW, n = 264), and invasive cancer with lymph node metastasis (ICLNM, n = 215). The SOX12 expression levels were as follows: 15.6% in ANTs, 42.2% in DCIS, 59.1% in ICW, and 70.2% in ICLNM. Positive SOX12 expression gradually increased with disease progression. Compared with ANTs, all other subtypes showed increased SOX12 expression with breast cancer progression (Figure 2A, DCIS vs. ANTs, P < 0.001; ICW vs. ANTs, P < 0.001; ICLNM vs. ANTs, P < 0.001; ICW vs. DCIS, P = 0.035; ICLNM vs. DCIS, P < 0.001; ICLNM vs. ICW, P = 0.011). Moreover, the median IRS levels of SOX12 staining in ANTs, DCIS, ICW, and ICLNM were gradually elevated; significant differences between groups were found (Figure 2B). Representative images of SOX12 staining in ANTs, DCIS, ICW, and ICLNM are presented in Figure 2C. The IRS of SOX12 expression in the invasive carcinoma subgroup was higher than that in the DCIS subgroup independent of ER expression; meanwhile, the IRS of SOX12 expression was increased in both the invasive carcinoma and DCIS subgroups with ER-positive expression (Figure 2D). The results further verified that SOX12 was related to ER expression in breast cancer.
Figure 2.
SOX12 promotes breast cancer progression. A. Comparison of the proportions of positive SOX12 expression of adjacent normal tissues (ANTs), ductal carcinoma in situ (DCIS), invasive carcinoma without lymph node metastasis (ICW), and invasive carcinoma with lymph node metastasis (ICLNM) tissues. Gradual increases in SOX12 expression levels with breast cancer progression. B. Markedly increased median immunoreactive score (IRS) with breast cancer progression. C. Representative SOX12 immunohistochemistry (IHC) staining micrographs of ANTs, DCIS, ICW, and ICLNM. Increased staining intensities in invasive carcinoma tissues. D. Higher median IRS in invasive carcinoma (IC) than in DCIS under the same estrogen receptor (ER) status. E. Comparison of the percentages of positive SOX12 expression in TNM stages I, II, and III. Gradual increases in the proportion of positive SOX12 expression as the cancer progresses. F. Increase in median SOX12 IRS as the extent of cancer increases. G. Representative SOX12 staining micrographs of stages I, II, and III.
Subsequently, the association between SOX12 expression and the TNM stage was evaluated. The positive rate of SOX12 in stage I was 47.3%, which increased to 66.5% and 77.9% in stages II and III, respectively (Figure 2E). Significant differences in SOX12 IRS among the subgroups at each TNM stage were indicated (Figure 2F). Representative photomicrographs of SOX12 immunohistochemical staining at each TNM stage are presented in Figure 2G. Overall, SOX12 expression plays a vital part in breast cancer development.
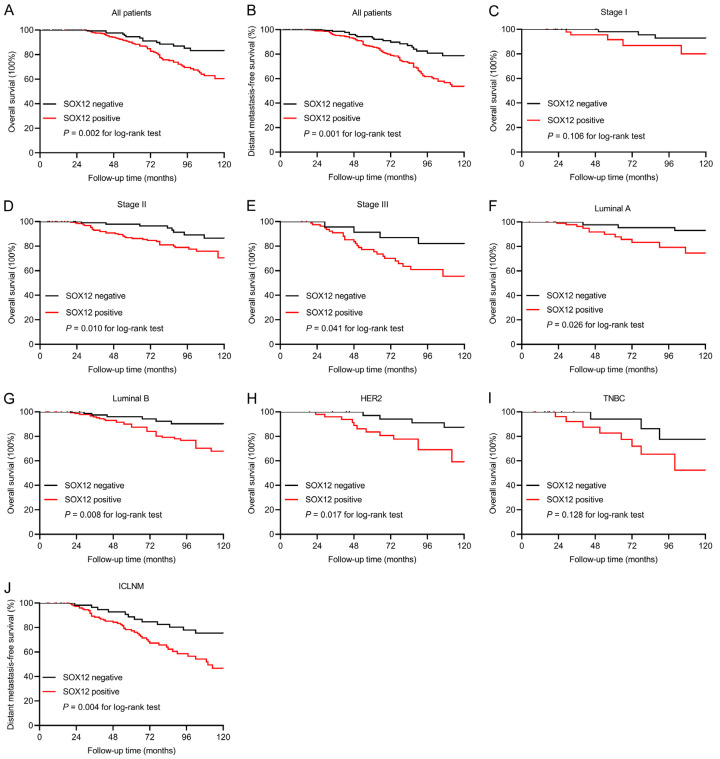
SOX12 expression correlated with poor patient prognosis
The median follow-up periods in the SOX12-negative and -positive subgroups were 10.6 and 10.1 y, respectively. The effect of SOX12 expression on clinical outcome was assessed using the Kaplan-Meier estimator. During the follow-up period, 81 (15.5%) cases died-65 (19.2%) of 339 patients from the SOX12-positive group and 16 (8.6%) of 185 cases from the SOX12 negative-group. The 10-year OS was 83.3% in the SOX12-negative group and 60.5% in the SOX12-positive group (univariate Cox regression HR 3.129, 95% CI 1.386-7.024, P = 0.006; Figure 3A; Table 3). In multivariate survival analysis, positive SOX12 expression proved to be an independent prognostic indicator for poor OS (HR 2.532, 95% CI 1.154-5.586, P = 0.023; Table 3).
Figure 3.
Prognostic significance of SOX12 in patients with breast cancer. A, B. Kaplan-Meier analysis suggesting that positive SOX12 expression is associated with poor overall survival (OS, P = 0.002) and distant metastasis-free survival (DMFS, P = 0.001). C-E. Kaplan-Meier curves for OS in breast cancer patients with different TNM staging. F-I. Kaplan-Meier curves for OS in breast cancer patients with different intrinsic molecular subtypes. J. Relation of SOX12 expression to a high risk of distant metastasis in invasive carcinoma with lymph node metastasis (ICLNM, P = 0.004).
Table 3.
Univariate and multivariate analyses of overall survival in breast cancer patients
| Variables | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age (Years) | > 35 vs. ≤ 35 | 1.225 | 0.543-2.612 | 0.628 | |||
| Tumor size | T2, T3 vs. T1 | 1.440 | 0.678-3.095 | 0.341 | |||
| Histological grade | Grade 3 vs. grade 1, 2 | 1.617 | 0.694-4.382 | 0.285 | |||
| ER | Negative vs. positive | 3.018 | 1.408-6.836 | 0.005 | 2.742 | 1.276-6.230 | 0.012 |
| PR | Negative vs. positive | 2.020 | 0.949-4.364 | 0.076 | |||
| HER2 | Positive vs. negative | 3.890 | 1.625-9.919 | 0.003 | 4.626 | 1.873-12.032 | 0.001 |
| Lymph node metastasis | Positive vs. negative | 1.775 | 0.656-6.190 | 0.284 | |||
| TNM stage | III vs. I + II | 2.936 | 1.211-7.485 | 0.017 | 1.703 | 0.579-5.548 | 0.349 |
| Ki-67 status | ≥ 20% vs. < 20% | 1.071 | 0.404-2.628 | 0.884 | |||
| P53 status | Negative vs. positive | 1.595 | 0.647-3.821 | 0.302 | |||
| SOX12 | Positive vs. negative | 3.129 | 1.386-7.024 | 0.006 | 2.532 | 1.154-5.586 | 0.023 |
HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis; SOX12, sex-determining region Y-box 12; P-values that reach significance are in bold.
Distant metastasis occurred in 101 cases during the follow-up period-84 (24.8%) of 339 cases from the SOX12-positive group and 17 (9.2%) of 185 cases from the SOX12-negative group. The 10-year DMFS was 80.1% in the SOX12-negative group and 53.8% in the SOX12-positive group (univariate survival analysis HR 1.907, 95% CI 1.238-2.943, P = 0.003; Figure 3B; Table 4). Multivariate analysis indicated that coupled with lymph node metastasis, positive-SOX12-expression was considered as an independent indicator for DMFS (HR 1.724, 95% CI 1.028-2.636, P = 0.012; Table 4).
Table 4.
Univariate and multivariate analyses of distant metastasis-free survival in breast cancer patients
| Variables | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age (Years) | > 35 vs. ≤ 35 | 1.194 | 0.827-1.721 | 0.343 | |||
| Tumor size | T2, T3 vs. T1 | 1.208 | 0.793-1.841 | 0.379 | |||
| Histological grade | Grade 3 vs. grade 1, 2 | 1.094 | 0.720-1.665 | 0.676 | |||
| ER | Negative vs. positive | 1.705 | 1.117-2.605 | 0.014 | 1.248 | 1.054-1.915 | 0.031 |
| PR | Negative vs. positive | 1.184 | 0.985-1.424 | 0.073 | |||
| HER2 | Positive vs. negative | 1.048 | 0.683-1.604 | 0.830 | |||
| Lymph node metastasis | Positive vs. negative | 2.473 | 1.584-3.879 | < 0.001 | 1.837 | 1.129-2.994 | 0.019 |
| TNM stage | III vs. I + II | 1.360 | 0.654-2.857 | 0.429 | |||
| Ki-67 status | ≥ 20% vs. < 20% | 1.585 | 0.552-4.544 | 0.405 | |||
| P53 status | Negative vs. positive | 1.061 | 0.370-3.042 | 0.926 | |||
| SOX12 | Positive vs. negative | 1.907 | 1.238-2.943 | 0.003 | 1.724 | 1.028-2.636 | 0.012 |
HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis; SOX12, sex-determining region Y-box 12; P-values that reach significance are in bold.
The influence of SOX12 expression on OS was further stratified by the TNM stage and molecular subtypes to improve the identification of high-risk patients. SOX12 expression was negatively related to OS in the stages II and III groups (P = 0.010, P = 0.041, respectively), instead of the stage I group (P = 0.106). This finding suggested that SOX12 is a potential indicator of poor prognosis in breast cancer in advanced stages (Figure 3C-E). In addition, in the subgroup analyses based on the intrinsic molecular subtype, SOX12 expression was correlated with shortened OS in subtypes luminal A (P = 0.026), luminal B (P = 0.008), and HER2 (P = 0.017) but not in TNBC (P = 0.128) (Figure 3F-I).
We then explored the association between SOX12 expression and DMFS in the ICLNM subgroup. ICLNM with positive SOX12 expression showed a high incidence of distant metastasis (P = 0.004) (Figure 3J). Our data suggested that SOX12 expression was an independent poor prognostic indicator for DMFS, particularly in ICLNM.
Discussion
Accumulating evidence indicates that SOX12 is associated with tumor progression in multiple types of malignancy, including renal cell carcinoma, lung cancer, gastric cancer, and hepatocellular carcinoma [20,29-32]. Our results showed that SOX12 expression increased gradually, from normal, DCIS, ICW, to ICLNM, exhibiting a typical pattern of breast cancer progression [33]. Moreover, SOX12 expression gradually increased with tumor staging. Therefore, SOX12 is related to tumor progression and plays an active role in breast cancer development.
Previous research has shown that SOX12 is related to poor prognosis in hepatocellular carcinoma, gastric cancer, and colorectal cancer [19,20,29]. The present study is the first to indicate that SOX12 expression is associated with poor postoperative prognosis in breast cancer, as demonstrated by OS and DMFS. We also found that SOX12 expression was an independent indicator for poor survival, together with ER, HER2 status, and lymph node metastasis. Further subgroup analysis revealed that patients with positive SOX12 expression are more likely to develop distant metastasis in ICLNM than those with negative SOX12 expression. The ICLNM group showed the highest percentage of SOX12-positive staining by IHC. These results reveal the critical effect of SOX12 on metastasis in breast cancer.
SOX12 was also related to poor prognosis in HER2-positive patients. HER-2 amplification is detected in about 25%-30% of patients with primary breast cancer [34,35]. Overexpression of HER2 is related to more aggressive clinical behaviors and poor prognosis in breast cancer [36,37]. Owing to the overexpression of IGF and its receptors, IGF signaling promotes cell proliferation and induces metastasis, and plays an essential role in the development of breast cancer [38]. The activation of IGF signaling induced by IGF1 led to the formation of the heterodimer IGF1R-HER2. With the existence of such a heterodimer, trastuzumab lost the ability to prevent cell growth in HER2-positive breast cancers, referred to as trastuzumab resistance [39]. A recent study reported that SOX12 could directly bind to the IGF1 promoter to promote its transcription in gastric cancer cells [20]. In our study, SOX12 was associated with HER2 expression. Moreover, positive SOX12 expression was related to poor OS in HER2-positive patients. Thus, we hypothesized that trastuzumab resistance induced by the SOX12-IGF1 regulatory axis could potentially lead to the poor prognosis in HER2-positive breast cancer. The intrinsic molecular mechanism requires further research.
In the current study, the abnormal expression and tumor-promoting effects of SOX12 on breast cancer have been demonstrated, but the specific mechanisms mediating the elevated SOX12 expression in breast cancer remain unclear. We determined that SOX12 was correlated with ER expression in DCIS and invasive carcinoma. Positive SOX12 expression was markedly related to reduced patient survival in luminal breast cancer. Mutations of PI3K/AKT signaling are the most common genetic changes in ER-positive breast cancers as well as recurrent and metastatic cases [40]. These mutations often result in the activation of PI3K/AKT signaling and resistance to endocrine treatment [41]. A recent study suggested that the cAMP response element-binding protein to be activated by PI3K/AKT signaling binds to the specific regions of the SOX12 promoter to transactivate its expression [20]. Deleting SOX12 in breast cancer cells MCF-7 and BT-474, which belong to luminal A and B subtypes, respectively, significantly inhibited cell proliferation, migration, and invasion [22]. These findings suggest that SOX12 overexpression is critical in promoting progression and reducing survival in breast cancer, particularly in the ER-positive group. Our results suggest that SOX12 expression can potentially distinguish ER-positive patients who benefit from more aggressive treatment strategies, and others with less intervention may be needed.
SOX12 expression was not related to prognosis in TNBC cases. Notably, SOX12 deletion induced by shRNA in stable cells showed that the proliferation of breast cancer cells with multiple intrinsic subtypes depend on SOX12, including TNBC [22,42]. The current study might merely be inadequate to determine the influence of SOX12 in TNBC because of the limited number of TNBC patients in our study. TNBC may be less dependent on SOX12 owing to other steady molecular mechanisms of survival and metastasis. Further research may be necessary to determine SOX12 as a prognostic indicator in TNBC. Other related regulatory factors affecting survival and prognosis should also be explored.
The molecular mechanisms by which SOX12 exerts its effect on cancer progression and prognosis are not fully defined. SOX12 deletion resulted in mediated alteration of genes and proteins involved in EMT (Twist1 and E-cadherin), apoptosis (Bax and Bcl-2), invasion (MMP9), and cell proliferation (Cyclin E and PCNA) in lung cancer cells [31]. A recent study suggested that SOX12 induced by forkhead box Q1 (FoxQ1) promoted migration and invasion by regulating FGFBP1 and Twist1 expression in hepatocellular carcinoma [29]. SOX12 functions as an oncogene to regulate Wnt/β-catenin signaling to promote the growth of multiple myeloma cells [43]. Dequet et al. found that SOX12 positively regulated endogenous WNT-TCF signaling to repress pulmonary metastases in colorectal cancer cells and SOX12 knockdown increased metastatic growth [44]. The specific role of SOX12 in the progression and metastasis in various types of cancer requires further study.
The current study has certain limitations. First, the number of patients with breast cancer in some subgroups was limited, which could affect the accuracy and authenticity of our results. Second, the pathologic determination of SOX12 expression in breast cancer patients by using IRS in IHC should be further examined in a large-scale cohort study, and a more suitable number of DCIS cases should be included. Third, patients with stage IV breast cancer were not covered in the present cohort. Finally, the high expression levels of SOX4 and SOX11, the other two members of the SOXC group, correlate with poor prognosis in patients with breast cancer [15,17]. However, we did not eliminate the interference of SOX4 and SOX11 in the present study, and thus we cannot exclude the influence of SOX4 and SOX11 on breast cancer. We will include SOX4, SOX11, and SOX12 in our following study to determine the prognostic values of the individual and combined expression of these three molecules in breast cancer.
In summary, SOX12 is markedly elevated in breast cancer and correlated with cancer progression. Notably, our findings prove that SOX12 expression is an independent prognostic indicator in breast cancer. Examining the molecular mechanism of SOX12 in breast cancer progression can elucidate the pathophysiological effect of SOX12. We speculate that SOX12 can potentially be used to prevent progression and metastasis in breast carcinoma and other SOX12-overexpressing malignant tumors.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81670595, 81970568), Shanghai Natural Science Funds (16ZR1428200), and the Excellent Youth Medical Talents Program of Shanghai General Hospital (06N1702011).
Disclosure of conflict of interest
None.
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Jemal A, Wender RC, Gansler T, Ma J, Brawley OW. An assessment of progress in cancer control. CA Cancer J Clin. 2018;68:329–339. doi: 10.3322/caac.21460. [DOI] [PubMed] [Google Scholar]
- 5.Demicheli R, Dillekås H, Straume O, Biganzoli E. Distant metastasis dynamics following subsequent surgeries after primary breast cancer removal. Breast Cancer Res. 2019;21:57. doi: 10.1186/s13058-019-1139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 7.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 8.She ZY, Yang WX. SOX family transcription factors involved in diverse cellular events during development. Eur J Cell Biol. 2015;94:547–563. doi: 10.1016/j.ejcb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 10.Hou L, Srivastava Y, Jauch R. Molecular basis for the genome engagement by Sox proteins. Semin Cell Dev Biol. 2017;63:2–12. doi: 10.1016/j.semcdb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Castillo SD, Sanchez-Cespedes M. The SOX family of genes in cancer development: biological relevance and opportunities for therapy. Expert Opin Ther Targets. 2012;16:903–919. doi: 10.1517/14728222.2012.709239. [DOI] [PubMed] [Google Scholar]
- 12.Penzo-Méndez AI. Critical roles for SoxC transcription factors in development and cancer. Int J Biochem Cell Biol. 2010;42:425–428. doi: 10.1016/j.biocel.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dy P, Penzo-Méndez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins--Sox4, Sox11 and Sox12--exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schübeler D, van Nimwegen E, Christofori G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Song GD, Sun Y, Shen H, Li W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumour Biol. 2015;36:4167–4173. doi: 10.1007/s13277-015-3051-9. [DOI] [PubMed] [Google Scholar]
- 16.Zvelebil M, Oliemuller E, Gao Q, Wansbury O, Mackay A, Kendrick H, Smalley MJ, Reis-Filho JS, Howard BA. Embryonic mammary signature subsets are activated in Brca1-/- and basal-like breast cancers. Breast Cancer Res. 2013;15:R25. doi: 10.1186/bcr3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd JH, Uray IP, Mazumdar A, Tsimelzon A, Savage M, Hilsenbeck SG, Brown PH. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget. 2016;7:13106–13121. doi: 10.18632/oncotarget.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliemuller E, Kogata N, Bland P, Kriplani D, Daley F, Haider S, Shah V, Sawyer EJ, Howard BA. SOX11 promotes invasive growth and ductal carcinoma in situ progression. J Pathol. 2017;243:193–207. doi: 10.1002/path.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du F, Chen J, Liu H, Cai Y, Cao T, Han W, Yi X, Qian M, Tian D, Nie Y, Wu K, Fan D, Xia L. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019;10:239. doi: 10.1038/s41419-019-1481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du F, Feng W, Chen S, Wu S, Cao T, Yuan T, Tian D, Nie Y, Wu K, Fan D, Xia L. Sex determining region Y-box 12 (SOX12) promotes gastric cancer metastasis by upregulating MMP7 and IGF1. Cancer Lett. 2019;452:103–118. doi: 10.1016/j.canlet.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang KK, Xu HM, Huang JY, Guo YX, Wang ZN. Low SOX12 expression is correlated with poor prognosis in patients with gastric cancer. Technol Cancer Res Treat. 2020;19:1533033819901126. doi: 10.1177/1533033819901126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding H, Quan H, Yan W, Han J. Silencing of SOX12 by shRNA suppresses migration, invasion and proliferation of breast cancer cells. Biosci Rep. 2016;36:e00389. doi: 10.1042/BSR20160053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10:2293–2301. doi: 10.2217/fon.14.110. [DOI] [PubMed] [Google Scholar]
- 24.Elebro K, Bendahl PO, Jernström H, Borgquist S. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res Treat. 2017;165:645–657. doi: 10.1007/s10549-017-4343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 27.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 29.Huang W, Chen Z, Shang X, Tian D, Wang D, Wu K, Fan D, Xia L. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61:1920–1933. doi: 10.1002/hep.27756. [DOI] [PubMed] [Google Scholar]
- 30.Gu W, Wang B, Wan F, Wu J, Lu X, Wang H, Zhu Y, Zhang H, Shi G, Dai B, Ye D. SOX2 and SOX12 are predictive of prognosis in patients with clear cell renal cell carcinoma. Oncol Lett. 2018;15:4564–4570. doi: 10.3892/ol.2018.7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Hu F, Shen S, Xiao H, Li G, Wang M, Mei J. Knockdown of SOX12 expression inhibits the proliferation and metastasis of lung cancer cells. Am J Transl Res. 2017;9:4003–4014. [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan P, Meng L, Wang N. SOX12 upregulation is associated with metastasis of hepatocellular carcinoma and increases CDK4 and IGF2BP1 expression. Eur Rev Med Pharmacol Sci. 2017;21:3821–3826. [PubMed] [Google Scholar]
- 33.Hong YK, McMasters KM, Egger ME, Ajkay N. Ductal carcinoma in situ current trends, controversies, and review of literature. Am J Surg. 2018;216:998–1003. doi: 10.1016/j.amjsurg.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 36.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 37.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 39.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augereau P, Patsouris A, Bourbouloux E, Gourmelon C, Lacourtoisie SA, Rigaud DB, Soulié P, Frenel JS, Campone M. Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy. Ther Adv Med Oncol. 2017;9:335–346. doi: 10.1177/1758834017693195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umeh-Garcia M, Simion C, Ho PY, Batra N, Berg AL, Carraway KL, Yu A, Sweeney C. A novel bioengineered miR-127 prodrug suppresses the growth and metastatic potential of triple-negative breast cancer cells. Cancer Res. 2020;80:418–429. doi: 10.1158/0008-5472.CAN-19-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y, Li L, Hou L, Niu B, Ru X, Zhang D. SOX12 promotes the growth of multiple myeloma cells by enhancing Wnt/β-catenin signaling. Exp Cell Res. 2020;388:111814. doi: 10.1016/j.yexcr.2020.111814. [DOI] [PubMed] [Google Scholar]
- 44.Duquet A, Melotti A, Mishra S, Malerba M, Seth C, Conod A, Ruiz i Altaba A. A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT-TCF pathway modulators TMED3 and SOX12. EMBO Mol Med. 2014;6:882–901. doi: 10.15252/emmm.201303799. [DOI] [PMC free article] [PubMed] [Google Scholar]