Abstract
Objectives: Elderly patients often suffer from cognitive dysfunction following surgery. However, the mechanisms underlying this phenomenon still remain unclear. This study investigated the critical part of Sirtuin-1 (SIRT1)-mediated autophagy and apoptosis in surgery-induced cognitive impairment. Methods: The aged (16-month-old) male C57BL/6 mice underwent anesthesia and surgery. Some mice received intraperitoneal injections of resveratrol, which is an activator of SIRT1, prior to exposure to splenectomy. To examine learning and memory behavior in different sets, the study performed a Morris water maze. Tissues from the hippocampus were harvested 1, 3 and 7 days after surgery. Western blotting and immunofluorescence analysis determined the expression of autophagy- and apoptosis- associated protein. Results: This article demonstrated surgery but not anesthesia considerably affected memory behavior and downregulated SIRT1 expression in the aged mice. Interestingly, rescue of hippocampal SIRT1 expression ameliorated the cognitive impairment in the elderly mice under splenectomy. In addition, surgical trauma decreased Beclin-1 protein levels and the LC3-II/LC3-I ratio, while expression of p62, Bax and cleaved caspase-3 in hippocampal neurons increased. However, rescue of hippocampal SIRT1 expression considerably attenuated the surgery-induced downregulation of Beclin-1, increased the ratio of LC3-II/LC3-I, and decreased expression of p62, Bax, and cleaved caspase-3. Conclusion: These findings suggest that surgery-induced downregulation of hippocampal SIRT1 participates in cognitive impairment after surgery by inhibiting the autophagy process and activating apoptosis.
Keywords: SIRT1, POCD, autophagy, apoptosis, Beclin1, cleaved caspase-3
Introduction
Cognitive dysfunction following surgery is a common postoperative complication among elderly patients (≥ 65 years) [1]. Postoperative cognitive dysfunction (POCD) reflects in concentration, memory, attention, learning, language comprehension, executive function, verbal fluency, and visual spatial performance [2]. POCD increases length of hospitalization, morbidity and mortality, and results in an overall worse quality of life [3,4]. This symptom is typically transient or self-limited; however, occasionally it is long-term or even permanent [5,6]. The incidence of POCD depends on many risk factors, including patient age, level of education, comorbidities, type of procedure, neurological disease, and metabolism, of which age is a predominant risk factor [5,7]. Between 25% and 40% of elderly patients experience confusion and cognitive disturbances at the point of discharge [6,8]. Though many studies have revealed that hippocampal neuroapoptosis [9], central autophagy [10], neuroinflammation [11] and reactive oxygen species [12] may contribute to the pathogenesis of POCD, the pathophysiological mechanisms underlying POCD are still poorly understood.
Autophagy and apoptosis are two essential biologic procedures for nerve cells to maintain their homeostasis and proper function [13]. Autophagy is a conservative process consisting of a series of events, which eliminates misfolded proteins and damaged organelles, including initiation, elongation, maturation, and, finally, degradation [14]. Previous studies reported that autophagosome formation and autophagic degradation were imbalanced in age-related neuro degeneration [15,16]. Ning et al. revealed that autophagy referred to propofol-related cognitive dysfunction in aged rats [10]. However, it is unclear whether autophagy is associated with POCD. In addition to autophagy disturbances, the imbalances of apoptosis are associated with cognitive disorder. Apoptosis is characterized as cells rounded, membrane bubble, cell structure disappearance, cytoplasmic concentration, deeply stained nuclei, solid reduction, chromosome concentration, membrane-encapsulated apoptotic bodies formation, and eventually engulfed by macrophages [13,17]. Zhang et al. showed that neuroinflammation and neuroapoptosis were responsible for POCD in response to activated brain mast cells [18]. Furthermore, Ge et al. demonstrated that exposure to isoflurane induced neuroapoptosis and resulted in cognitive impairment [19]. However, it is unclear whether apoptosis contributes to POCD, and the mechanism remains unknown.
Sirtuins, could protect against energy metabolism and senescence by deacylation of substrates in mammalian cells [20]. Sirtuin-1 (SIRT1) is the most evolutionally conservative subtype and is also involved in apoptosis, autophagy, development, metabolism [21,22], and circadian rhythms [23]. Recently, the neuroprotective effects of SIRT1 have attracted great interest. A previous study suggested that SIRT1 could modulate cognition, learning and memory associated with many molecular events, deacetylation, mitochondrial dysfunction, and inflammation included [24]. Michán et al. showed that SIRT1 knockout mice exhibit significant deficits in cognitive function, characterized by immediate memory, classical conditioning, and spatial learning [25]. A large number of studies demonstrated that SIRT1 has particular importance in the modulation of cell apoptosis and autophagy [26,27]. However, it is not yet known whether and how the SIRT1-mediated autophagy and apoptosis participate in POCD. To this end, we carried out the surgery in elderly mice to determine the role of SIRT1 in surgery-induced cognitive dysfunction and define the mechanisms underlying this action.
Experimental procedures
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University (protocol number: SYXK2015-0009), which operates according to the recommendations for the care and use of experimental mice. C57BL/6 male mice (aged 16 months) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. and raised in a standard and nutritional condition (22 ± 1°C and 55-75% humidity, 12:12 h light/dark cycle).
Experimental design and surgery model
Mice were randomly assigned to five groups (n = 30/group): Group C (mice not exposed to anesthesia or surgery or any drugs), group A (mice exposed to 1% pentobarbital alone), group S (mice exposed to splenectomy), group SD (mice were pretreated with DMSO before surgery), and group SR (mice were pretreated with resveratrol before surgery). Previous studies showed that resveratrol can cross the blood-brain barrier [28,29]. Mice in group SR were intraperitoneally (i.p.) injected daily for 5 days with 10 mg/kg resveratrol (Selleck, USA), and the equal volume of DMSO was given instead to mice in Group SD.
Mice were anesthetized with 1% pentobarbital (7 ml/kg, i.p.) and analgesia with Penicillin lidocaine gel (New Asiatic Pharmaceutical, China). Briefly, after disinfection of the abdomen, a 1.5 cm longitudinal incision was made on the abdominal midline under the xiphoid. The spleen was excised from the left upper abdominal quadrant. Once no bleeding was confirmed, the abdomen was closed by utilizing 4/0 Prolene sutures (Ethicon LLC, USA). Temperature of mice was maintained at 37 ± 0.5°C in the insulation blanket until recovery of consciousness. Mice in group A were exposed to anesthesia alone, and mice in group C served as naive controls.
Open field test
An open field test was conducted to detect the emotional responses and spontaneous locomotor activity. Each mouse was released into chamber, which was divided into 16 squares. Mice were permitted to travel for 10 min. The crossing frequency and total distance were recorded, and the chamber was sprayed with 75% alcohol to avoid smell.
Morris water maze test
The mice were given Morris water maze (MWM) raining for five consecutive days (4 trials per day) before treatment. Resveratrol was injected intraperitoneally 6 h before MWM training day by day. MWM includes a circular pool (diameter, 120 cm; height, 30 cm) filled with opaque water (24-26°C) and divided into four quadrants. A hidden platform (6 cm diameter) was placed in one of the quadrants submerged 1 cm beneath water. The mice were released in the water facing the wall at one of four starting quadrants to locate the hidden platform in 60 s. If a mouse failed to do so, it would be hand directed to it and allowed to remain on it for 15 s. The learning parameters during the training trials were recorded by a video tracking system. At 24 h after the MWM training, splenectomy or anesthesia alone was performed. The probe trials were carried out days 1, 3 and 7 after treatment to determine spatial learning and memory. After removing platform, each mouse was allowed to swim freely for 60 s. The latency for the first time to reach the platform, swimming speed, time percentage of target quadrant, times crossing the platform and the swimming trajectory during the probe trials were recorded. The mice were sacrificed after the MWM test and brain tissue was harvested for further study.
Western blotting
For western blotting analysis, 40 μg of protein from the hippocampus was separated through SDS-PAGE gel and transferred onto PVDF membranes (Merck Millipore, Germany). After sealed in 10% skim milk at 37°C, the membranes were immunoblotted overnight using specific antibody (Table 1) at 4°C. After rinses in TBST, membranes were probed with the secondary antibody (Biosharp, China) for 1 h at 20-22°C and detected using ECL reagents (Affinity, USA). β-Actin was regarded as the internal control.
Table 1.
Primary Antibodies for western blot
| Name | Dilution | Isotype | Supplier |
|---|---|---|---|
| β-actin | 1:3000 | Mouse IgG | Cell Signaling Technology |
| SIRT1 | 1:1000 | Mouse IgG | Abcam |
| Beclin1 | 1:3000 | Rabbit IgG | Cell Signaling Technology |
| P62 | 1:5000 | Rabbit IgG | Abcam |
| LC3β | 1:2000 | Rabbit IgG | Abcam |
| Cleaved caspase3 | 1:1000 | Rabbit IgG | Cell Signaling Technology |
| Bax | 1:500 | Mouse IgG | Santa Cruz |
Immunofluorescence
After the MWM test, the mice were anesthetized with pentobarbital and infused with 50 ml of 0.9% cold saline slowly followed by 100 ml of 4% paraformaldehyde (PFA). Brain tissues were then isolated and fixed with 4% PFA at 4°C for 1 d, at which time 4% PFA was replaced by 30% sucrose solution and embedded in optimal cutting temperature compound (Solarbio, China) at -80°C. Coronal sections (8 µm) of the brains were made in a cryostat. Slices were then sealed for 45 min in 5% donkey serum (Solarbio, China) at 20-22°C and then immunoblotted overnight with the specific antibody (Mouse anti-SIRT1: 1:200, Rabbit anti-p62: 1:400, Rabbit anti-cleaved caspase3: 1:400) at 4°C. After three 5 min washes with PBS the next day, sections were probed with the secondary antibody (Biosharp, China) for 1 h at 20-22°C and then supplemented with DAPI (Abcam, UK) for 5 min. Images were acquired with a fluorescence microscope (Leica, Germany).
Quantitative real-time PCR and standard curve
For quantitative real-time PCR, hippocampi were used for purification of total RNA with TRNzol Reagent (ThermoFisher, USA) according to the manufacturer’s instruction, and immediately reverse-transcribed to cDNA utilizing a Reverse Transcription kit (ThermoFisher, USA). All qPCR was performed with triplicate cDNAs on the iCycler real-time PCR detection system (Bio-Rad, USA) with SYBR Green (TOYOBO, Japan). The primers used for qPCR were as follows: β-actin (forward 5’-GGGAATGGGTCAGAAGGACT-3’ and reverse 5’-TTTGATGTCACGCACGATTT-3’) and SIRT1 (forward 5’-TGATTGGCACCGATCCTCG-3’ and reverse 5’-CCACAGCGTCATATCATCCAG-3’).
Statistical analysis
Statistical analysis was done using SPSS and the data was expressed utilizing the GraphPad software. Significances between two groups were performed by utilizing a student’s t test, and multiple comparisons were evaluated with one-way analysis of variance (ANOVA). P < 0.05 was defined as statistically significant.
Results
Surgery but not anesthesia led to cognitive impairment in aged mice
In the open field test, there were no significant variances in the crossing frequency and total distance among group C, group A and group S during the postoperative period (Figure 1A, 1B). These results indicate that anesthesia and surgery did not significantly affect the motor ability.
Figure 1.
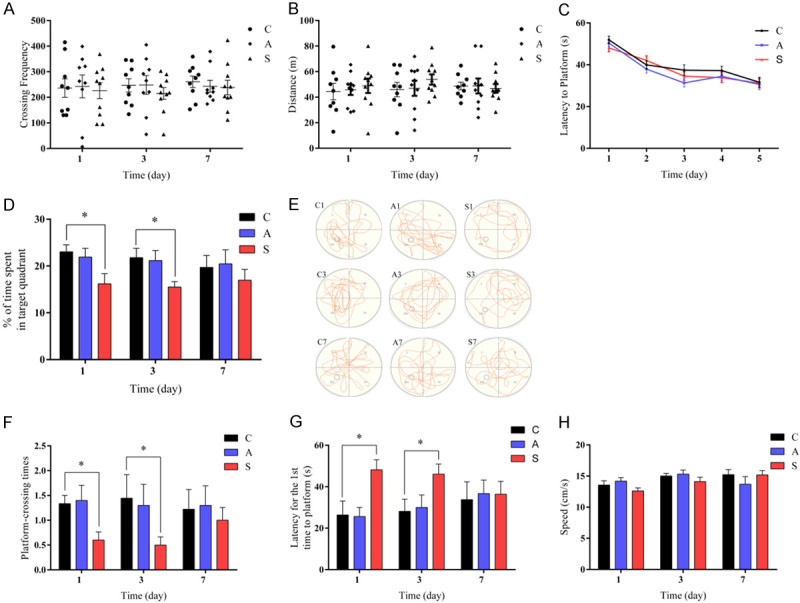
Surgery but not anesthesia induced cognitive dysfunction in aged mice. (A) The crossing frequency and (B) total distance during the open field test. (C) Latency to platform (time to find the hidden platform) in the MWM training trials. (D) The percentage of time spent in the target quadrant. (E) The swimming trajectory of mice. (F) The platform crossing times. (G) Latency for the first time to platform. (H) Swimming speed during the MWM probe trials. Data were presented as mean ± SEM. *P < 0.05.
In the MWM training trials, the mice in three groups showed similar improvement in spatial learning and memory over five consecutive training days before splenectomy (Figure 1C). During the probe trials, group S displayed less time spend in the goal quadrant, lower platform-crossing frequency, and longer first time to the platform on postoperative days 1 and 3 compared with group C. Performance returned to normal on postoperative day 7 (Figure 1D-G). However, there was no major variation in the performance on postoperative days 1, 3 and 7 between group C and group A (Figure 1D-G). These data suggest that surgery caused impairment in spatial learning and memory, while anesthesia alone did not produce any significant effect on the cognitive function. No difference in swimming speed was observed among the three groups (Figure 1H). This indicates that the poorer performance in group S was not due to their decreased locomotor ability or postoperative pain.
Surgery but not anesthesia downregulated SIRT1 expression in the hippocampus
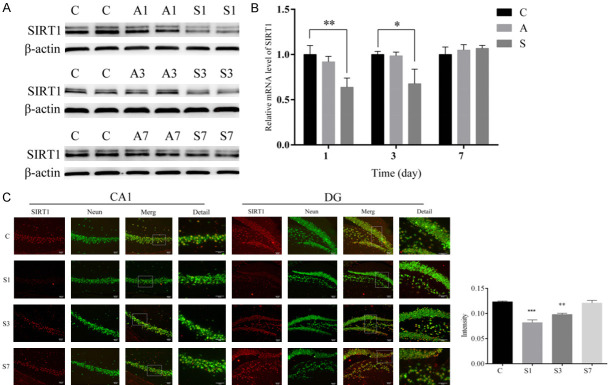
A previous study demonstrated that SIRT1 was expressed mainly in neurons and that its levels in the brain, especially in the hippocampus, were significantly higher than those found in other mammalian tissues [30]. In order to assess the effects of anesthesia and surgery on the expression of SIRT1 in mice, we measured the expression level of SIRT1. Compared with group C, expression of SIRT1 mRNA and protein levels were downregulated in group S on postoperative days 1 and 3 (Figure 2A, 2B). The protein and mRNA levels of hippocampal SIRT1 were unchanged under the normal condition after anesthesia alone (Figure 2A, 2B). There were no differences in the protein and mRNA levels of hippocampal SIRT1 on postoperative day 7 among the three groups (Figure 2A, 2B). Furthermore, our immunofluorescence staining indicated that the mice in the surgery group displayed a smaller number of SIRT1-labeled neurons in the hippocampus compared to the control group, mainly in the CA1 and DG regions, on postoperative days 1 and 3 (Figure 2C). As expected, there were no differences in the number of SIRT1-labled neurons in the surgery group on postoperative day 7 among the three groups (Figure 2C). These results indicate that SIRT1 expression levels in the hippocampus of elderly mice were decreased after surgery but not after anesthesia.
Figure 2.
Surgery but not anesthesia downregulated SIRT1 expression in the hippocampus. (A) Image of western blot protein bands for SIRT1 and (B) relative expression of SIRT1 mRNA in the hippocampus in each group, with β-actin as an endogenous control. (C) Immunofluorescence analysis detected SIRT1 protein levels in the CA1 and DG region of the hippocampus (scale bar = 50 µm). Data were presented as mean ± SEM. *P < 0.05, **P < 0.01.
Rescuing expression of hippocampal SIRT1 improved cognitive dysfunction induced by splenectomy in elderly mice
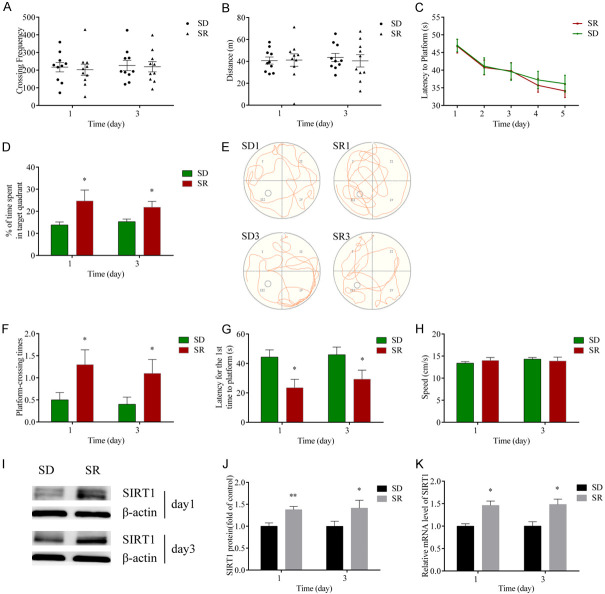
To explore the potential role of SIRT1 on POCD in aged mice, we performed an open field test and Morris water maze test on group SD (surgery plus DMSO) and group SR (surgery plus SIRT1 activator resveratrol [31,32]).
The crossing frequency and total distance in the open field test were comparable between the two groups (Figure 3A, 3B), indicating that resveratrol and surgery did not dramatically alter motor ability in the mice. Mice in group SD and group SR showed similar improvement in learning function (latency to platform) over five consecutive days during the water maze test training (Figure 3C). In the probe trial, both the time in the target area and platform-crossing times in the group SD were less than those in the group SR on postoperative days 1 and 3 (Figure 3D-F). The latency for the first time to platform in group SD was longer than that of group SR on days 1 and 3 postoperatively (Figure 3G). There were no statistical differences in the speed after surgery between the two groups (Figure 3H). These results indicate that the memory function in group SD was worse than that of group SR, and, ultimately, the poorer performance did not result from the reduced motor ability.
Figure 3.
Upregulation of SIRT1 improved surgery-induced cognitive dysfunction in aged mice. (A) The crossing frequency and (B) total distance between the two groups during the open field test. (C) The latency to platform (time to find the hidden platform) in the MWM training trials. (D) The percentage of time spent in the target quadrant. (E) The swimming trajectory of mice. (F) The platform crossing times. (G) Latency for the first time to platform and (H) the swimming speed in each group during the MWM probe trials. (I) Image of western blot protein bands. (J) Quantification for SIRT1. (K) Relative expression of SIRT1 mRNA in the hippocampus. Data were presented as mean ± SEM. *P < 0.05, **P < 0.01.
Consistent with the improvement of neurobehavioral function in group SR, the protein and mRNA levels of SIRT1 in group SR were obviously higher than those in the group SD (Figure 3I-K). These data indicate that rescuing expression of hippocampal SIRT1 through resveratrol administration restored the surgery-associated cognitive reduction in elderly mice.
Rescuing expression of hippocampal SIRT1 blocked the inhibition of the autophagy process induced by splenectomy in elderly mice
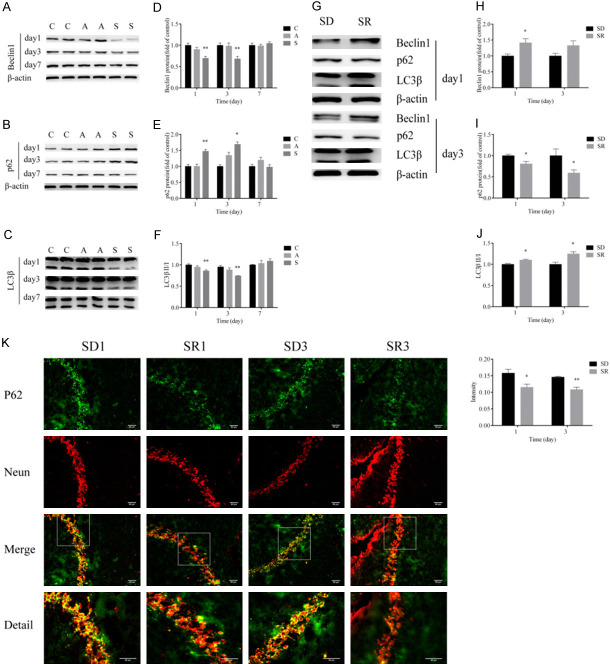
To explore the underlying mechanisms through which rescuing expression of hippocampal SIRT1 improves POCD in aged mice, we first examined expression of autophagy-related protein in the hippocampus. We found that surgical trauma dramatically reduced Beclin-1 expression, decreased the ratio of LC3-II/LC3-I, and upregulated the level of p62 in the hippocampus of aged mice on postoperative days 1 and 3 (Figure 4A-F). These changes were not seen on postoperative day 7, however (Figure 4A-F). Rescuing downregulation of SIRT1 through resveratrol administration considerably attenuated the surgery-induced downregulation of Beclin-1, decreased the ratio of LC3-II/LC3-I, and increased p62 in the hippocampus on postoperative days 1 and 3 (Figure 4G-J). Furthermore, the immunofluorescence analysis demonstrated that the number of p62-postive cells increased markedly in the group SD compared with the SR group on days 1 and 3 postoperatively (Figure 4K). The above results indicate that rescuing expression of hippocampal SIRT1 restored the impaired autophagy after surgery.
Figure 4.
SIRT1 reversed the suppressed autophagy process induced by surgery. A-F. Western blot images and quantification of Beclin-1, p62 and LC3β at postoperative days 1, 3 and 7 in groups C, A and S. G-J. Western blot images and quantification of Beclin-1, p62 and LC3β at postoperative days 1 and 3 in groups SD and SR. K. Immunofluorescence analysis detected p62 protein levels at postoperative days 1 and 3 in groups SD and SR (scale bar = 50 µm). Group C (control), group A (anesthesia alone), group S (surgery), group SD (surgery + DMSO), group SR (surgery + SIRT1 activator resveratrol). Data were presented as mean ± SEM. *P < 0.05, **P < 0.01.
Rescuing expression of hippocampal SIRT1 blocked the promotion of apoptosis induced by splenectomy in aged mice
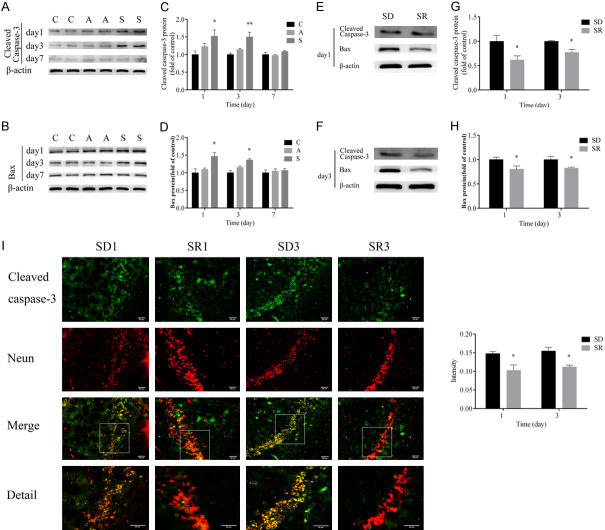
Finally, we examined the expression of Bax and cleaved caspase-3, which were well-recognized indicators of apoptosis. Splenectomy significantly increased the levels of Bax and cleaved caspase-3 on postoperative days 1 and 3, but not on day 7 (Figure 5A-D). These increases were significantly ameliorated by rescuing SIRT1 downregulation through administration of resveratrol on postoperative days 1 and 3 (Figure 5E-H). Moreover, results of the immunofluorescence analysis showed that the number of cleaved caspase-3-postive cells increased considerably in group SD compared with group SR on postoperative days 1 and 3 (Figure 5I). These data demonstrate that rescuing expression of hippocampal SIRT1 blocked the surgery-induced apoptosis.
Figure 5.
SIRT1 reversed the activated apoptosis process induced by surgery. A-D. Western blot images and quantification of cleaved caspase-3 and Bax at postoperative days 1, 3 and 7 in groups C, A and S. E-H. Western blot images and quantification of cleaved caspase-3 and Bax at postoperative days 1 and 3 in groups SD and SR. I. Immunofluorescence analysis detected cleaved caspase-3 protein levels at postoperative days 1 and 3 in groups SD and SR (scale bar = 50 µm). Group C (control), group A (anesthesia alone), group S (surgery), group SD (surgery + DMSO), group SR (surgery + SIRT1 activator resveratrol). Data were presented as mean ± SEM. *P < 0.05, **P < 0.01.
Discussion
In the present study, we have presented evidence that SIRT1 plays a protective role in POCD. A large number of studies claim that the hippocampus is the primary cognitive region and that hippocampal formation is responsible for learning and memory function [33]. This study demonstrated that splenectomy, but not anesthesia alone, downregulated expression of hippocampal SITR1 and led to cognitive deficits in aged mice. This downregulation corresponded to autophagy inhibition and apoptosis promotion in the hippocampus of aged mice. Rescuing downregulation restored the autophagy, reduced apoptosis in the hippocampus, and, overall, improved cognitive function. These findings indicate that hippocampal SIRT1 downregulation was a key contributor to the occurrence of POCD. Furthermore, our results may help decide the potential treatment of SIRT1 in surgery-induced neurobehavioral deficits.
A growing body of studies indicates that SIRT1 plays pivotal roles in cognitive function. Previous research indicates that overexpression of SIRT1 in the hippocampus of mice enhances cognitive function through proteostatic and neurotrophic mechanisms, and improves the isoflurane associated memory deterioration following hyperbaric oxygen pretreatment of middle-aged mice [34]. In addition, Jun Gao et al. acclaimed SIRT1 participates in synaptic plasticity and memory formation via a microRNA-mediated mechanism [35]. In our surgical mouse model, surgical trauma led to cognitive deficits, which were accompanied by downregulation of hippocampal SIRT1 expression. Rescuing this downregulation significantly blocked the cognitive deficits. These data indicate that SIRT1 has a protective effect in surgery-induced cognitive deficits.
Autophagy is a phenomenon that exists widely in eukaryotic cells and exhibits a neuroprotective effect [14]. Autophagy-related genes (ATGs) control major steps in the autophagic pathway by performing important roles in autophagy. Recent studies demonstrated that deacetylation of ATGs by SIRT1 was necessary for the induction of autophagosome formation [36-38]. The present study demonstrates that surgical stress inhibits SIRT1 expression and subsequently suppresses the autophagy process significantly through downregulation of LC3-II/LC3-I, decreases in Beclin-1 expression and increases in p62 accumulation in the hippocampus. Moreover, rescuing hippocampal SIRT1 expression not only improved POCD in aged mice, but also enhanced autophagy activity in the hippocampus of the aged mice subjected to surgery. Collectively, our findings and those of previous studies [36-39] indicate that SIRT1 improves POCD by enhancing autophagy in the aged mice.
Regarding to the interaction about autophagy and apoptosis in central nervous system, there are individual views. Morphological evidence showed that they occur in the same cells [40,41]. Our data reveal that rescuing hippocampal SIRT1 expression inhibits apoptosis in surgical mice, ameliorates surgery-induced elevation of Bax and cleaved caspase-3, and attenuates surgery-induced negative regulation of Bcl-2 in the hippocampus of the aged mice. Beclin-1 is the homologue of the mammals yeast protein and is a significant positive regulator of autophagy [14]. It promotes the formation of pre-autophagosomal structures and recruits the ATG5 complex and LC3 from the cytoplasm to enhance the expansion of autophagosomes [42]. Beclin-1 contains 450 amino acids and four specific domains and interacts with substrates to produce various biological effects [42]. Interaction with antiapoptotic factor Bcl-2 could lead to inhibition of apoptosis [43]. Recent research demonstrated that Beclin-1 is regarded as a rising deacetylation substrate of SIRT1 and increases autophagy through this mechanism [36,44]. Furthermore, SIRT1 exerts its anti-apoptosis effect via regulation of p53 deacetylation [45,46]. Acetylation of p53 changes the conformation of DNA-binding domain, which makes it easier to induce apoptosis through activation of Bax transcription [47]. Consistent with these studies, we observed that rescue of hippocampal SIRT1 downregulation restored Beclin-1 expression, increased the ratio of LC3-II/LC3-I and Bcl-2 expression, and decreased Bax, caspase-3 and p62 in the hippocampi of surgically elderly mice. These results suggest that SIRT1 promotes autophagy and inhibits apoptosis in the hippocampus. This supports the standpoint that SIRT1 attenuates neuronal apoptosis by deacetylatingautophagy [37,48].
Overall, the present study indicates that SIRT1 accelerates neurologic function recovery through activation of autophagy and inhibition of apoptosis in the aged mice with POCD. Our results indicate a novel molecular mechanism for neuroprotective function and a clinical application of SIRT1 in POCD therapy. However, our study has some limitations. First, we only used male mice in the present study. Though the fluctuating concentration of estrogen and progesterone interferes with learning and memory in female mice, the effects of SIRT1 on POCD in female animals will be examined in the future. Second, we only focused on the role of SIRT1 in autophagy and apoptosis in POCD. As the NAD-dependent deacetylase, SIRT1 may have other functions, such as anti-inflammatory and antioxidant effects. These potential mechanisms involved in the role of SIRT1 in POCD need to be studied in more detail.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study. This study was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY17H090015) and National Natural Science Foundation of China (No: 81271204). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Steinmetz J, Rasmussen LS. Peri-operative cognitive dysfunction and protection. Anaesthesia. 2016;71(Suppl 1):58–63. doi: 10.1111/anae.13308. [DOI] [PubMed] [Google Scholar]
- 2.Hood R, Budd A, Sorond FA, Hogue CW. Peri-operative neurological complications. Anaesthesia. 2018;73(Suppl 1):67–75. doi: 10.1111/anae.14142. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 4.Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259–272. doi: 10.1016/s1521-6896(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 5.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 6.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 7.Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54:951–956. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 8.Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Qi Z, Tianbao Y, Yanan L, Xi X, Jinhua H, Qiujun W. Pre-treatment with nimodipine and 7.5% hypertonic saline protects aged rats against postoperative cognitive dysfunction via inhibiting hippocampal neuronal apoptosis. Behav Brain Res. 2017;321:1–7. doi: 10.1016/j.bbr.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Yang N, Li L, Li Z, Ni C, Cao Y, Liu T, Tian M, Chui D, Guo X. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci Lett. 2017;649:85–92. doi: 10.1016/j.neulet.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu LL, Luo D, Zhang H, Shi YS, Li YJ, Wu D, Chen J, Ji MH, Yang JJ. Nox-2-mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Front Aging Neurosci. 2016;8:234. doi: 10.3389/fnagi.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Metaxakis A, Ploumi C, Tavernarakis N. Autophagy in age-associated neurodegeneration. Cells. 2018;7:37. doi: 10.3390/cells7050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Nah J, Yuan J, Jung YK. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelyshev IuA, Cherepnev GV, Saĭtkulov KI. Apoptoz v nervnoĭ sisteme [Apoptosis in the nervous system] . Ontogenez. 2001;32:118–129. [PubMed] [Google Scholar]
- 18.Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S, Qian Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav. 2015;151:16–23. doi: 10.1016/j.physbeh.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagao K, Jinnouchi T, Kai S, Yanagita T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J Nutr Biochem. 2017;43:151–155. doi: 10.1016/j.jnutbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee HJ, Yang SJ. Aging-related correlation between serum sirtuin 1 activities and basal metabolic rate in women, but not in men. Clin Nutr Res. 2017;6:18–26. doi: 10.7762/cnr.2017.6.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G. Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J Biol Chem. 2016;291:23318–23329. doi: 10.1074/jbc.M116.737114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Yan Z, Zhou T, Wang G. SIRT1 regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. J Diabetes Res. 2017;2017:7121827. doi: 10.1155/2017/7121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Luo H, Wang H, Cai J, Zhang Y. SIRT1 induces resistance to apoptosis in human granulosa cells by activating the ERK pathway and inhibiting NF-κB signaling with anti-inflammatory functions. Apoptosis. 2017;22:1260–1272. doi: 10.1007/s10495-017-1386-y. [DOI] [PubMed] [Google Scholar]
- 27.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–9. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 29.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS Alzheimer’s Disease Cooperative Study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–91. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, Torres G. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec (Hoboken) 2010;293:1024–32. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 32.Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, Xu RM. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015;29:1316–25. doi: 10.1101/gad.265462.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu HQ, Shu MQ, Xue F, Liu SS, Cao HJ, Hou WG, Yan WJ, Peng ZW. Sirt1 mediates improvement of isoflurane-induced memory impairment following hyperbaric oxygen preconditioning in middle-aged mice. Physiol Behav. 2018;195:1–8. doi: 10.1016/j.physbeh.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–9. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Pan Z, Fang Z, Lin W, Wu S, Yang F, Li Y, Fu H, Gao H, Li S. Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J Neuroinflammation. 2018;15:310. doi: 10.1186/s12974-018-1345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun T, Li X, Zhang P, Chen WD, Zhang HL, Li DD, Deng R, Qian XJ, Jiao L, Ji J, Li YT, Wu RY, Yu Y, Feng GK, Zhu XF. Acetylation of Beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat Commun. 2015;6:7215. doi: 10.1038/ncomms8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, Liu W. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456–66. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Corpas R, Revilla S, Ursulet S, Castro-Freire M, Kaliman P, Petegnief V, Giménez-Llort L, Sarkis C, Pallàs M, Sanfeliu C. SIRT1 overexpression in mouse hippocampus induces cognitive enhancement through proteostatic and neurotrophic mechanisms. Mol Neurobiol. 2017;54:5604–5619. doi: 10.1007/s12035-016-0087-9. [DOI] [PubMed] [Google Scholar]
- 40.Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG. The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol. 2015;39:63–69. doi: 10.1016/j.semcdb.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Loos B, Genade S, Ellis B, Lochner A, Engelbrecht AM. At the core of survival: autophagy delays the onset of both apoptotic and necrotic cell death in a model of ischemic cell injury. Exp Cell Res. 2011;317:1437–53. doi: 10.1016/j.yexcr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Jin S, Tian S, Chen Y, Zhang C, Xie W, Xia X, Cui J, Wang RF. USP19 modulates autophagy and antiviral immune responses by deubiquitinating Beclin-1. EMBO J. 2016;35:866–80. doi: 10.15252/embj.201593596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz KJ, Ademi C, Bertram S, Schmid KW, Baba HA. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J Surg Oncol. 2016;14:189. doi: 10.1186/s12957-016-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu G, Li X, Wei C, Che X, He S, Lu J, Wang S, Pang K, Fan L. The prognostic role of SIRT1-autophagy axis in gastric cancer. Dis Markers. 2016;2016:6869415. doi: 10.1155/2016/6869415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 46.Kim WJ, Rivera MN, Coffman EJ, Haber DA. The WTX tumor suppressor enhances p53 acetylation by CBP/p300. Mol Cell. 2012;45:587–597. doi: 10.1016/j.molcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–51. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]