Abstract
Concerns about the potential neurotoxicity of general anesthesia to the developing brain have been increasing in recent years. Animal studies have shown that neonatal exposure to general anesthesia causes both acute neurotoxicity and behavioral abnormalities later in life. In the present study, we observed over-activation of neuronal apoptosis in the brain of neonatal mice after a single exposure to anesthesia with sevoflurane for 6 hours at the age of 7 days. More importantly, we found that insulin administered through intranasal delivery prior to anesthesia prevented anesthesia-induced over-activation of neuronal apoptosis. This study provides experimental evidence for a potential effective, yet simple, method to prevent anesthesia-induced neurotoxicity in children, especially in infants.
Keywords: General anesthesia, sevoflurane, apoptosis, developing brain, insulin
Introduction
General anesthesia is essential for many surgical procedures in children, but the impact of anesthesia on the developing brain is poorly understood. Clinical studies have shown that general anesthesia in children younger than 3 years of age increases the risk of developmental disorders and deficits in language/abstract reasoning [1-3]. Anesthesia in neonatal mice has been reported to induce apoptosis, neuroinflammation, and alterations of synaptic proteins in the brain in addition to respiratory and metabolic changes, as well as behavioral deficits later in life [4-13]. However, no strategy is available for preventing the developing brain from anesthesia-induced damage.
We previously reported that anesthesia of adult and aged mice with sevoflurane, a commonly used inhalation anesthetic, induces brain changes and spatial memory deficits and that pre-anesthesia intranasal administration of insulin can prevent such anesthesia-induced abnormalities [14-16]. Intranasal administration can bypass the blood-brain barrier and deliver insulin directly to the brain [17]. Intranasal administration of insulin is currently being investigated in clinical trials for its efficacy to treat Alzheimer’s disease [18-21]. In a recent study, we found that intranasal administration of insulin prior to anesthesia can reduce chronic behavioral abnormalities and neuronal apoptosis induced by repeated exposure to anesthesia in neonatal mice [22]. Here, we report that a single 6-hr exposure of neonatal mice to general anesthesia with sevoflurane, the most commonly used inhalation anesthetic for pediatric anesthesia, also induced marked activation of neuronal apoptosis and, more importantly, that a single dose of insulin administered intranasally prior to anesthesia prevented the over-activation of neuronal apoptosis.
Materials and methods
Materials and reagents
Sevoflurane was purchased from Henry Schein, Inc. (Melville, NY, USA), and insulin (Humulin R U-100) from Eli Lily (Indianapolis, IN, USA). Primary antibodies used in this study are listed in Table 1. Peroxidase-conjugated anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The enhanced chemiluminescence (ECL) kit was from Pierce (Rockford, IL, USA). Other chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Table 1.
Primary antibodies used in this study
| Antibody | Type | Source (Catalog#) |
|---|---|---|
| Anti-synapsin | Polyclonal | Enzo Life Sciences, Inc. (ADI-VAP-SV060) |
| Anti-synaptophysin | Monoclonal | Millipore (MAB5258) |
| Anti-PSD95 | Monoclonal | Cell Signaling Technology (3450S) |
| Anti-Iba1 | Polyclonal | Abcam (ab5076) |
| Anti-GFAP | Monoclonal (Rabbit) | Sternberger (SM122) |
| Anti-cleaved aspase-3 | Monoclonal (Rabbit) | Cell Signaling Technology (#9664) |
| Anti-GAPDH | Polyclonal | Sigma-Aldrich (G9545) |
Animals and animal treatments
The breeding pairs of C57BL/6J mice were initially obtained from Jackson Laboratory (New Harbor, ME, USA). The mice were bred in our air-conditioned animal facility and housed with a 12/12 hr light/dark cycle and with ad libitum access to food and water. The housing, breeding, and animal experiments were approved by the Institutional Animal Care and Use Committee of the New York State Institute for Basic Research in Developmental Disabilities and were in accordance with the PHS Policy on Human Care and Use of Laboratory Animals (revised March 15, 2010).
Induction of anesthesia was carried out by placing neonatal mice at the age of postnatal (P) days 7 in an anesthesia chamber (25 cm × 15 cm × 13 cm) filled with 5% sevoflurane in a mixture of O2 and N2 (50%/50%). The sevoflurane concentration was reduced to 2.5% after the induction period of 3 min and was maintained for 6 hr. The air flow rate was 0.9-1.0 L/min during anesthesia. A small petri dish of water was placed into the anesthesia chamber to maintain moisture. At the end of anesthesia, the sevoflurane was turned off, and the mouse pups were kept in the same chamber with O2 and N2 for one hour to allow their recovery from anesthesia. A warm pad was placed in the anesthesia chamber to maintain the body temperature of the neonatal mice to 35-36°C during the procedure. After they awakened from anesthesia, the mouse pups were returned to their parents’ cages. Neonatal mice of control groups were removed from the parents’ cages and left in the experiment room for the same periods of time as the anesthetized group.
Neonatal mice received a total of 7.0 μl insulin (140 mU/mouse) or saline treatment through intranasal delivery 30 min before the beginning of anesthesia. The manual intranasal administration method was modified from that for adult mice reported previously [23]. Briefly, the P7 mouse pups were held in a supine position in hand, and 1.0 μl insulin or saline was delivered into the left nare by using a 2.5-μl Eppendorf pipette. The pups were given 15-20 sec to allow the fluid to be inhaled before repeating the administration six times.
Neonatal mice (P7, both male and female) from various litters were randomly assigned into four groups: (1) control (Con) group, which received intranasal administration of saline instead of insulin and were not anesthetized; (2) sevoflurane (Sevo) group, which received intranasal saline followed by anesthesia with sevoflurane; (3) sevoflurane plus insulin (Sevo+Ins) group, which received both; and (4) control insulin (Ins) group, which received insulin but not sevoflurane. Mouse pups at the age of P7 with body weight less than 3.0 grams were excluded from the study. A total of 10 and 6 mouse pups were included in each group and time point for Western blot analyses and immunohistochemistry, respectively. To eliminate any potential bias caused by litter variations, a similar number of mouse pups from each litter was assigned to each group, and each group included pups from several litters.
Western blot analysis
The mouse pups were sacrificed by decapitation, and the forebrains were removed and homogenized in pre-chilled buffer containing 50 mM Tris-HCl (pH 7.4), 50 mM GlcNAc, 20 µM UDP, 2.0 mM EGTA, 2.0 mM Na3VO4, 50 mM NaF, 20 mM glycerophosphate, 0.5 mM AEBSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 4 µg/ml pepstatin A. Protein concentrations of the homogenates were determined by using the Pierce 660-nm Protein Assay (Rockford, IL, USA). The homogenate samples were resolved by 10% SDS-PAGE and electro-transferred onto Immobilon-P membrane (Millipore, Bedford, MA, USA). The blots were then probed with primary antibodies and developed with the corresponding horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescent kit.
Immunofluorescence
Mouse brains were immersion-fixed in 4% paraformaldehyde at 4°C for 24 hr, followed by dehydration in 30% sucrose at 4°C for 48 hr. Coronal brain sections (40-µm thick) were cut by using a freezing sliding microtome. The sections were stored in antifreeze solution, consisting of glycerol, ethylene glycol, and phosphate-buffered saline (PBS) at the ratio of 3:3:4, at -20°C till immunofluorescence staining at a later time.
Coronal mouse brain sections at the same plate, as evidenced by the identical hippocampal size and structure in the sections, were chosen for immunofluorescence studies. The brain sections were first washed with PBS three times, 15 min each, followed by incubation in 0.5% Triton X-100 in PBS for 20 min. The sections were then washed with PBS for another 10 min and blocked in PBS containing 5% normal goat serum and 0.1% Triton X-100 for 30 min, followed by incubation overnight at 4°C with antibody against cleaved caspase-3. After washing with PBS again, the sections were incubated with Alexa 488-conjugated goat anti-mouse IgG (1:1000) at room temperature for 2 hr. The sections were washed for a last time, mounted, and cover-slipped by using Prolong ® gold anti-fade mountant (Invitrogen, Carlsbad, CA, USA). The immunostaining was analyzed by using a laser scanning confocal microscope (PCM 200, Nikon). The immuno-positive cells were counted manually from three sections per mouse brain and six brains per group.
Statistical analysis
The quantitative data were analyzed by one-way ANOVA plus post hoc test, if applicable, by using Graphpad. All data are presented as means ± SEM, and P < 0.05 was considered statistically significant.
Results
Anesthesia of neonatal mice with sevoflurane have been shown to induce synaptic abnormalities [7,8,11,22,24], neuroinflammation [6], neuroapoptosis [4,5,22], and behavioral deficits at a later age [4,6-11,22], but inconsistent results were often reported. Thus, we first verified the above changes in the brains of neonatal mice immediately and 6 hr after awakening from 6-hr anesthesia with sevoflurane. The presynaptic proteins synapsin and synaptophysin and the postsynaptic protein PSD95 were used to study the synaptic changes. Neuroinflammation markers included microglial marker Iba1 and astrocyte marker GFAP. Apoptosis was assessed by the level of cleaved/activated caspase-3. Western blots of the brain homogenates indicated a marked increase in cleaved caspase-3 level at 6 hr, but not immediately (0 hour), post anesthesia (Figure 1). These results indicate a marked activation of apoptosis in neonatal mouse brains hours after sevoflurane exposure. Except for a mild increase in Iba1 level immediately post anesthesia, we did not observe any significant changes in the synaptic proteins or neuroinflammation markers post anesthesia (Figure 1). These results indicate that anesthesia in neonatal mice with sevoflurane for 6 hr induces immediate mild neuroinflammation and a marked increase of apoptosis hours later.
Figure 1.
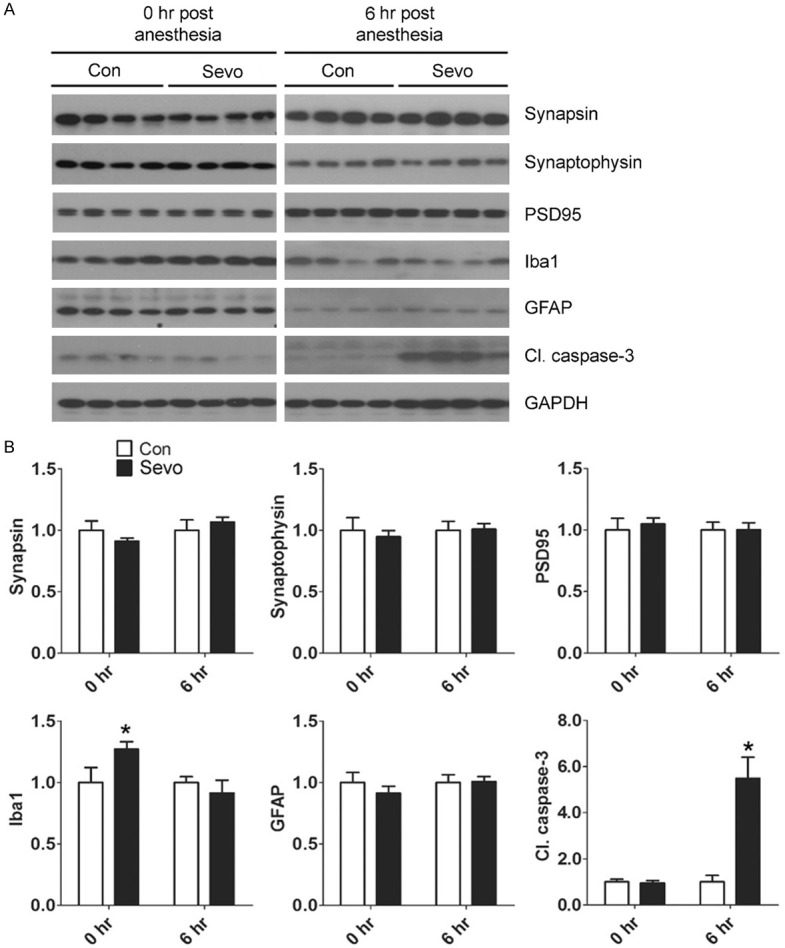
Effect of sevoflurane on synaptic proteins, glial markers, and apoptosis marker in the neonatal mouse brain. P7 mice were sacrificed at the end of anesthesia (0 hr post anesthesia) or at 6 hr post anesthesia with 2.5% sevoflurane (Sevo) for 6 hr. The mouse forebrains were dissected, homogenized, and analyzed by Western blots developed with antibodies indicated at the right side of the blots (A). The blots were then quantified, and the relative levels of each protein (means ± SEM) are shown (B). *, P < 0.05 vs. control as analyzed using one-way ANOVA (n = 10 mice/group).
To investigate whether pretreatment of neonatal mice with insulin can prevent sevoflurane-induced brain changes, we administered insulin intranasally 30 min before anesthesia with sevoflurane and then determined the levels of Iba1 and cleaved caspase-3, both of which were found to be changed after exposure to sevoflurane under the used conditions. We also studied PSD95 in this cohort because a decrease in its level after anesthesia of neonatal mice with sevoflurane was previously reported [7,22]. We found that the insulin treatment did not prevent the transient increase in Iba1 induced by sevoflurane (Figure 2), but it prevented the sevoflurane-induced elevation of cleaved caspase-3. As a control, intranasal insulin did not significantly alter the level of cleaved caspase-3 in the brains of control neonatal mice without anesthesia. Again, the brain PSD95 level was not affected by either anesthesia or insulin treatment in this cohort (Figure 2). These results suggest that administration of insulin before anesthesia can prevent sevoflurane-induced activation of apoptosis in the neonatal brain.
Figure 2.
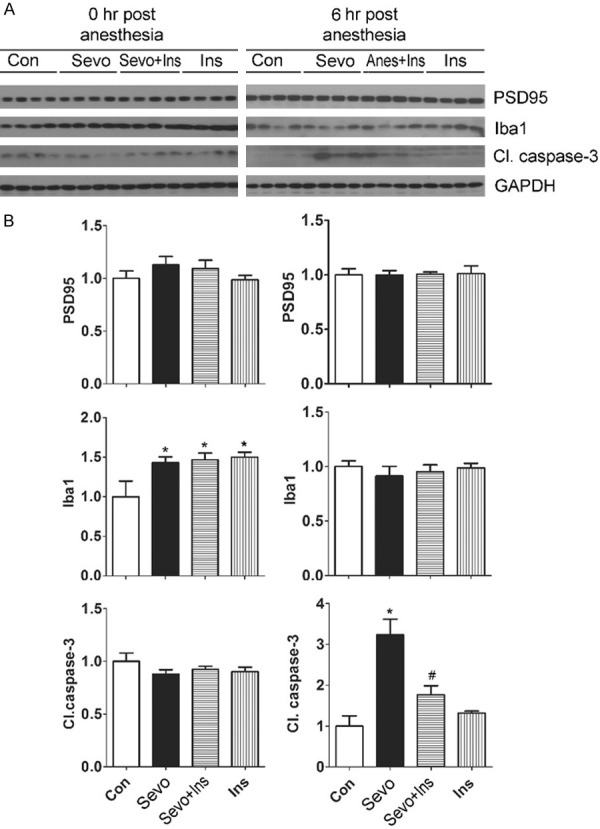
Effect of intranasal insulin on PSD95, Iba1, and apoptosis marker in neonatal mouse brains exposed to sevoflurane. P7 mice received intranasal administration of insulin (Ins) or, as a control, saline, followed by inhalational anesthesia with 2.5% sevoflurane (Sevo) for 6 hr beginning 30 min after intranasal administration. Some mouse pups were sacrificed at the end of anesthesia (0 hr post anesthesia), and the others at 6 hr post anesthesia. The mouse brains were homogenized and analyzed by Western blots developed with antibodies indicated at the right side of the blots (A). The blots were then quantified, and the relative levels of each proteins (means ± SEM) are shown (B). *, P < 0.05 vs. control; #, P < 0.05 vs. Sevo group, as analyzed using one-way ANOVA plus post hoc tests (n = 10 mice/group).
To verify the sevoflurane-induced apoptosis activation and to learn the topographic distribution of the apoptosis activation, we immunostained coronal sections of neonatal mouse brains with antibody against the cleaved/activated caspase-3. We found that, as expected for developing brain, there were immuno-positive apoptotic cells scattered throughout the brains of neonatal mice without exposure to sevoflurane. A marked increase in the number of immuno-positive apoptotic cells was seen in the brains of neonatal mice after exposure to sevoflurane (Figure 3). The apoptotic cells in the neonatal brains after exposure to sevoflurane were mainly distributed in the outer layers of the neocortex, followed by the hippocampus, amygdala, and hypothalamus, as represented by the red dots in Figure 3A. Examination of the immuno-positive cells under high magnification indicated that most, if not all, of the apoptotic cells were neurons based on their morphology and size (Figure 3A, enlarged c’ and h’). Quantification of the immuno-positive neurons in the brain sections indicated > 1-fold and > 20-fold increase of the apoptotic neurons in the neocortex and the hippocampus, respectively, after anesthesia with sevoflurane (Figure 3B, 3C). Importantly, the pre-treatment of neonatal mice with intranasal insulin reduced the sevoflurane-induced increase in apoptotic neurons remarkably both in the neocortex and the hippocampus (Figure 3B, 3C). These results are consistent with the above Western blot data.
Figure 3.
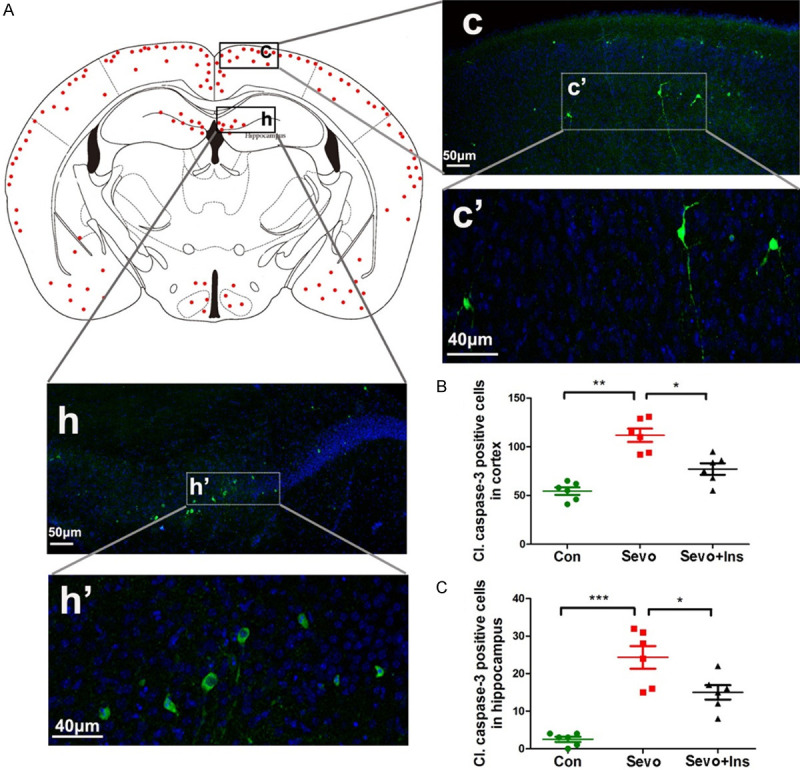
Immunofluorescence of cleaved caspase-3 of neonatal mouse brains after sevoflurane exposure and intranasal insulin treatment. (A) P7 mice received intranasal administration of insulin or, as a control, saline, followed by inhalational anesthesia with 2.5% sevoflurane for 6 hr, beginning 30 min after intranasal administration. The mice were sacrificed at 6 hr post anesthesia, and the brains were fixed and immunostained by using antibody against cleaved caspase-3 (green) and counter-stained with nuclear marker TO-PRO (blue). The red dots in the mouse brain diagram in (A) represent the relative intensities of cleaved caspase-3-positive cells in various regions of the mouse brains after exposure to sevoflurane. (B, C) Neurons positive to cleaved caspase-3 in the cerebral cortex (B) and in the hippocampus (C) were counted separately and are shown as cleaved caspase-3-positive neurons per section (mean ± SEM, n = 6 mice/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, as analyzed using one-way ANOVA plus post hoc tests.
Discussion
Potential damage of general anesthesia to the developing brain has received considerable attention in recent years. Many animal studies have demonstrated neurotoxicity of anesthetics to the neonatal brain [4,6-12,22]. Human studies also have alerted to the potential adverse impact of pediatric anesthesia [1-3]. By studying a single exposure of P7 neonatal mice to anesthesia with sevoflurane for 6 hr, we found here that the most noticeable acute brain change post anesthesia was activation of neuronal apoptosis. These results are consistent with those from our recent studies of neonatal mice post-repeated anesthesia, which also showed mild reduction of brain PSD95 and activation of microglia marker Iba1 [22]. More importantly, we found that intranasal insulin administered prior to anesthesia can prevent sevoflurane-induced over-activation of neuronal apoptosis.
The molecular mechanisms by which sevoflurane induces increased neuronal apoptosis and insulin prevents it are not understood at present. Apoptosis is a natural process for development of the mammalian central nervous system. Many factors can affect neuronal apoptosis. However, increased neuronal apoptosis after sevoflurane exposure appears to be a result of anesthesia or sevoflurane itself rather than other factors, such as stress or fasting, because these factors were also present in the control group. Anesthesia of neonatal mice with sevoflurane or isoflurane can induce severe, reversible hypoglycemia [12,13,25], but it is unlikely to be responsible for the increased neuronal apoptosis, because similar apoptosis was observed when blood glucose was maintained with injection of dextrose [25]. Previous studies suggest that sevoflurane-induced neuronal apoptosis may involve a downregulation of cAMP/CREB and BDNF/TrkB signaling [26] and of neuropeptide Y expression [27]. The present study does not provide mechanistic information about how insulin prevents sevoflurane-induced elevation of neuronal apoptosis. Because insulin delivered into the brain through intranasal administration is expected to promote brain insulin signaling, leading to PI3K/Akt/mTOR activation, and because this signaling is involved in the prevention of sevoflurane-induced apoptotic activation with epigallocatechin-3-gallate in neonatal mouse brains [26], the preventive action of insulin observed in the present study might be through promotion of brain insulin signaling.
Although most biochemical and immunohistochemical studies of the immediate impact of anesthesia on the developing brain found some changes [4-8,11,12,22,24], the results of the majority of these studies are not consistent. The inconsistency appears to result from different species/strains of animals, different ages, anesthetics, anesthesia regimens used, and the time points when the brains were investigated. When we anesthetized P7 neonatal mice with sevoflurane for 2-3 hr and studied the brain levels of synaptic proteins and markers of neuroinflammation and neuroapoptosis by Western blots, we did not find any significant changes after anesthesia (data not shown). Continuous anesthesia with sevoflurane for 6 hr induced marked activation of neuronal apoptosis detectable 6 hr post anesthesia, but this activation was not detectable immediately post anesthesia. The present study, together with previous studies of neonatal rodents, suggests that (1) the anesthesia-induced brain damage, such as over-activation of neuronal apoptosis, is dynamic, which may be detectable only during small time windows, and (2) the adverse impact of anesthesia for a shorter period (e.g., < 3 hr) may be limited, and anesthesia for a longer period increases the risk of neurotoxicity to the developing brain. Nevertheless, the temporary brain damage induced by anesthesia exposure during the neonatal period appears to have a long-term impact on the brain, because behavioral impairments are detectable after the mice reach adulthood [22].
Neuronal apoptosis is critical for normal brain development and maturation. Any disruption of the normal level of apoptosis and its dynamics would interfere with normal brain development, which can lead to the long-term consequences seen in the adult age. For instance, deficient apoptosis in the anterior neural ridge during early development results in brain malformations [28]. Over-activation of apoptosis in the developing brain such as widespread apoptotic death of neurons and oligodendroglia triggered by alcohol during gestation, is associated with brain changes, including overall or regional reductions in brain mass, and long-term neurobehavioral disturbances [29]. Over-activation of neuronal apoptosis induced by general anesthesia in the developing brain might underlie the long-term behavioral and cognitive impairment observed at adulthood in these animals [4,5,22,30,31].
The most important finding of the present study is the prevention of sevoflurane-induced activation of apoptosis with a single dose of intranasal insulin administration. Recent studies have demonstrated many important roles of insulin in the brain, including neurotrophic and neuroprotective activities, regulation of neural development and plasticity, and a role in learning and memory [32-34]. Neurons in the mammalian brain can synthesize insulin [35,36], but the majority of brain insulin is believed to derive from the periphery via receptor-mediated transport [37]. It is obviously challenging to administer insulin into the brain because the peripheral administration could lead not only to hypoglycemia, but also to very limited amounts in the brain. Intranasal administration appears to be an effective and practical method for delivering insulin directly into the brain without detectable hypoglycemia. We have recently reported that intranasal insulin can restore insulin signaling, increase the levels of synaptic proteins, and reduce Aβ level and microglia activation in adult 3xTg-AD mouse brains, as well as prevent anesthesia-induced cognitive impairment and chronic neurobehavioral changes in adult mice [15,16,38].
Potential prevention of anesthesia-induced neurotoxicity and behavioral deficits has been reported in a few animal studies. Yonamine et al. reported that the sevoflurane-induced neuroapoptosis and subsequent behavioral deficits can be suppressed by co-administration of hydrogen gas as part of the carrier gas mixture in mice [4]. Boscolo et al. reported that the anti-oxidative agents EUK-134 and R(+)-pramipexole can reduce anesthesia-induced neuronal loss in neonatal rats [39]. Anti-inflammatory treatment of neonatal mice may also ameliorate sevoflurane-induced cognitive impairment [6]. Treatment of neonatal mice with erythropoietin, a potent neuroprotective agent, immediately after exposure to sevoflurane was reported to reduce both activation of neural apoptosis and cognitive impairment [5]. However, there have been no follow-up studies or clinical studies testing these potential preventive approaches against anesthesia-induced neurotoxicity to the neonatal brain.
In conclusion, we found that a single anesthesia exposure with sevoflurane of P7 neonatal mice, which is within the period of brain development spurt, induced marked over-activation of neuronal apoptosis in the brain and that prior administration of intranasal insulin prevented the over-activation. Because the well-regulated neuronal apoptosis is critical to normal brain development, effective prevention of anesthesia-induced over-activation of neural apoptosis may be able to prevent the anesthesia-induced neurotoxicity in the developing brain. These findings, together with our recent findings in neonatal mice after repeated exposure to anesthesia [22], provide an initial indication for the development of a simple and effective preventive method against anesthesia-induced neurotoxicity and probably also against the increased risk of developing learning disabilities in children.
Acknowledgements
This work was supported in part by the New York State Office for People With Developmental Disabilities, Albany, NY, and a scholarship (to H.L.) from Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China. We thank Jeffrey Goodman, PhD, and Ms. Maureen Marlow of the New York State Institute for Basic Research in Developmental Disabilities, Staten Island, NY, USA, for the use of his laboratory’s gas anesthesia equipment and for copy-editing of the manuscript, respectively.
Disclosure of conflict of interest
K.I. serves on the scientific advisory board of AXON Neuroscience, has received research grants from Ever NeuroPharma and Signum Biosciences, and holds several patents on treatment of Alzheimer disease and related conditions. C.-X.G. serves on the scientific advisory board of Alectos Therapeutics. K.I., F.L. and C.-X.G hold a patent on intranasal insulin administration for the minimization of anesthesia-induced memory loss.
References
- 1.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 4.Yonamine R, Satoh Y, Kodama M, Araki Y, Kazama T. Coadministration of hydrogen gas as part of the carrier gas mixture suppresses neuronal apoptosis and subsequent behavioral deficits caused by neonatal exposure to sevoflurane in mice. Anesthesiology. 2013;118:105–113. doi: 10.1097/ALN.0b013e318275146d. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini L, Bennis Y, Velly L, Grandvuillemin I, Pisano P, Bruder N, Guillet B. Erythropoietin protects newborn rat against sevoflurane-induced neurotoxicity. Paediatr Anaesth. 2014;24:749–759. doi: 10.1111/pan.12372. [DOI] [PubMed] [Google Scholar]
- 6.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SQ, Fang F, Xue ZG, Cang J, Zhang XG. Neonatal sevoflurane anesthesia induces long-term memory impairment and decreases hippocampal PSD-95 expression without neuronal loss. Eur Rev Med Pharmacol Sci. 2013;17:941–950. [PubMed] [Google Scholar]
- 8.Xiao H, Liu B, Chen Y, Zhang J. Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int J Dev Neurosci. 2016;48:38–49. doi: 10.1016/j.ijdevneu.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Liu Y, Xu S, Zhao Q, Guo X, Shen R, Wang F. Early life exposure to sevoflurane impairs adulthood spatial memory in the rat. Neurotoxicology. 2013;39:45–56. doi: 10.1016/j.neuro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Huang Y, Jiang J, Hu R, Yang Y, Jiang H, Yan J. Neuronal apoptosis may not contribute to the long-term cognitive dysfunction induced by a brief exposure to 2% sevoflurane in developing rats. Biomed Pharmacother. 2016;78:322–328. doi: 10.1016/j.biopha.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Shen F, Xu D, Zhao X. A lasting effect of postnatal sevoflurane anesthesia on the composition of NMDA receptor subunits in rat prefrontal cortex. Int J Dev Neurosci. 2016;54:62–69. doi: 10.1016/j.ijdevneu.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SC, Pan A, Sun GX, Freed A, Stokes JC, Bornstein R, Witkowski M, Li L, Ford JM, Howard CRA, Sedensky MM, Morgan PG. Relevance of experimental paradigms of anesthesia induced neurotoxicity in the mouse. PLoS One. 2019;14:e0213543. doi: 10.1371/journal.pone.0213543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Q, Li J, Dai CL, Li H, Iqbal K, Liu F, Gong CX. Anesthesia with sevoflurane or isoflurane induces severe hypoglycemia in neonatal mice. PLoS One. 2020;15:e0231090. doi: 10.1371/journal.pone.0231090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Run X, Liang Z, Zhao Y, Dai CL, Iqbal K, Liu F, Gong CX. Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front Aging Neurosci. 2014;6:100. doi: 10.3389/fnagi.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Dai CL, Wu Z, Iqbal K, Liu F, Zhang B, Gong CX. Intranasal insulin prevents anesthesia-induced cognitive impairment and chronic neurobehavioral changes. Front Aging Neurosci. 2017;9:136. doi: 10.3389/fnagi.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX. Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in Mice. Sci Rep. 2016;6:21186. doi: 10.1038/srep21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 19.Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH 2nd, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 2010;95:E468–472. doi: 10.1210/jc.2010-0744. [DOI] [PubMed] [Google Scholar]
- 21.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, Craft S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Dai CL, Gu JH, Peng S, Li J, Yu Q, Iqbal K, Liu F, Gong CX. Intranasal administration of insulin reduces chronic behavioral abnormality and neuronal apoptosis induced by general anesthesia in neonatal mice. Front Neurosci. 2019;13:706. doi: 10.3389/fnins.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez V, Feinstein SD, Lunardi N, Joksovic PM, Boscolo A, Todorovic SM, Jevtovic-Todorovic V. General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology. 2011;115:992–1002. doi: 10.1097/ALN.0b013e3182303a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loepke AW, Istaphanous GK, McAuliffe JJ 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 26.Ding ML, Ma H, Man YG, Lv HY. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can J Physiol Pharmacol. 2017;95:1396–1405. doi: 10.1139/cjpp-2016-0333. [DOI] [PubMed] [Google Scholar]
- 27.Kang W, Lu D, Yang X, Ma W, Chen X, Chen K, Xu X, Zhou X, Zhou L, Feng X. Sevoflurane induces hippocampal neuronal apoptosis by altering the level of neuropeptide Y in neonatal rats. Neurochem Res. 2020;45:1986–1996. doi: 10.1007/s11064-020-03028-9. [DOI] [PubMed] [Google Scholar]
- 28.Nonomura K, Yamaguchi Y, Hamachi M, Koike M, Uchiyama Y, Nakazato K, Mochizuki A, Sakaue-Sawano A, Miyawaki A, Yoshida H, Kuida K, Miura M. Local apoptosis modulates early mammalian brain development through the elimination of morphogen-producing cells. Dev Cell. 2013;27:621–634. doi: 10.1016/j.devcel.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Creeley CE, Olney JW. Drug-induced apoptosis: mechanism by which alcohol and many other drugs can disrupt brain development. Brain Sci. 2013;3:1153–1181. doi: 10.3390/brainsci3031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Y, Xu H, Jia C, Hu X, Kang Y, Yang X, Xue Q, Tao G, Yu B. Tanshinone IIA attenuates sevoflurane neurotoxicity in neonatal mice. Anesth Analg. 2017;124:1244–1252. doi: 10.1213/ANE.0000000000001942. [DOI] [PubMed] [Google Scholar]
- 31.Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 33.Blazquez E, Velazquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 2014;5:161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Deng Y, Zhang B, Gong CX. Deregulation of brain insulin signaling in Alzheimer’s disease. Neurosci Bull. 2014;30:282–294. doi: 10.1007/s12264-013-1408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schechter R, Holtzclaw L, Sadiq F, Kahn A, Devaskar S. Insulin synthesis by isolated rabbit neurons. Endocrinology. 1988;123:505–513. doi: 10.1210/endo-123-1-505. [DOI] [PubMed] [Google Scholar]
- 36.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- 37.Miller DW, Keller BT, Borchardt RT. Identification and distribution of insulin receptors on cultured bovine brain microvessel endothelial cells: possible function in insulin processing in the blood-brain barrier. J Cell Physiol. 1994;161:333–341. doi: 10.1002/jcp.1041610218. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, Liu F, Gong CX. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol. 2014;261:610–619. doi: 10.1016/j.expneurol.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Boscolo A, Starr JA, Sanchez V, Lunardi N, DiGruccio MR, Ori C, Erisir A, Trimmer P, Bennett J, Jevtovic-Todorovic V. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2012;45:1031–1041. doi: 10.1016/j.nbd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


