ABSTRACT
Objective:
The objective of this study was to compare in vitro the antifungal efficacy of the essential oil of Cinnamomum zeylanicum (Canela) (EOC) at 25%, 50%, 75%, and 100% against strains of Candida albicans ATCC 10231.
Materials and Methods:
The design was experimental, in vitro, prospective, and longitudinal study, having a sample of n = 30 petri dishes per six groups. The test was conducted in the microbiology laboratory of the Universidad Nacional Federico Villarreal. The essential oil was prepared by steam distillation, which means that the pressure steam enters in connection with the plant cells and breaks them, releasing the essence and trapping it in drops of water. Cinnamon essential oil was obtained using the hydrodistillation method, subsequently the oil obtained was dehydrated with sodium sulfate and then filtered at 0.22 µm. Then the vials were stored at a temperature of 4°C. Finally, Candida albicans ATCC 10231 was used as the biological material. Antifungal efficacy was measured by the Kirby–Bauer method (disk diffusion).
Results:
It was found that in the 24-h group the concentration that had the greatest antifungal effect was 100% EOC with a mean of 22.1 ± 11 mm; however, the lowest antifungal activity was seen in the 25% EOC with 17.9 ± 1.6 mm. On the contrary, in the 48-h group, it was shown that the highest antifungal efficacy was also observed in the 100% EOC with an average of 31.2 ± 3.2 mm, but the lowest antifungal activity was in the 25% EOC with 22.6 ± 1.7 mm. Although in both groups, both at 24 and 48h, nystatin was the one with the lowest antifungal efficacy 15.1 ± 1.0 and 19.9 ± 0.1 mm, respectively.
Conclusions:
EOC had a better statistically significant antifungal effect compared to nystatin. Otherwise, on analysis of the results in different concentrations, the EOC showed a directly proportional antifungal effectiveness as the concentration against the strains of C. albicans ATCC 10231 increased, compared to nystatin, suggesting its potential use as a possible attractive therapeutic alternative for the control of diseases caused by strains of C. albicans resistant to nystatin.
KEYWORDS: Antifungal efficacy, Candida albicans, Canela, Cinnamomum zeylanicum, essential oil
INTRODUCTION
The oral cavity has multiple diseases such as bacterial, viral, parasitic, or fungal infections. Of the latter, candidiasis is the main species associated with oral mycosis. Today its incidence is increasing in the most advanced countries due to different predisposing factors harmful to humans because the prevalence of candida infections remains a challenge for medicine despite the important advances in the field of antifungal therapy.[1]
Opportunistic microorganisms such as fungi of the genus Candida find a favorable environment in the oral cavity, considering that four of every thousand patients who go to a dental office have symptoms of candidiasis infection.[2,3,4,5] The application of natural medicine has shown excellent results but there is still a great demand for the use of synthetic products, due to the lack of experimentation and investigation of the properties that they possess several medicinal plants, being their utility wasted and reduced. The use of medicinal substances such as essential oils is an application that is currently experiencing a new momentum. The great variety of natural resources such as Cinnamomum zeylanicum (Canela) has empirically and methodologically shown its effectiveness on a number of microorganisms.[6,7]
Since ancient times certain benefits of medicinal plants for healing purposes are known. Natural remedies, and especially medicinal plants, were the primary and even the only remedy available to doctors. Phytotherapy is known as the science that studies the application of products of plant origin with a rehabilitative purpose, be it for prevention, mitigate, or heal a pathological condition. It also refers to the intervention to recover health through the use of herbs with medicinal properties or their derivatives.[8,9]
Traditionally, essential oil is obtained by the steam drag distillation method through solvent extraction and/or maceration and extraction, for which the natural resource must previously be completely dry and clean. Essential oils have a potential use in medicine because they are a complex combination of volatile substances, made from the secondary metabolites of plants, which is integrated in the plant, in a nonuniform way, only in some of its parts, for example, in flowers, seeds, bark, wood, roots, or rhizomes, but more frequently in leaves and stems, and which are obtained from plants by steam dragging, steam distillation, and hydrodistillation of plant material.[7,10,11] For this reason, given the incidence of oral candidiasis, there is an immediate need to work in the prevention and treatment, so we must have therapeutic alternatives with effective and safe effects as attributed to medicinal plants.
Therefore, the aim of this in vitro study was to determine the antifungal efficacy of four different concentrations of the essential oil of C. zeylanicum (Canela) (EOC) against Candida albicans ATCC 10231.
MATERIALS AND METHODS
STUDY DESIGN
The design was an experimental in vitro, longitudinal, and comparative study. The unit of analysis consisted of a petri dish inoculated with strains of C. albicans ATCC 10231. The sample size was determined using the mean comparison formula, for which the Stata 15 Statistical Software (StataCorp, College Station, Texas) was used with an α of 0.05 and a β of 0.8. An n = 30 discs were determined for each experimental group.
Finally, this research was carried out at the microbiology laboratory of the Faculty of Dentistry of the Universidad Nacional Federico Villarreal, and the following groups were formed:
Group 1: Petri dishes with 25% EOC inoculated with C. albicans
Group 2: Petri dishes with 50% EOC inoculated with C. albicans
Group 3: Petri dishes with 75% EOC inoculated with C. albicans
Group 4: Petri dishes with 100% EOC inoculated with C. albicans
Group 5: Nystatin (Mycostatin) oral suspension 100.000 U/mL
Group 6: Physiological serum 0.9% Baxter (ClearFlex) solution for infusion as negative control
CHEMICALS, MEDIUMS, AND MICROORGANISM
C. albicans ATCC 10231 strain was obtained from Laboratory Gen Lab from Peru S.A.C. Sabouraud dextrose, Mueller Hinton agar, and sodium sulfate (Na2SO4) anhydrous (Merck, Darmstadt, Germany) were used, which were prepared according to the same manufacturer’s standards.
PLANT COLLECTION
Cinnamon was imported from the Democratic Socialist Republic of Sri Lanka (Latitude: 7°52’ 38.72” N and longitude: 80°42’1.23”E). So, 1000 g of cinnamon bark was collected, identified, and verified that there were no signs of any morphological alteration [Figure 1]. Later it was transported to the pharmacy and biochemistry laboratory of the Universidad Nacional Mayor San Marcos and Universidad Nacional Federico Villarreal, Peru, with voucher no. 301-2018-DA-FO-UNFV, where it was stored in Kraft paper bags to avoid an influence of the environment on its properties.
Figure 1.

Cinnamomum zeylanicum (Canela) powdered
PREPARATION OF ESSENTIAL OIL
Cinnamon essential oil was obtained using the hydrodistillation method, subsequently the oil obtained was dehydrated with Na2SO4 and then filtered at 0.22 µm. Then the vials were stored at a temperature of 4°C. All the analyses were obtained at an initial temperature of 50°C with helium gas as a vehicle. The hydrolate, the result of the distillate, was separated, taking into account its properties of immiscibility and density difference between water and essential oil, using a glass pear-shaped separating funnel, dehydrating water, and essential oil impurities with Na2SO4 anhydrous; the pure oil was filtered and stored at −20°C until use. The essential oil concentration preparations were made using the Tween solution also known as polysorbate because they are polyoxyethylene sorbitan esters, whose main characteristics are brownish yellow, oily liquid and water-soluble, anhydrous ethanol and methanol.With a density of 1.10 g/ml; HLB (Hydrophilic-Lipophilic Balance) of 16.7; and a molecular weight of 1227.5. Then the concentrations of cinnamon essential oils were placed in a water bath at 37°C to avoid solidification.
FUNGAL CULTURE
Subsequently, from the result of the extraction of the essential oil, 25%, 50%, 75%, and 100% dilutions were prepared, placed in different bottles and properly labeled. The sowings were carried out in Saboraud dextrose agar for the cultivation of fungi (yeasts and dermatophytes). Once the cultures were done, they were stored in the incubator at 37°C for a period of 24 hours. After having achieved the growth of bacterial colonies, sensitivity tests were carried out. The petri dishes were conditioned with the Mueller Hinton agar, in which each plate was seeded with C. albicans ATCC 10231. Next, five wells were made in each petri dish: four wells for the four concentrations of the EOC and the last one for the nystatin. Finally, the inhibition halos were measured with a Mitutoyo calibrator, at 24- and 48-h intervals.
ANTIFUNGAL ACTIVITY
Direct observation was made, and the measurement of the inhibitory halo (Kirby–Bauer method)[6,8,9,10] of four concentrations at 25%, 50%, 75%, and 100% of the EOC and nystatin on strains of Candida albicans ATCC 10231 was taken; the data obtained was recorded in a data collection sheet where the inhibition halos measurements in millimeters, formed in each of the discs embedded with the EOC. In the period of 24 and 48h of having made the sowing, the readings and filling of the collection sheet were made, taking into account the identification of the sample, identification of the concentration used, and the measurement in millimeters of the halo of inhibition formed [Figure 2].
Figure 2.
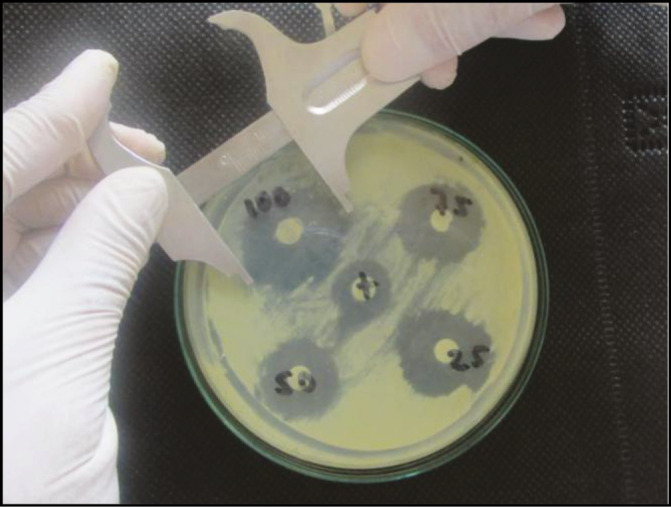
Measurement of halos inhibiting the antifungal efficacy of Cinnamomum zeylanicum (Canela)
STATISTICAL ANALYSIS
Data was analyzed using Stata® 15 statistical program (College Station, Texas 77845 USA). The normality test was first applied through the Shapiro–Wilk test. To perform the bivariate analysis, the Student t test and analysis of variance (ANOVA) were used for multiple comparisons, and thus obtain a prospective result of a before and after. The level of statistical significance used was P < 0.05.
RESULTS
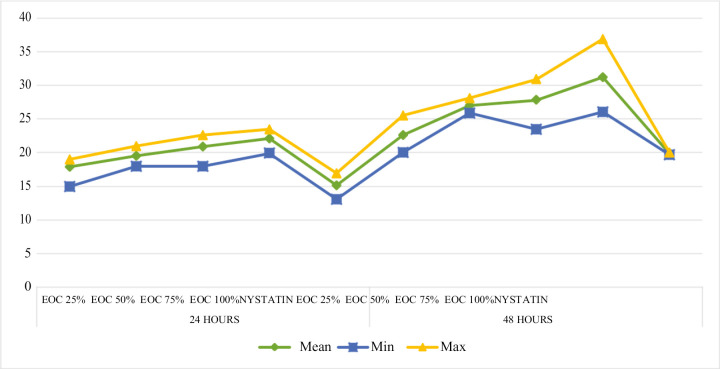
According to Table 1, it was found that in the 24-h group, the concentration that had the greatest antifungal effect was 100% EOC with a mean of 22.1 ± 11 mm; however, the lowest antifungal activity was seen in the 25% EOC with 17.9 ± 1.6 mm. On the contrary, in the 48-h group, it was shown that the highest antifungal efficacy was also observed in the 100% EOC with an average of 31.2 ± 3.2 mm, but the lowest antifungal activity was in the 25% EOC with 22.6 ± 1.7 mm Although in both groups, both at 24 and 48h, nystatin was the one with the lowest antifungal efficacy 15.1 ± 1.0 and 19.9 ± 0.1 mm, respectively [Graph 1].
Table 1.
Antifungal efficacy of the different concentrations of essential oil of Cinnamomum zeylanicum according to time
| Time | Group | Mean | SD | Min | Max | P* |
|---|---|---|---|---|---|---|
| 24 h | EOC 25% | 17.9 | 1.6 | 15.0 | 19.0 | |
| EOC 50% | 19.5 | 1.1 | 18.0 | 21.0 | ||
| EOC 75% | 20.9 | 1.1 | 18.0 | 22.6 | 0.05 | |
| EOC 100% | 22.1 | 1.1 | 19.9 | 23.5 | ||
| Nystatin | 15.1 | 1.0 | 13.1 | 16.9 | ||
| 48 hours | EOC 25% | 22.6 | 1.7 | 20.0 | 25.5 | |
| EOC 50% | 27.0 | 0.8 | 25.9 | 28.1 | ||
| EOC 75% | 27.8 | 2.0 | 23.5 | 30.9 | 0.05 | |
| EOC 100% | 31.2 | 3.2 | 26.0 | 36.9 |
*Shapiro–Wilk test; All groups presented normal distribution, so it was decided to use parametric tests in inferential statistics. The physiological serum group was excluded from any statistical analysis because it did not present any antifungal effect
Graph 1.
Descriptive analysis of the effectiveness of essential oil of Cinnamomum zeylanicum
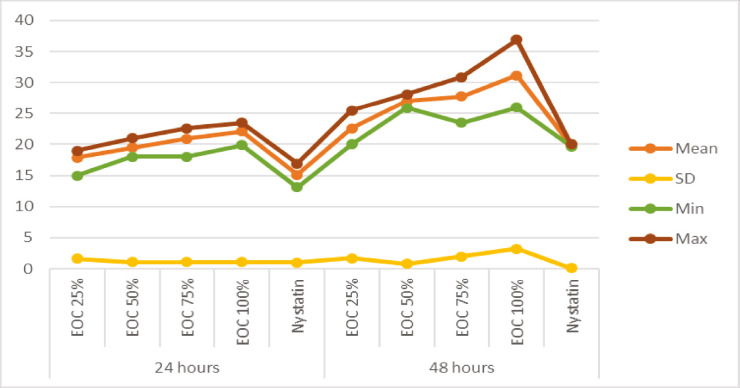
Table 2 shows that when comparing the antifungal efficacy at both 24 and 48h of the four concentrations of EOC (25%, 50%, 75%, and 100%) with nystatin, it was found that there were statistically significant differences (P < 0.001). Otherwise, when comparing the efficacy of each concentration independently at 24 versus 48 hours, statistically significant differences were also found with a P < 0.001 [Graph 2].
Table 2.
In vitro comparison of the antifungal efficacy of the different concentrations of essential oil of Cinnamomum zeylanicum according to concentrations and time
| Time | EOC 25% | EOC 50% | EOC 75% | EOC 100% | Nystatin | P** |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 24 h | 17.9 ± 1.6 | 19.5 ± 1.1 | 20.9 ± 1.1 | 22.1 ± 1.1 | 15.1 ± 1.0 | <0.001 |
| 48 h | 22.6 ± 1.7 | 27.8 ± 0.8 | 27.8 ± 2.0 | 31.2 ± 3.2 | 19.9 ± 0.1 | <0.001 |
| P* | <0.001 | <0.001 | <0.001 | 0.001 | 0.008 |
EOC = Essential oil of Cinnamomum zeylanicum
*Student t test, **ANOVA test; significance level (P < 0.05)
The values were measured through the halos of inhibition (in millimeters) by the Kirby–Bauer technique. The physiological serum group was excluded from any statistical analysis because it did not present any antifungal effect
Graph 2.
In vitro comparison of the antifungal efficacy of the four concentrations of essential oil of Cinnamomum zeylanicum
Table 3 shows that in the post hoc in vitro comparison of the antifungal efficacy of the different concentrations of EOC, statistically significant differences were observed between all the groups evaluated (EOC 25%, EOC 50%, EOC 75%, and EOC 100%) with a P < 0.001.
Table 3.
Post hoc in vitro comparison of the antifungal efficacy of the different concentrations of essential oil of Cinnamomum zeylanicum
| Groups assessed | Concentration | P |
|---|---|---|
| EOC 25% | EOC 50% | 0.001 |
| EOC 75% | 0.001 | |
| EOC 100% | 0.001 | |
| EOC 75% | EOC 25% | 0.001 |
| EOC 50% | 0.001 | |
| EOC 100% | 0.001 | |
| EOC 100% | EOC 25% | 0.001 |
| EOC 50% | 0.001 | |
| EOC 75% | 0.001 | |
| Nystatin | EOC 25% | 0.001 |
| EOC 50% | 0.001 | |
| EOC 75% | 0.001 |
EOC = essential oil of Cinnamomum zeylanicum
*Student t test, and **Bonferroni test; significance level (P < 0.05)
The values were measured through the halos of inhibition (in mm) by the Kirby–Bauer technique. The physiological serum group was excluded from any statistical analysis because it did not present any antifungal effect
DISCUSSION
One of the drawbacks in the success of the treatment of diseases is the resistance that microorganisms develops against the use of conventional medicines, so the need arises to develop antifungal compounds with high activity and low toxicity. For this reason, research is initiated on plants and their components as potential antifungal agents.[12,13,14] Traditionally, it has been discovered that the essential oils of aromatic plants are excellent therapeutic agents for treating fungal ailments. Candida species resistant to certain antifungals are an emerging problem.[15]
When contrasting the results of this research with the literature, it was evident that there are studies that explored the properties of the EOC against different microorganisms associated with infections.[16,17,18,19,20] For example, in the trial of Farisa Banu et al.,[1] they evaluated the growth curve for Candida spp. and thus corroborated the antifungal efficacy, and found that there was a significant reduction in fungus growth after 24h of incubation. Similarly, Shahina et al.[2] showed that cinnamon bark extract (C. zeylanicum) had inhibitory activity against C. albicans, although more studies are needed to understand this phenomenon. This is probably due to the fact that the essential oil used in that investigation was composed of terpenoids, phenols, and aldehydes, which could suggest that essential oils can serve as new antifungal substances to fight resistant germs.
However, a study by Rangel et al.[5] showed the presence of ergosterol in the case of Candida tropicalis species; the EOC has a weak activity on the permeability of the cell membrane. This effect was not found in the C. albicans strains because the presence of ergosterol was not associated with the increase in the minimum inhibitory concentration (MIC). Also, the essential oil extracted from the leaves of C. zeylanicum Blume had eugenol as a component, which gave him his antifungal action on Candida spp., although the performance of phase I and II clinical trials should be tested on oral fungi so scientifically conclude. The study highlighted the anti-candida potency of the essential oil of Thymus capitatus against Candida species sensitive to fluconazole.
On the contrary, the essential oil of Pelargonium graveolens and Cinnamomum verum showed a synergistic effect with the antifungal drug fluconazole against the strain of C. albicans resistant to fluconazole. These combinations could be effective in increasing the efficacy of fluconazole. The synergistic activity of the essential oil of C. verum and P. graveolens with fluconazole could involve multiple sites in Candida cells. P. graveolens is directed to cell wall synthesis by inhibiting fatty acid biosynthesis and improving fluconazole entry into Candida cells that lead to ergosterol inhibition.[3]
According to Wang et al.,[12] who investigated the antifungal mechanisms and healing effects of the cinnamon oil and pogostemon oil complexes toward intestinal infections by Candida, the average MIC values of the fungal complexes were found to be 0.064mg/mL (cinnamon oil), 0.032mg/mL (pogostemon oil) for C. albicans; therefore, it was concluded that the cinnamon oils and pogostemon oil had strong antifungal effects against C. albicans, C. tropicalis, and Candida krusei, and they impacted the morphology and sub-microstructures of the fungus at 48–72h, and finally denatured and killed the cells. As a result of this investigation, they observed that the aqueous cinnamon extract had no antifungal effect, whereas the EOC had positive results on C. albicans. These results together with that of other studies[1,2,5,7] are consistent with our research, because the essential oil of C. zeylanicum (Canela) obtained a greater antifungal effectiveness, forming a greater halo of inhibition compared with nystatin in the 24-h period.
In this research, it was decided to use nystatin as positive control group because it’s one of the medications more prescribed due their great scientific support, for this reason for comparative purposes with our results, also used in this medicine as a control group. The main limitation of this research was that only antifungal efficacy was shown against one strain and against a single positive control group; however, it is important to compare the efficacy of this natural resource against other antifungals. Another limitation was that only the antifungal efficacy of cinnamon was evaluated, and it was not verified whether there is a synergism with other Peruvian natural resources[21,22] that can also be easily obtained in the national territory. However, despite all these limitations, this study was performed successfully. Finally, it is recommended to conduct studies with other antifungal medications, to contrast the behavior when compared with the EOC. In addition, the EOC should be assessed against other fungal strains resistant to traditional medicines. Finally, “in vivo” studies should be carried out to assess the effectiveness and toxicity that the active components of C. zeylanicum (cinnamon) can provide and to check if the “in vitro” results are similar.
CONCLUSION
According to the results found in this study, it is concluded that the EOC has a better statistically significant antifungal effect compared to nystatin. Also, on analysis of the results in different concentrations, the EOC showed a directly proportional antifungal effectiveness as the concentration against the strains of C. albicans ATCC 10231 increased, compared to nystatin, suggesting its potential use as a possible attractive therapeutic alternative for the control of diseases caused by strains of C. albicans resistant to nystatin.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
None to declare.
AUTHORS CONTRIBUTIONS
Study conception (RH, NP), data collection (RH, NP, FM, WG), data acquisition and analysis (DAT, FMT, LV), data interpretation (WG, FM, FMT), manuscript writing (FMT, DAT, FM, LV).
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
This project is exempted from ethical approval due to it was an experimental in vitro study.
PATIENT DECLARATION OF CONSENT
Not applicable.
DATA AVAILABILITY STATEMENT
The data that support the study results are available from the author (Dr. Frank Mayta-Tovalino, e-mail: fmaytat@ucientifica.edu.pe) on request.
ACKNOWLEDGEMENTS
We acknowledge the Universidad Cientifica del Sur (UCSUR) for constantly supporting us in the elaboration of the manuscript.
REFERENCES
- 1.Farisa Banu S, Rubini D, Shanmugavelan P, Murugan R, Gowrishankar S, Karutha Pandian S, et al. Effects of patchouli and cinnamon essential oils on biofilm and hyphae formation by Candida species. J Mycol Med. 2018;28:332–9. doi: 10.1016/j.mycmed.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Shahina Z, El-Ganiny AM, Minion J, Whiteway M, Sultana T, Dahms TES. Cinnamomum zeylanicum bark essential oil induces cell wall remodelling and spindle defects in Candida albicans. Fungal Biol Biotechnol. 2018;5:3. doi: 10.1186/s40694-018-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essid R, Hammami M, Gharbi D, Karkouch I, Hamouda TB, Elkahoui S, et al. Antifungal mechanism of the combination of Cinnamomum verum and Pelargonium graveolens essential oils with fluconazole against pathogenic Candida strains. Appl Microbiol Biotechnol. 2017;101:6993–7006. doi: 10.1007/s00253-017-8442-y. [DOI] [PubMed] [Google Scholar]
- 4.Pires RH, Montanari LB, Martins CH, Zaia JE, Almeida AM, Matsumoto MT, et al. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia. 2011;172:453–64. doi: 10.1007/s11046-011-9448-0. [DOI] [PubMed] [Google Scholar]
- 5.Rangel ML, de Aquino SG, de Lima JM, Castellano LR, de Castro RD. In vitro effect of Cinnamomum zeylanicum Blume essential oil on Candida spp. involved in oral infections. Evid Based Complement Alternat Med. 2018;2018:4045013. doi: 10.1155/2018/4045013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poma-Castillo L, Espinoza-Poma M, Mauricio F, Mauricio-Vilchez C, Alvítez-Temoche D, Mayta-Tovalino F. Antifungal activity of ethanol-extracted Bixa orellana (L) (Achiote) on Candida albicans, at six different concentrations. J Contemp Dent Pract. 2019;20:1159–63. [PubMed] [Google Scholar]
- 7.Soares IH, Loreto ÉS, Rossato L, Mario DN, Venturini TP, Baldissera F, et al. In vitro activity of essential oils extracted from condiments against fluconazole-resistant and -sensitive Candida glabrata. J Mycol Med. 2015;25:213–7. doi: 10.1016/j.mycmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Mayta-Tovalino F, Gamboa E, Sánchez R, Rios J, Medina R, García M, et al. Development and formulation of the experimental dentifrice based on Passiflora mollissima (Tumbo) with and without fluoride anion: Antibacterial activity on seven antimicrobial strains. Int J Dent. 2019;2019:9056590. doi: 10.1155/2019/9056590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderon A, Salas J, Dapello G, Gamboa E, Rosas J, Chávez J, et al. Assessment of antibacterial and antifungal properties and in vivo cytotoxicity of Peruvian Passiflora mollisima. J Contemp Dent Pract. 2019;20:145–51. [PubMed] [Google Scholar]
- 10.Mayta-Tovalino F, Sedano-Balbin G, Romero-Tapia P, Alvítez-Temoche D, Álvarez-Paucar M, Gálvez-Calla L, et al. Development of new experimental dentifrice of Peruvian Solanum tuberosum (Tocosh) fermented by water stress: Antibacterial and cytotoxic activity. J Contemp Dent Pract. 2019;20:1206–11. [PubMed] [Google Scholar]
- 11.Miller AB, Cates RG, Lawrence M, Soria JA, Espinoza LV, Martinez JV, et al. The antibacterial and antifungal activity of essential oils extracted from Guatemalan medicinal plants. Pharm Biol. 2015;53:548–54. doi: 10.3109/13880209.2014.932391. [DOI] [PubMed] [Google Scholar]
- 12.Wang GS, Deng JH, Ma YH, Shi M, Li B. Mechanisms, clinically curative effects, and antifungal activities of cinnamon oil and pogostemon oil complex against three species of Candida. J Tradit Chin Med. 2012;32:19–24. doi: 10.1016/s0254-6272(12)60026-0. [DOI] [PubMed] [Google Scholar]
- 13.Pozzatti P, Scheid LA, Spader TB, Atayde ML, Santurio JM, Alves SH. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can J Microbiol. 2008;54(11):950–6. doi: 10.1139/w08-097. [DOI] [PubMed] [Google Scholar]
- 14.López P, Sánchez C, Batlle R, Nerín C. Development of flexible antimicrobial films using essential oils as active agents. J Agric Food Chem. 2007;55:8814–24. doi: 10.1021/jf071737b. [DOI] [PubMed] [Google Scholar]
- 15.Quale JM, Landman D, Zaman MM, Burney S, Sathe SS. In vitro activity of Cinnamomum zeylanicum against azole resistant and sensitive Candida species and a pilot study of cinnamon for oral candidiasis. Am J Chin Med. 1996;24:103–9. doi: 10.1142/S0192415X96000153. [DOI] [PubMed] [Google Scholar]
- 16.Salma U, Saha SK, Sultana S, Ahmed SM, Haque SD, Mostaqim S. The antibacterial activity of ethanolic extract of cinnamon (Cinnamomum zeylanicum) against two food borne pathogens: Staphylococcus aureus and Escherichia coli. Mymensingh Med J. 2019;28:767–72. [PubMed] [Google Scholar]
- 17.Kerekes EB, Vidács A, Takó M, Petkovits T, Vágvölgyi C, Horváth G, et al. Anti-biofilm effect of selected essential oils and main components on mono- and polymicrobic bacterial cultures. Microorganisms. 2019;7:E345. doi: 10.3390/microorganisms7090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abeysekera WPKM, Arachchige SPG, Abeysekera WKSM, Ratnasooriya WD, Medawatta HMUI. Antioxidant and glycemic regulatory properties potential of different maturity stages of leaf of ceylon cinnamon (Cinnamomum zeylanicum Blume) in vitro. Evid Based Complement Alternat Med. 2019;2019:2693795. doi: 10.1155/2019/2693795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latti P, Ramanarayanan S, Prashant GM. Antifungal efficacy of spice extracts against candida albicans: An in vitro study. Indian J Community Med. 2019;44:77–80. doi: 10.4103/ijcm.IJCM_140_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel N, Rohilla H, Singh G, Punia P. Antifungal activity of cinnamon oil and olive oil against Candida spp. isolated from blood stream infections. J Clin Diagn Res. 2016;10:DC09–11. doi: 10.7860/JCDR/2016/19958.8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina-Flores D, Ulloa-Urizar G, Camere-Colarossi R, Caballero-García S, Mayta-Tovalino F, del Valle-Mendoza J. Antibacterial activity of Bixa orellana L. (Achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pacific J Tropical Biomed. 2016;6:400–3. [Google Scholar]
- 22.Camere-Colarossi R, Ulloa-Urizar G, Medina-Flores D, Caballero-García S, Mayta-Tovalino F, del Valle-Mendoza J. Antibacterial activity of Myrciaria dubia (Camu Camu) against Streptococcus mutans and Streptococcus sanguinis. Asian Pacific J Tropical Biomed. 2016;6:740–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the study results are available from the author (Dr. Frank Mayta-Tovalino, e-mail: fmaytat@ucientifica.edu.pe) on request.