Abstract
Background
Keloids are characterized by disturbance of fibroblast proliferation and apoptosis, deposition of collagen, and upregulation of dermal inflammation cells. This benign dermal fibro-proliferative scarring condition is a recognized skin inflammation disorder. Chronic inflammation is a well-known contributor to bone loss and its sequelae, osteoporosis. They both shared a similar pathogenesis through chronic inflammation. We assessed whether keloids increase osteoporosis risk through using National Health Insurance Research Database.
Methods
The 42,985 enrolled patients included 8597 patients with keloids but no history of osteoporosis; 34,388 controls without keloids were identified from the general population and matched at a one-to-four ratio by age, gender. Kaplan-Meier method was applied to determine cumulative incidence of osteoporosis. Cox proportional hazard regression analysis was performed after adjustment of covariates to estimate the effect of keloids on osteoporosis risk.
Results
Of the 8597 patients with keloids, 178 (2.07%) patients were diagnosed with osteoporosis while in the 34,388 controls, 587 (1.71%) were diagnosed with osteoporosis. That is, the keloids patients had 2.64-fold higher risk of osteoporosis compared to controls after adjustment for age, gender, Charlson Comorbidity Index and related comorbidities. The association between keloids and osteoporosis was strongest in patients younger than 50 years (hazard ratio = 7.06%) and in patients without comorbidities (hazard ratio = 4.98%). In the keloids patients, a high incidence of osteoporosis was also associated with advanced age, high Charlson Comorbidity Index score, hyperlipidemia, chronic liver disease, stroke, and depression.
Conclusions
Osteoporosis risk was higher in patients with keloids compared to controls, especially in young subjects and subjects without comorbidities.
Keywords: Epidemiology, Inflammation, Keloid, Population-based study, Osteoporosis
Background
Keloids, which are an abnormal response to cutaneous wound healing [1], result from proliferative scars characterized by dermal fibrosis and collagen accumulation [2, 3]. Keloids often grow beyond the surrounding healthy skin and are often refractory to treatment [4]. Keloids are prone to occur in dark-skinned individuals; e.g., the estimated incidence is 4–6% in the general population but is as high as 16% in African cohorts [5]. Although its mechanisms are largely unknown, genetic susceptibility is considered the main factor in the development of this disease due to its high prevalence in certain ethnicities such as the Han-Chinese [6].
“Osteoporosis” is derived from the Greek words for “porous bone”. Osteoporosis is the most common bone disease worldwide and affects millions of people [7]. This disease is characterized by low bone mineral density (BMD) as well as deteriorated bone architecture resulting from breakup of bone homeostasis [8, 9]. Osteoporosis mostly affects middle-aged and elderly or post-menopausal women [10]. Therefore, people with osteoporosis are predisposed to bone fragility and susceptibility to osteoporotic fractures [11] resulting in substantial morbidity and mortality [12]. Additionally, osteoporosis threatens senile health; hence, osteoporosis is a worldwide public health concern.
Growing evidence shows that fibrotic diseases such as pulmonary fibrosis or scleroderma can decrease BMD. In a case-control study of a hospital database, Xie et al [13].reported that pulmonary fibrosis is a risk factor for osteoporosis independent of possible confounding factors. According to recent reports, keloids share some pathobiological features with scleroderma, including inflammation and excessive collagen synthesis [14]. Di Munno et al. and La Montagna et al. reported lower than normal BMD in a study of Caucasian patients with systemic sclerosis [15, 16]. As well, Amira et al reported that patients with systemic sclerosis tended to develop osteoporosis at the distal radium and osteopenia in the lumbar area. Through chronic inflammation, impairment of bone microarchitecture and further bone mass loss develop. Hence, keloids and osteoporosis share the possible common pathophysiology by the release of inflammatory signals.
Until now, no large epidemiological studies have investigated the relationship between keloids and osteoporosis. Therefore, this study used patient data contained in the Taiwan National Health Insurance Research Database (NHIRD) to investigate whether keloid contributes to osteoporosis.
Methods
Data sources
Data were collected from the NHIRD Longitudinal Health Insurance Database (LHID) 2010, a subset of the NHIRD. The LHID2010 comprises data for 1 million beneficiaries randomly selected from all Taiwan National Health Insurance (NHI) beneficiaries enrolled in the NHIRD in 2010. The Taiwan NHI is a single-payer health insurance program implemented in March, 1995. It covers approximately 99% of the 23.74 million residents of Taiwan. The original medical claims in the NHIRD are available to researchers and have been used extensively for epidemiological studies in Taiwan [17]. This study of data from an encrypted secondary database was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (II) 20,160,016) and complied with Declaration of Helsinki guidelines.
All disease diagnoses were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. The analysis included 8597 patients with keloids (ICD9-CM code: 701.4) diagnosed by a dermatologist or plastic surgeon for the first time [18, 19]. The occurrence of keloids was defined as > 2 diagnoses of keloids in ambulatory visits or > 1 diagnoses of keloids in inpatient care. The index date for keloids was defined as the first date of a keloids diagnosis. The analysis excluded patients aged less than 20 years old at the time of diagnosis and patients who had a history of osteoporosis (ICD-9-CM code 733) diagnosed by orthopedics with at least one BMD exam before the index date. For enhanced power in statistical analyses, particularly stratified analysis, a control group of 34,388 patients without keloids were randomly identified and matched at a ratio of 1:4 for age, gender, and index year.
Outcome and definitions of comorbidities
All enrolled participants were followed up until the first diagnosis of osteoporosis, the end of the observation time, or the end of 2010. The occurrence of osteoporosis was defined as > 2 diagnoses of osteoporosis in ambulatory visits or > 1 diagnoses of osteoporosis in inpatient care. The impact of numerous relevant comorbidities and Charlson Comorbidity Index (CCI) scores were analyzed. A relevant comorbidity was defined as a history of diagnosis of any of the following comorbidities in the claims records data before the index date: hyperlipidemia (ICD-9-CM code 272), hypertension (ICD-9-CM codes 401–405), diabetes mellitus (ICD-9-CM code 250), chronic liver disease (ICD-9-CM codes 571.2, 571.4–571.6, 456.0–456.21, 572.2–572.8), chronic kidney disease (ICD-9-CM codes 582,583,585,586 and 588), chronic pulmonary disease (ICD-9-CM codes 490–496), hyperthyroidism (ICD-9-CM code 242), hyperparathyroidism (ICD-9-CM code 252), stroke (ICD-9-CM codes 430–438), depression (ICD-9-CM codes 296.2, 296.3, 300.4 and 311;), alcohol attributed diseases (ICD-9-CM codes 291.0–9, 303, 305.0, 357.5, 425.5, 535.3, 571.0–3, 980.0 and V11.3), obesity (ICD-9-CM code 278), tobacco use disorder (ICD-9-CM code 350.1). Based on the CCI scores, the severity of comorbidities was classified as 0, 1, 2 or > 3, where 0 and > 3 were defined as the lowest and highest severity of comorbidity, respectively.
Statically analysis
First demographic data for the enrolled population were analyzed. Student t test and Wilcoxon rank-sum test were utilized to estimate continuous variables, including mean age and follow-up time (y) while Chi-square test was utilized to examine categorical variables in clinical characteristics between the two cohorts. Kaplan-Meier method was employed to measure cumulative incidence of osteoporosis, and 2-tailed log rank test was utilized to analyze between-group differences. In keloids patients, the survival period was calculated from the index date of keloids until the occurrence of an ambulatory visit or hospitalization for osteoporosis, or the end of the study period (December 31, 2010), whichever came first. Incidence rates of osteoporosis were expressed in 1000 person-years and compared by Poisson regression analyses. Cox proportional hazard regression models were applied to derive the hazard ratio (HR) with corresponding 95% confidence interval (CI) for the association between keloids and the risk of developing osteoporosis after adjusting covariates including age, gender, CCI score, and relevant comorbidities (hyperlipidemia, hypertension, diabetes mellitus, chronic liver disease, chronic kidney disease, chronic pulmonary disease, hyperthyroidism, hyperparathyroidism, stroke, depression, alcohol attributed diseases, obesity, and tobacco use disorder). All analyses in the study were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-tailed p-value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 compares the baseline characteristics of the keloids group and the control group. In total, 8597 keloids patients and 34,388 controls without keloids (mean age 34.6 + 13.5 and 34.7+ 13.7 years, respectively) were enrolled for analysis. Females comprised 62.37% of all participants and approximately half (50.04%) were less than 30 years old. Compared to the control group, the keloids group had a higher CCI score and significantly more patients with related comorbidities.
Table 1.
Baseline characteristics of the keloids group and the control group
| Variables | Keloid | P value | |
|---|---|---|---|
| Yes | No | ||
| N=8597 | N=34,388 | ||
| Osteoporosis patients, n (%) | 178 (2.07) | 587 (1.71) | < 0.05 |
| Period of developing osteoporosis median (IQRa), years | 3.0 (1.2–5.7) | 6.7 (4.1–10.2) | < 0.001 |
| Mean age of osteoporosis (SDb), years | 56.3 (14.0) | 65.5 (14.8) | < 0.001 |
| Age mean (SDb), years | 34.6 (13.5) | 34.7 (13.7) | 0.299 |
| Age group, n (%) | |||
| 20–29 | 4302 (50.04) | 17,208 (50.04) | |
| 30–39 | 1939 (22.55) | 7756 (22.55) | |
| 40–49 | 1194 (13.89) | 4776 (13.89) | |
| 50–59 | 617 (7.18) | 2468 (7.18) | |
| 60–69 | 309 (3.59) | 1236 (3.59) | |
| > 70 | 236 (2.75) | 944 (2.75) | 1.000 |
| Sex, n (%) | |||
| Males | 3235 (37.63) | 12,940 (37.63) | |
| Females | 5362 (62.37) | 21,448 (62.37) | 1.000 |
| Charlson Comorbidity Index, n (%) | |||
| 0 | 2494 (29.01) | 16,286 (47.36) | |
| 1 | 3963 (46.10) | 13,710 (39.87) | |
| 2 | 1389 (16.16) | 3018 (8.78) | |
| ≥3 | 751 (8.74) | 1374 (4.00) | < 0.001 |
| Co-morbidity, n (%) | |||
| Hyperlipidemia | 1771 (20.60) | 4197 (12.20) | < 0.001 |
| Hypertension | 1513 (17.60) | 3818 (11.10) | < 0.001 |
| Diabetes mellitus | 1049 (12.20) | 2533 (7.37) | < 0.001 |
| Chronic liver disease | 2186 (25.43) | 4947 (14.39) | < 0.001 |
| Chronic kidney disease | 568 (6.61) | 1352 (3.93) | < 0.001 |
| Chronic pulmonary disease | 2887 (33.58) | 8370 (24.34) | < 0.001 |
| Hyperthyroidism | 643 (7.48) | 1424 (4.14) | < 0.001 |
| Hyperparathyroidism | 19 (0.22) | 35 (0.10) | 0.005 |
| Stroke | 262 (3.05) | 530 (1.54) | < 0.001 |
| Depression | 1066 (12.40) | 2274 (6.61) | < 0.001 |
| Alcohol attributed disease | 232 (2.70) | 632 (1.84) | < 0.001 |
| Obesity | 227 (2.64) | 591 (1.72) | < 0.001 |
| Tobacco use disorder | 65 (0.76) | 122 (0.35) | < 0.001 |
a IQR Interquartile range; b SD Standard deviation;
Furthermore, the keloids group had a significantly (P< 0.05) higher incidence of osteoporosis compared to the control group. Out of 8597 patients in the keloids group, 178 (2.07%) had osteoporosis. In contrast, out of the 34,388 patients in the control group, 587(1.71%) had osteoporosis. Osteoporosis developed significantly faster in the keloids group (3.0 years) compared to the control group (6.7 years) during subsequent periods. Osteoporosis were diagnosed at a significantly younger age in the keloids group compared to the control group (56.3 vs. 65.5 years, respectively; P< 0.001).
Figure 1 presents the 15-year probability for osteoporosis associated with keloids. Kaplan–Meier survival analysis with a log rank test revealed that keloids was significantly associated with subsequent osteoporosis (P < 0.001).
Fig. 1.
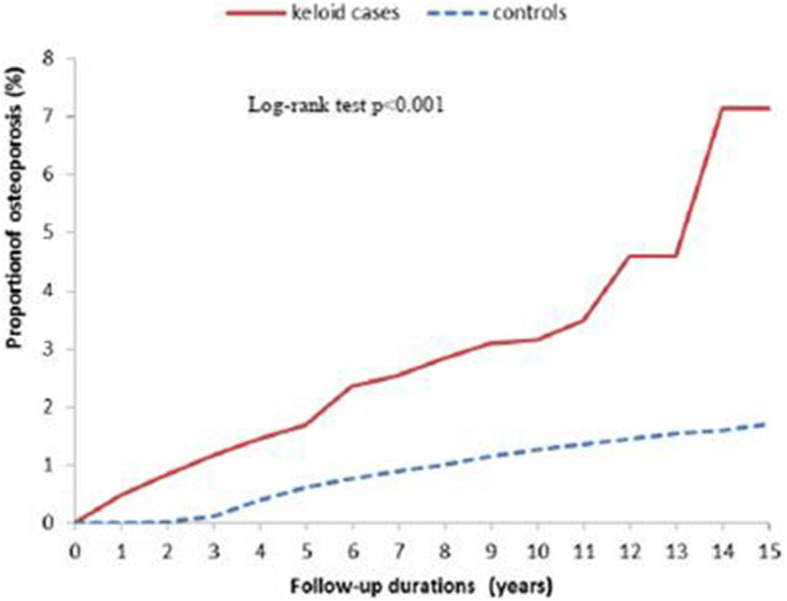
Cumulative incidence of osteoporosis among patients with keloids and the control cohort
Table 2 stratifies the osteoporosis incidence and risk in keloids patients by age, gender and comorbidities.
Table 2.
Keloids associated with osteoporosis stratified by age, gender and comorbidities
| Variables | Patients with keloid | Patients without keloid | Compared with non-keloid | |||||
|---|---|---|---|---|---|---|---|---|
| Osteoporosis | PYs | Rate | Osteoporosis | PYs | Rate | IRR (95% CI) | Adjusted HRa (95% CI) | |
| All | 178 | 49,037.42 | 3.63 | 587 | 511,351.32 | 1.48 | 3.16 (2.67–3.74)* | 2.64 (2.21–3.17)* |
| Gender | ||||||||
| Men | 43 | 18,664.24 | 2.30 | 144 | 193,172.89 | 0.75 | 3.09 (2.19–4.34)* | 2.97 (2.04–4.33)* |
| Women | 135 | 30,373.18 | 4.44 | 443 | 318,178.43 | 1.39 | 3.19 (2.63–3.87)* | 2.56 (2.08–3.15)* |
| Age | ||||||||
| 20–49 | 61 | 43,388.67 | 1.41 | 64 | 445,735.38 | 0.14 | 9.79 (6.89–13.90)* | 7.06 (4.93–10.11)* |
| > 50 | 117 | 5648.75 | 20.71 | 523 | 65,615.94 | 7.97 | 2.59 (2.13–3.18)* | 2.06 (1.67–2.54)* |
| Comorbidity | ||||||||
| No | 9 | 16,842.54 | 0.53 | 40 | 279,314.00 | 0.14 | 3.73 (1.81–7.69)* | 4.98 (2.40–10.29)* |
| Yes | 169 | 32,194.88 | 5.25 | 547 | 232,037.32 | 2.36 | 2.23 (1.87–2.65)* | 2.54 (2.11–3.06)* |
PYs Person-year; Rate, incidence rate in per 1000 person-years; IRR Incidence rate ratio in per 1000 person-years; 95% CI 95% Confidence interval; HR Hazard ratio
a Model adjusted for age, gender, Charlson Comorbidity Index and relevant comorbidities
* P< 0.001
In the keloids group, 178 individuals had osteoporosis, which was an incidence density of 3.63 per 1000 person-years. In the control group, 587 individuals had osteoporosis, which was an incidence density of 1.48 per 1000 person-years. The keloids group had a 2.64-fold greater risk of osteoporosis compared to controls after adjustment for age, gender, CCI score, and related comorbidities.
Gender-specific analyses showed that the incidence of osteoporosis was higher in women than in men in both the keloids group (4.44 vs. 2.30 per 1000 person-years, respectively) and the control group (1.39 vs. 0.75 per 1000 person-years, respectively). Furthermore, osteoporosis risk was significantly higher in the keloids group compared to the control group in both women (adjusted HR=2.56, 95% CI: 2.08–3.15, P< 0.001) and men (adjusted HR=2.97, 95% CI: 2.04–4.33, P< 0.001).
Age-stratified analyses revealed that the incidence of osteoporosis consistently increased with age in both cohorts. Patients younger than 50 years (adjusted HR=7.06, 95% CI=4.93–10.11, P< 0.001) and the elderly aged 50 years and older (adjusted HR=2.06, 95% CI=1.67–2.54,
P< 0.001) were prone to developing osteoporosis. Nevertheless, younger patients were at significantly higher risk than were older patients.
The comorbidity-stratified analyses revealed that the keloids group had a higher risk of osteoporosis compared to the control group. The osteoporosis risk contributed by keloids was decreased in the presence of comorbidity.
Table 3 depicts the risk of developing osteoporosis in keloids patients.
Table 3.
Significant predictors of osteoporosis after keloid
| Variables | Adjusted HR a | (95% CI) | P |
|---|---|---|---|
| Age (in 10-year interval) | 2.07 | (1.85–2.33) | < 0.001 |
| Female gender | 4.17 | (2.91–5.98) | < 0.001 |
| High Charlson Comorbidity Index | 1.53 | (1.23–1.89) | < 0.001 |
| Hyperlipidemia | 1.79 | (1.27–2.53) | < 0.001 |
| Stroke | 1.79 | (1.21–2.66) | < 0.01 |
| Chronic liver disease | 1.54 | (1.12–2.11) | < 0.01 |
| Depression | 1.57 | (1.12–2.19) | < 0.01 |
HR Relative hazard ratio; 95% CI 95% Confidence interval
a Model adjusted for age, gender, Charlson Comorbidity Index and relevant comorbidities
A Cox regression model was used to identify potential risk factors for osteoporosis in keloids patients. Table 3 shows that predictive factors included older age, female gender, high CCI scores, hyperlipidemia, stroke, chronic liver disease, and depression.
The effects of comorbidities on the association between keloids and osteoporosis risk were further explored by adjusting these covariates and stratifying risk by these comorbidities. A comparison of the keloids group and the control group revealed that risk factors for osteoporosis were hyperlipidemia, chronic liver disease, stroke and depression (Table 4).
Table 4.
The osteoporosis incidence and risk in keloids patients with comorbidity
| Variables | Patients with keloids | Patients without keloids | Compared with non-keloids controls | ||||
|---|---|---|---|---|---|---|---|
| Osteoporosis | PY | Rate | Osteoporosis | PY | Rate | Adjusted HR(95% CI)a | |
| Hyperlipidemia | |||||||
| No | 60 | 38,378.15 | 1.56 | 251 | 451,138.90 | 0.57 | 2.48 (1.85–3.33)* |
| Yes | 118 | 10,659.27 | 11.07 | 336 | 60,212.42 | 5.58 | 2.74 (2.20–3.42)* |
| Stroke | |||||||
| No | 143 | 47,551.35 | 3.01 | 501 | 504,095.08 | 0.99 | 2.59 (2.13–3.16)* |
| Yes | 35 | 1486.07 | 23.55 | 86 | 7256.23 | 11.85 | 2.90 (1.95–4.33)* |
| Chronic liver disease | |||||||
| No | 81 | 35,472.65 | 2.28 | 338 | 439,113.30 | 0.77 | 2.34 (1.82–3.01)* |
| Yes | 97 | 13,564.77 | 7.15 | 249 | 72,238.01 | 3.45 | 2.99 (2.34–3.81)* |
| Depression | |||||||
| No | 125 | 42,773.81 | 2.92 | 467 | 478,243.51 | 0.98 | 2.45 (1.98–3.02)* |
| Yes | 53 | 6263.61 | 8.46 | 120 | 33,107.81 | 3.62 | 3.30 (2.37–4.61)* |
PYs Person-year; Rate, incidence rate in per 1000 person-years; 95% CI 95% Confidence interval; HR Hazard ratio
a Model adjusted for age, gender, Charlson Comorbidity Index and relevant comorbidities
* P< 0.001
Table 5 shows the effect of keloids and comorbidities on the risk of osteoporosis development. The analyses suggested that keloids and comorbidities jointly affected the subsequent development of osteoporosis.
Table 5.
Joint effect of keloid and comorbidities on the risk of osteoporosis
| Variables | N | Osteoporosis | Rate | Adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| Keloid | Hyperlipidemia | ||||
| No | No | 30,191 | 251 | 0.56 | 1.00 (Reference) |
| No | Yes | 4197 | 336 | 5.58 | 1.37 (1.13–1.65)** |
| Yes | No | 6826 | 60 | 1.56 | 2.48 (1.85–3.33)** |
| Yes | Yes | 1771 | 118 | 11.07 | 3.75 (2.93–4.80)** |
| Keloid | Chronic liver disease | ||||
| No | No | 29,441 | 338 | 0.77 | 1.00 (Reference) |
| No | Yes | 4947 | 249 | 3.45 | 1.19 (1.00–1.43)# |
| Yes | No | 6411 | 81 | 2.28 | 2.34 (1.82–3.01)** |
| Yes | Yes | 2186 | 97 | 7.15 | 3.56 (2.77–4.58)** |
| Keloid | Stroke | ||||
| No | No | 33,858 | 501 | 0.99 | 1.00 (Reference) |
| No | Yes | 530 | 86 | 11.85 | 1.45 (1.14–1.85)* |
| Yes | No | 8335 | 143 | 3.01 | 2.59 (2.13–3.16)** |
| Yes | Yes | 262 | 35 | 23.55 | 4.21 (2.94–6.03)** |
| Keloid | Depression | ||||
| No | No | 32,114 | 467 | 0.98 | 1.00 (Reference) |
| No | Yes | 2274 | 120 | 3.62 | 1.18 (0.96–1.46)# |
| Yes | No | 7531 | 125 | 2.92 | 2.45 (1.98–3.02)** |
| Yes | Yes | 1066 | 53 | 8.46 | 3.92 (2.90–5.29)** |
a Rate, incidence rate in per 1000 person-years; 95% CI 95% Confidence interval; HR Hazard ratio
* P< 0.01, **P< 0.001, # non-significant
Discussion
The results of this study indicated that keloids were associated with an increased sequential risk of osteoporosis. To the best of our belief, this is the first study designed to examine the association between keloids and osteoporosis in an Asian population. Compared to the control group, the keloids group had a 2.64-fold higher osteoporosis risk after adjusting for covariates. As expected, the incidence of subsequent osteoporosis in keloids patients was higher in females than in males (4.44 vs. 2.30 per 1000 person-years, respectively). The association between keloids and osteoporosis was much stronger in patients younger than 50 years compared to those older than 50 years (HR= 7.06%) and was much stronger in patients without comorbidities compared to those with comorbidities (HR = 4.98%). In keloids patients, old age, female gender, high CCI score, hyperlipidemia, chronic liver disease, stroke and depression were associated with an increased incidence of osteoporosis.
Several possible mechanisms could underlie the increased osteoporosis risk observed in the keloids group. First, 1,25-dihydroxyvitamin D (1,25 (OH)2 D3) is the active metabolite of vitamin D, which is critical in cell proliferation and differentiation, collagen synthesis and degradation [20], hormone secretion, calcium homeostasis as well as bone remodeling [21]. Recent studies show an association between vitamin D and keloids [22]. Zhang et al [23].reported that vitamin D and its metabolites can decrease fibrosis in keloids through vitamin D receptors (VDR) while 1,25 (OH)2 D3 inhibits extracellular matrix deposition and matrix-metalloproteinase activity induced by transforming growth factor (TGF)-β. In 2013, Yu et al [24].reported the presence of TagI gene polymorphisms of VDR and lower levels of serum circulating 1,25 (OH)2 D3 in Chinese patients with keloids. Carriers of the TagI CC genotype had a higher keloids risk and significantly lower serum 1,25 (OH)2 D3 compared to carriers of other genotypes. Gong et al [25]. also reported that high levels of plasminogen activator inhibitor-1 and low levels of VDR expressions were significantly associated with keloids development. Since vitamin D is important for maintaining bone health, vitamin D insufficiency and hyperparathyroidism are the main causes of osteoporosis in men and pre-menopausal women [26]. Low vitamin D levels are independently associated with a 7.5-fold increase in osteoporosis risk [27]. Patients who have prolonged vitamin D deficiency are predisposed to osteoporosis. Therefore, decreased serum vitamin D level may also contribute to osteoporosis risk in keloids patients.
Second, chronic inflammation caused by keloids inhibits osteoblast growth, which can lead to osteoporosis [28]. Arima et al. indicated that hypertension is associated with severity of keloids. In a study of 340 keloids patients, ordinal logistic regression analyses revealed that blood pressure correlated positively with both the number and size of keloids. The results implied that hypertension may aggravate keloids by increasing inflammatory responses in tissues while hypertension damages blood vessels [29, 30]. Moreover, histology studies show that keloids illustrates proliferation of fibroblasts, excess production of collagen, formation of new vessels, and inflammation of dermal cells, which reveals continuous inflammation in abnormal wound healing processes [31, 32]. In comparison with normal skin, keloids have a larger number of T cells, a higher CD4/CD8 ratio [14], and more persistent peripheral blood lymphocytes, which accelerate collagen production by dermal fibroblasts [33]. The release of proinflammatory cytokines (interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α) in keloid tissues increases sensitivity to injury and upregulates these cytokines compared to the general population [34, 35] . Redlich et al [36]. reported that chronic inflammation in the body could influence bone tissue metabolism and eventually cause bone loss. These cytokines inhibit bone collagen synthesis and are potent stimulators of osteoclast-induced bone resorption, which are central in the context of chronic inflammation [37]. Accordingly, keloids increase osteoporosis risk via cytokines produced by chronic inflammation.
Third, micro-RNA (miRNA), a class of non-coding small RNA, can control gene expression by binding target mRNAs and are crucial in cellular hemostasis [38, 39]. Aberrant miRNA expression reportedly contributes to both the development of keloids and the pathogenesis of osteoporosis [40–46]. Additionally, miR-21 has a central role in wound healing [47]. After tissue injury, miR-21 expression is induced by TGF-β1 [48]. The miR-21 is up-regulated in keloid tissues and can regulate keloidal fibroblast proliferation and apoptosis [49–51]. In 2014, Seeliger et al. identified significantly higher than normal expressions of miR-21 in serum and bone tissues in patients with osteoporosis. Additionally, miR-21 reportedly promotes osteoclastogenesis by participating in the RANKL-induced differentiation of osteoclasts [52]. Sugatani et al [53]. also reported that miR-21 has a crucial role in estrogen-controlled osteoclastogenesis. Consequently, micro-RNA has essential contributing roles in the occurrence of keloids and osteoporosis.
Finally, several reports indicate that major depression and depressive symptoms adversely affect bone density, which increases bone fracture risk. The relationship between depression and BMD has also been illustrated in elderly Caucasian women and in Asian men [54, 55]. In recent Taiwan studies, Lee et al. reported that depression patients had a 1.30-fold greater chance of developing osteoporosis compared to controls without depression, and Huang et al. reported that patients with post-traumatic stress disorder had a 2.66-fold higher likelihood of developing osteoporosis compared to controls without this disorder [56]. Previous studies suggest that depression can cause osteoporosis through dysregulation of the hypothalamic-pituitary-adrenocortical axis, parathyroid hormones and cytokines. In Furtado et al. [57], the Hospital Depression and Anxiety Scale administered before and after surgery revealed that psychological stress influenced recurrence of keloids. Therefore, depression is a significant risk factor for osteoporosis in keloids patients, which is consistent with the results of our analyses.
There are several clinical implications for human health in our study. First, physicians might assess keloids patients for osteoporosis from diagnosis to follow-up due to the increased risk. Second, measuring BMD is an easily-performed noninvasive technique and gives accessory glues to assess future fracture risk, which can remind physicians to early recognition of osteoporosis. As keloids increased the risk of osteoporosis, particularly in keloids and comorbidities (hyperlipidemia, chronic liver disease, stroke and depression), physician may arrange the osteoporosis survey for those susceptible patients with complaints to improve outcome and quality of life.
A notable strength of this study is the use of a large-population database, which provided sufficient statistical power. Moreover, use of administrative data eliminated the potential for volunteer or selection bias. Finally, since the majority of patients in the two cohorts were ethnic Chinese, in which human leukocyte antigen polymorphism has a strong impact on keloid susceptibility with different ethnics, further analyses of data for this population may reveal genetic factors in the development of keloids and their role in osteoporosis. However, several limitations of this study should be addressed. First, the database lacked personal information such as tobacco use, dietary supplements, calcium intake, body mass index, physical activity, socioeconomic status, and laboratory data, which could have biased the analyses. Second, the severity (size or number), location and the nature of keloids were not disclosed in the database. Hence, this study could not determine how these factors affect the occurrence of osteoporosis. A further prospective study is needed to validate this association. Third, the information of detailed medication was incomplete in the NHIRD database. The association of keloids and osteoporosis might be confounded by treatment of keloid unless patients receive large cumulative dosage of corticosteroids. However, the total amount of corticosteroids is hard to identified and is usually too small to induce osteoporosis. Therefore, the weight of the confounder by medication to keloids could be diluted by other factors causing by keloids itself.
Conclusions
This study is the first to analyze the association between keloids and osteoporosis risk in an Asian population. The analyses showed that keloids patients had a 2.64-fold higher osteoporosis risk compared to controls, suggesting keloids, a common disorder in Asian, would be an early predictor of osteoporosis. Additionally, among patients with younger age (less than 50 years) and those without comorbidities, the probability of osteoporosis was much higher than in the counterpart. In the keloids group, risk factors for osteoporosis included old age, female gender, high CCI score, hyperlipidemia, chronic liver disease, stroke and depression. Hence, counseling and management are suggested for helping keloids patients improve osteoporosis symptoms (e.g., backache and joint pain) and for preventing known complications (e.g., fractures).
Acknowledgements
Not applicable.
Abbreviations
- BMD
Bone mineral density
- CCI
Charlson comorbidity index
- ICD-9-CM
International classification of diseases, ninth revision, clinical modification
- IL
Interleukin
- LHID
Longitudinal health insurance database
- miRNA
Micro-RNA
- NHIRD
National health insurance research database
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- VDR
Vitamin D receptors
Authors’ contributions
CCL, YYL and CHW conceptualized the study and obtained the research databases. CCL drafted the manuscript. HQ, ZHZ and CLZ reviewed the literatures and interpreted the data. YYL and CHW performed the data analysis, revised the manuscript and supervised the whole study (equal contributors). All authors have read and approved the final manuscript.
Funding
This research work was supported by grants from the Taiwan Ministry of Science and Technology (MOST107–2314-B-075B-009; MOST108–2314-B-075B-001-MY3), Kaohsiung Veterans General Hospital (VGHKS-109-124), Kaohsiung Medical University Hospital (KMUH104- 4R16, KMUH106- 6 T09 and KMUH108-8 M23) and Kaohsiung Medical University (KMU-Q108029). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the Taiwan Personal Information Protection Act, but are available from the corresponding author on reasonable request. All relevant data to support the study findings are provided in this article.
Ethics approval and consent to participate
The NHIRD files contain de-identified secondary data, and encrypt patient personal information to protect privacy. Informed consents from the participants are therefore waived, which does not affect the rights and welfare of the participants. This study was also approved by ethical review board of Kaohsiung Medical University Hospital and was performed according to Declaration of Helsinki guidelines. (KMUHIRB-EXEMPT (II) 20160016). According to the IRB regulations, the need for informed consent was also waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying-Yi Lu and Chieh-Hsin Wu were equal contributors in this study.
Contributor Information
Ying-Yi Lu, Email: actinp@hotmail.com.
Chieh-Hsin Wu, Email: wujoeys@gmail.com.
References
- 1.Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–114. doi: 10.2147/CCID.S35252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray JC, Pollack SV, Pinnell SR. Keloids: a review. J Am Acad Dermatol. 1981;4:461–470. doi: 10.1016/S0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- 3.Wolfram D, Tzankov A, Pulzl P, Piza-Katzer H. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 4.Halim AS, Emami A, Salahshourifar I, Kannan TP. Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg. 2012;39:184–189. doi: 10.5999/aps.2012.39.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Gao JH, Yan X, Song M, Liu XJ. Location of predisposing gene for one Han Chinese keloid pedigree. Zhonghua Zheng Xing Wai Ke Za Zhi. 2007;23:137–140. [PubMed] [Google Scholar]
- 7.Pavone V, Testa G, Giardina SMC, Vescio A, Restivo DA, et al. Pharmacological therapy of osteoporosis: a systematic current review of literature. Front Pharmacol. 2017;8:803. doi: 10.3389/fphar.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W, Shen G, Ren H, Liang D, Yu X, et al. Therapeutic potential of microRNAs in osteoporosis function by regulating the biology of cells related to bone homeostasis. J Cell Physiol. 2018;233:9191–9208. doi: 10.1002/jcp.26939. [DOI] [PubMed] [Google Scholar]
- 9.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-95. [DOI] [PubMed]
- 10.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 11.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Lyles CR, Schafer AL, Seligman HK. Income, food insecurity, and osteoporosis among older adults in the 2007-2008 National Health and nutrition examination survey (NHANES) J Health Care Poor Underserved. 2014;25:1530–1541. doi: 10.1353/hpu.2014.0174. [DOI] [PubMed] [Google Scholar]
- 13.Xie Z, He Y, Sun Y, Lin Z, Yang M, et al. Association between pulmonary fibrosis and osteoporosis in the elderly people: a case-control study. Medicine (Baltimore) 2016;95:e5239. doi: 10.1097/MD.0000000000005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R, et al. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol. 2012;167:1053–1066. doi: 10.1111/j.1365-2133.2012.11190.x. [DOI] [PubMed] [Google Scholar]
- 15.La Montagna G, Vatti M, Valentini G, Tirri G. Osteopenia in systemic sclerosis. Evidence of a participating role of earlier menopause. Clin Rheumatol. 1991;10:18–22. doi: 10.1007/BF02208027. [DOI] [PubMed] [Google Scholar]
- 16.Di Munno O, Mazzantini M, Massei P, Ferdeghini M, Pitaro N, et al. Reduced bone mass and normal calcium metabolism in systemic sclerosis with and without calcinosis. Clin Rheumatol. 1995;14:407–412. doi: 10.1007/BF02207673. [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Lu YY, Lu CC, Su YF, Tsai TH, et al. Osteoporosis in adult patients with atopic dermatitis: A nationwide population-based study. PLoS One. 2017;12:e0171667. doi: 10.1371/journal.pone.0171667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu YY, Lu CC, Yu WW, Zhang L, Wang QR, et al. Keloid risk in patients with atopic dermatitis: a nationwide retrospective cohort study in Taiwan. BMJ Open. 2018;8:e022865. doi: 10.1136/bmjopen-2018-022865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun LM, Wang KH, Lee YC. Keloid incidence in Asian people and its comorbidity with other fibrosis-related diseases: a nationwide population-based study. Arch Dermatol Res. 2014;306:803–808. doi: 10.1007/s00403-014-1491-5. [DOI] [PubMed] [Google Scholar]
- 20.Humbert PG, Dupond JL, Rochefort A, Vasselet R, Lucas A, et al. Localized scleroderma--response to 1,25-dihydroxyvitamin D3. Clin Exp Dermatol. 1990;15:396–398. doi: 10.1111/j.1365-2230.1990.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 21.Bikle DD. Vitamin D and bone. Curr Osteoporos Rep. 2012;10:151–159. doi: 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DE, Trowbridge RM, Ayoub NT, Agrawal DK. High-mobility group box Protein-1, matrix Metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast Reconstr Surg Glob Open. 2015;3:e425. doi: 10.1097/GOX.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang GY, Cheng T, Luan Q, Liao T, Nie CL, et al. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol. 2011;164:729–737. doi: 10.1111/j.1365-2133.2010.10130.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu D, Shang Y, Luo S, Hao L. The TaqI gene polymorphisms of VDR and the circulating 1,25-dihydroxyvitamin D levels confer the risk for the keloid scarring in Chinese cohorts. Cell Physiol Biochem. 2013;32:39–45. doi: 10.1159/000350121. [DOI] [PubMed] [Google Scholar]
- 25.Gong ZH, Ji JF, Yang J, Xiang T, Zhou CK, et al. Association of plasminogen activator inhibitor-1 and vitamin D receptor expression with the risk of keloid disease in a Chinese population. Kaohsiung J Med Sci. 2017;33:24–29. doi: 10.1016/j.kjms.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 27.Graat-Verboom L, Smeenk FW, van den Borne BE, Spruit MA, Jansen FH, et al. Progression of osteoporosis in patients with COPD: a 3-year follow up study. Respir Med. 2012;106:861–870. doi: 10.1016/j.rmed.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 29.Arima J, Huang C, Rosner B, Akaishi S, Ogawa R. Hypertension: a systemic key to understanding local keloid severity. Wound Repair Regen. 2015;23:213–221. doi: 10.1111/wrr.12277. [DOI] [PubMed] [Google Scholar]
- 30.Snyder AL, Zmuda JM, Thompson PD. Keloid associated with hypertension. Lancet. 1996;347:465–466. doi: 10.1016/S0140-6736(96)90042-2. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed]
- 32.Shi C, Zhu J, Yang D. The pivotal role of inflammation in scar/keloid formation after acne. Dermatoendocrinol. 2017;9:e1448327. doi: 10.1080/19381980.2018.1448327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postlethwaite AE, Smith GN, Mainardi CL, Seyer JM, Kang AH. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984;132:2470–2477. [PubMed] [Google Scholar]
- 34.Chen W, Fu X, Sun X, Sun T, Zhao Z, et al. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003;113:208–216. doi: 10.1016/S0022-4804(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 35.Dong X, Mao S, Wen H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (review) Biomed Rep. 2013;1:833–836. doi: 10.3892/br.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 37.De Benedetti F, Rucci N, Del Fattore A, Peruzzi B, Paro R, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006;54:3551–3563. doi: 10.1002/art.22175. [DOI] [PubMed] [Google Scholar]
- 38.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 39.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Yang D, Xiao Z, Zhang M. miRNA expression profiles in keloid tissue and corresponding normal skin tissue. Aesthet Plast Surg. 2012;36:193–201. doi: 10.1007/s00266-011-9773-1. [DOI] [PubMed] [Google Scholar]
- 41.Kashiyama K, Mitsutake N, Matsuse M, Ogi T, Saenko VA, et al. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol. 2012;132:1597–1604. doi: 10.1038/jid.2012.22. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Bai Y, Liu H, Zuo X, Yao H, et al. Comparative study of microRNA profiling in keloid fibroblast and annotation of differential expressed microRNAs. Acta Biochim Biophys Sin (Shanghai) 2013;45:692–699. doi: 10.1093/abbs/gmt057. [DOI] [PubMed] [Google Scholar]
- 43.Yu X, Li Z, Chan MT, Wu WK. microRNA deregulation in keloids: an opportunity for clinical intervention? Cell Prolif. 2015;48:626–630. doi: 10.1111/cpr.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu ZY, Lu L, Liang J, Guo XR, Zhang PH, et al. Keloid microRNA expression analysis and the influence of miR-199a-5p on the proliferation of keloid fibroblasts. Genet Mol Res. 2014;13:2727–2738. doi: 10.4238/2014.April.14.2. [DOI] [PubMed] [Google Scholar]
- 45.De-Ugarte L, Yoskovitz G, Balcells S, Guerri-Fernandez R, Martinez-Diaz S, et al. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genet. 2015;8:75. doi: 10.1186/s12920-015-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J Cell Physiol. 2013;228:1506–1515. doi: 10.1002/jcp.24306. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Feng Y, Sun H, Zhang L, Hao L, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181:1911–1920. doi: 10.1016/j.ajpath.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Wang X, Yang D, Xiao Z, Chen X. MicroRNA-21 affects proliferation and apoptosis by regulating expression of PTEN in human keloid fibroblasts. Plast Reconstr Surg. 2014;134:561e–573e. doi: 10.1097/PRS.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 50.Zhu HY, Li C, Bai WD, Su LL, Liu JQ, et al. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PLoS One. 2014;9:e97114. doi: 10.1371/journal.pone.0097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Li Y, Li N, Teng W, Wang M, et al. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Rep. 2016;6:32231. doi: 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugatani T, Vacher J. Hruska. KAA microRNA expression signature of osteoclastogenesis. Blood. 2011;117:3648–3657. doi: 10.1182/blood-2010-10-311415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia Palacios V, Robinson LJ, Borysenko CW, Lehmann T, Kalla SE, et al. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 Cells by estrogen and phytoestrogens. J Biol Chem. 2005;280:13720–13727. doi: 10.1074/jbc.M410995200. [DOI] [PubMed] [Google Scholar]
- 54.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001;49:732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 55.Wong SY, Lau EM, Lynn H, Leung PC, Woo J, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong) Osteoporos Int. 2005;16:610–615. doi: 10.1007/s00198-004-1730-2. [DOI] [PubMed] [Google Scholar]
- 56.Huang WS, Hsu JW, Huang KL, Bai YM, Su TP, et al. Post-traumatic stress disorder and risk of osteoporosis: a nationwide longitudinal study. Stress Health. 2018;34:440–445. doi: 10.1002/smi.2806. [DOI] [PubMed] [Google Scholar]
- 57.Furtado F, Hochman B, Farber PL, Muller MC, Hayashi LF, et al. Psychological stress as a risk factor for postoperative keloid recurrence. J Psychosom Res. 2012;72:282–287. doi: 10.1016/j.jpsychores.2011.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the Taiwan Personal Information Protection Act, but are available from the corresponding author on reasonable request. All relevant data to support the study findings are provided in this article.


