Abstract
Background
Emotional cognition and effective interpretation of affective information is an important factor in social interactions and everyday functioning, and difficulties in these areas may contribute to aetiology and maintenance of mental health conditions. In younger people with depression and anxiety, research suggests significant alterations in behavioural and brain activation aspects of emotion processing, with a tendency to appraise neutral stimuli as negative and attend preferentially to negative stimuli. However, in ageing, research suggests that emotion processing becomes subject to a ‘positivity effect’, whereby older people attend more to positive than negative stimuli.
Aims
This review examines data from studies of emotion processing in Late-Life Depression and Late-Life Anxiety to attempt to understand the significance of emotion processing variations in these conditions, and their interaction with changes in emotion processing that occur with ageing.
Method
We conducted a systematic review following PRISMA guidelines. Articles that used an emotion-based processing task, examined older persons with depression or an anxiety disorder and included a healthy control group were included.
Results
In Late-Life Depression, there is little consistent behavioural evidence of impaired emotion processing, but there is evidence of altered brain circuitry during these processes. In Late-Life Anxiety and Post-Traumatic Stress disorder, there is evidence of interference with processing of negative or threat-related words.
Conclusions
How these findings fit with the positivity bias of ageing is not clear. Future research is required in larger groups, further examining the interaction between illness and age and the significance of age at disease onset.
Keywords: Emotion processing, older adults, cognition, late-life depression, late-life anxiety
Emotional cognition involves a range of functions, including ‘perceiving, interpreting, managing, and generating responses to socially relevant stimuli, such as intentions and behaviour of others’.1 Being able to undertake these functions efficiently is an important part of interpersonal relationships and social interactions. Interpretation of affective information is vital to these interactions as it influences emotional states and governs behavioural responses in social situations. Difficulties in social situations and avoidance of these, in the context of mental disorders, may maintain clinical levels of distress in patients and hinder attempts to treat their disorder. Further, emotional material can impair efficient cognitive processing by inducing biases in attention or decision-making, and by interfering with efficient cognitive processing, thereby impairing other aspects of cognition.
Emotion Processing in Depression
The cognitive neuropsychological hypothesis of depression2 posits that in depression there is both behavioural and neurocircuitry evidence of a bias toward negative emotional stimuli. It is suggested that those who are more vulnerable to depression tend to perceive social cues as more negative and attend to, and recall more, negative information.3 These biases may play a role in precipitating depression, maintaining depression and conferring susceptibility to relapse. Antidepressants may reverse this bias relatively quickly, but it takes some time for this change to be translated, via improved social interactions, into a reduction in depressive symptoms.4,5 Multiple paradigms have been used to investigate emotion processing, and several, when studied in groups of younger individuals with depression, show differences broadly supporting the cognitive neuropsychological theory. For example, using a dot probe task, several studies have shown a bias toward sad faces compared with neutral faces in patients experiencing a major depressive episode.6 In addition, there is a bias in individuals with depression toward perceiving positive (happy), neutral or ambiguous facial expressions as more negative or less happy, compared with those in healthy control groups.7,8 In more complex social processing, examined with the Reading the Mind in the Eyes Task (RMET) and other complex tasks, people with depression perform significantly worse than control participants.9
Emotion Processing in Anxiety
In younger adults with anxiety disorders, there is the tendency for a bias toward threat-related stimuli, which is hypothesised to maintain a heightened sense of threat. For example, in facial expression recognition (FER) tasks, evidence suggests that individuals with social anxiety are not significantly different from healthy controls in identifying facial expressions,10,11 but show a tendency to misidentify neutral facial expressions as angry.12 Attentional bias toward threat-related stimuli has been reported in Generalised Anxiety Disorder (GAD), when using a dot probe task.13 There is also evidence of increased response latencies to both anxiety-related words and generally negative words when using emotional Stroop (eStroop) tasks across anxiety disorders, suggesting an attentional bias toward threat-related stimuli.14 In younger adults, changes in emotion processing similar to those in other anxiety disorders have also been seen in Post-Traumatic Stress Disorder (PTSD). For example, a meta-analysis of eStroop task performance in PTSD15 found that individuals with PTSD, compared with healthy controls, showed impairments in the eStroop task when processing trauma-related and generally threatening, but not positive, information.
Changes in Emotion Processing in Ageing
Multiple experimental paradigms examining different aspects of attention and memory, as well as meta-analysis of attention and memory in younger compared with older adults,16 have suggested a ‘positivity bias’ in older adults.17 For example, older participants have been shown to be more likely to remember positive images compared with negative images, whereas a preference for negative images was observed in young adults.18 This positivity bias has been demonstrated in multiple other emotion processing paradigms, including those examining autobiographical memory,19,20 working memory,21 attention to emotional faces22 and recall of facial expressions.23 Evidence also shows that older adults are slower and less accurate in studies investigating the effects of ageing on face perception by using tasks such as face detection,24 face identification25,26 and emotion recognition.27–29
Several theories attempt to explain this bias. Socioemotional selectivity theory, suggests that as time to the end of life becomes shorter, goals change focus and reflect a preference for emotional meaning and satisfaction.30 This change in goals then affects how we process emotions. An alternative theory is dynamic integration theory.31 Since processing of negative affect may be more cognitively demanding,32 this theory suggests that as we age, diminishing cognitive capacity (reduced capacity for processing) makes it more difficult to accept and integrate negative feelings, and therefore older adults disconnect from negative feelings, resulting in a positivity bias.33 However, if this theory is accurate then it is argued that this effect would be greatest in those with poorer or impaired cognitive function, whereas in fact, the opposite appears to be the case.34,35 Furthermore, depression is associated with cognitive impairment but, as noted, seems to increase bias for negative material – although not necessarily the integration and processing of this.36
A further theory, the aging brain model, suggests that the changes in emotional cognition seen in older adults may be associated with age-related changes in adrenergic and amygdala function,37 which leads to an impairment in the processing of negative stimuli. Studies do show differences in brain activation associated with emotion processing as ageing occurs, indirectly suggesting a decline in processing capacity in emotion processing areas. For example, older adults show reduced limbic and greater cortical activation (e.g. insula, frontal cortex) during processing of emotional faces.38,39 Some of these changes have been shown to correlate with the positivity bias. Sakaki et al,40 found increased negative coupling between the medial prefrontal cortex (MPFC) and amygdala and enhanced MPFC activity when learning emotional faces. This increase in MPFC activity may indicate an attempt to overcome an age related decline in capacity of MPFC processing areas. In general, a reduction in activity of prefrontal cortical processing areas, with concomitant increased limbic activation (amygdala, basal ganglia), has also been shown in studies of depression both in young and older individuals during emotion processing,41 in particular in response to sad faces.42
Research in Late-Life Depression (LLD) is further complicated by the issue of age at disease onset. Onset of depression at a later age is associated with greater neuropsychological abnormality, white matter hyperintensities and a higher rate of dementia at follow-up.43–45 The fact that late-onset depression has not been associated with frequent episodes across the lifespan may also be important in determining emotion processing. It may therefore be that emotion processing deficits, if they exist, are different between early- and late-onset depression.
To date, research examining emotion processing in older adults with mood and anxiety disorders has not been systematically synthesised. This review aims to examine this literature to understand the interactions between depression and anxiety disorders, cognitive decline and the positivity bias in older adults, and their effects on emotion processing.
Based on the factors discussed above, our questions were as follows:
In LLD, Late-Life Anxiety (LLA) and PTSD, does the positivity bias in old age mitigate the emotion processing abnormalities that might be expected given the evidence of negativity bias in younger people with depression or anxiety disorders?
Are changes in emotion processing circuitry in LLD, LLA and PTSD similar to those seen in younger people with depression or anxiety?
Are behavioural and brain changes different in people with late-onset depression compared with early-onset depression, and what is the relationship between these changes and age-related cognitive decline?
Method
Protocol and registration
Details of the protocol for this systematic review were registered with the Prospective Register of Systematic Reviews (registration identifier: CRD42020124980; accessed at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020124980).
Search strategy
Up to December 2019, a systematic review of electronic databases was carried out for relevant papers, using PubMed and Web of Science. In the initial search, the search terms used were ‘depression’, ‘anxiety’, ‘PTSD’, ‘bipolar’, ‘emotion processing’, ‘older persons’ and ‘elderly’, in different permutations. Reference lists of all relevant papers were then checked to ensure inclusion of all pertinent articles. Citations of relevant articles were then followed using Web of Science to allow for capture of any missed articles.
Inclusion criteria
Peer-reviewed articles involving assessment with an emotion-based processing task and comparison of a clinical sample with a healthy control sample were included in the review. Studies examining all psychiatric disorders were to be considered; however, studies were only found for depression, anxiety and PTSD populations. Sample participants were to be aged over 60 years and samples were to be categorised as ‘older adult’ or similar.
Exclusion criteria
Reasons for exclusion were comorbid major medical or neurological disorder in either group in the study, and studies involving participants with mild or greater levels of cognitive impairment (Mini-Mental State Examination score <25 or equivalent). All studies were limited to English-language publications.
Full study review
This review was undertaken with recommended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and using the PRISMA statement to guide the search, screening and extraction process.46 Articles were screened by one of the reviewers, who independently reviewed the titles and abstracts of studies, to accept or reject for full-text review. The same reviewer then examined the full texts of the studies that had passed initial screening, to determine if they still met inclusion criteria. If inclusion of a paper was unclear, all three co-authors discussed this, to achieve a consensus. For each study, the following data was extracted: characteristics of the sample, including sample size, average age and baseline depression/anxiety severity; study design; cognitive tests used during assessment and study outcomes.
Results
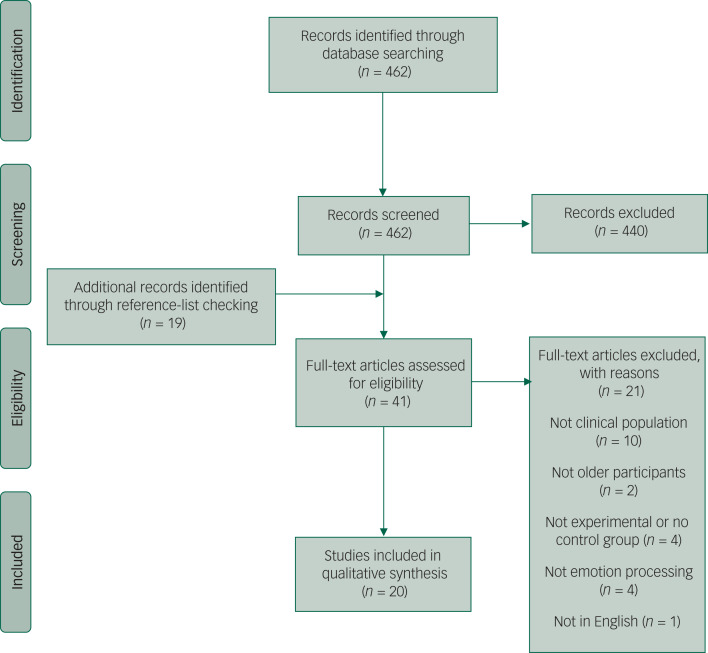
The initial search for this review found 462 articles (Fig. 1). After a title and abstract review, 440 of these were excluded. The full text of the remaining 22 studies were obtained and reviewed. Nineteen additional papers were found through examination of the reference sections of the full-text studies. Of these 41 studies, 21 were then excluded for not being clinical or experimental studies, not including older participants or emotion processing, or not being in English. The remaining 20 papers are reviewed as follows. All of the studies included clinically diagnosed populations, unless otherwise stated. In this area of research, there is little consistency regarding terminology. LLD, depression and late- or early-onset depression are all used, with varying intended meaning; as such, we have deferred to the original authors and used the terminology that was present in the original article.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies retrieved for the review.
Depression
Table 1 displays characteristics and main findings of studies examining behavioural data of samples with depression.
Table 1.
Selected demographic characteristics of included studies examining behavioural data of samples with depression
| Author | Arm | N | Gender | Age (s.d.) | Task | Outcomes |
|---|---|---|---|---|---|---|
| Dudley et al47 | LLD | 12 | 9 Female | 74.4 (7) | Emotional Stroop (interference) | Participants with LLD had longer response times across blocks than control participants. Older participants with LLD showed a specific increase in response time to negative words. |
| Control | 12 | 9 Female | 72.9 (8) | No difference in response time across any word valence. | ||
| Broomfield et al48 | LLD | 16 | 9 Female | 73 (6.23) | Emotional Stroop (interference) | Older patients with LLD had lower reaction times across all trial types compared with controls. Older patients with LLD had significantly longer reaction times to negative words relative to neutral compared with controls. |
| Control | 19 | 9 Female | 72.05 (5.56) | No change in reaction time for any valence of words. | ||
| Callahan and Hudon49 | LLD | 10 | Not provided | Not provided | Emotional Stroop (interference) | Patients with LLD were slower than controls across all blocks. |
| Control | 15 | Not provided | Not provided | Controls showed longer reaction times on negative compared with neutral blocks. | ||
| Huang et al50 | LLD | 55 | 38 Female | 66.36 (5.42) | Emotional Stroop (interference) | Both positive and negative words were associated with longer latencies in both groups. There was, however, no group×emotion interaction on any of the measures. |
| Control | 40 | 25 Female | 68.10 (5.30) | Control participants were more accurate and had shorter response latencies. | ||
| Mah and Pollock51 | LLD | 11 | 7 Female | 73 (8.4) | Emotion regulation task (interference) | Overall response latencies did not differ between LLD and control groups. Difference was found for emotional stimuli compared with negative stimuli for the LLD group. |
| Control | 11 | 8 Female | 75 (6.9) | Response latencies for all emotions were significantly longer compared with response to neutral stimuli. | ||
| Zhou et al52 | LLD | 14 | 9 Female | 66.36 (5.20) | Primed face emotion recognition task (interference) | Reaction times for happy and sad primes were significantly longer than for ambiguous primes for all participants. |
| Control | 14 | 9 Female | 65.64 (3.93) | |||
| Zhou et al52 (Event-Related Potential) |
LLD | 14 | 9 Female | 66.36 (5.20) | Primed face emotion recognition task (interference) | Participants with LLD had smaller event-related potential amplitudes overall, compared with controls for all priming stimuli, irrespective of valence. No differences were seen in event-related potential amplitudes between the different prime valences. |
| Control | 14 | 9 Female | 65.64 (3.93) | No differences found between happy and ambiguous primes, but there were significant differences between happy (larger) and sad and ambiguous (larger) and sad primes within the control group. | ||
| Mah and Pollock51 | LLD | 11 | 7 Female | Emotion Perception task (recognition) | Participants with LLD were significantly more likely to misidentify a neutral face compared with control participants. | |
| Control | 11 | 9 Female | ||||
| Savaskan et al53 | LLD | 18 | 14 Female | 76.2 (1.8) | Face portrait recognition test (recognition) | Participants with LLD recalled fewer faces overall compared with controls. This lower recall was especially pronounced for recall of happy faces. There were no differences in recall for participants with LLD between happy and angry faces. Treatment with escitalopram did not improve memory recognition for happy faces, but did improve memory for faces with an angry expression. |
| Control | 22 | 16 Female | 76.9 (1.8) | Participants in the control group had better memory for happy over angry faces. | ||
| Brassen et al54 | LLD | 13 | 13 Female | 66.4 (6.1) | Verbal emotion recognition task (memory and recognition) | No differences in correct responding, reaction time or misattribution of emotions were seen between the LLD and control groups at baseline or follow-up. |
| Control | 13 | 13 Female | 65.6 (6.1) | |||
| Callahan et al55 | LLD | 19 | 15 Female | 72.4 (9.0) | Memory for emotionally valenced words (memory) | At delayed recall, participants with LLD recalled significantly fewer words compared with control participants. At immediate recall, participants with LLD recalled more negative than neutral words. At delayed recall, participants with LLD recalled more positive and negative words than neutral words. |
| Control | 28 | 21 Female | 72.1 (8.1) | No differences seen in immediate word recall between LLD and control groups. At both immediate and delayed recall control participants recalled more positive and negative words than neutral words overall. |
||
| Szanto et al57 | Suicidal/LLD | 24 | 38% Male | 68.2 (8.7) | Reading the Mind in the Eyes Task (social cognition) | Participants with LLD who had attempted suicide had significantly poorer emotion recognition compared with controls. This difference was not maintained when global cognition was accounted for. |
| Non-suicidal LLD | 38 | 34% Male | 70.2 (7.7) | Participants with LLD who had not attempted suicide had an emotion recognition ability, which fell between the control group and those with LLD who had attempted suicide, but was not significantly different from either. | ||
| Control | 28 | 61% Male | 69.6 (6.3) |
LLD, Late-Life Depression.
Emotion interference
The emotion processing tasks discussed in this section involve inhibition of emotional information to carry out a cognitive task. This is most commonly a modified version of the Stroop paradigm (eStroop), which uses words that are of a positive, negative or neutral valence. Participants are required to name the colour of the presented word, but to ignore the word itself. A pilot study by Dudley et al47 (12 depressed, 12 controls) showed an interaction between group and word valence, which was explained by a greater interference (longer time to colour name) of depression-related words in the depressed group, with an effect size difference compared with controls of 0.9 (Cohen's d). Broomfield et al48 (16 depressed, 19 controls) also showed a group×valence interaction in participants with depression compared with controls, with participants with depression being slower to respond to negative words compared with neutral words. The group×valence interaction persisted when anxiety was controlled for. Using a similar eStroop paradigm, Callahan and Hudon49 found those with LLD (n = 10) were generally slower than controls (n = 15), but with no difference seen across valences. No group×valence analysis was reported. Finally, in 55 people with LLD and 40 controls, using an eStroop,50 controls were more accurate and had shorter latencies. There was no effect of emotion on accuracy, but there was on latency, with both positive and negative words associated with longer latencies. There was, however, no group×emotion interaction on any of the measures. Apart from the study by Dudley et al47 , no estimates of effect size difference between depressed and control groups are given.
Mah and Pollock51 used a facial emotion-based paradigm to measure emotion inhibition in 11 participants with depression and 11 controls. In this paradigm, participants were presented with faces displaying different emotions (happy, sad, fearful and neutral) and were asked to answer questions about a non-affective aspect of the face (inhibition). There was a significant group×valence interaction, whereby overall latencies were similar in both groups, but were significantly longer for all emotion-laden stimuli (not only negative emotions) compared with neutral stimuli in the control group and did not vary by emotion in the depressed group.
Zhou et al52 studied event-related potentials (ERP), in response to emotional faces, in 14 older adults with depressive symptoms (but specifically not meeting criteria for a DSM-IV diagnosis of depression), and compared this group with 14 controls. The study was included in the review, after discussion, since the patient group had significant depressive symptoms despite no formal diagnosis (Centre for Epidemiologic Studies Depression Scale mean group score of 20.21 ± 5.65). Participants were presented with an emotional prime (facial expressions: happy, sad, ambiguous) and then asked to identify the emotion of the target which followed. Older adults with depressive symptoms had smaller ERP amplitudes compared with controls, regardless of valence of the priming stimulus. Older adults with depressive symptoms showed no differences in amplitude between the different prime valences. In control participants, there were no differences found between happy and ambiguous primes, but there were significant differences between happy and sad (larger ERP) primes, and between ambiguous and sad (larger ERP) primes. However, it was notable that the group×prime interaction was not statistically significant (p = 0.07), making further analyses and conclusions very tentative. Behavioural data showed no differences between groups.
Emotion recognition and memory
Most commonly used is an FER task,4 in which participants are presented with various facial expressions (usually happy, sad, angry, disgusted, fearful, surprised and neutral) and asked to identify the emotion portrayed. Mah and Pollock51 used an FER task in which participants were presented with happy, sad, fearful or neutral faces. Although overall accuracy was similar between depressed (n = 11) and control (n = 11) groups, there was a significantly increased probability of participants with depression incorrectly identifying neutral faces.
Savaskan et al53 used a memory for faces paradigm that included both happy and angry faces. At baseline, patients with depression (n = 18) recalled fewer faces overall compared with controls (n = 22). There was no group×valence interaction, although the authors suggested that compared with controls, patients with depression showed lower ability to recognise previously viewed happy facial expressions after a delay. Following 4 weeks of treatment, the patient group showed a significant reduction in depressive symptoms and a significant improvement in general cognitive function. Further, their memory for angry faces significantly improved from baseline, whereas no effect was seen for happy faces.
Two studies examined emotion recognition and memory for emotional material, using verbal stimuli. Brassen et al54 used an emotion recognition task with positive, neutral and negative words to examine neural responses in 13 antidepressant-naïve female patients with LLD versus 13 controls. Participants were shown a positive, negative or neutral adjective, which was then replaced by a response screen where they indicated the valence of the word. No significant differences in response correctness, response time or misattributions of emotion were found at either time point between the two groups.
Callahan et al55 examined recall of neutral, positive and negative words from a list including 12 of each. At immediate recall, control participants (n = 28) displayed better recall of positive and negative words compared with neutral words, whereas participants with depression (n = 19) recalled more negative than neutral words. However, performance of the two groups was not directly compared. At delayed recall, both groups generally showed better recall of emotional compared with neutral words. During recognition, all participants were more likely to report false recognition for emotional than neutral words. Once again, there was no group comparison.
Social cognition
The RMET56 requires individuals to identify complex or social emotions from images portraying only the eyes of the face. Szanto et al57 examined the RMET in older adults with depression who had (n = 24) or had not (n = 38) attempted suicide. A control group was also examined (n = 28). Individuals who had attempted suicide performed significantly worse than healthy controls. The performance of participants with depression who had not attempted suicide fell between controls and those who had attempted suicide, but was not significantly different from either. Further analysis showed that when global cognitive function was accounted for (Mattis Dementia Rating Scale58), the significant difference found for individuals who had attempted suicide was not maintained, suggesting that the group's reduced ability to recognise social emotions may have been attributable to global cognitive impairment, rather than a specific impairment in social cognition.
Functional magnetic resonance imaging studies
Table 2 displays characteristics and main findings of studies examining neuroimaging data of samples with depression.
Table 2.
Selected demographic characteristics of included studies examining neuroimaging data of samples with depression
| Author | Arm | N | Gender | Age (s.d.) | Task | Outcomes |
|---|---|---|---|---|---|---|
| Aizenstein et al59 | LLD | 33 | 21 Female | 67.7 (5.2) | Faces and Shapes task (recognition) | Greater limbic activation for participants with LLD than controls during affective processing |
| Control | 27 | 19 Female | 71.6 (7.5) | |||
| Huang et al50 | LLD | 55 | 38 Female | 66.36 (5.42) | Emotional Stroop (interference) | LLD showed reduced activation in middle frontal gyrus and left dorsolateral prefrontal cortex, and increased activation in anterior cingulate cortex, compared with controls. This was mediated by cognitive reserve such that greater cognitive reserve correlated with greater middle frontal gyrus activation. |
| Control | 40 | 25 Female | 68.10 (5.30) | |||
| Brassen et al54 | LLD | 13 | 13 Female | 66.4 (6.1) | Verbal emotion recognition task (memory and recognition) | Participants with LLD showed an attenuated neural response in the ventromedial prefrontal cortex in response to negative stimuli when compared with positive stimuli. At 7-month follow-up, when participants symptoms had significantly improved, this attenuated response was found to have normalised. |
| Control | 13 | 13 Female | 65.6 (6.1) | |||
| Briceño et al60 | LLD | 26 | 12 Female | Not provided | Emotion recognition task (recognition) | In older participants, women with LLD showed hypoactivation in emotion processing circuits, particularly the right prefrontal cortex, when compared with healthy controls. In contrast, older men with LLD showed hyperactivation in these areas when compared with the control group. |
| Control | 25 | 12 Female | Not provided | |||
| Vanyukov et al61 | LLD and suicide | 18 | 6 Female | 67.44 (7.0) | Face matching task (recognition) | LLD and suicide attempts did not predict a different response to angry faces than healthy controls. |
| LLD | 13 | 9 Female | 68.15 (6.1) | |||
| Control | 18 | 11 Female | 70.05 (7.7) |
LLD, Late-Life Depression.
Aizenstein et al59 used a facial expression affective-reactivity task. Participants with depression (n = 33) showed greater subgenual cingulate activity during affective processing compared with controls (n = 27) with a significant correlation between white matter hyperintensity and activity. Huang et al50 (55 participants with LLD, 40 controls) showed reduced activation in middle frontal gyrus and left dorsolateral prefrontal cortex, and increased activation in anterior cingulate cortex, in the LLD group compared with controls in a study examining activation during an eStroop task. This was mediated by cognitive reserve such that greater cognitive reserve correlated with greater middle frontal gyrus activation in the LLD group. Brassen et al54 reported that in comparison with controls (n = 13), female patients with LLD (n = 13) showed attenuated neural response in the ventromedial prefrontal cortex in response to negative compared with positive words. When correlated with depression severity in the depressed group (Geriatric Depression Scale), reduced activation in the medial orbito-frontal cortex was correlated with higher depression scores. Increased activation in the superior medial frontal cortex, for positive compared with negative words, was also correlated with higher depression scores. At the 7-month follow-up, when patients’ symptoms had significantly improved, this attenuated response was found to have normalised.
Briceño et al60 examined the effects of age and gender on neural circuits in an FER paradigm that involved four emotions (happiness, sadness, fear and anger). The study included participants in younger and older age groups, as well as participants with and without depression (older participants with depression, n = 26; older controls, n = 25). When depressed and non-depressed groups were not separated by age and gender, no overall effects were detected between groups. However, when separated by age and gender, older women with depression showed hypoactivation in emotion processing circuits, particularly the right prefrontal cortex, when compared with older controls. In contrast, older males with depression showed hyperactivation in these areas when compared with the control group.
Vanyukov et al61 used the faces and shapes task,62 in which participants are required to match a target face to one of two presented faces. Authors used faces showing anger and fear during the face trials. In neutral trials, shapes were used as non-affective controls. The study examined patients with depression (n = 13), patients with depression who had attempted suicide (n = 18) and controls (n = 18). There was no difference in response to angry faces in either depressed group compared with controls. Responses to fear-related stimuli were not discussed.
Anxiety disorders
Table 3 presents characteristics and key findings from reviewed studies examining samples with anxiety disorders.
Table 3.
Selected demographic characteristics of included studies examining anxiety and post-traumatic stress disorder
| Author | Arm | N | Gender | Age (s.d.) | Task | Outcomes |
|---|---|---|---|---|---|---|
| Price et al63 (Behavioural) |
High (late-life GAD) | 20 | 16 Female | 67.2 (6.2) | Emotional Stroop (interference) | Reaction time for the threat related words was longer than neutral words. Reaction time for positive words was shorter than for neutral words. |
| Mid | 19 | 12 Female | 68.5 (6.4) | Low- and medium-worry groups had faster reaction time for threat words compared with neutral, and slower reaction time for positive words compared with neutral. | ||
| Low | 21 | 15 Female | 68.3 (5.6) | |||
| Mohlman et al65 (Behavioural) |
GAD | 34 | 68% Female | 66.67 (4.56) | Dot probe task (interference) | A bias away from positive information in positive-neutral pairs was seen across all participants. |
| Control | 28 | 71% Female | 67.37 (5.53) | No significant differences in reaction time overall or between word types between the two groups. | ||
| Price et al66 | GAD | 16 | 11 Female | 63.1 (3.1) | Emotional Stroop (interference) | Slower reaction times and decreased prefrontal cortex activation when identifying negative versus neutral words, compared with controls. |
| Control | 12 | 8 Female | 67.2 (7.6) | |||
| Wu et al67 | GAD | 16 | 9 Female | 67.38 (5.78) | Faces and Shapes task (recognition) | No significant differences between the GAD group and the controls in either connectivity in the amygdala or BNST. |
| Control | 20 | 10 Female | 67.8 (7.88) | |||
| Karim et al68 | GAD | 17 | 10 Female | 64 (6) | Faces and Shapes task (recognition) | No significant differences between GAD and controls in brain activation. |
| Control | 20 | 10 Female | 67.5 (8.5) | |||
| Wittekind et al69 | PTSD | 22 | 20 Female | 72.73 (2.27) | Spatial cueing paradigm (interference) | No attentional bias for trauma-related stimuli in PTSD group. |
| No PTSD | 26 | 17 Female | 73.00 (2.00) | |||
| Control | 22 | 15 Female | 73.73(2.98) | |||
| Wittekind et al70 | PTSD | 20 | 18 Female | 72.75 (2.31) | Emotional Stroop (interference) | PTSD group had longer response latencies to trauma and depression related words compared with healthy controls. |
| No PTSD | 26 | 17 Female | 73.00 (2.00) | |||
| Control | 21 | 15 Female | 73.86 (2.99) | Within-group slowing for trauma compared with neutral words found in all groups in the study. |
GAD, Generalised Anxiety Disorder; BNST, bed nucleus of the stria terminalis; PTSD, Post-Traumatic Stress Disorder.
Price et al63 divided 60 community recruited adults into high-, medium- and low-trait anxiety, based on responses to the Penn State Worry Questionnaire (PSWQ). A total of 90% of participants in the high-worry group (n = 20) scored ≥50 on the PSWQ, which has been noted as a cut-off for late-life GAD.64 The remaining participants (n = 40) formed comparison groups. An eStroop task containing positive, threat-related, and neutral words was used. In the high-worry group, response time for threat-related words was longer than neutral words (Cohen's d = 0.76). The high-worry group also showed faster reaction times for positive words than neutral. The low- and medium-worry groups showed the opposite pattern: faster response time for threat-related words compared with neutral, and slower response time for positive words compared with neutral. Between-group comparisons of estimated marginal means showed a difference between the high-worry and low-worry group, with bias toward threat-related words being greater (Cohen's d = 0.67).
Mohlman et al65 used a dot probe task which included depression, threat, positive and neutrally valenced words to examine attentional biases. There was no significant between-group differences in reaction time overall or between word types in GAD (n = 34) versus controls (n = 28). Using differences between probe-target congruent word pairs and incongruent pairs, bias scores were calculated. No significant differences in bias scores between GAD and control groups were found. A bias away from positive information in positive–neutral pairs was seen across all participants in the study. No significant change in performance following treatment (cognitive–behavioural therapy versus waitlist) was found.
Functional magnetic resonance imaging studies
Price et al66 examined functional magnetic resonance imaging responses to performance on an eStroop task in older adults with late-life GAD. Participants with GAD (n = 16) had slower reaction times when responding to negative versus neutral words, compared with controls (n = 12) (Cohen's d = 0.85), and also showed less activation in the prefrontal cortex in response to negative compared with neutral words. An increase in activation was seen in the GAD group in the left amygdala, compared with the control group.
Two studies67,68 examined functional connectivity associated with emotional reactivity in late-life GAD compared with healthy controls. Neither study found any significant differences between the GAD group and the controls, using a faces and shapes task. Both studies examined the data using the factor of worry, as measured by the PSWQ. Wu et al67 found that there was a significant interaction between group and ‘worry’ on connectivity between the left amygdala and left orbitofrontal cortex, MPFC and both anterior cingulate cortices, and on connectivity between the bed nucleus of the stria terminalis and the left orbitofrontal cortex. When worry was examined across groups, there was a U-shaped curve, whereby connectivity between limbic and cortical areas was optimal at medium levels of ‘worry’. The effect sizes of these curves varied from r2 = 0.21 to r2 = 0.25. Karim et al68 found that across the groups, increased global anxiety, measured by the Hamilton Anxiety and Depression Scale, was associated with greater activation in the parahippocampal area and precuneus. In contrast, worry (PSWQ) was associated with decreased precuneus and prefrontal activation. Complex mediation analyses broadly suggested that the mediation between increased white matter hyperintensity burden and anxiety symptoms are mediated by increased activation of limbic and paralimbic structures, and decreased activation of regulatory regions, such as the ventromedial prefrontal cortex.
PTSD
One study, published as two papers, examined emotion processing in older populations with PTSD. Wittekind et al69 used a spatial cueing task involving priming with an emotional facial stimulus (anxiety, depression, trauma and neutral), followed by a spatial (left or right) decision, in response to a non-affective target (26 participants with PTSD, 22 controls). Authors found no attentional biases within the PTSD group for trauma-related stimuli. However, post hoc redistribution of participants into those who did or did not meet the criteria for depression showed that participants in the depressed sample had longer reaction times to depressive stimuli (Cohen's d = 1.5).
The same participants also completed an eStroop task70 containing words related to depression, trauma, anxiety or neutral words. Participants with PTSD showed longer response latencies to trauma- and depression-related words compared with healthy controls, but no differences in latency for neutral and anxious words. Slowing for trauma compared with neutral words was found across all groups in this study.
Discussion
The main findings of the review were as follows. First, at a behavioural level, evidence regarding differences in emotion processing between individuals with LLD and controls is inconsistent. This is the case for interference of emotional material in cognitive processes (eStroop), memory for emotional compared with other material, and the explicit process of recognition of emotional facial expressions. Second, there are few studies in LLA. Studies suggest interference with processing by threat related words in anxiety and by trauma-related words in PTSD, but there are no replication studies. Finally, studies show differences in activation in emotional processing circuitry in LLD, with the general pattern of increased limbic but reduced prefrontal activity, as in younger individuals with depression.
The review more specifically examined three main questions as below.
How does the positivity bias seen in older persons interact with biases toward negative or threat-related emotional material in depression or anxiety?
There are no consistent findings regarding any of the aspects of emotion processing studied. This was the case both for implicit processes (such as the eStroop) and for explicit processes (such as emotion recognition). Not all of these phenomena have been consistently replicated in younger people with depression either. For example, results on the eStroop have not been found to be consistent.71 The phenomenon seen most consistently in younger depression is the misinterpretation of, or attentional bias toward, neutral faces.6,72 In LLD, this was only examined in three studies and was not seen consistently.
The lack of consistent evidence of bias toward negative emotional stimuli in LLD may reflect a situation in which, on average, negative bias is less in LLD than in younger depression. It could be hypothesised that this relates to the positivity bias associated with ageing, which counteracts the biases seen in depression. However, the inconsistencies in the data are more likely to be related to the small numbers of studies and limited power of most studies. Further issues with the data are that there are no studies directly comparing emotion processing between younger patients with depression and LLD. Similarly no comparisons across the lifespan have been conducted in anxiety disorders. Therefore, the interaction of age with depression or anxiety cannot be fully evaluated. Finally, studies generally did not examine possible complicating factors, such as concomitant cognitive impairment, age at disease onset and the effects of medication.
Are changes in emotion processing circuitry similar to those seen in younger people with depression or anxiety?
In younger people with depression, studies have generally shown a reduction in activity of dorsal-cognitive structures combined with increased activity of ventral-affective structures during emotion processing.73 The studies in LLD are broadly in line with this pattern with the largest studies showing increased activation of limbic structures or decreased activation of prefrontal structures in LLD compared with controls, in line with a general pattern of reduced top-down processing.50,54,59
Of interest, there was also evidence of an interaction between depression and both white matter lesions59 and cognitive reserve50 in determining patterns of activation during emotional processing. White matter changes were associated with an exaggeration of the increase in subgenual cingulate activation seen during emotion processing in LLD.59 In the study of Huang et al50, severity of depression was associated with reduced medial frontal activation during emotion processing, but this was attenuated by having greater cognitive reserve (measured using years of education and verbal fluency). Both findings suggest a situation in which if processing capacity is reduced for a variety of possible reasons, this may impair efficient emotion processing, resulting in processing being driven to a greater extent by limbic structures.
Are behavioural and brain changes different in people with late-onset compared with early-onset depression, and what is the relationship between these changes and age-related cognitive decline?
Of note, our review excluded studies in which participants had a Mini-Mental State Examination score < 25. The rationale was that although we were interested in the interaction between mood and anxiety, cognitive ability and the relationship of these to emotion processing and positivity bias, we wanted to restrict the review to examining LLD and LLA and not to extend this into dementia. Four studies did examine the relationship between emotional processing and other aspects of cognitive functioning. Callahan et al55 examined the influence of depression on emotion processing in mild cognitive impairment (MCI), based on the suggestion that MCI plus depression is particularly likely to progress to dementia, and therefore constitutes a prognostically important subtype of MCI.74,75 Consistent with the hypothesis that negative information requires greater capacity to process, Callahan et al55 showed that for patients with MCI, immediate recall was better for positive words than negative words; however, this was not the case for MCI with depression, healthy controls or LLD. A caveat to this conclusion is the lack of an analysis of group×valence interaction. Furthermore, a similar effect was not seen in a separate examination of effects on an eStroop test.49 Although Huang et al50 did not find the hypothesised eStroop effect in LLD, there was more preserved middle frontal gyrus activity during eStroop performance in people with greater cognitive reserve. This suggests that cognitive reserve (measured using a combination of years of education and an executive task) mediates a more top-down emotional regulation, i.e. preserved processing capacity in those with greater cognitive reserve. Szanto et al57 examined social cognition in LLD. Those who attempted suicide had lower scores than controls but, interestingly, this did not survive covarying for general cognitive function, suggesting that the two functions are related, at least in depression.
Specific issues in late-life anxiety disorders
Five studies examining emotion processing in LLA disorders were identified. Those using an eStroop task both showed increased latency for negatively valenced words.63,66 In one of the studies which examined brain activation,66 the hypothesised difference from controls was seen, with an increase in activation of part of the amygdala, accompanied by a decrease in activation of the dorsal lateral prefrontal cortex in older patients with GAD. Two further studies showed no difference between patients with GAD and controls,67,68 in brain activation and connectivity. However, in one there was a complex relationship between worry and connectivity, suggesting that connectivity between limbic and cortical areas was maximal at an intermediate level of worry.66 In the other study,67 anxiety was associated with greater activation in parahippocampal areas and precuneus. In general this is in keeping with attenuation of activity in, and connectivity with, processing areas seen in younger patients.76
Data regarding interference by trauma-related words in eStroop tasks has been consistently demonstrated in younger adults with PTSD.15 Both in LLA and PTSD, studies examining simple attention bias toward negative stimuli have not shown a difference from controls.65,69 This may suggest that the basic focus of attention is not altered, but that negative emotional stimuli do interfere with processing.
Limitations and recommendations for future research
This review has limitations both directly related to the methodology and to the content of the studies reviewed. Related directly to the review, it considered English-language papers only. Although this is standard practice for an English-language review, it may mean that some relevant studies have been missed. Second, meta-analysis has not been possible given the heterogeneity of paradigms investigated in the studies examined, and in the variety of ways the data has been analysed and presented.
Limitations of the data, which can be translated into recommendations for the field are, as follows. First, although studies are in ‘older adults’, the majority have mean ages from 65 to 75 years, with the lower age cut-off being 60 years in most studies. This may mean that effects of age that might have been seen, for example, in 70–80 year olds, are washed out by there being relatively less effect in the lower age range. This could, of course, be overcome by studies being adequately powered to examine the effects of age in a linear fashion – possibly even over a larger age range so that the effects of age and its interaction with depression or anxiety could be examined. This would better reflect the fact that risk factors and neurobiology likely change in a linear fashion across the lifespan.77 Second, most studies did not analyse the effects of having early-onset compared with late-onset depression. Once again the issue is mainly one of power and as such this should be examined with sufficiently large studies. Third, a variety of paradigms have been used to study emotion processing even within similar processes. This makes it generally difficult to pool or synthesise results from different studies. Consensus on the most useful and clinically relevant paradigms would aid progress in the field. Fourth, studies have rarely attempted to determine the extent to which decline in non-emotional cognitive processes, such as executive function, may be affecting emotional processing directly. Future studies should consider undertaking testing of non-emotional memory and executive function and examining the relationship between this and emotion processing. Fifth, critically, studies have tended to be very small. Future studies should be adequately powered to show differences at least as small as 0.5 s.d. between groups. They should also, ideally, be large enough to take into account the possible effects of varying medication, late compared with early onset and age as a longitudinal factor on emotion processing. Finally, in reporting results, most studies use analysis of variance but do not report estimated marginal means and s.d., making it impossible to calculate the magnitude of differences between groups. These should be reported or effect sizes calculated.
In conclusion, the ability to correctly process and interpret emotions is an important part of social interactions, something that becomes especially important as we age. The cognitive neuropsychological hypothesis of depression also suggests that these interactions are an important part of the aetiology of depression and may provide a target for treatment. Indeed, packages of emotion recognition training are being developed to address this issue (e.g. the study by Penton-Voak et al78).
The review has highlighted the fact that there are relatively few large studies of emotion processing in older adults with depression and anxiety disorders. We have provided recommendations for future research.
Given the lack of studies that examine emotion processing in depression or anxiety across the life cycle, it is not possible to determine the interaction of abnormalities in these conditions with the ageing positivity bias. In general, there have been findings of a bias toward negative stimuli, and concomitant alteration of brain activity to a pattern of greater limbic and reduced prefrontal cortex activation in younger people with depression and anxiety. Similar patterns have been shown in studies in older persons, although not consistently in some aspects of emotion processing.
Author contributions
V.G. conducted the systematic review of papers and prepared the first draft of the manuscript. R.J.P. and K.M.D. supervised the systematic review and reviewed and updated subsequent drafts.
Funding
We are grateful to the University of Otago for financial support, by providing V.G. with the Gilbert M. Tothill Scholarship in Psychological Medicine.
Declaration of interest
V.G. has nothing to disclose. K.M.D. uses software for cognitive remediation intervention research that is provided free of charge by Scientific Brain Training PRO. R.J.P. uses computer software at no cost, provided by SBT-Pro, for research. He has received support for travel to educational meetings from Servier and Lundbeck.
References
- 1.Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry 2012; 69(12): 1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren MB, Pringle A, Harmer CJ. A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc B Biol Sci 2015; 370(1677): 20140213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011; 12(8): 467–77. [DOI] [PubMed] [Google Scholar]
- 4.Harmer CJ, Bhagwagar Z, Perrett DI, Völlm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology 2003; 28(1): 148–52. [DOI] [PubMed] [Google Scholar]
- 5.Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Prog Neuro-Psychopharmacol Biol Psychiatry 2011; 35(7): 1586–92. [DOI] [PubMed] [Google Scholar]
- 6.Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry 2010; 44(8): 681–96. [DOI] [PubMed] [Google Scholar]
- 7.Hale WW III, Jansen JHC, Bouhuys AL, van den Hoofdakker RH. The judgment of facial expressions by depressed patients, their partners and controls. J Affect Disord 1998; 47(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 8.Leppänen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Res 2004; 128(2): 123–33. [DOI] [PubMed] [Google Scholar]
- 9.Bora E, Berk M. Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord 2016; 191: 49–55. [DOI] [PubMed] [Google Scholar]
- 10.Philippot P, Douilliez C. Social phobics do not misinterpret facial expression of emotion. Behav Res Ther 2005; 43(5): 639–52. [DOI] [PubMed] [Google Scholar]
- 11.Stevens S, Gerlach AL, Rist F. Effects of alcohol on ratings of emotional facial expressions in social phobics. J Anxiety Disord 2008; 22(6): 940–8. [DOI] [PubMed] [Google Scholar]
- 12.Bell C, Bourke C, Colhoun H, Carter F, Frampton C, Porter R. The misclassification of facial expressions in generalised social phobia. J Anxiety Disord 2011; 25(2): 278–83. [DOI] [PubMed] [Google Scholar]
- 13.Bradley BP, Mogg K, White J, Groom C, De Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol 1999; 38(3): 267–78. [DOI] [PubMed] [Google Scholar]
- 14.Joyal M, Wensing T, Levasseur-Moreau J, Leblond J, Sack AT, Fecteau S. Characterizing emotional Stroop interference in posttraumatic stress disorder, major depression and anxiety disorders: a systematic review and meta-analysis. PLoS ONE 2019; 14(4): e0214998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cisler JM, Wolitzky-Taylor KB, Adams TG Jr, Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev 2011; 31(5): 817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed AE, Chan L, Mikels JA. Meta-analysis of the age-related positivity effect: age differences in preferences for positive over negative information. Psychol Aging 2014; 29(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 17.Carstensen LL, DeLiema M. The positivity effect: a negativity bias in youth fades with age. Curr Opin Behav Sci 2018; 19: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charles ST, Mather M, Carstensen LL. Aging and emotional memory: the forgettable nature of negative images for older adults. J Exp Psychol Gen 2003; 132(2): 310–24. [DOI] [PubMed] [Google Scholar]
- 19.Cuddy LL, Sikka R, Silveira K, Bai S, Vanstone A. Music-evoked autobiographical memories (MEAMs) in Alzheimer disease: evidence for a positivity effect. Cog Psychol 2017; 4(1): 1277578. [Google Scholar]
- 20.Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychol Sci 2004; 15(3): 208–14. [DOI] [PubMed] [Google Scholar]
- 21.Mikels JA, Larkin GR, Reuter-Lorenz PA, Carstensen LL. Divergent trajectories in the aging mind: changes in working memory for affective versus visual information with age. Psychol Aging 2005; 20(4): 542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychol Sci 2003; 14(5): 409–15. [DOI] [PubMed] [Google Scholar]
- 23.Sava A-A, Krolak-Salmon P, Delphin-Combe F, Cloarec M, Chainay H. Memory for faces with emotional expressions in Alzheimer's disease and healthy older participants: positivity effect is not only due to familiarity. Aging. Neuropsychol Cogn 2017; 24(1): 1–28. [DOI] [PubMed] [Google Scholar]
- 24.Norton D, McBain R, Chen Y. Reduced ability to detect facial configuration in middle-aged and elderly individuals: associations with spatiotemporal visual processing. J Gerontol B Psychol Sci Soc Sci 2009; 64(3): 328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformations that link facial identity across views. Vision Res. 2008; 48(1): 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megreya AM, Bindemann M. Developmental improvement and age-related decline in unfamiliar face matching. Perception 2015; 44(1): 5–22. [DOI] [PubMed] [Google Scholar]
- 27.Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, et al. Facial expression recognition across the adult life span. Neuropsychologia 2003; 41(2): 195–202. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt A, Wilhelm O, Herzmann G, Sommer W. Face and object cognition across adult age. Psychol Aging 2013; 28(1): 243–48. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrandt A, Wilhelm O, Schmiedek F, Herzmann G, Sommer W. On the specificity of face cognition compared with general cognitive functioning across adult age. Psychol Aging 2011; 26(3): 701–15. [DOI] [PubMed] [Google Scholar]
- 30.Carstensen LL. The influence of a sense of time on human development. Science 2006; 312(5782): 1913–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labouvie-Vief G. Dynamic integration: affect, cognition, and the self in adulthood. Curr Dir Psychol Sci 2003; 12(6): 201–6. [Google Scholar]
- 32.Pratto F, John OP. Automatic vigilance: the attention-grabbing power of negative social information. J Pers Soc Psychol 1991; 61(3): 380–92. [DOI] [PubMed] [Google Scholar]
- 33.Labouvie-Vief G, Diehl M, Jain E, Zhang F. Six-year change in affect optimization and affect complexity across the adult life span: a further examination. Psychol Aging 2007; 22(4): 738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychol Aging 2008; 23(3): 585–94. [DOI] [PubMed] [Google Scholar]
- 35.Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging 2005; 20(4): 554–70. [DOI] [PubMed] [Google Scholar]
- 36.Porter RJ, Robinson LJ, Malhi GS, Gallagher P. The neurocognitive profile of mood disorders–a review of the evidence and methodological issues. Bipolar Disord 2015; 17: 21–40. [DOI] [PubMed] [Google Scholar]
- 37.Cacioppo JT, Berntson GG, Bechara A, Tranel D, Hawkley LC. Could an aging brain contribute to subjective well-being? The value added by a social neuroscience perspective In Oxford Series in Social Cognition and Social Neuroscience. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind (eds Todorov A, Fiske ST, Prentice DA): 249–62. Oxford University Press, 2011. [Google Scholar]
- 38.Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Bäckman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett 2005; 386(2): 99–104. [DOI] [PubMed] [Google Scholar]
- 39.Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiolf Aging 2003; 24(2): 285–95. [DOI] [PubMed] [Google Scholar]
- 40.Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. J Cogn Neurosci 2013; 25(8): 1206–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007; 61(2): 198–209. [DOI] [PubMed] [Google Scholar]
- 42.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57(3): 201–9. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Young RC, Meyers BS. Geriatric depression: age of onset and dementia. Biol Psychiatry 1993; 34(3): 141–5. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulos GS, Young RC, Shindledecker RD. Brain computed tomography findings in geriatric depression and primary degenerative dementia. Biol Psychiatry 1992; 31(6): 591–9. [DOI] [PubMed] [Google Scholar]
- 45.Jacoby RJ, Levy R. Computed tomography in the elderly 3. Affective disorder. Br J Psychiatry 1980; 136(3): 270–5. [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley R, O'Brien J, Barnett N, McGuckin L, Britton P. Distinguishing depression from dementia in later life: a pilot study employing the emotional Stroop task. Int J Geriatr Psychiatry 2002; 17(1): 48–53. [DOI] [PubMed] [Google Scholar]
- 48.Broomfield NM, Davies R, MacMahon K, Ali F, Cross SMB. Further evidence of attention bias for negative information in late life depression. Int J Geriatr Psychiatry 2007; 22(3): 175–80. [DOI] [PubMed] [Google Scholar]
- 49.Callahan BL, Hudon C. Emotional Stroop performance: further evidence for distinct cognitive patterns in depressed and non-depressed aMCI. Alzheimer's & Dement 2014; 10(suppl 4): P434. [Google Scholar]
- 50.Huang C-M, Fan Y-T, Lee S-H, Liu H-L, Chen Y-L, Lin C, et al. Cognitive reserve-mediated neural modulation of emotional control and regulation in people with late-life depression. Soc Cogn Affect Neurosci 2019; 14(8): 849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mah L, Pollock BG. Emotional processing deficits in late-life depression. Am J Geriatr Psychiatry 2010; 18(7): 652–6. [DOI] [PubMed] [Google Scholar]
- 52.Zhou H, Dai B, Rossi S, Li J. Electrophysiological evidence for elimination of the positive bias in elderly adults with depressive symptoms. Front Psychiatry 2018; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savaskan E, Müller SE, Böhringer A, Schulz A, Schächinger H. Antidepressive therapy with escitalopram improves mood, cognitive symptoms, and identity memory for angry faces in elderly depressed patients. Int J Neuropsychopharmacol 2008; 11(3): 381–8. [DOI] [PubMed] [Google Scholar]
- 54.Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buechel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol Psychiatry 2008; 64(4): 349–55. [DOI] [PubMed] [Google Scholar]
- 55.Callahan BL, Simard M, Mouiha A, Rousseau F, Laforce R, Hudon C. Impact of depressive symptoms on memory for emotional words in mild cognitive impairment and late-life depression. J Alzheimers Dis 2016; 52(2): 451–62. [DOI] [PubMed] [Google Scholar]
- 56.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘Reading the Mind in the Eyes’ test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 2001; 42(2): 241–51. [PubMed] [Google Scholar]
- 57.Szanto K, Dombrovski AY, Sahakian BJ, Mulsant BH, Houck PR, Reynolds CF 3rd, et al. Social emotion recognition, social functioning, and attempted suicide in late-life depression. Am J Geriatr Psychiatry 2012; 20(3): 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattis S. Dementia Rating Scale (DRS): Professional Manual. Psychological Assessment Resources, 1988. [Google Scholar]
- 59.Aizenstein H, Andreescu C, Edelman K, Cochran J, Price J, Butters M, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry 2011; 168(10): 1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briceño EM, Rapport LJ, Kassel MT, Bieliauskas LA, Zubieta J-K, Weisenbach SL, et al. Age and gender modulate the neural circuitry supporting facial emotion processing in adults with major depressive disorder. Am J Geriatr Psychiatry 2015; 23(3): 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanyukov PM, Szanto K, Siegle GJ, Hallquist MN, Reynolds CF III, Aizenstein HJ, et al. Impulsive traits and unplanned suicide attempts predict exaggerated prefrontal response to angry faces in the elderly. Am J Geriatr Psychiatry 2015; 23(8): 829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hariri AR, Mattay VS, Tessitore A, Kolachana B, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297(5580): 400–3. [DOI] [PubMed] [Google Scholar]
- 63.Price RB, Siegle G, Mohlman J. Emotional Stroop performance in older adults: effects of habitual worry. Am J Geriatr Psychiatry 2012; 20(9): 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb SA, Diefenbach G, Wagener P, Novy DM, Kunik M, Rhoades HM, et al. Comparison of self-report measures for identifying late-life generalized anxiety in primary care. J Geriatr Psychiatry Neurol 2008; 21(4): 223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohlman J, Price RB, Vietri J. Attentional bias in older adults: effects of generalized anxiety disorder and cognitive behavior therapy. J Anxiety Disord 2013; 27(6): 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry 2011; 1: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu M, Mennin DS, Ly M, Karim HT, Banihashemi L, Tudorascu DL, et al. When worry may be good for you: worry severity and limbic-prefrontal functional connectivity in late-life generalized anxiety disorder. J Affect Disord 2019; 257: 650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karim H, Tudorascu DL, Aizenstein H, Walker S, Good R, Andreescu C. Emotion reactivity and cerebrovascular burden in late-life GAD: a neuroimaging study. Am J Geriatr Psychiatry 2016; 24(11): 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wittekind CE, Muhtz C, Jelinek L, Moritz S. Depression, not PTSD, is associated with attentional biases for emotional visual cues in early traumatized individuals with PTSD. Front Psychol 2015; 5: 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wittekind CE, Muhtz C, Moritz S, Jelinek L. Performance in a blocked versus randomized emotional Stroop task in an aged, early traumatized group with and without posttraumatic stress symptoms. J Behav Ther Exp Psychiatry 2017; 54: 35–43. [DOI] [PubMed] [Google Scholar]
- 71.Epp AM, Dobson KS, Dozois DJ, Frewen PA. A systematic meta-analysis of the Stroop task in depression. Clin Psychol Rev 2012; 32(4): 316–28. [DOI] [PubMed] [Google Scholar]
- 72.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 2018; 195(2): 102–8. [DOI] [PubMed] [Google Scholar]
- 73.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 2012; 37(1): 117–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21(7): 685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 2008; 25(2): 115–26. [DOI] [PubMed] [Google Scholar]
- 76.Goossen B, van der Starre J, van der Heiden C. A review of neuroimaging studies in generalized anxiety disorder: ‘So where do we stand?’. J Neural Transm 2019; 126(9): 1203–16. [DOI] [PubMed] [Google Scholar]
- 77.Schaakxs R, Comijs HC, van der Mast RC, Schoevers RA, Beekman AT, Penninx BW. Risk factors for depression: differential across age? Am J Geriatr Psychiatry 2017; 25(9): 966–77. [DOI] [PubMed] [Google Scholar]
- 78.Penton-Voak IS, Adams S, Button KS, Fluharty M, Dalili M, Browning M, et al. Emotional recognition training modifies neural response to emotional faces but does not improve mood in healthy volunteers with high levels of depressive symptoms. Psychol Med [Epub ahead of print] 17 Feb 2020. Available from: 10.1017/S0033291719004124. [DOI] [PubMed] [Google Scholar]