Many countries around the world have seen a sharp rise in COVID-19 cases since the beginning of October due to the second wave of the pandemic. A decline in the antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was reported exclusively in the early month, increases the risk of reinfection for convalescent individuals. There is a current need to follow the maintenance of specific antibodies against SARS-CoV-2.
Twenty patients who had recovered from COVID-19 were included in our cohort. Blood samples were obtained in February and October, corresponding to a median of 25 (range 5–33 days) and 230 (range 221–248 days) days after symptom onset (Fig. 1a). Enzyme-linked immunosorbent assay was performed to evaluate the presence of anti-SARS-CoV-2 spike (S) receptor-binding domain (RBD) IgG over 8 months. A preliminary positive cutoff was set with the mean value of negative controls above 3 standard deviations.1 Neutralizing antibodies (NAbs) were measured by pseudovirus-based assays associated with two SARS-CoV-2 strains (S-D614 and S-G614) in 293T-ACE2 cells. The 50% inhibitory dose (ID50) was calculated as the NAb titer.
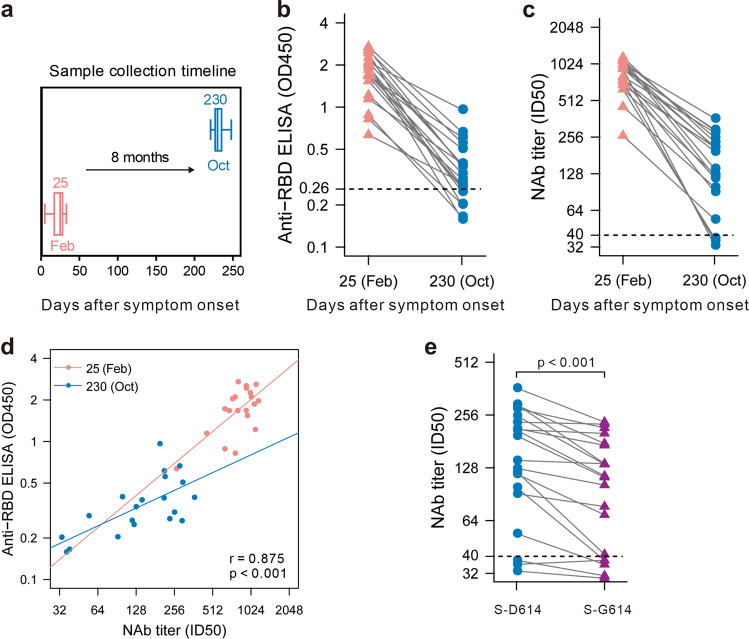
Fig. 1.
Maintenance of the humoral response to SARS-CoV-2 in convalescent patients over 8 months. a Blood samples were collected in February and October. Enzyme-linked immunosorbent assays (ELISAs) (b) and pseudovirus-based neutralizing assays (c) were performed to detect IgG levels and neutralizing antibody (NAb) titers against SARS-CoV-2. The thresholds of detection were 0.26 for the OD450 value and 1:40 for the ID50. d Correlation of IgG and NAb levels. e Neutralizing activities of convalescent plasma against SARS-CoV-2 S-D614 or S-G614 mutant at 8 months after symptom onset
Of the convalescent patients, there were 9 women and 11 men. The median age was 51.5 years (range 45–65). Except for two cases with severe symptoms, 90% of the infected patients had mild symptoms (Supplementary Table 1).
In all 20 participants, antibodies against the SARS-CoV-2 spike RBD decreased from a mean OD450 value of 1.78 (range 0.55–2.72) to 0.38 (range 0.15–1.01) over 8 months. When the OD450 value was <0.26, the specimen was considered seronegative. At follow-up time point 2 (in October), the IgG level of five participants (25%) had became negative (Fig. 1b). A similar decline was observed in the pseudovirus neutralization assay. Indeed, NAb titers decreased from a mean ID50 value of 836.55 (range 263–1160) to 170.30 (range 33–365). Among them, the NAb titers of three participants (15%) were lower than the threshold at 8 months after symptom onset (Fig. 1c). Moreover, NAb titers correlated significantly with IgG levels (p < 0.001) (Fig. 1d). The cross-protective role of NAbs at 8 months after symptom onset was evaluated by a pseudovirus-based neutralization assay using SARS-CoV-2 S-G614, which is currently the dominant strain worldwide. The NAb titers against the S-G614 mutant pseudovirus of five participants (25%) decreased below the threshold. Moreover, there was a statistically significant difference in the neutralizing efficacy of convalescent plasma against SARS-CoV-2 S-D614 and S-G614 mutant pseudoviruses (Fig. 1e).
Herein, we report changes in the humoral immunity response in SARS-CoV-2 convalescent patients over 8 months. In agreement with previous follow-up studies within a shorter time frame, declines in both IgG and NAb were observed.1–3 Furthermore, the better significant correlation between IgG and NAb levels in February than in October indicates that the anamnestic immune response and other protective immunity should be evaluated within the context of low levels of NAbs.4
Facing the challenge of the second wave of SARS-CoV-2 infection, the risk of reinfection among convalescent patients by the currently dominant strain (SARS-CoV-2 S-G614) is worth considering. Weaker neutralizing activity against the S-G614 mutant pseudovirus has been demonstrated. In two samples, NAb titers even quickly decreased from 1:99 or 1:122 to near the limit of detection. This might be a warning about the possible loss of protective capacity for convalescent plasma with lower titers against the SARS-CoV-2 S-G614 variant, similar to the reinfection case reported in Hong Kong.5 Therefore, more data about the longevity of humoral immunity are needed to evaluate the effectiveness of herd immunity.
Supplementary information
Acknowledgements
We acknowledge funding support from the Key Laboratory of Infectious Diseases (CQMU, 202005), the Emergency Project from the Science & Technology Commission of Chongqing (cstc2020jscx-fyzx0053), the Emergency Project for Novel Coronavirus Pneumonia from Chongqing Medical University (CQMUNCP0302), the Leading Talent Program of CQ CSTC (CSTCCXLJRC201719), and a Major National Science & Technology Program grant (2017ZX10202203) from the Science & Technology Commission of China.
Author contributions
A.H., N.T., K.W., P.P. and J.H. developed the conceptual ideas and designed the study. P.P. and J.H. performed the experiments. B.L. and L.F. provided the samples. H.D. performed the statistical analysis. All authors provided scientific expertise and interpreted the data. P.P. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. All authors reviewed and approved the final version of the report.
Funding
Our work has received funding support from the Key Laboratory of Infectious Diseases (CQMU, 202005), the Emergency Project from the Science & Technology Commission of Chongqing (cstc2020jscx-fyzx0053), the Emergency Project for Novel Coronavirus Pneumonia from Chongqing Medical University (CQMUNCP0302), the Leading Talent Program of CQ CSTC (CSTCCXLJRC201719), and a Major National Science & Technology Program Grant (2017ZX10202203) from the Science & Technology Commission of China.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The licence information was missing from this article and should have been CC-BY.
These authors contributed equally: Pai Peng, Jie Hu
Change history
8/30/2022
A Correction to this paper has been published: 10.1038/s41423-022-00915-9
Contributor Information
Kai Wang, Email: wangkai@cqmu.edu.cn.
Ni Tang, Email: nitang@cqmu.edu.cn.
Ai-long Huang, Email: ahuang@cqmu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-00605-4) contains supplementary material.
References
- 1.Ripperger TJ, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low prevalence communities and reveal durable humoral immunity. Immunity. 2020 doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MM, et al. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020 doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell. 2020 doi: 10.1016/j.cell.2020.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To KK-W, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.