Abstract
Atherogenesis is a chronic inflammatory process, closely related to high morbidity and mortality. Circular RNAs (circRNAs) were reported to function in atherosclerosis. However, the functional impact of circRNA ubiquitin-specific Protease 36 (circ_USP36) on atherosclerosis and the possible mechanism are still unclear. Serum specimens were collected from atherosclerosis patients and healthy volunteers. Human umbilical vein smooth muscle cells (HUVSMCs) exposed with 25 μg/mL oxidized low-density lipoprotein (ox-LDL) were utilized to simulate atherosclerosis. Expression of circ_USP36, microRNA (miR)-182-5p and Kruppel-like factor 5 (KLF5) was determined via quantitative real-time polymerase chain reaction or western blot assay. Cell viability and apoptosis were evaluated by Cell Counting Kit-8 and flow cytometry. Cell metastasis, including migration and invasion, was assessed via Transwell assay. Biomarker protein was analyzed by western blot. The relationship among circ_USP36, miR-182-5p and KLF5 was confirmed by dual-luciferase reporter and RNA pull-down assays. Circ_USP36 and KLF5 were up-regulated, while miR-182-5p was down-regulated in atherosclerosis patients and ox-LDL-induced HUVSMCs. Circ_USP36 knockdown inhibited proliferation and metastasis of ox-LDL-induced HUVSMCs by up-regulating miR-182-5p. MiR-182-5p targeted KLF5, and ameliorated ox-LDL-mediated injury of HUVSMCs. Circ_USP36 knockdown down-regulated KLF5 expression by sponging miR-182-5p. Knockdown of circ_USP36 alleviated ox-LDL-mediated injury of HUVSMCs by modulating miR-182-5p/KLF5 axis, potentially providing a treatment target for atherosclerosis.
Keywords: Atherosclerosis, ox-LDL, human umbilical vein smooth muscle cells, circ_USP36, miR-182-5p, KLF5
Introduction
Atherosclerosis, one of underlying causes of cardiovascular diseases (CVDs), is a chronic disease existing in arterial wall, bringing about a huge amount of deaths all over the world [1,2]. The promotion of atherosclerosis was benefited by the imbalance between endothelium damage and repair [3]. Oxidized low-density lipoprotein (ox-LDL), a biologic marker of CVDs, could contribute to the inflammatory environment and lipid sedimentation in the arterial wall during atherogenesis [4]. Here, in this study, human umbilical vein smooth muscle cells (HUVSMCs) treated by ox-LDL to mimic atherosclerosis in vitro to explore its pathological mechanism, so as to search potential therapy target.
Circular RNAs (circRNAs), highly expressed in the eukaryotic transcriptome, are a novel category of non-coding RNAs, featured by covalently closed-loop structure [5,6]. Emerging reports highlighted the significant role of circRNAs in CVDs, including atherogenesis [7]. For instance, knockdown of circRNA circCHFR could negatively regulate the proliferation and migration abilities of vascular smooth muscle cells [8]; Circular RNA ciRS-7 facilitated proliferation tube formation and migration of microvascular endothelial cells, acting as an oncogene in atherogenesis [9]; CircANRIL was demonstrated to trigger nucleolar stress and p53 activation, contributing to cell apoptosis, thereby affecting atherosclerosis [10]. Apart from this, circRNA ubiquitin-specific Protease 36 (circ_USP36), also named as circ_0003204, was up-regulated in human umbilical vein endothelial cells treated with ox-LDL, with potential to affect the progression of AS [11]. However, the functional mechanism of circ_USP36 in atherogenesis remains to be investigated thoroughly.
Unlike circRNAs, microRNAs (miRNAs) were a neotype subgroup of short non-coding RNAs, with just about 21 nucleotides, and were closely associated to many human diseases [12]. Li et al. announced that miRNAs could function in important cellular behaviors of vascular smooth muscle cells, thus altering the formation of atherosclerosis [13]. MiR-210 took part in the regulation of atherosclerosis progression through promoting endothelial cell apoptosis [14]. MiR-99a-5p could alleviate atherosclerosis via targeting Homeobox A1, showing as its inhibitory impact on proliferation, migration, and invasion of human aortic smooth muscle cells [15]. As for miR-182-5p, a potential target gene of circ_USP36 (predicted by Starbase 3.0), was well-studied oncogenic miRNA in human prostate cancer [16], bladder cancer [17] and breast cancer [18]. Qin et al. proved that miR-182-5p played a crucial role in atherosclerosis by decreasing TLR4 expression [19]. Here, we tried to figure out other probable mechanisms by which miR-182-5p participating in atherosclerosis.
Starbase 3.0 predicted that Kruppel-like factor 5 (KLF5) was a downstream mRNA of miR-182-5p. Former studies substantiated KLF5 could serve as a new therapeutic target for cancer treatment [20]. Besides, KLF5 was a key transcription factor during cardiovascular remodeling and an underlying therapy target of CVDs [21].
In the current study, the upregulated expression of circ_USP36 in atherosclerosis patients and ox-LDL-induced HUVSMCs was detected. We further explored the action mechanism of circ_USP36 in atherosclerosis, hoping to expand basis for the application of circRNAs in atherosclerosis treatment.
Materials and methods
Collection of clinical samples
All experimental procedures were ratified by the Ethics Committee of Henan Provincial Chest Hospital. Blood samples were acquired from 45 patients diagnosed as atherosclerosis through clinical symptoms and coronary angiography without any therapy, as well as 45 healthy volunteers registered at Henan Provincial Chest Hospital. After isolation from blood samples through centrifugation, serum specimens were stored at -80°C at once. Besides, all above participators provided written informed consents.
Cell culture and ox-LDL disposition
Human umbilical vein smooth muscle cells (HUVSMCs; CP-H084) purchased from Procell (Wuhan, China) and 293T cells (CRL-1573) purchased from American Type Culture Collection (Manassas, VA, USA) were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Carlsbad, CA, USA) added with 10% fetal bovine serum (FBS; Invitrogen) and 1% Penicillin/Streptomycin (Invitrogen) in an incubator containing 5% CO2 at 37°C.
HUVSMCs were treated with ox-LDL (Solarbio, Beijing, China) at different concentrations (0 μg/mL, 25 μg/mL, 50 μg/mL or 75 μg/mL) for 0 h, 6 h, 12 h, 24 h or 48 h to explore the optimum concentration and handling time to mimic atherosclerosis in vitro.
Measurement of reactive oxygen species (ROS) concentration, Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)
For analysis of the level of ROS in HUVSMCs exposed with 0 μg/mL or 25 μg/mL ox-LDL, the 2’,7’-dichlorofluorescin diacetate (DCFDA) Cellular ROS Assay Kit (ab113851; Abcam Shanghai, China) was applied. In brief, cell suspension was incubated with DCFDA solution at 37°C, then the fluorescence (Ex/Em = 485/535 nm) was measured using a flow cytometer (FACScan; BD Biosciences, Franklin Lakes, NJ, USA).
Additionally, the concentration (pg/mL) of IL-6 and TNF-α in ox-LDL-induced HUVSMCs was detected using IL-6 Human Enzyme-linked immunosorbent assay (ELISA) Kit (#KHC0061; Invitrogen) and TNF alpha Human ELISA Kit (#KHC3014; Invitrogen) referring to the manufacturer’s instruction, respectively.
Cell transfection
Small interfering RNA (siRNA) against circ_USP36 (si-circ, 5’-GCATGGGGCTGTGTCACCTGC-3’) and its negative control si-NC (5’-UUCUCCGAACGUGUCACGUTT-3’), miR-182-5p mimic (5’-AGUGUGAGUUCUAC-CAUUGCCAAA-3’) and its negative control mimic NC (5’-UUCUCCGAACGUGUCACGUTT-3’), miR-182-5p inhibitor (5’-UCACACUCAAGAUG-GUAACGGUUU-3’) and its negative control inhibitor NC (5’-CAGUACUUUUGUGUAGUACAA-3’), as well as overexpression vector of KLF5 (oe-KLF5, constructed by introducing the full sequence of KLF5 into pcDNA 3.1 vector) and its negative control pcDNA 3.1 vector (Invitrogen) were all supplied by GenePharma Co. Ltd. (Shanghai, China). Then, constructed oligonucleotides (40 nM) or plasmids (2 μg) were introduced into HUVSMCs treated with 25 μg/mL ox-LDL using Lipofectamine 3000 (Invitrogen) referring to the protocols supplied by the manufacturer.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA derived from serum specimens or treated HUVSMCs was extracted with the aid of TRIzol Reagent (Invitrogen) based on the user’s manual. Then 1 μg RNA was subjected for complementary DNA (cDNA) synthesis utilizing M-MLV Reverse Transcriptase (Invitrogen) or miRNA First-Strand cDNA Synthesis Kit (Agilent, Santa Clara, CA, USA). To detect circ_USP36, KLF5 and LOX-1 (ox-LDL receptor) expression, following quantitative PCR assay was performed using SYBR Green Real-time PCR Master Mix (Solarbio), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. For evaluating the relative expression of miR-182-5p, TaqMan miRNA assays (Applied Biosystems Inc., Foster City, CA, USA) was employed, with U6 snRNA serving as the endogenous normalization standard. All quantitative PCR primers were as follows: circ_0003204, 5’-GATCCAGAGGCCATGGAAGAGT-3’ (forward) and 5’-AGCAGGTGACACAGCCCCATGC-3’ (reverse); miR-182-5p, 5’-TTAGGAACCCTCCTCTCTC-3’ (forward) and 5’-ACTTTCGTTCTTGAGGAATG-3’ (reverse); KLF5, 5’-ATCGAGATGTTCGCTCGTGC-3’ (forward) and 5’-TTTAAAGGCAGACACTGAGTCAG-3’ (reverse); LOX-1, 5’-GAAACCCTTGCTCGGAAGCTGA-3’ (forward) and 5’-CAGATCCAGTCTTGCGGACAAG-3’ (reverse); GAPDH, 5’-CCATGGGGAAGGTGAAGGTC-3’ (forward) and 5’-TGATGACCCTTTTGGCTCCC-3’ (reverse); U6, 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse). In addition, the relative expression of genes was assessed using the 2-ΔΔCt approach.
Cell counting Kit-8 (CCK-8) assay
The determination of the cell viability was implemented via CCK-8 assay. In brief, treated HUVSMCs were placed into 96-well plates, and each well contained 5×103 cells. At 24 h, 48 h or 72 h post routine culture, 10 μL CCK-8 solution (Beyotime, Shanghai, China) was instilled into each well. After additional 2 h, the absorbance of each well at 450 nm was recorded using a microplate reader (Bio-Rad, Hercules, CA, USA).
Transwell assay
Transwell assay was executed to examine the migration and invasion abilities of treated HUVSMCs using Transwell chamber (8 µm size; Corning Inc., Corning, NY, USA). For invasion capacity determination, 5×104 HUVSMCs suspended in FBS-free RPMI-1640 medium (about 200 μL) were placed onto the upper chamber pre-coated with the Matrigel (Corning Inc.). Corresponding lower chamber contained 600 μL with 20% FBS. After culture at 37°C for 48 h, HUVSMCs invaded to lower chamber were subjected for fixation using 4% paraformaldehyd, dyeing using 1% crystal violet (Beyotime), and counting in 6 randomly selected fields under the microscope (100×; Olympus, Tokyo, Japan).
For migration ability analyzation, 1×104 HUVSMCs were seeded upper chamber (without Matrigel) containing 200 μL FBS-free medium, the other procedures were the same to those of the invasion assay.
Flow cytometry
Flow cytometry was conducted to analyze the apoptotic rate of treated HUVSMCs. When cell confluence reached 80%, the culture medium was discarded. After washing trypsin digestion, and additional washing, cells were re-suspended into 500 μL binding buffer and treated with 5 μL Annexin V-fluorescein isothiocyanate (FITC) reagent (Beyotime), then treated with 5 μL propidium iodide (PI) for 10 min in dark place. Apoptotic cells were monitored by means of a flow cytometer and analysis of data was carried out utilizing CellQuest software (BD Biosciences). The apoptotic rate represents the proportion of cells at Annexin V+/PI- and Annexin V+/PI+ quadrants.
Western blot assay
Protein samples were extracted from HUVSMCs taking advantage of Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime), then quantified exploiting bicinchoninic acid assay kit (Beyotime). After mixed with loading buffer, 40 µg protein samples were subjected for electrophoresis through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretic transfer onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Subsequently, at 37°C, membranes were blocked with 5% fat-free milk (overnight), incubated with primary antibodies (4 h) and secondary antibody (1 h), followed by visualization of protein bolts using electrochemiluminescent system (PerkinElmer Life Science, Waltham, MA, USA). Finally, Quantity One software (version 4.5; Bio-Rad) was used to analyze the density.
The antibodies used in this assay were purchased from Abcam: anti-N-cadherin (anti-N-cad; ab76011, 1:1,500), anti-E-cadherin (anti-E-cad; ab40772, 1:2,000), anti-BCL2-Associated X (Bax) (anti-Bax; ab32503, 1:1,000), anti-matrix metalloproteinase 9 (anti-MMP9; ab38898, 1:2,000), anti-Proliferating Cell Nuclear Antigen (anti-PCNA; ab92552, 1:2,000), anti-KLF5 (ab24331, 1:1,000), anti-GAPDH (181602, 1:3,000) and goat anti-rabbit IgG/HRP-linked secondary antibody (ab205718, 1:5000).
Target genes prediction and confirmation
The potential target miRNAs of circ_USP36 were predicted using online software Starbase 3.0 (http://starbase.sysu.edu.cn/index.php), and miR-182-5p could bind with circ_USP36. Meanwhile, the downstream genes of miR-182-5p were forecasted by Starbase 3.0. MiR-182-5p also contained binding region with the 3’ untranslated region (3’UTR) of KLF5.
Dual-luciferase reporter assay was carried out to validate above prediction. The wild type luciferase vectors of circ_USP36 and KLF5 3’UTR (circ_USP36 wt: 5’-CUGGGGAGCCCCACGUGUUGCCAAG-3’ and KLF5 3’UTR wt: 5’-AGAAUAAAUAAGCAAAAUGCCAAA-3’), as well as their corresponding mutant (circ_USP36: 5’-CUGGGGAGCCCCACGUGAACGGUUG-3’ and KLF5 3’UTR mut: 5’-AGAAUAAAUAAGCAAAAACGGUUA-3’) were established by RIBOBIO Co. Ltd. (Guangzhou, China). Above luciferase vector and mimic NC or miR-182-5p mimic were co-transfected into 293T cells. Whereafter, the luciferase activity was determined using Dual-Luciferase Reporter detection System (Promega Corp., Madison, WI, USA) strictly referring to the specifications.
RNA pull-down assay was performed to confirm the interaction between miR-182-5p and circ_USP36 or KLF5. In brief, HUVSMCs were lysed in lysis buffer (Ambion, Carsland, CA, USA). Then, cell lysate was incubated with Biotinylated miR-182-5p (Bio-miR-182-5p) or its negative control (Bio-NC), which were synthesized by GenePharma Co. Ltd., followed by the addition of streptavidin magnetic beads (Invitrogen). Finally, bound RNA was purified using TRIzol Reagent, and then the enrichment of circ_USP36 and KLF5 was determined by qRT-PCR assay.
Statistical analysis
All experiments in this project were performed triple times. Data were presented as mean ± standard deviation (SD) after analysis using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Unpaired Student’s t-test was applied for comparison of data between two groups, and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test or Bonferroni post hoc test was exploited to analyze difference among three or more groups. Besides, correlation among circ_USP36, miR-182-5p and KLF5 in serum specimens of 45 atherosclerosis patients was evaluated by Pearson correlation analysis. The statistically significant difference indicates P < 0.05.
Results
Circ_USP36 was up-regulated in serum of atherosclerosis patients and ox-LDL-induced HUVSMCs
We first detected the expression level of circ_USP36 in serum samples of atherosclerosis patients and healthy volunteers. QRT-PCR assay manifested that circ_USP36 level was higher in serum of atherosclerosis patients than that in healthy control (Figure 1A). HUVSMCs were treated with ox-LDL at different concentrations for different time. And we found that the circ_USP36 expression was gradually elevated in a concentration-dependent manner (Figure 1B) and in a time-dependent manner (Figure 1C). What’s more, HUVSMCs were treated with 25 μg/mL ox-LDL to stimulate atherosclerosis in vitro. For HUVSMCs exposed with ox-LDL at this concentration exhibited high levels of ROS production, IL-6, TNF-α and LOX-1 mRNA (Supplementary Figure 1), indicating ox-LDL treatment induced oxidative stress and inflammation. Collectively, circ_USP36 was up-regulated in atherosclerosis patients and ox-LDL-induced HUVSMCs.
Figure 1.
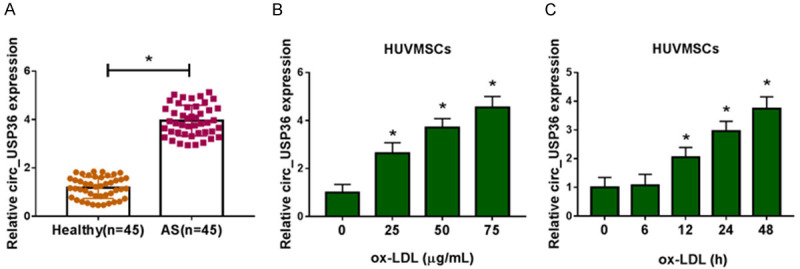
Circ_USP36 was up-regulated in serum of atherosclerosis patients and ox-LDL-induced HUVSMCs. A. QRT-PCR assay for circ_USP36 expression in serum samples of atherosclerosis patients and healthy volunteers (n=45). Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. B. QRT-PCR assay for circ_USP36 expression in HUVSMCs treated with ox-LDL at different concentrations (0 μg/mL, 25 μg/mL, 50 μg/mL or 75 μg/mL) for 48 h. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. C. QRT-PCR assay for circ_USP36 expression in HUVSMCs treated with 25 μg/mL ox-LDL for 0 h, 6 h, 12 h, 24 h or 48 h. Repeated measures of ANOVA were applied for comparison of data at different time points, followed by Bonferroni post hoc test. *P < 0.05.
Circ_USP36 knockdown inhibited proliferation, migration and invasion of ox-LDL-induced HUVSMCs
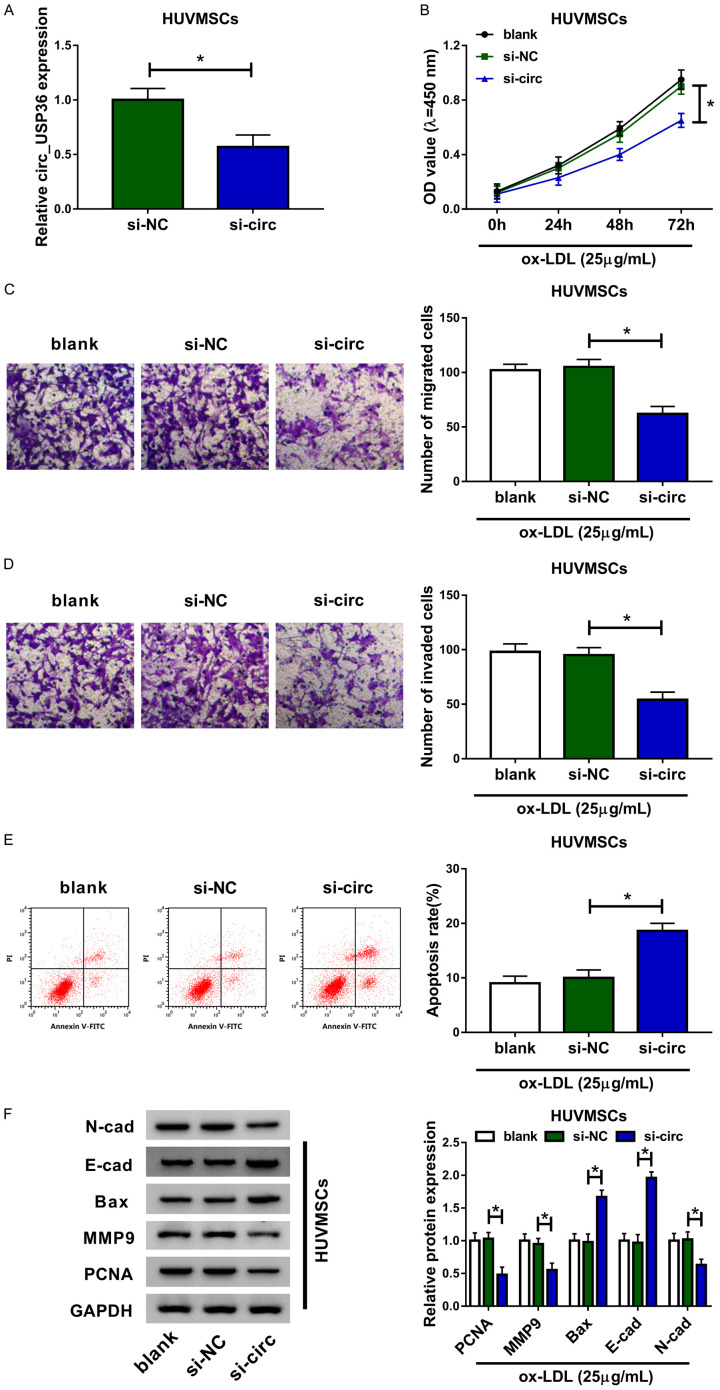
To identify the effects of circ_USP36 on the ox-LDL-induced HUVSMCs, we used si-circ to knockdown its expression, the knockdown efficiency was displayed in Figure 2A. Transfection with si-circ resulted in about 40% reduction of circ_USP36 expression in HUVSMCs, relative to cells transfected with si-NC. Afterwards, functional analysis of circ_USP36 was executed. The CCK-8 assay revealed that, compared to si-NC group, ox-LDL-induced HUVSMCs in si-circ group showed lower cell viability (Figure 2B). Apart from this, silencing of circ_USP36 seemed to promote cell apoptosis of ox-LDL-induced HUVSMCs (Figure 2E). Following Transwell assay demonstrated that circ_USP36 knockdown obviously suppressed migration and invasion abilities of ox-LDL-induced HUVSMCs (Figure 2C, 2D). We then performed western blot assay to examine the impact of circ_USP36 on the levels of N-cad, E-cad and MMP9 (metastasis biomarkers), Bax (apoptosis biomarker) and PCNA (proliferation biomarker). Compared to si-NC, si-circ triggered the down-regulation of N-cad, MMP9 and PCNA proteins, as well as the up-regulation of E-cad and Bax proteins in ox-LDL-induced HUVSMCs (Figure 2F). From above data, we concluded that circ_USP36 knockdown inhibited proliferation, migration and invasion of ox-LDL-induced HUVSMCs.
Figure 2.
Circ_USP36 knockdown inhibited proliferation, migration and invasion of ox-LDL-induced HUVSMCs. HUVSMCs treated with 25 μg/mL ox-LDL were transfected with blank, si-NC or si-circ. A. QRT-PCR assay for circ_USP36 expression in ox-LDL-induced HUVSMCs after transfection. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. B. CCK-8 assay for cell viability of ox-LDL-induced HUVSMCs after transfection. C, D. Transwell assay for cell migration and invasion of ox-LDL-induced HUVSMCs after transfection. E. Flow cytometry for cell apoptosis of ox-LDL-induced HUVSMCs after transfection. F. Western blot assay for levels of N-cad, E-cad, Bax, MMP9 and PCNA proteins in ox-LDL-induced HUVSMCs after transfection. B-F. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. *P < 0.05.
MiR-182-5p was targeted by circ_USP36
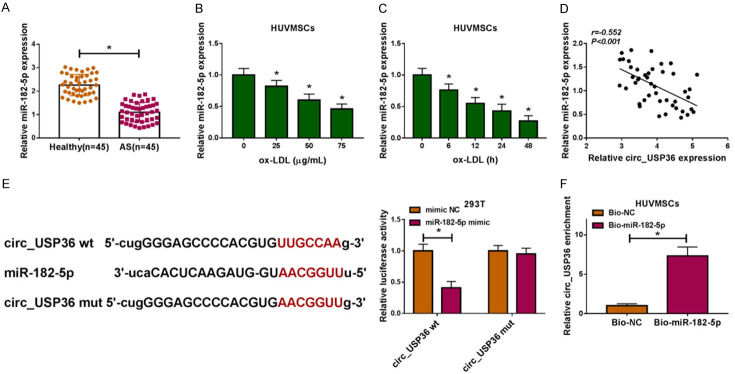
QRT-PCR assay uncovered the down-regulation of miR-182-5p in serum samples of atherosclerosis patients (Figure 3A) and ox-LDL-induced HUVSMCs (Figure 3B, 3C). In addition, miR-182-5p expression in serum of atherosclerosis patients was negatively correlated with circ_USP36 level, demonstrated by Pearson correlation analysis (Figure 3D). Bioinformatics analysis for circ_USP36 utilizing Starbase 3.0 revealed that miR-182-5p had complementary binding sites with circ_USP36. Dual-luciferase reporter assay results indicated that introduction of miR-182-5p reduced the luciferase activity of circ_USP36 wt group rather than circ_USP36 mut group, in contrast to 293T cells co-transfected with mimic NC (Figure 3E). The results of RNA pull-down assay revealed that Bio-miR-182-5p-enriched circ_USP36 content was apparently higher than Bio-NC-enriched one (Figure 3F). Taken together, miR-182-5p was down-regulated in atherosclerosis and ox-LDL-induced HUVSMCs, and was a potential target of circ_USP36.
Figure 3.
MiR-182-5p was targeted by circ_USP36. A. QRT-PCR assay for miR-182-5p expression in serum samples of atherosclerosis patients and healthy volunteers (n=45). Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. B. QRT-PCR assay for miR-182-5p expression in HUVSMCs treated with ox-LDL at different concentrations (0 μg/mL, 25 μg/mL, 50 μg/mL or 75 μg/mL) for 48 h. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. C. QRT-PCR assay for miR-182-5p expression in HUVSMCs treated with 25 μg/mL ox-LDL for 0 h, 6 h, 12 h, 24 h or 48 h. Repeated measures of ANOVA were applied for comparison of data at different time points, followed by Bonferroni post hoc test. D. Pearson correlation analysis for the correlation between the expression levels of circ_USP36 and miR-182-5p in serum samples of atherosclerosis patients (r=-0.552, P < 0.001). E. Dual-luciferase reporter assay for the luciferase activity of 293T cells in circ_USP36 wt and circ_USP36 mut groups. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. F. RNA pull-down and qRT-PCR assays for the enrichment of circ_USP36 in HUVSMCs at Bio-NC and Bio-miR-182-5p groups. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. *P < 0.05.
Circ_USP36 knockdown exerted inhibitory effects on proliferation and metastasis of ox-LDL-induced HUVSMCs by up-regulating miR-182-5p
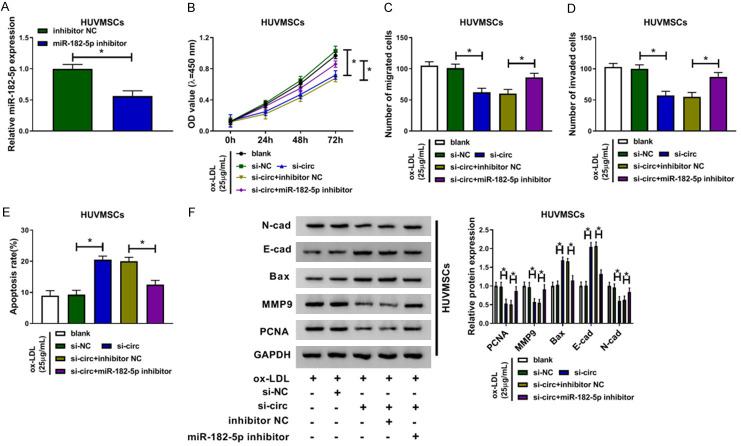
To clarify whether miR-182-5p participating in silencing of circ_USP36-mediated proliferation and metastasis inhibition of ox-LDL-induced HUVSMCs, we constructed cells with miR-182-5p down-regulation, with respect to cells transfected with inhibitor NC (Figure 4A). Following functional assays showed that miR-182-5p deficiency largely weakened circ_USP36 knockdown-mediated reduction of cell viability (Figure 4B), migration (Figure 4C) and invasion (Figure 4D), as well as promotion of cell apoptosis (Figure 4E) of ox-LDL-induced HUVSMCs. Likewise, the down-regulation of N-cad, MMP9 and PCNA proteins, as well as the up-regulation of E-cad and Bax proteins in ox-LDL-induced HUVSMCs with circ_USP36 knockdown were recovered by additional miR-182-5p inhibitor (Figure 4F). Therefore, circ_USP36 knockdown exerted inhibitory effects on proliferation and metastasis of ox-LDL-induced HUVSMCs by positively regulating miR-182-5p expression.
Figure 4.
Circ_USP36 knockdown exerted inhibitory effects on proliferation and metastasis of ox-LDL-induced HUVSMCs by up-regulating miR-182-5p. A. QRT-PCR assay for miR-182-5p expression in 25 μg/mL ox-LDL-induced HUVSMCs transfected with inhibitor NC or miR-182-5p inhibitor. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. B-F. Ox-LDL-induced HUVSMCs were transfected with blank, si-NC, si-circ, si-circ + inhibitor NC or si-circ + miR-182-5p inhibitor. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. B. CCK-8 assay for cell viability of ox-LDL-induced HUVSMCs after transfection. C, D. Transwell assay for cell migration and invasion of ox-LDL-induced HUVSMCs after transfection. E. Flow cytometry for cell apoptosis of ox-LDL-induced HUVSMCs after transfection. F. Western blot assay for levels of N-cad, E-cad, Bax, MMP9 and PCNA proteins in ox-LDL-induced HUVSMCs after transfection. *P < 0.05.
KLF5 was a target of miR-182-5p
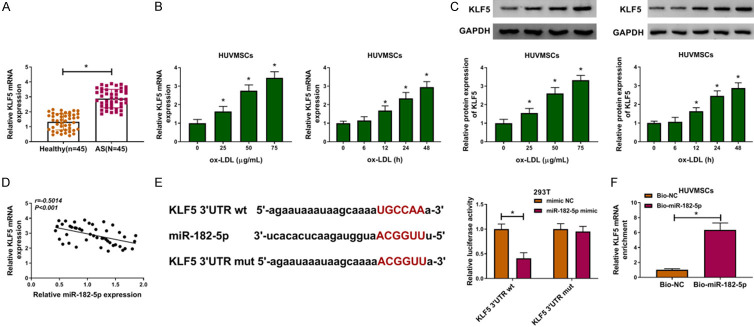
Subsequently, we explored the relative expression of KLF5 in atherosclerosis patients and ox-LDL-induced HUVSMCs. As depicted in Figure 5A, KLF5 mRNA was up-regulated in serum samples of atherosclerosis patients in reference to those of healthy controls. The up-regulation of KLF5 at mRNA and protein levels was also observed in ox-LDL-induced HUVSMCs, which was detected by qRT-PCR and western blot assays (Figure 5B, 5C). The inverse correlation between the expression levels between miR-182-5p and KLF5 mRNA in serum of atherosclerosis patients was demonstrated (Figure 5D). According to the prediction of Starbase 3.0, KLF5 3’UTR had binding region with miR-182-5p, and the target interaction between miR-182-5p and KLF5 was validated by dual-luciferase reporter assay (Figure 5E). RNA pull-down assay further confirmed that miR-182-5p could directly bind with KLF5 (Figure 5F). Collectively, KLF5 was a target of miR-182-5p.
Figure 5.
KLF5 was a target of miR-182-5p. (A) QRT-PCR assay for KLF5 mRNA expression in serum samples of atherosclerosis patients and healthy volunteers (n=45). Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. (B and C) QRT-PCR and western blot assays for KLF5 mRNA (B) and protein (C) expression in HUVSMCs treated with 0 μg/mL, 25 μg/mL, 50 μg/mL or 75 μg/mL ox-LDL for 0 h, 6 h, 12 h, 24 h or 48 h. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. Repeated measures of ANOVA were applied for comparison of data at different time points, followed by Bonferroni post hoc test. (D) Pearson correlation analysis for the correlation between the expression levels of miR-182-5p and KLF5 mRNA in serum samples of atherosclerosis patients (r=-0.5014, P < 0.001). (E) Dual-luciferase reporter assay for the luciferase activity of 293T cells in KLF5 3’UTR wt and KLF5 3’UTR mut groups. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. (F) RNA pull-down assay for the binding ability between miR-182-5p and KLF5 in HUVSMCs. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. *P < 0.05.
MiR-182-5p could repress the proliferation and metastasis of ox-LDL-induced HUVSMCs by targeting KLF5
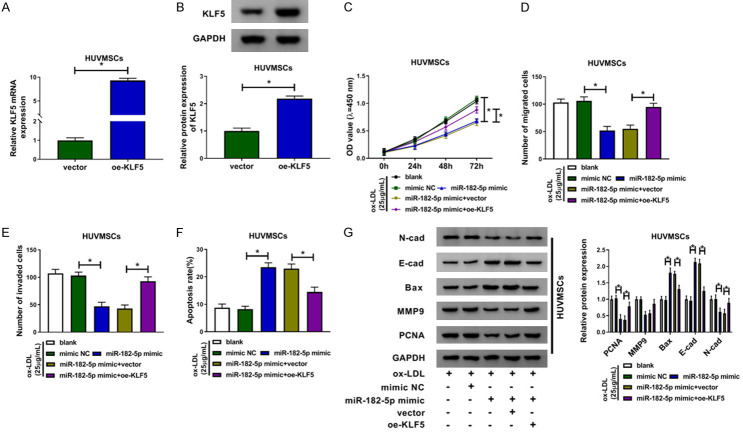
Having known that circ_USP36 could target miR-182-5p to alter the cellular behaviors of ox-LDL-induced HUVSMCs, we then investigated the regulatory effects of miR-182-5p/KLF5 on ox-LDL-induced HUVSMCs. KLF5 mRNA was overexpressed in HUVSMCs by introduction with oe-KLF5, with respect to cells transfected with vector (Figure 6A, 6B). As shown in Figure 6C-F, compared with mimic NC group, miR-182-5p up-regulation significantly restrained cell viability, migration and invasion, but contributed to cell apoptosis of ox-LDL-induced HUVSMCs, which all reversed by additional oe-KLF5. Moreover, gain of miR-182-5p-induced the down-regulated N-cad, MMP9 and PCNA proteins, as well as the up-regulated E-cad and Bax proteins in ox-LDL-treated HUVSMCs were all attenuated via co-transfection with oe-KLF5 (Figure 6G). Above results suggested that KLF5 overexpression mitigated the repressed effects of miR-182-5p on ox-LDL-stimulated HUVSMCs.
Figure 6.
MiR-182-5p could repress the proliferation and metastasis of ox-LDL-induced HUVSMCs by targeting KLF5. (A and B) QRT-PCR and western blot assays for KLF5 mRNA (A) and protein (B) expression in HUVSMCs transfected with vector or oe-KLF5. Unpaired t-test was used to compare data with normal distribution and equal variance between two groups. (C-G) Ox-LDL-induced HUVSMCs were transfected with blank, mimic NC, miR-182-5p mimic, miR-182-5p mimic + vector or miR-182-5p mimic + oe-KLF5. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. (C) CCK-8 assay for cell viability of ox-LDL-induced HUVSMCs after transfection. (D and E) Transwell assay for cell migration and invasion of ox-LDL-induced HUVSMCs after transfection. (F) Flow cytometry for cell apoptosis of ox-LDL-induced HUVSMCs after transfection. (G) Western blot assay for levels of N-cad, E-cad, Bax, MMP9 and PCNA proteins in ox-LDL-induced HUVSMCs after transfection. *P < 0.05.
Circ_USP36 regulated KLF5 expression by antagonizing miR-182-5p
As circ_USP36 targeted miR-182-5p and KLF5 was a target of miR-182-5p, we then analyzed the impact of circ_USP36 on KLF5 expression level. As displayed in Figure 7A, 7B, circ_USP36 knockdown down-regulated the mRNA and protein levels of KLF5, while silencing of miR-182-5p reverted it. Collectively, circ_USP36 positively regulated KLF5 expression by sponging miR-182-5p.
Figure 7.
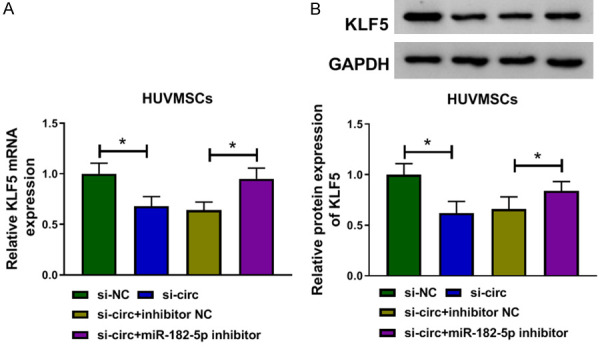
Circ_USP36 regulated KLF5 expression by antagonizing miR-182-5p. QRT-PCR and western blot assays for KLF5 mRNA (A) and protein (B) expression in HUVSMCs transfected with si-NC, si-circ, si-circ + inhibitor NC or si-circ + miR-182-5p inhibitor. One-way ANOVA was applied for comparison of data among multiple groups, followed by Tukey’s post hoc test. *P < 0.05.
Discussion
In this study, we found that circ_USP36 expression was up-regulated in atherosclerosis patients and was induced by ox-LDL in HUVSMCs. Depletion of circ_USP36 or introduction of miR-182-5p blocked proliferation and metastasis of ox-LDL-induced HUVSMCs. Meanwhile, circ_USP36 targeted miR-182-5p to up-regulate KLF5 abundance. Circ_USP36/miR-182-5p/KLF5 axis played a vital part in atherosclerosis development.
The key steps of pathogenesis of atherosclerosis contain vascular smooth muscle cell migration and proliferation, and accumulation of LDL [11,22]. Ox-LDL could contribute to atherosclerosis progression, which was usually used to simulate atherosclerosis in vitro [23]. Extensive works testified the important role of circRNAs in atherosclerosis progression [9,24]. What’s more, the dysregulation of circ_USP36 in human umbilical vein endothelial cells exposed with ox-LDL [11,25] promoted us to investigate the functional effects of circ_USP36 on atherosclerosis evolvement. In this study, HUVSMCs treated with 25 μg/mL ox-LDL were subjected for functional assays. From our experimental data, the up-regulation of circ_USP36 was also detected in atherosclerosis patients and ox-LDL-induced HUVSMCs. In addition, depletion of circ_USP36 triggered the proliferation and metastasis inhibition of ox-LDL-induced HUVSMCs, in keeping with preceding report [26]. As a consequence, we inferred that circ_USP36 acted stimulated role in atherosclerosis.
Mechanistically, many circRNAs could serving as sponges of miRNAs to regulate the biological behaviors of mammalian cells [27]. More than that, circRNA-miRNA-mRNA axis was reported to partake in the pathological evolvement of CVDs, including atherosclerosis [28]. Circ_USP36 was identified as a potential biomarker for cerebral atherosclerosis, and it stimulated ectopic inactivation in endothelial cells by modulating miR-370/TGFβR2/phospho-SMAD3 axis [26]. In the current project, miR-182-5p was identified as a target miRNA of circ_USP36, as demonstrated by online forecast and experiment validation.
MiR-182-5p is a mature form of miR-182, recognized as an oncology-related miRNA closely associated with the initiation and development of many human tumors [29,30]. Besides, miR-182-5p could alter the chemoresistance [31] or chemosensitivity [32] of tumor cells. MiR-182-5p was involved in with unprotected left main coronary artery disease [33] and myocardial infarction [34]. Nevertheless, investigation paper about the function of miR-182-5p in atherosclerosis is few now. Only Qin et al. reported that miR-182-5p had important regulatory effect on ox-LDL induced atherosclerosis [19]. Similarly, we also discovered that miR-182-5p enrichment was declined in atherosclerosis patients, inversely correlated with circ_USP36 level. MiR-182-5p was also down-regulated in ox-LDL-induced HUVSMCs, and its inhibitors largely undermined circ_USP36 knockdown-mediated proliferation and metastasis reduction of ox-LDL-induced HUVSMCs. Thus, we speculated that circ_USP36 sponged miR-182-5p to affect the cellular behaviors of ox-LDL-induced HUVSMCs.
Based on miRNA targeting rules, miRNAs could target mRNAs and induce translational repression to exert their function [35]. MiR-182-5p was reported to function in chondrogenesis and idiopathic pulmonary fibrosis by targeting PTHLH and Smad7, respectively [36,37]. In ox-LDL induced atherosclerosis, miR-182-5p hindered oxidative stress and apoptosis by decreasing TLR4 expression [19]. In this study, KLF5 was predicted and validated to be a downstream mRNA of miR-182-5p.
KLF5 is a crucial member of KLF family, a group of DNA-binding transcriptional regulators, with various functions in multiple cellular processes during cancers and cardiovascular diseases [38]. KLF5 was confirmed to be a target gene of miR-152 and miR-145 to partake in the regulation of malignant progression of atherosclerosis [39,40]. In this study, we detected the up-regulation of KLF5 in atherosclerosis patients and ox-LDL-induced HUVSMCs, in consistent with earlier reports [39-41]. And KLF5 almost reversed the miR-182-5p-mediated inhibitory influence of the proliferation and metastasis of ox-LDL-induced HUVSMCs. In addition, circ_USP36 positively regulated KLF5 expression by antagonizing miR-182-5p. So, circ_USP36 modulated ox-LDL induced atherosclerosis through regulating miR-182-5p/KLF5 axis.
As a consequence, circ_USP36 was up-regulated in atherosclerosis. As the first discovery, knockdown of circ_USP36 sponged miR-182-5p to reduce KLF5 expression, thereby attenuating ox-LDL-induced injury of HUVSMCs, including the inhibitory effects on cell proliferation, migration and invasion, as well as the enhanced impact on cell apoptosis. Therefore, circ_USP36 might be a potential molecular therapeutic target for atherosclerosis.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology. 2008;59:69S–72S. doi: 10.1177/0003319708320761. [DOI] [PubMed] [Google Scholar]
- 4.Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 5.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol. 2019;234:5588–5600. doi: 10.1002/jcp.27384. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Yang F, Zhao H, Wang M, Zhang Y. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–441. doi: 10.1016/j.omtn.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui M, Shen W, Qin W, Wang X, Li Y, Xu F, Xin Z. Circular RNA ciRS-7 promotes tube formation in microvascular endothelial cells through downregulation of miR-26a-5p. J Biochem Mol Toxicol. 2020;34:e22468. doi: 10.1002/jbt.22468. [DOI] [PubMed] [Google Scholar]
- 10.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CY, Ma L, Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother. 2017;95:1514–1519. doi: 10.1016/j.biopha.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Li Y, Tang C. The role of microRNAs in the involvement of vascular smooth muscle cells in the development of atherosclerosis. Cell Biol Int. 2019;43:1102–1112. doi: 10.1002/cbin.11164. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Yang C, Zhang L, Yang P. MicroRNA-210 induces endothelial cell apoptosis by directly targeting PDK1 in the setting of atherosclerosis. Cell Mol Biol Lett. 2017;22:3. doi: 10.1186/s11658-017-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Guan Y, Liu B, Lin Y, Yan Y, Wang H, Wang H, Jing B. MicroRNA-99a-5p alleviates atherosclerosis via regulating Homeobox A1. Life Sci. 2019;232:116664. doi: 10.1016/j.lfs.2019.116664. [DOI] [PubMed] [Google Scholar]
- 16.Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013;8:e55502. doi: 10.1371/journal.pone.0055502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata H, Ueno K, Shahryari V, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder cancer. PLoS One. 2012;7:e51056. doi: 10.1371/journal.pone.0051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao YS, Yang WC, Xin HW, Han JX, Ma SG. MiR-182-5p knockdown targeting PTEN inhibits cell proliferation and invasion of breast cancer cells. Yonsei Med J. 2019;60:148–157. doi: 10.3349/ymj.2019.60.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin SB, Peng DY, Lu JM, Ke ZP. MiR-182-5p inhibited oxidative stress and apoptosis triggered by oxidized low-density lipoprotein via targeting toll-like receptor 4. J Cell Physiol. 2018;233:6630–6637. doi: 10.1002/jcp.26389. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Ding Y, Chen H, Chen H, Zhou J. Targeting kruppel-like factor 5 (KLF5) for cancer therapy. Curr Top Med Chem. 2015;15:699–713. doi: 10.2174/1568026615666150302105052. [DOI] [PubMed] [Google Scholar]
- 21.Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005;3:1569–1576. doi: 10.1111/j.1538-7836.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 22.Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84:1–7. doi: 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Shen L, Hu Y, Lou J, Yin S, Wang W, Wang Y, Xia Y, Wu W. CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Mol Med Rep. 2019;19:3923–3932. doi: 10.3892/mmr.2019.10011. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Ma X, Mao Z, Shen M, Zhu J, Chen F. Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell Signal. 2020;70:109595. doi: 10.1016/j.cellsig.2020.109595. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Song G, Yuan J, Qiao S, Xu S, Si Z, Yang Y, Xu X, Wang A. Circular RNA circ_0003204 inhibits proliferation, migration and tube formation of endothelial cell in atherosclerosis via miR-370-3p/TGFbetaR2/phosph-SMAD3 axis. J Biomed Sci. 2020;27:11. doi: 10.1186/s12929-019-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017;14:992–999. doi: 10.1080/15476286.2016.1220473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Duan L, Li Y, Liu B. Long noncoding RNA/circular noncoding RNA-miRNA-mRNA axes in cardiovascular diseases. Life Sci. 2019;233:116440. doi: 10.1016/j.lfs.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 29.Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang Q, Cheng P, Tang ZH, Huang F. Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene. 2013;527:26–32. doi: 10.1016/j.gene.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Huynh C, Segura MF, Gaziel-Sovran A, Menendez S, Darvishian F, Chiriboga L, Levin B, Meruelo D, Osman I, Zavadil J, Marcusson EG, Hernando E. Efficient in vivo microRNA targeting of liver metastasis. Oncogene. 2011;30:1481–1488. doi: 10.1038/onc.2010.523. [DOI] [PubMed] [Google Scholar]
- 31.Huang XX, Zhang Q, Hu H, Jin Y, Zeng AL, Xia YB, Xu L. A novel circular RNA circFN1 enhances cisplatin resistance in gastric cancer via sponging miR-182-5p. J Cell Biochem. 2020 doi: 10.1002/jcb.29641. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Li X, Yang B, Ren H, Xiao T, Zhang L, Li L, Li M, Wang X, Zhou H, Zhang W. Hsa_circ_0002483 inhibited the progression and enhanced the Taxol sensitivity of non-small cell lung cancer by targeting miR-182-5p. Cell Death Dis. 2019;10:953. doi: 10.1038/s41419-019-2180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Chen T, Ye W, Wang JY, Zhou JP, Li ZY, Li CC. Circulating miR-182-5p and miR-5187-5p as biomarkers for the diagnosis of unprotected left main coronary artery disease. J Thorac Dis. 2019;11:1799–1808. doi: 10.21037/jtd.2019.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Fang J, Ma H. Inhibition of miR-182-5p protects cardiomyocytes from hypoxia-induced apoptosis by targeting CIAPIN1. Biochem Cell Biol. 2018;96:646–654. doi: 10.1139/bcb-2017-0224. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Chang HR, Baek D. Rules for functional microRNA targeting. BMB Rep. 2017;50:554–559. doi: 10.5483/BMBRep.2017.50.11.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai M, Yin H, Zhao J, Li Y, Wu Y. miR-182-5p overexpression inhibits chondrogenesis by down-regulating PTHLH. Cell Biol Int. 2019;43:222–232. doi: 10.1002/cbin.11047. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Zhang Q, Zhou Y, Yang Z, Tan M. Inhibition of miR-182-5p attenuates pulmonary fibrosis via TGF-beta/Smad pathway. Hum Exp Toxicol. 2020;39:683–695. doi: 10.1177/0960327119895549. [DOI] [PubMed] [Google Scholar]
- 38.Tetreault MP, Yang Y, Katz JP. Krüppel-like factors in cancer. Nat Rev Cancer. 2013;13:701–713. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Zhang Y, Wang L, Li J, Li Y, Yang X, Wu Y. mircroRNA-152 prevents the malignant progression of atherosclerosis via down-regulation of KLF5. Biomed Pharmacother. 2019;109:2409–2414. doi: 10.1016/j.biopha.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Zhang YN, Xie BD, Sun L, Chen W, Jiang SL, Liu W, Bian F, Tian H, Li RK. Phenotypic switching of vascular smooth muscle cells in the normal region’ of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med. 2016;20:1049–1061. doi: 10.1111/jcmm.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng B, Zheng CY, Zhang Y, Yin WN, Li YH, Liu C, Zhang XH, Nie CJ, Zhang H, Jiang W, Liu SF, Wen JK. Regulatory crosstalk between KLF5, miR-29a and Fbw7/CDC4 cooperatively promotes atherosclerotic development. Biochim Biophys Acta Mol Basis Dis. 2018;1864:374–386. doi: 10.1016/j.bbadis.2017.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.