Abstract
Shikonin, as a traditional Chinese herbal medicine with a role of anti-cancer, anti-inflammatory, anti-bacterial and other effects. However, there are few studies on the effect of shikonin on osteoporosis. Therefore, the purpose of this study aims to investigate the role and mechanism of shikonin on differentiation of BMSCs and BMMs into osteoblasts and osteoclasts formation. In our study, we treated the cells with different concentrations of shikonin, and then illuminated its effect on oteogenesis and osteoclast differentiation by ALP/alizarin red staining, ALP activity, qRT-PCR, immunofluorescence, Western blot, and TRAP staining. The result showed that shikonin may promote BMSCs differentiate into osteoblasts through the Wnt/β-catenin signaling pathway. At the same time, it may also inhibit the formation of osteoclasts mediated by RANK/RANKL/OPG pathway in vitro. Our research explains excellently the mechanism of shikonin alleviating osteoporosis in vitro, which maybe contributing to the exploration of a new way to prevent osteoporosis.
Keywords: Shikonin, mesenchymal stem cells, osteoporosis, Wnt, β-cantenin, RANKL, OPG
Introduction
Osteoporosis (OP) affects millions of patients and is considered to be the most common skeletal disease worldwide. Due to the risk of cardiovascular damage, breast cancer and uterine cancer, estrogen is not recommended as a treatment and preventive therapy for osteoporosis [1-4]. Parathyroid hormone (PTH), which approved by the Food and Drug Administration, promotes bone resorption and induces bone formation. Due to the potential risk of osteosarcoma, the therapy cannot exceed 2 years [1,2,5]. Currently, osteoporosis drugs are mainly aimed at inhibiting bone resorption, but there is a lacking of drugs that promote bone formation [1,3]. Therefore, further exploration of potential bone synthesis drugs with fewer side effects has far-reaching significance for better treatment of OP.
Osteoblasts are mainly differentiated from BMSCs and the lineage is strictly regulated by many osteogenic signals, especially the Wnt/β-catenin signaling pathway [6-8]. Research has shown that bone formation and osteogenic differentiation are closely related to the Wnt signaling pathway and its related factors [6]. The extracellular proteins of the canonical Wnt signaling pathway include wnt 1, wnt 2, wnt 3, wnt 3a, wnt 8, and wnt 8b, which activate the Wnt/β-catenin signaling pathway by binding to cell membrane receptors, respectively. Moreover, Wnt/β-catenin pathway can induce the expression of OPG in osteoblasts by regulating the differentiation of osteoclasts and affecting the function of osteoclasts, and ultimately affects the bone resorption process [6]. The activation of Wnt/β-catenin signaling pathway is critical to bone development [9]. Previous research have illuminated that shikonin can stimulate the differentiation of ME3T3 cells into osteoblasts through the BMP-2/smads signaling pathway [10]. Therefore, we speculated whether shikonin could play a role in osteogenic differentiation through Wnt/β-catenin signaling pathway.
Osteoblasts and BMSCs express RANKL, which binds to osteoclast progenitor cells or RANK on the osteoclast surface and, through activating the transcription factors which regulated osteoclast formation, thereby promoting the formation and differentiation of osteoclasts and inhibiting the apoptosis of osteoclasts [11,12]. OPG secreted by osteoblasts can bind to RANKL and competitively inhibit the binding between RANKL and RANK, thus inhibiting the proliferation and differentiation of osteoclasts and ultimately inhibiting bone absorption.
Shikonin is an effective component of shikonin which extracted from Lithospermum erythrorhizon and belongs to the gentianaceae family [13]. Brockman and Liebigs clarified the structure of the molecule as (5, 8-dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-en-1-yl] naphthalene-1, 4-dione) in 1936 (Figure 1A), with a molecular weight of 288. In recent years, pharmacological studies have shown that shikonin has obvious roles of anti-inflammatory, anti-fungal and anti-tumor [14]. However, the effect of shikonin on OP has not been illuminated exactly. Our experiment aimed to illuminate the effect of shikonin on osteoblasts and osteoclasts, we also elucidated the underlying molecular mechanisms in vitro.
Figure 1.
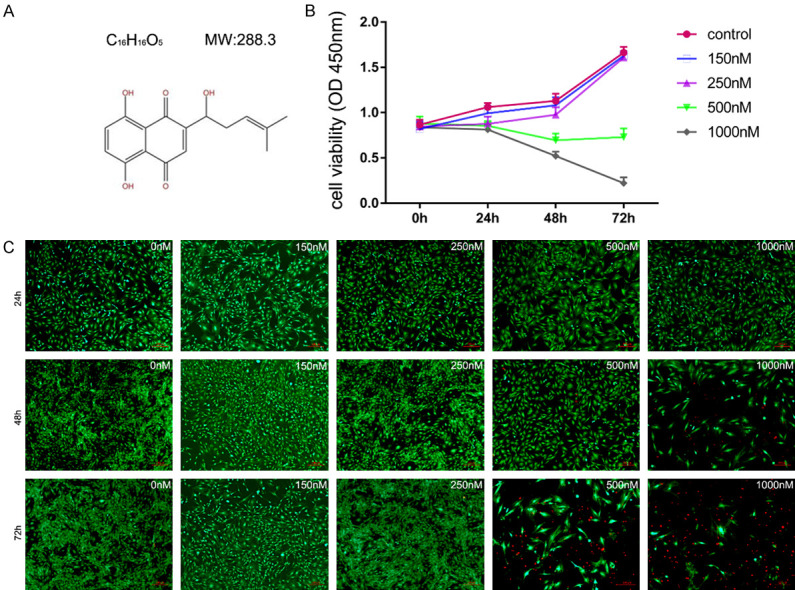
(A) The molecular structure of shikonin. High concentration of shikonin restrained the proliferation of BMSCs. Primary BMSCs were treated with shikonin before measuring cell viability. The CCK-8 assay (B) and live/dead staining (C) showed that the number of dead BMSCs decreased significantly after 48 hours of shikonin treatment at a concentration higher than 250 nM. Cytotoxicity (live/dead) assay images show the live (green) and dead cells (red) in a cellulose sample.
Materials and methods
Media, antibodies, and reagents
Shikonin, Alizarin Red kits, InvitrogenTRIzol reagent and TRAP staining kits were purchased from Sigma-Aldrich Corporation. shikonin was stored at -20°C. α-MEM was obtained from corning company, Wnt/β-catenin inhibitor ICG-001 was purchased from Selleckchem Corporation. FBS, trypsin and viability/cytotoxicity Kit were obtained from Thermo Fisher Scientific company. Cck-8 obtained from Dojindo laboratories, ALP Staining kit, Rhodamine-conjugated phalloidin for F-actin staining, DAPI were purchased from Beyotime Institute of Biotechnology. Anti-wnt 1, β-catenin, anti-ALP, anti-col-1, anti-OCN, anti-Runx2, anti-RANKL, anti-OPG, anti-ALP, anti-β-actin, anti-GSK-3β and anti-p-GSK-3β were purchased from Abcam Corporation. Primer sequences were obtained from the literature and purchased from golden wisdom biotechnology Co. LTD (SuZhou, China).
Cell culture
BMSCs and BMMs were extracted from femur and tibia of SD rats at 4-6 weeks. The primary cells were cultured in α-MEM containing 1% penicillin/streptomycin and 10% FBS at 37°C in 5% CO2 [15]. BMMs were cultured in complete medium with 30 ng/ml M-CSF at 37°C in 5% CO2 until they reached 80% confluence. The medium was replaced every other day.
Cytotoxicity test
The cytotoxic effects of shikonin on BMSCs and BMMs were determined using the CCK-8 assay. Briefly, BMSCs (3.0*103 cells/well) were plated in 96-well plates and cultured with different concentrations of shikonin (150 nM, 250 nM, 500 nM, 1000 nM) for 24 h, 48 h or 72 h, five parallel control (0 nM) well were set in each group. Similarly, BMMs (1.0*104 cells per well) were incubated with different concentration of shikonin (50 nM, 150 nM, 250 nM) for 24 h, 48 h or 72 h. Afterward, 10 μL of CCK-8 buffer was added to each well at 37°C and incubated in dark for 2 hours. The absorbance was measured at 465 nm wavelength on an absorbance microplate reader.
Live death staining
To measure the viability of shikonin-treated BMSCs and BMMs, the Viability/Cytotoxicity Kit was used. BMSCs were planted in 24-well plates, 2.0*104 cells/well and three parallel control wells were set. In short, BMSCs were incubated with different concentrations of shikonin (0 nM, 150 nM, 250 nM, 500 nM, 1000 nM) for 24 h, 48 h or 72 h. Similarly, BMMs were incubated with different concentrations of shikonin (0 nM, 50 nM, 150 nM, 250 nM) for 24 h, 48 h or 72 h. Liquid A and liquid B in the Live/Dead kit were prepared with working liquid according to the concentration indicated in the instructions. Afterward, working liquid was added to each well, and cells were incubated for the additional 30 minutes at 37°C. Images were acquired through a fluorescence microscope.
Osteoblast differentiation
For the determination of osteoblast differentiation in vitro, BMSCs were plated at 1.0×104 cells per well. At 80% confluence, osteogenic medium (0.04 mg/ml dexamethasone, 50 mg/ml ascorbic acid and 10 mM β-glycerol-phosphate) with different concentrations of shikonin or without it were replaced.
ALP and alizarin red staining
In the early stage of osteogenic differentiation, ALP staining kit was used for ALP staining after 7 days. Briefly, cells were fixed with paraformaldehyde (4%) for 5 min and washed by PBS. After that, cells were stained with the ALP staining solution in the dark for 20 min. Stained cells were washed and then examined under an inverted fluorescence microscope, measured with the absorbance of 405 nm. To investigate the effect of shikonin on mineralization, Alizarin Red S staining was performed after 14 days in the late stage of osteogenic differentiation. In a word, the cells were fixed with paraformaldehyde for 20 min, then stained with 0.4% Alizarin Red S solution for 15 min. The absorbance was measured at 570 nm for quantitation.
ALP activity
We used the ALP activity detection kit in order to evaluate the ALP activity of BMSCs, Briefly, the BMSCs cultured in OM with different concentrations of shikonin or without it for 7 days then lysed with RIPA lysis buffer on ice. Then, the samples were centrifuged at 148,000 r/min for 30 min and ALP activity was measured following with instructions.
TRAP staining
BMMs were cultured in 96 well plates at the density of 2.0×104 cells/well in α-medium with 30 ng/ml M-CSF. After 24 h, the BMMs were incubated with 50 ng/mL RANKL and different concentrations of shikonin (50 nM, 100 nM, 150 nM) for direct study. On the other hand, BMSCs were incubated with different concentrations of shikonin (0 nM, 50 nM, 150 nM or 250 nM) for two days then were cultured in complete medium without shikonin. Two days later, 20% supernatant of every well was extracted, and BMMs were incubated with 30 ng/mL M-CSF, 50 ng/mL RANKL, and supernatant (20%) for indirect study. TRAP staining was performed after 5 days. Untreated cells were included as controls. Briefly, cells were fixed in formaldehyde (4%) for 5 min and stained with a tartaric phosphate kit. Trap-positive cells with more than five nuclei were considered osteoclasts. TRAP-positive multinucleated cells in each well were visualized and counted under light microscopy.
Immunofluorescence assay
After incubated with different doses of shikonin for 7 days, BMSCs in every group were fixed with paraformaldehyde (4%) for 15 min at room temperature and then permeabilized with 0.5% Triton X-100 for 20 min. Cells were incubated with BSA (5%) to block the nonspecific antibody binding sites for 20 min and then incubated with primary β-catenin antibodies (1:200) overnight at 4°C. After washing 3 times with PBS, cells were incubated with a 1:500 dilution of an APC-labeled rabbit secondary antibody for 1 h. Next, BMSCs were stained with DAPI and images were acquired through Image Manager software.
Similarly, BMMs were treated as previously described. Next, the cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.3% Triton X-100 for 5 min, followed by incubation with 2% BSA for 1 h in order to block nonspecific binding of antibodies. The cells were stained with phalloidin for 30 min at 25°C, and nuclei were counterstained with DAPI subsequently. Images were acquired through fluorescence microscope.
qRT-PCR assay
We used qRT-PCR to detect gene expression levels during the formation of osteoblast and osteoclast. BMSCs were seeded in 6-well plates at a density of 2.0×104 cells/well and cultured in the OM with different doses of shikonin. Similarly, BMMs were seeded in 6-well plates at a density of 1.0×105 cells/well and cultured in the α-MEM supplemented with 50 ng/ml RANKL and 30 ng/ml M-CSF. Cells were treated with 150 nM shikonin for 48 h. Then the cells were washed with PBS and RNA was extracted using TRIzol reagent. Next, cDNA was synthesized using the Biometra TGradient First-Strand Synthesis System (Whatman Biometra Germany). QRT-PCR was performed using the Real-Time PCR System and SYBR Green SuperMix according to the manufacturer’s instructions. The primers of qRT-PCR are shown in Table 1. All data are displayed as means ± SD of three independent experiments.
Table 1.
The primers used for quantitative PCR
| Gene | Sequence |
|---|---|
| RANKL | forward 5’-CGATGGTGGATGGCTCATG-3’ |
| reverse 5’TGAGCAAAAGGCTGAGCTTCA-3’ | |
| OPG | forward 5’-CGGCACATTGGACATGCTA-3’ |
| reverse 5’-TCCCGGTAAGCTTTCCATCA-3’ | |
| Runx2 | forward 5’-GTTCCCAGGCATTTCATCCC-3’ |
| reverse 5’-AAGGTGGCTGGATAGTGCAT-3 | |
| ALP | forward 5’-CAAGGATGCTGGGAAGTCCG-3’ |
| reverse 5’-CTCTGGGCGCATCTCATTGT-3’ | |
| col-1 | forward 5’-TAA AGGGTCATCGTGGCTTC-3’ |
| reverse 5’-ACTCTCCGCTCT TCCAGTCA-3’ | |
| OCN | forward 5’-CTTGAAGACCGCCTACAAAC-3’ |
| reverse 5’-GCTGCTGTGACATCCATAC-3’ | |
| β-cantenin | forward 5’-CCTGTACGCCAACACAGTGC-3’ |
| reverse 5’-ATACTCCTGCTTGCTGATCC-3’ | |
| Wnt 1 | forward 5’-ACAGCGTTCATCTTCGCAATCACC-3’ |
| reverse 5’-AAATCGATGTTGTCACTGCAGCCC-3’ | |
| CTSK | forward 5’-TTCTGCTGCTACCCATGGTG-3’ |
| reverse 5’-TGCACGTATTGGAAGGCAGT-3’ | |
| TRAP | forward 5’-TCCTGGCTCAAAAAGCAGTT-3’ |
| reverse 5’-ACATAGCCACACCGTTCTC-3’ | |
| c-Fos | forward 5’-CCAGTCAAGAGCATCAGCAA-3’ |
| reverse 5’-AAGTAGTGCAGCCCGGAGTA-3’ | |
| NFATc1 | forward 5’-GGGTCAGTGTGACCGAAGAT-3’ |
| reverse 5’-GGAAGTCAGAAGTGGGTGGA-3’ | |
| GAPDH | forward 5’-ACCCAGAAGACTGTGGATGG-3’ |
| reverse 5’-CACATTGGGGGTAGGAACAC-3’ |
Western blot
To determine the activity of osteoblast/osteoclast formation and signaling pathways affected by shikonin, treated BMSCs and BMMs were lysed and total proteins were extracted using RIPA lysis buffer. The protein samples were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked with Blocking Buffer (Beyotime, China) for 1 h and incubated with the primary antibodies ALP, col-1, OCN, wnt 1, Runx2, GSK-3β, p-GSK-3β, β-catenin, RANKL, OPG, TRAP, c-Fos, NFATc1 and β-actin overnight at 4°C, according to the manufacturer’s instructions. After incubation with the secondary antibodies in blocking buffer at room temperature for 1 h, protein bands were visualized using ChemiDocTM Touch Imaging System (Bio-Rad Laboratories, USA).
Statistical analysis
Experiments were performed in triplicate and repeated more than three times independently. The data were presented as mean ± standard error of the mean and analyzed using GraphPad Prism 5.0. Difference between 2 groups was compared by using Two-way student’s t-test. One-way ANOVA was exploiting to explore the significant differences between multiple groups and P-values < 0.05 were considered statistically significant.
Results
Treatment with a high concentration of shikonin restrained the proliferation of BMSCs
Cck-8 assay was performed to analyze the potential cytotoxicity against BMSCs. As shown in Figure 1B, 250 nM of shikonin did not affect cell viability of BMSCs. Subsequently, Calcein AM (labeled living cells, green) and PI (labeled dead cells, red) staining assays illuminated that the proliferation of BMSCs were not affected by treatment with shikonin at the indicated concentrations (0 nM, 150 nM, 250 nM) (Figure 1C). The dose of 250 nM was defined as the sublethal concentration of BMSCs treated with shikonin.
Shikonin promoted osteogenesis differentiation in BMSCs
To determine the effect of different concentrations of shikonin on the osteogenic differentiation of BMSCs, ALP/Alizarin red staining and ALP activity were evaluated. Figure 2A shows that shikonin dose-dependently increased the number of ALP-positive cells on 7 days, as evidenced by ALP staining and quantitative analysis of the ALP activity (Figure 2D). In addition, we also used alizarin red staining to investigate the effects of shikonin on formation of calcium nodules. More plaque calcified extracellular matrices were found following the treatment with 150 and 250 nM shikonin, compared with those cells in OM and 50 nM shikonin after 14 days (Figure 2B, 2C). Similarly, the level of mRNA expression of ALP, col-I and OCN were increased with the treatment of shikonin in a dose-dependent manner, as determined by qRT-PCR (Figure 2E-G).
Figure 2.
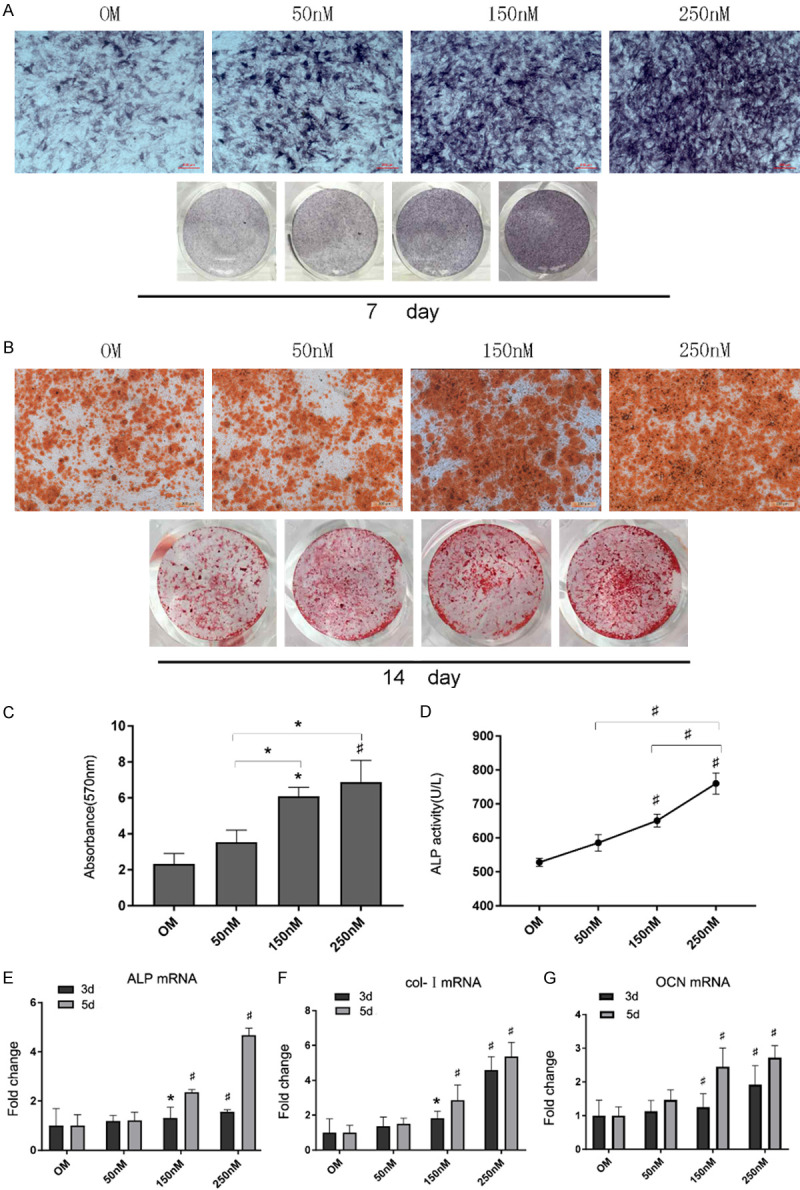
Effect of shikonin on osteogenic differentiation in BMSCs. A. BMSCs were cultured with OM (control) and shikonin. All the assays were performed using ALP staining assay after 7 days. B, C. BMSCs were cultured with OM (control), with or without shikonin for 14 days. All the assays were performed and quantified by Alizarin Red S staining. *P < 0.05 and #P < 0.01 vs the control group (n=3). D. ALP activity was tested on BMSCs cultured with OM and shikonin for 7 days. *P < 0.05 and #P < 0.01 vs the control group (n=3). E-G. BMSCs cultured with OM (control), shikonin (50, 150, 250 nM) were harvested on 3 day and 5 day. The mRNA expression levels of ALP, col-1 and OCN were assessed by qRT-PCR and quantified *P < 0.05 and #P < 0.01 vs the control group.
Shikonin promotes osteoblasts differentiation through Wnt/β-catenin signaling pathway and downregulated the ratio of RANKL/OPG
The Wnt/β-catenin pathway plays an important role in stimulating osteoblastogenesis. To clarify the mechanisms underlying shikonin-induced promotion of osteogenic differentiation, the protein expression levels of wnt 1, β-catenin, GSK-3β, p-GSK-3β, ALP, col-1 and OCN were detected by WB. Here, results demonstrated that shikonin promoted ALP, col-1 and OCN expression during osteoblast formation (Figure 3C, 3F) through promoting the expression of wnt 1, β-catenin and p-GSK-3β protein in a dose and time-dependent manner, however there is no obvious effect on GSK-3β (Figure 3A, 3B). Our finding indicates that shikonin significantly promotes the mRNA expression of Runx2 and β-catenin in a dose-dependent manner (Figure 3D). Further immunofluorescence showed the promotion of β-catenin both in the nuclei and cytoplasm following the treatment with 150 nM and 250 nM shikonin, compared with those in the controls and cells treated with 50 nM (Figure 3E).
Figure 3.
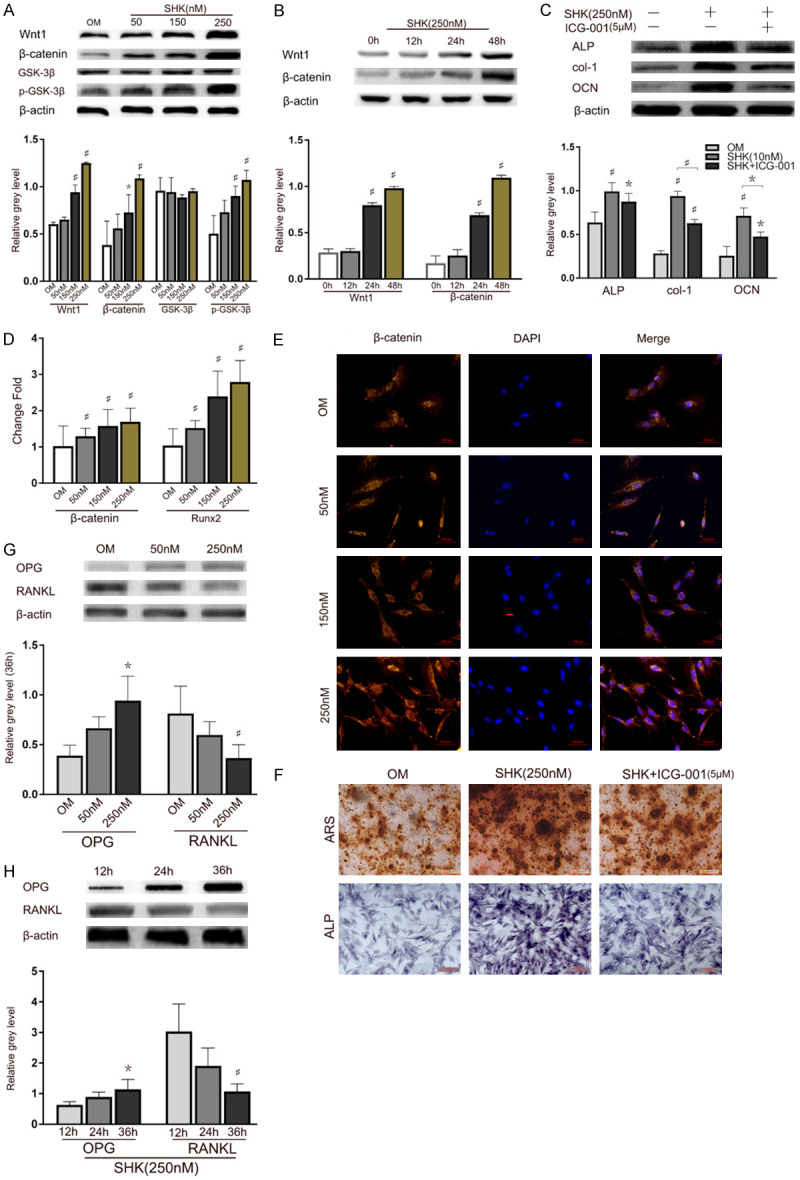
Shikonin stimulated the osteogenesis differentiation in BMSCs through Wnt/β-catenin signalling pathway. A. BMSCs cultured with OM (control), shikonin (50, 150, 250 nM) were harvested on days 3. The protein expression levels of wnt 1, β-catenin, GSK-3β and p-GSK-3β were assessed by WB and quantified *P < 0.05 and #P < 0.01 vs the control group. B. The protein expression levels of wnt 1 and β-catenin were quantified *P < 0.05 and #P < 0.01 vs the control group. C. BMSCs cultured with OM (control), with or without shikonin (250 nM) or ICG-001 were harvested on 48 h, the protein expression of ALP, col-1 and OCN were quantified. *P < 0.05 and #P < 0.01 vs the control group. D. BMSCs cultured with OM (control), with or without shikonin were harvested at 48 h. The mRNA expression levels of β-catenin and Runx2 were assessed by qRT-PCR and quantified. *P < 0.05 and #P < 0.01 vs the control group. E. Immunofluorescence detection of β-catenin translocation in cultured. BMSCs were treated with OM (control) or shikonin (50, 150, 250 nM). β-catenin expressed in both the cytoplasm and nucleus, as well as the fluorescent density and intensity was increased dose dependently for 5 days. The nuclei were stained with DAPI and were shown as blue fluorescence. Scale bar =100 µm. F. BMSCs cultured with OM (control), with or without shikonin or ICG-001 were harvested on 48 h, ALP staining and Alizarin red S staining were assessed. G. BMSCs treated with OM (control), shikonin were harvested on 48 h. The protein expression levels of RANKL and OPG were assessed by WB and quantified *P < 0.05 and #P < 0.01 vs the control group. H. BMSCs treated with shikonin (250 nM) were harvested on 12, 24 or 36 h. The protein expression levels of RANKL and OPG were assessed by WB and quantified *P < 0.05 and #P < 0.01 vs the control group.
The ratio of OPG/RANKL is important for osteoclastogenesis. We found that shikonin could downregulate RANKL expression of BMSCs, while it could upregulate OPG expression. The WB results (Figure 3G, 3H) showed that the protein expression levels of RANKL decreased in both time and dose-dependent manner. By contrast, OPG is expressed in an opposite manner.
Shikonin restrained proliferation and osteoclast differentiation of rankl-induced BMMs in a direct manner
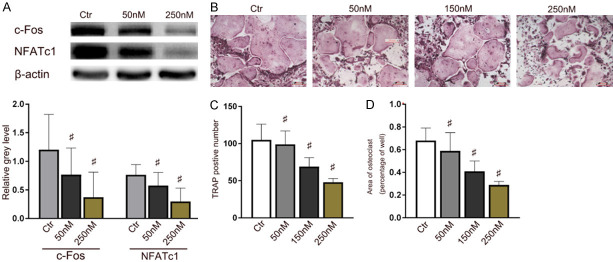
Cell viability assay was performed to analyze the potential cytotoxicity of shikonin against BMMs. The result illuminated that shikonin did not reveal poisonousness toward BMMs at the investigated concentrations (0-150 nM) (Figure 4A). Live/dead staining also demonstrated that BMMs showed obvious apoptosis compared with the control group when the shikonin concentration was higher than 150 nM (Figure 4B). Since shikonin can increase the ratio of OPG/RANKL, BMMs treated without shikonin were used as controls. As shown in Figure 4C-E, the formation of TRAP-positive cells was suppressed following with the shikonin treatment in a dose-dependent manner (38.8 ± 5.39 cells/well following the treatment with 150 nM shikonin) in comparison with the control group (97.5 ± 4.47 cells/well). c-Fos and NFATc1 are vital transcription factors in osteoclast differentiation which can initiate the expression of TRAP. The expression of genes, including TRAP, NFATc1, CTSK and c-Fos were inhibited in a time-dependent manner (Figure 4F, 4G), which indicated that shikonin suppressed osteoclastogenesis in vitro directly.
Figure 4.
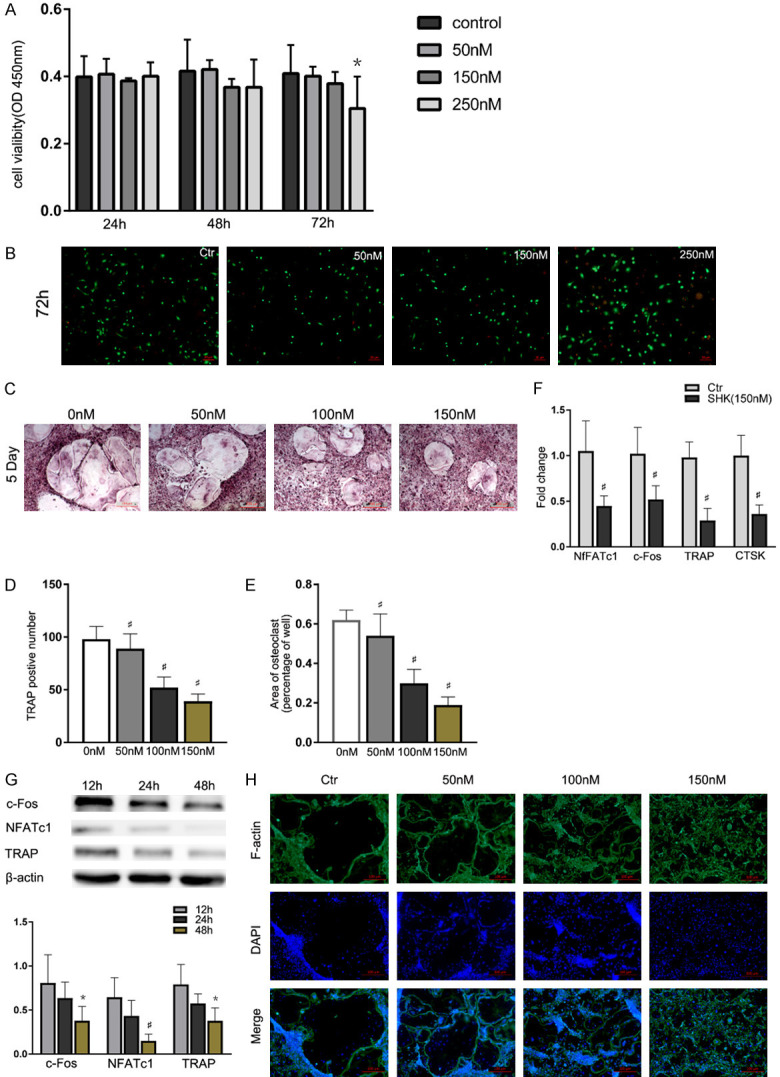
Shikonin restrained the proliferation and differentiation of BMMs directly. A. Effects of shikonin on BMMs viability at 24, 48 or 72 h. B. Live/dead staining obtained for the activity of shikonin against BMMs at 72 h. *P < 0.05 and #P < 0.01 vs the control group. C-E. TRAP-positive BMMs treated with different concentrations of shikonin followed by the stimulation with M-CSF and RANKL for 5 days. Quantification of TRAP-positive multinuclear cells, area of osteoclasts. F. NFATc1, c-Fos, CTSK and TRAP expression in BMMs treated with the indicated shikonin concentrations for 2 days and quantified. *P < 0.05 and #P < 0.01 vs the control group. G. NFATc1, c-Fos and TRAP expression levels in BMMs treated with shikonin for 12 h, 24 h or 48 h. *P < 0.05 and #P < 0.01 vs the control group. H. Immunofluorescence detection of TRAP-positive BMMs in cultured for 5 days.
Shikonin restrained osteoclast differentiation of rankl-induced BMMs in a indirect manner
The supernatant extracted from the medium of BMSCs which treated with shikonin inhibited the specific gene expression of osteoclast (Figure 5A) and the formation of TRAP-positive cells (Figure 5B-D). Thus, we found that shikonin dose-dependently inhibit RANKL-mediated osteoclastogenesis in an indirect manner.
Figure 5.
Shikonin inhibited the differentiation of BMMs indirectly. A. NFATc1 and c-Fos expression in BMMs treated with the indicated concentrations of supernatant for 48 h and quantified. *P < 0.05 and #P < 0.01 vs the control group. B-D. TRAP-positive BMMs treated with indicated concentrations of supernatant followed by the stimulation with M-CSF and RANKL for 5 days. Quantification of TRAP-positive multinuclear cells, area of osteoclasts.
Discussion
Recently, it was reported that the ethanol (EtOH) extracts of Lithospermum erythrorhizon Sieb. et Zucc (LES) affected the activity of OSX and Runx2 in osteoblastogenesis [16]. Additionally, shikonin also stimulated the osteogenic differentiation of MC3T3-E1 cells through the BMP-2/Smad5 signaling pathway [10]. Moreover, studies have shown that the administration of shikonin prevented reductions in the bone mass density and trabecular bone volume in adjuvant-induced arthritic mice [17].
After activation of the canonical Wnt signaling pathway, the process of bone formation is regulated by extracellular antagonists (SOST, DKK1 and SFRP) transmembrane receptors and intracellular components [18]. Studies have found that the Wnt signalling pathway can be restrained in the absence of the transmembrane receptor TCF/LEF-1 or Axin GSK-3β-adenoma polyp protein complex [19]. Intracellular, the β-catenin degradation complex is composed of GSK-3β, APC, Axin and casein kinase 1 (CK1) to promote the degradation of β-catenin in the inactivated state [20]. However, when wnt binds to the receptors of FRZ and lrp5/6, it activates the wnt signal in the cell and results in the accumulation of β-catenin in the cytoplasm. When the amount of β-catenin is accumulated, it can enter the nucleus and promote the expression of target genes by interacting with TCF/LEF. In the Wnt signaling pathway, GSK-3β is an upstream gene and an important member of the Axin complex, acting together with other proteins to participate in the degradation of β-catenin and affecting the expression of the Wnt signaling pathway. ICG-001 selectively antagonizes Wnt/β-catenin/TCF-mediated transcription and specifically binds to CREB-binding protein [21]. After we added ICG-001 to antagonize Wnt/β-catenin/TCF mediated transcription, calcification was reduced and the expression of osteogenic markers decreased. The results showed that shikonin could promote osteogenic differentiation by stimulating the Wnt/β-catenin signaling pathway in a dose and time-dependent manner. Our study also expounded that shikonin had no significant effect on the protein expression levels of GSK-3β, but can enhance the expression levels of p-GSK-3β in BMSCs. Moreover, we also illuminated that shikonin enhanced the mRNA expression level of β-catenin and Runx2, which suggested that shikonin may promote the expression of β-catenin and transfer it into the nucleus.
β-catenin can induce the expression of osteoclast inhibitor OPG on osteoblasts by regulating osteoclast differentiation and affecting osteoclast function, affecting bone resorption ultimately [22]. Wei found that activating β-catenin could inhibit osteoclast differentiation in mice [23]. It has been reported that adipose-derived stem cells could be promoted to differentiate into osteoblasts by regulating Wnt/β-catenin signals [24]. Other studies have confirmed that β-catenin can inhibit the adhesion and aggregation of osteoclasts on the bone surface, but the specific mechanism remains unclear [23].
The OPG/RANK/RANKL system is a set of cytokines that regulate osteoclast differentiation and activation. Bone marrow stromal cells and osteoblasts express RANKL, which binds to osteoclast progenitor cells or RANK on the osteoclast surface and, through activating the transcription factors which regulated osteoclast formation, thereby promoting the formation and differentiation of osteoclasts and inhibiting the apoptosis of osteoclasts. OPG secreted by BMSCs and osteoblast can bind to RANKL and competitively inhibit the binding between RANKL and RANK, thereby inhibiting the proliferation and differentiation of osteoclasts and ultimately inhibiting bone resorption. Our study found that shikonin can inhibit the expression of RANKL and promote the expression of OPG through direct/indirect manners, so as to inhibit the differentiation of BMMs into osteoclasts and the formation of osteoclasts in vitro.
In summary, shikonin (range from 50-150/250 nM) can promote BMSCs differentiation into osteoblasts through the Wnt/β-catenin signaling pathway, and inhibit BMMs differentiation into osteoclasts by down-regulating RANKL and up-regulating OPG expression in vitro. Although therapeutic index seemed to low, shikonin is still expected to be a new bidirectional drug to inhibit osteoporosis, and it’s in vivo experiment will be the further research direction of this experiment.
Acknowledgements
We would like to thank Yingkang Huang and Yu Zhang for communicating data and information.
Disclosure of conflict of interest
None.
Abbreviations
- SHK
shikonin
- BMSCs
bone marrow mesenchymal stem cells
- BMMs
bone marrow macrophages
- CCK-8
cell counting kit-8
- FBS
Fetal bovine serum
- ALP
alkaline phosphatase
- RANKL
receptor activator of nuclear factor-κB ligand
- RANK
receptor activator of nuclear factor-κB
- OPG
osteoprotegerin
- qRT-PCR
real-time fluorescence quantitative-Polymerase Chain Reaction
- PBS
phosphate buffered solution
- DEPC
diethylpyrocarbonate
- M-CSF
macrophage colony-stimulating factor
- OCN
osteocalcin
- FITC
fluorescein isothiocyannate
- DAPI
diamidine-2’-phenylindole dihydrochloride
- IF
Immunofluorescence
- NFATc1
nuclear factor of activated T-cells-cytoplasm 1
- TRAP
tartrate-resistant acid phosphatase
- Runx2
runt-related transcription factor
- col-1
collage I
- wnt
wingless-type MMTV integration site
References
- 1.Kawai M, Modder UI, Khosla S, Rosen CJ. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov. 2011;10:141–156. doi: 10.1038/nrd3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Connor E, Grady D, Stefanick ML. The rise and fall of menopausal hormone therapy. Annu Rev Public Health. 2005;26:115–140. doi: 10.1146/annurev.publhealth.26.021304.144637. [DOI] [PubMed] [Google Scholar]
- 4.Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone. 2013;53:42–50. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang OH, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109:E2197–2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Lin CW, Jiang X, Dai ZQ, Guo XZ, Weng TJ, Wang J, Li YH, Feng GY, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Yu H, Sun X, Tang P, Jie Z, Chen S, Wang J, Qin A, Fan S. A novel diterpenoid suppresses osteoclastogenesis and promotes osteogenesis by inhibiting ifrd1-mediated and ikappabalpha-mediated p65 nuclear translocation. J Bone Miner Res. 2018;33:667–678. doi: 10.1002/jbmr.3334. [DOI] [PubMed] [Google Scholar]
- 10.Fang T, Wu Q, Mu S, Yang L, Liu S, Fu Q. Shikonin stimulates MC3T3-E1 cell proliferation and differentiation via the BMP-2/Smad5 signal transduction pathway. Mol Med Rep. 2016;14:1269–1274. doi: 10.3892/mmr.2016.5363. [DOI] [PubMed] [Google Scholar]
- 11.Keating SE, Maloney GM, Moran EM, Bowie AG. IRAK-2 participates in multiple toll-like receptor signaling pathways to NFkappaB via activation of TRAF6 ubiquitination. J Biol Chem. 2007;282:33435–33443. doi: 10.1074/jbc.M705266200. [DOI] [PubMed] [Google Scholar]
- 12.Yasui T, Kadono Y, Nakamura M, Oshima Y, Matsumoto T, Masuda H, Hirose J, Omata Y, Yasuda H, Imamura T, Nakamura K, Tanaka S. Regulation of RANKL-induced osteoclastogenesis by TGF-beta through molecular interaction between Smad3 and Traf6. J Bone Miner Res. 2011;26:1447–1456. doi: 10.1002/jbmr.357. [DOI] [PubMed] [Google Scholar]
- 13.Andujar I, Rios JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta Medica. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, You J. Immune modulation of shikonin in a rat allergic rhinitis model. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30:1626–1629. doi: 10.13201/j.issn.1001-1781.2016.20.011. [DOI] [PubMed] [Google Scholar]
- 15.Benzhi C, Limei Z, Ning W, Jiaqi L, Songling Z, Fanyu M, Hongyu Z, Yanjie L, Jing A, Baofeng Y. Bone marrow mesenchymal stem cells upregulate transient outward potassium currents in postnatal rat ventricular myocytes. J Mol Cell Cardiol. 2009;47:41–48. doi: 10.1016/j.yjmcc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Choi YH, Kim GS, Choi JH, Jin SW, Kim HG, Han Y, Lee DY, Choi SI, Kim SY, Ahn YS, Lee KY, Jeong HG. Ethanol extract of Lithospermum erythrorhizon Sieb. et Zucc. promotes osteoblastogenesis through the regulation of Runx2 and Osterix. Int J Mol Med. 2016;38:610–618. doi: 10.3892/ijmm.2016.2655. [DOI] [PubMed] [Google Scholar]
- 17.Kim YO, Hong SJ, Yim SV. The efficacy of shikonin on cartilage protection in a mouse model of rheumatoid arthritis. Korean J Physiol Pharmacol. 2010;14:199–204. doi: 10.4196/kjpp.2010.14.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudin E, Fijalkowski I, Piters E, Van Hul W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum. 2013;43:220–240. doi: 10.1016/j.semarthrit.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 20.Kimelman D, Xu W. beta-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 21.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] . Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei W, Zeve D, Suh JM, Wang XQ, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan YH. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol. 2011;31:4706–4719. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandara N, Gurusinghe S, Lim SY, Chen HY, Chen SF, Wang DW, Hilbert B, Wang LX, Strappe P. Molecular control of nitric oxide synthesis through eNOS and caveolin-1 interaction regulates osteogenic differentiation of adipose-derived stem cells by modulation of Wnt/beta-catenin signaling. Stem Cell Res Ther. 2016;7:182. doi: 10.1186/s13287-016-0442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]