Abstract
Recent studies show decreasing prostate-specific antigen utilization and increasing incidence of metastatic prostate cancer in the United States after national recommendations against screening in 2012. Yet, whether the increasing incidence of metastatic prostate cancer is consistent in magnitude with the expected impact of decreased screening is unknown. We compared observed incidence of metastatic prostate cancer from the Surveillance, Epidemiology, and End Results program and published effects of continued historical screening and discontinued screening starting in 2013 projected by 2 models of disease natural history, screening, and diagnosis. The observed rate of new metastatic prostate cancer cases in 2017 was 44%-60% of the projected increase under discontinued screening relative to continued screening. Thus, the observed increase in incident metastatic prostate cancer is consistent with the expected impact of reduced screening. Although this comparison does not establish a causal relationship, it highlights the plausible role of decreased screening in the observed trend.
Over the 2 decades following the adoption of prostate-specific antigen (PSA) screening in the United States, there was a 66% decline in the incidence of newly diagnosed metastatic prostate cancer among men aged 50-84 years (1). This decline in metastatic disease suggested that PSA screening detected many cancers early while they were still localized and could be treated with curative intent. A pooled analysis of large randomized screening trials confirmed that early detection statistically significantly reduces prostate cancer deaths (2). Now, the nation faces the opposite situation, with a declining utilization of PSA screening and increasing incidence of metastatic prostate cancers.
Because of concerns about overdetection, treatment morbidity, and limited short-term absolute mortality benefit (3), the US Preventive Services Task Force (USPSTF) recommended against PSA screening for men aged 75 years and older in 2008 and for all ages in 2012 (4,5). According to the National Health Interview Survey, between 2008 and 2015 there was a nearly 10% decrease in the proportion of men aged 50 years and older who said they had been screened in the prior 12 months (6). Jemal and colleagues recently estimated an annual 5% increase in incident metastatic prostate cancer from 2010 to 2016 among men aged 50 years and older (7). Contemporary data from the Surveillance, Epidemiology, and End Results (SEER) program demonstrate that newly diagnosed metastatic prostate cancer cases increased by 39% in the 5 years after the 2012 USPSTF recommendation (8).
In this brief communication, we examined whether the rising incidence of advanced prostate cancer in the United States is consistent in magnitude with the predicted impact of reduced screening after the USPSTF recommendations in 2008 and 2012. Specifically, we reexamined metastatic incidence projections from 2 models of prostate cancer progression and detection published soon after the 2012 USPSTF recommendation (9).
The Fred Hutchinson Cancer Research Center and University of Michigan models estimate transitions from preclinical to clinical and from localized to advanced prostate cancer. Although they make different assumptions about underlying natural history, both models were calibrated to prostate cancer incidence rates from SEER under a reconstruction of historical PSA screening (10). The models previously projected new diagnoses of metastatic disease under 1) a continuation of historical PSA screening and 2) discontinued PSA screening starting January 1, 2013 (9). In this analysis, we superimpose observed metastatic incidence rates from SEER through 2017 over these model projections. For either model, we calculate the rise in observed metastatic incidence relative to these 2 extreme scenarios.
Figure 1 shows incidence of metastatic prostate cancer projected by the models under a continuation of historical PSA screening and under discontinued screening starting in 2013, with observed incidence from SEER superimposed. Neither model fully captured the drop in metastatic incidence after PSA screening started, possibly because of the models accounting only for the effects of screening and not for other influences on early detection, such as increasing awareness of prostate cancer by patients and providers (11). Yet, both models’ projections were close to observed rates in 2012.
Figure 1.
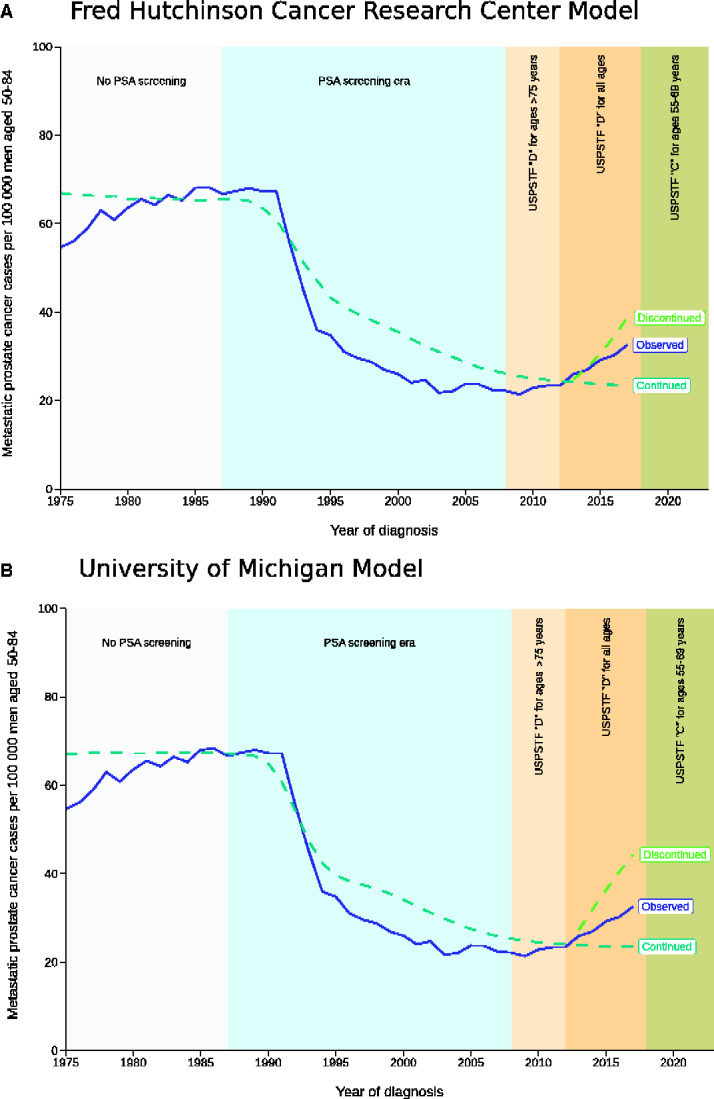
Metastatic prostate cancer incidence rates per 100 000 men aged 50-84 years over the period 1975-2017 from the Surveillance, Epidemiology and End Result program and projections from 2 prostate cancer models under a continuation of historical screening and discontinued screening beginning January 1, 2013. (A) This graph shows projections from the Fred Hutchinson Cancer Research Center model. (B) This graph shows projections from the University of Michigan model. PSA = prostate-specific antigen; USPSTF = US Preventive Services Task Force.
In 2017, the observed rate was 32.6 new cases per 100 000, a 39% increase over the 23.4 per 100 000 projected by both models under a continuation of historical screening. Under discontinued screening, the models projected 44.3 (University of Michigan) or 38.7 (Fred Hutchinson Cancer Research Center) new cases per 100 000. Thus, the observed rate was 44%-60% of the increase under discontinued screening relative to continued screening. Because it falls midway between the bounds projected under these extreme scenarios, we conclude that the observed incidence of metastatic prostate cancer is consistent with the expected effect of reduced PSA screening.
This comparison shows that recent rises in advanced prostate cancer correspond with the effects of decreased screening anticipated by 2 disease models. These findings do not establish causality. Nonetheless, they show that the magnitude of the recent rise in the incidence of advanced disease is consistent with the expected impact of reduced screening following national recommendations against routine PSA screening.
Whether the observed increase in incident metastatic prostate cancer is a public health concern depends on the population still being screened. Has screening been reduced in all men, or is it now more concentrated among the men most likely to benefit (12)? According to the National Health Interview Survey from 2008 to 2015, the percentage of men who received a PSA test in the prior 12 months decreased more among men at least 75 years old when compared with men aged 50-74 years; however, the percentage of men screened in the older group remains higher than that in the younger group (35% vs 30%) (6). Some older men, particularly those with long life expectancy and few prevalent comorbidities, may benefit from early detection and treatment. However, benefit is more likely in younger men, so the higher percentage of men screened at older ages than at younger ages is concerning.
Is the observed increase in incident metastatic prostate cancer an acceptable cost for the benefit of reducing overdiagnosis and overtreatment? This depends on the magnitudes of reduced overdetection and overtreatment—which are impossible to quantify with certainty—and whether this increase confers substantial increases in mortality. Quality-of-life and economic concerns should likewise be considered. Symptomatic metastatic prostate cancer is morbid and costly. The standard treatment—androgen deprivation—is associated with hot flashes, cardiovascular disease, cognitive impairment, and sexual side effects (13). And new first-line therapies for metastatic disease cost between $80 000 and $500 000 per treatment (14). Thus, increases in metastatic diagnosis have implications beyond potential increases in prostate cancer deaths.
In summary, the observed increase in incident metastatic prostate cancer is consistent with model predictions of the impact of decreased PSA screening on diagnosis. With the revised 2018 USPSTF recommendation for shared decision-making between men aged 55-69 years and their providers regarding PSA screening (15), epidemiological surveys and modeling studies will continue to help analyze and interpret trends. Our objective should be to determine whether we are successfully balancing the harms of screening with the harms of not screening, including the impactful harm of increasing incident metastatic prostate cancer.
Funding
This work was supported by a postdoctoral fellowship award from the Department of Defense (grant number CDMRP W81XWH1910577 to YAN) and by the National Cancer Institute at the National Institutes of Health (grant numbers U01 CA199338 to RE and R50 CA221836 to RG).
Notes
Role of the funder: The funding agencies had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors declare no potential conflicts of interest.
Author contributions: Conceptualization, YAN, RG, and RE; Methodology, RG and AT; Formal analysis, RG and AT; Writing - original draft, YAN and RG; Writing - review & editing, YAN, RG, JLG, and RE; Visualization, RG.
Data Availability
The data underlying this article are available in the Harvard Dataverse at https://doi.org/10.7910/DVN/QQQ6BY.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 9 Regs Research Data, Nov 2006 Sub (1973-2004). National Cancer Institute, DCCPS, Surveillance Research Program. http://ww.seer.cancer.gov. Released April 2007. Accessed May 22, 2020.
- 2. Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167(7):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shoag JE, Nyame YA, Gulati R, Etzioni R, Hu JC. Reconsidering the trade-offs of prostate cancer screening. N Engl J Med. 2020;382(25):2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185–191. [DOI] [PubMed] [Google Scholar]
- 5. Moyer VA; US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 6. Negoita S, Feuer EJ, Mariotto A, et al. Annual Report to the Nation on the Status of Cancer, part II: recent changes in prostate cancer trends and disease characteristics: recent changes in prostate cancer trends. Cancer. 2018;124(13):2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jemal A, Culp MB, Ma J, Islami F, Fedewa SA. Prostate cancer incidence 5 years after US Preventive Services Task Force Recommendations against Screening. JNCI J Natl Cancer Inst. 2020; djaa068. doi:10.1093/jnci/djaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 9 Registries, Nov 2019 Sub (1975-2017). National Cancer Institute, DCCPS, Surveillance Research Program, Released April 2020. http://ww.seer.cancer.gov. Accessed May 22, 2020.
- 9. Gulati R, Tsodikov A, Etzioni R, et al. Expected population impacts of discontinued prostate-specific antigen screening: impacts of discontinued PSA screening. Cancer. 2014;120(22):3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109(9):1877–1886. [DOI] [PubMed] [Google Scholar]
- 11. Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making. 2008;28(3):323–331. [DOI] [PubMed] [Google Scholar]
- 12. Etzioni R, Gulati R. Recent trends in PSA testing and prostate cancer incidence: a look at context. JAMA Oncol. 2016;2(7):955. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836. [DOI] [PubMed] [Google Scholar]
- 14. Wong SE, Everest L, Jiang DM, Saluja R, Chan KKW, Sridhar SS. Application of the ASCO value framework and ESMO magnitude of clinical benefit scale to assess the value of abiraterone and enzalutamide in advanced prostate cancer. J Clin Oncol Pract. 2020;16(2):e201–e210. [DOI] [PubMed] [Google Scholar]
- 15. Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the Harvard Dataverse at https://doi.org/10.7910/DVN/QQQ6BY.


