Abstract
Background
Malaria is a top cause of mortality on the island nation of Madagascar, where many rural communities rely on subsistence agriculture and livestock production. Understanding feeding behaviours of Anopheles in this landscape is crucial for optimizing malaria control and prevention strategies. Previous studies in southeastern Madagascar have shown that Anopheles mosquitoes are more frequently captured within 50 m of livestock. However, it remains unknown whether these mosquitoes preferentially feed on livestock. Here, mosquito blood meal sources and Plasmodium sporozoite rates were determined to evaluate patterns of feeding behaviour in Anopheles spp. and malaria transmission in southeastern Madagascar.
Methods
Across a habitat gradient in southeastern Madagascar 7762 female Anopheles spp. mosquitoes were collected. Of the captured mosquitoes, 492 were visibly blood fed and morphologically identifiable, and a direct enzyme-linked immunosorbent assay (ELISA) was used to test for swine, cattle, chicken, human, and dog blood among these specimens. Host species identification was confirmed for multiple blood meals using PCR along with Sanger sequencing. Additionally, 1,607 Anopheles spp. were screened for the presence of Plasmodium falciparum, P. vivax-210, and P. vivax 247 circumsporozoites (cs) by ELISA.
Results
Cattle and swine accounted, respectively, for 51% and 41% of all blood meals, with the remaining 8% split between domesticated animals and humans. Of the 1,607 Anopheles spp. screened for Plasmodium falciparum, Plasmodium vivax 210, and Plasmodium vivax 247 cs-protein, 45 tested positive, the most prevalent being P. vivax 247, followed by P. vivax 210 and P. falciparum. Both variants of P. vivax were observed in secondary vectors, including Anopheles squamosus/cydippis, Anopheles coustani, and unknown Anopheles spp. Furthermore, evidence of coinfection of P. falciparum and P. vivax 210 in Anopheles gambiae sensu lato (s.l.) was found.
Conclusions
Here, feeding behaviour of Anopheles spp. mosquitoes in southeastern Madagascar was evaluated, in a livestock rich landscape. These findings suggest largely zoophagic feeding behaviors of Anopheles spp., including An. gambiae s.l. and presence of both P. vivax and P. falciparum sporozoites in Anopheles spp. A discordance between P. vivax reports in mosquitoes and humans exists, suggesting high prevalence of P. vivax circulating in vectors in the ecosystem despite low reports of clinical vivax malaria in humans in Madagascar. Vector surveillance of P. vivax may be relevant to malaria control and elimination efforts in Madagascar. At present, the high proportion of livestock blood meals in Madagascar may play a role in buffering (zooprophylaxis) or amplifying (zoopotentiation) the impacts of malaria. With malaria vector control efforts focused on indoor feeding behaviours, complementary approaches, such as endectocide-aided vector control in livestock may be an effective strategy for malaria reduction in Madagascar.
Keywords: Blood meal, Cattle, Livestock, Plasmodium vivax, Mosquito, Ranomafana
Background
Human malaria, caused by an infection with Plasmodium parasites and transmitted by Anopheles mosquitoes, is one of the greatest causes of mortality in the world. Nearly half-a-million people die from malaria each year, with the greatest burden (> 90%) of morbidity and mortality occurring in sub-Saharan Africa [1]. Malaria control efforts have increased in recent years, with a resurgence in investment in research and practical prevention efforts such as targeted vector control strategies. These targeted vector control strategies use insecticide-treated bed nets (ITNs), as well as indoor residual spraying (IRS) to reduce vector populations and to protect humans from malaria transmission via mosquito bites [2]. The overwhelming focus on insecticides, in particular with ITNs and IRS, for malaria prevention over the past several decades has led to shifts in mosquito susceptibility to these insecticides [3–7], and may lead to facultative or long-term shifts in the behaviour of vector species [8–12]. Most populations of the primary malaria vector species in Africa, Anopheles gambiae sensu stricto (s.s.) and Anopheles funestus s.s., are now at least partially resistant to most pyrethroid insecticides [11, 12], while development of resistance to other commonly used classes of insecticides, such as carbamates and organophosphates, is ongoing [11, 13]. Certain populations of An. gambiae sensu lato (s.l.) and An. funestus s.l. also seem to have undergone behavioural changes following implementation of ITN campaigns, switching to feeding at times when people are less likely to be under bed nets [4, 14, 15] as well as displaying increased levels of exophagy, avoiding contact with insecticide while seeking a host [4, 6, 7]. In regions where Anopheles vectors are becoming increasingly exophagic, they also appear to be becoming increasingly zoophagic [3, 6, 7, 16, 17], supporting research which indicates that extrinsic factors such as host availability influence host blood meal preference, although methodological variables, such as indoor/outdoor trapping location may influence the type of blood meal detected [18, 19]. While measures such as ITNs are effective in reducing the bulk of human malaria cases caused by endophagic mosquitoes, residual transmission, facilitated by exophagic mosquitoes, remains an issue of concern [6, 16, 20–23]. In order to achieve long-term malaria control and to fight residual transmission, it is critical to consider mosquito ecology and to integrate alternative vector control strategies with the methods currently used [20, 24, 25].
The island nation of Madagascar represents an area of ongoing residual malaria transmission where alternative strategies may need to be integrated with current approaches to combat transmission. One hundred percent of Madagascar’s population lives in areas where malaria is endemic [26]. Despite successful control strategies in Madagascar in recent decades, the impact of ongoing efforts to distribute ITNs, as well as widespread application of IRS, has not been enough to halt the transmission of malaria-causing parasites. This is evident in the extensive outbreaks seen in recent years [27]. The continued malaria burden and impact on mortality in Madagascar suggests current vector control strategies need to be revised and improved. The high ratio of cattle to humans could create potential for a zooprophylactic approach, one in which livestock are used to divert malaria vectors from human populations. The success of such an approach depends on the importance of various vector species and their host feeding preferences.
The goals of this study were to: (1) determine community compositions of host-seeking malaria vector species in southeastern Madagascar, (2) determine host preference of Anopheles spp. collected in this area, where habitat alteration is common and livestock are prevalent, and (3) determine the proportion of Anopheles spp. in this area harbouring infective sporozoite stages of Plasmodium parasites Plasmodium falciparum, Plasmodium vivax VK210 (P. vivax 210) and P. vivax VK247 (P. vivax 247). Due to the high level of zoophagy demonstrated by Anopheles in other parts of sub-Saharan Africa where livestock are common, it was hypothesized that in this study area, mosquito species which are typically considered to be anthropophagic, such as An. gambiae s.l. and An. funestus s.l., would exhibit feeding preference toward livestock, such as cattle. It was further hypothesized that typically zoophagic species, such as Anopheles coustani and Anopheles squamosus/cydippis, would maintain preference for feeding on livestock. Additionally, it was hypothesized that these zoophagic vectors would exhibit Plasmodium sporozoite rates similar to those of the primary vectors, An. gambiae s.s. and An. funestus s.s. Finally, based on reported case data, it was hypothesized that P. vivax would be present, but its prevalence in the vector population would be lower than that of P. falciparum [26].
Methods
Mosquito sampling and identification
Sampling was conducted in the Ifanadiana district of southeastern Madagascar (21° 02′–21° 25′ S, 47° 18′–47° 37′ E), a location characterized by a highly seasonal environment. Mosquito collections were done during both dry and rainy seasons, spanning August – December 2016 in seven villages; Namhoaka, Kianjanoby, Ambatavory, Ampasipotsy, Ambasoary, Mangevo, and Amboditanimena. Elevation varied from 116 to 533 m above sea level. Villages where traps were placed were selected based on the presence of livestock and close proximity to habitat altered through slash-and-burn agricultural techniques. All villages were within 1 km of the boundary of Ranomafana National Park (RNP), a 42500 hectare protected montane rainforest. Mosquitoes were collected using CDC miniature light traps baited with field-produced CO2 made from a sugar-yeast-water mixture. Specifically, the CO2 mixture was made by combining 1 part yeast with 3 parts sugar, gently mixed for 30 min, followed by the addition of 1 part brown sugar in a recycled 1.5 L bottle (adapted from [28]).
Twelve light traps were placed in each of the seven villages for three consecutive nights in each site, for a total of 252 “trap nights”. Traps were placed at 16:00 and checked at 07:00 the following day. In each village, four traps were set in sites within < 10 m of livestock, four inside houses, and four in forested sites near (< 1 km) the village centre or nearby rice fields. Mosquito collection bags were removed and replaced with new ones each afternoon. Captured mosquitoes were morphologically identified in the field to the genus level and An. gambiae s.l., An. funestus s.l., and An. coustani using the key of Gillies and Coetzee [29]. Specimens were sorted into individual 1.5 mL tubes with desiccant and stored in a cool, dry place until they were brought to a laboratory and identified to species also using the Grjebine key [30]. Specimens within An. gambiae and An. funestus complexes were assayed using a protocol provided by Scott et al. [31] and Walker et al. [32]. All Anopheles spp. mosquitoes were dissected and stored separately as wings plus legs, abdomen, and head plus thorax. Mosquitoes that appeared to be blood fed using a stereomicroscope were identified for downstream blood meal analysis.
Direct enzyme-linked immunosorbent assays (ELISAs) for blood meal detection
The abdomens of blood fed mosquitoes were ground in 50 µL of phosphate buffer saline (PBS) using a motor-powered pestle. After grinding, the pestle was rinsed with 450 µl of PBS and the eluate was collected into the original tube for a final volume of approximately 500 µl. Samples were stored overnight at 4 °C and tested the following day. Positive and negative controls were stored on desiccant at − 20 °C and were prepared in the same manner as described above for blood fed abdomens. Positive controls were generated by feeding previously unfed An. gambiae (BEI Resources MR4, MRA-112) using commercially purchased blood (Hemostat) on a membrane apparatus under laboratory conditions at the US Centers for Disease Control and Prevention. One positive control was used per assay. No positive control for dog was used. Seven unfed An. gambiae s.l. abdomens were used in each assay as negative controls.
The specifics of the direct ELISA and blocking buffer protocol can be found in the paper by Beier et al. [33]. The assay was run with host specific markers for: pig, cattle, dog, chicken, human (SeraCare product number: 5220–0363, 5220–0361, 5220–0368, 5220–0373, 522–0330, respectively). Plates were covered for all incubations to prevent evaporation and held at room temperature. All washes were performed using approximately 200 µL of PBS-T (0.5% Tween 20 in PBS). Briefly, 50 µl of each prepared sample were placed into a separate well of a 96-well assay plate (Costar) and incubated for three hours. The wells were then washed twice and then incubated with 50 µl of conjugate per well for one hour. The conjugate was incubated for three hours at 4 °C before use and consisted of host-specific peroxidase-labelled antibody and undiluted non-host serum to help control non-specific cross-reactivity. The total volume in the wells was aspirated out, washed three times and incubated with 100 µl ABTS (SeraCare 5120–0032, 50 µL component A and 50µL component B) for 30 min. Following incubation, absorbance was immediately measured at 405 nm with a SpectraMax 340 spectrophotometer (Molecular Devices, LLC., San Jose, CA) using Softmax Pro 7 software.
DNA extraction from blood meal ELISA homogenate
Samples that included evidence of a blood meal from more than one host species using the direct ELISA were further analysed by multiplex PCR. DNA was extracted from the remaining mosquito abdomen ELISA homogenate according to Collins et al. [34]. This protocol typically starts with unprocessed mosquito tissue, thus a 2X concentrated extraction buffer was formulated resulting in a standard 1X concentration of all components when added to the abdomen homogenate. 200 µL of abdomen homogenate was combined with 200 µL of 2X Collins buffer and incubated at 65 °C for 90 min. Potassium acetate was added to a concentration of 1 M and samples were centrifuged at 2270g for 20 min at room temperature. The supernatant was transferred to a new tube mixed with 450 µL of ice cold 100% EtOH and incubated at 4 °C overnight. The next day, the tubes were centrifuged at 2270 g at 4 °C for 20 min, supernatant was discarded, and the remaining pellet was washed with 200µL of ice cold 70% EtOH. Following centrifugation at 2270g for 5 min and removal of supernatant, the tubes were placed in a speed vacuum concentrator for 20 min at room temperature to allow any remaining EtOH to evaporate. DNA was resuspended in 20µL of reagent-grade water and stored at 4 °C until use.
Multiplex polymerase chain reaction (PCR)
The samples with more than one positive results from the blood meal ELISA were evaluated with a multiplex PCR targeting a region of cytochrome B DNA [35] using the following primers: PIG573F (PigF) (5′-CCT CGC AGC CGT ACA TCT C-3′), DOG368F (DogF) (5′-GGA ATT GTA CTA TTA TTC GCA ACC AT-3′), COW121F (CowF) (5′-CAT CGG CAC AAA TTT AGT CG-3′), HUMAN741F (HumF) (5′-GGC TTA CTT CTC TTC ATT CTC TCC T-3′), and UNREV1025 (5′-GGT TGT CCT CCA ATT CAT GTT A-3′). Total PCR reaction volumes were 25 µL and contained 0.1 µM of each primer, 2 × AccuStart™ II GelTrack PCR SuperMix and 1.0 µL of extracted DNA. Thermal cycler conditions (Bio-Rad T100) consisted of an initial 5-min denaturation at 95 °C followed by 35 cycles at 95 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min and a final extension step of 72 °C for 7 min.
Circumsporozoite (cs) ELISA for the detection of Plasmodium parasites in Anopheles spp.
The proportion of captured mosquitoes with sporozoites (sporozoite rate) was determined by performing circumsporozoite (cs) ELISA. The heads and thorax from all morphologically identified mosquitoes were processed and assayed to detect antibodies against the circumsporozoite proteins of P. falciparum (Pf), P. vivax VK210 (Pv210) or P. vivax VK247 (Pv247) using the sandwich csELISA according to the protocol established by Wirtz et al., (1992) [36] and updated methods from the 2017 Malaria Resource Reagent Reference Center [37]. False positives on the csELISA assay are known to appear in Anopheles spp. with cattle and swine blood meals as a result of cross-reacting antigens [38]. To avoid potential false positives, homogenate was boiled at 100 °C for 10 min to denature any heat-unstable cross-reactive proteins and retested to confirm positives.
Results
A total of 7762 Anopheles were collected. Of these, 7.5% (n = 582) had abdomens that contained a blood meal, as determined by visual inspection with a stereomicroscope. An equal proportion of blood fed mosquitoes were identified from specimens collected in both rainy and dry seasons. The blood fed mosquitoes were morphologically identified as 82 An. gambiae s.l. (14.1%), 71 An. funestus s.l. (12.2%), 127 An. coustani (21.8%), 214 An. squamosus/cydippis (36.7%), and 88 unidentified Anopheles spp. (15.1%). The distribution of vectors was heterogeneous across all six survey sites, with greater diversity collected in the northern most and southern most points of Ranomafana National Park, and with An. funestus s.l. present in only five of the seven sites, An. coustani and An. sqamosus/cydippis collected in six of the seven sites, and An. gambiae s.l. captured in all seven. Specimens within An. gambiae and An. funestus complexes assayed using [32], and [34] to determine specific species were inconclusive due to the absence of positive amplicons for the targeted species specific sequences (Fig. 1).
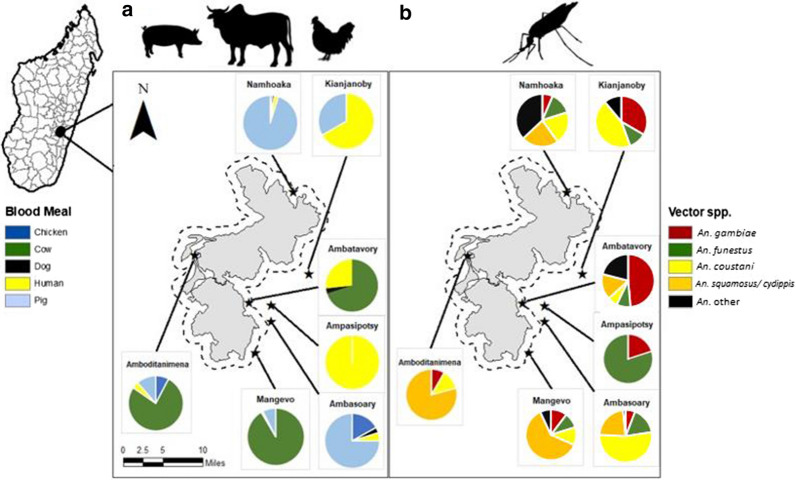
Fig. 1.
Mosquitoes were successfully assayed for blood meal composition (n = 480) were collected within Ranomafana National Park (park boundary in gray) or within 1 mile of the park boundary (represented by a dashed line) in the Ifanadiana District of Madagascar. a Blood meal source. Blood meals with multiple hosts identified are included in this figure as separate data points. b Vector species distribution
Blood meal identification
Our assays successfully identified bloodmeals from > 97% of all identified Anopheles spp. specimens assayed (n = 480). Among all Anopheles tested, a portion of all species tested displayed evidence of feeding on livestock hosts. Pigs and cattle were the source for most blood meals in villages close to the park, while humans accounted for a higher percentage of blood meals in villages further from the park boundaries. In total, > 92% of the blood meals taken by Anopheles collected in this study came from livestock and domestic animals, with cattle (245/480) and pigs (194/480) being the most common blood meal hosts. Human blood was detected in 7.5% (36/480) mosquito abdomens tested. Blood meals from cattle were detected in 71.8% of An. squamosus/cydippis (150/214), 56.4% of An. gambiae s.l. (44/78), 37.7% of An. funestus s.l. (26/69), and 20.2% of An. coustani (25/124; Fig. 2). Blood meals from pigs were second-most common among tested mosquitoes, with pig blood detected in 66.1% (82/124) of blood fed An. coustani, 43.4% of An. funestus s.l. (30/69), 33.5% of An. squamosus/cydippis (70/214), and 15.4% in An. gambiae s.l. (12/78). Finally, human blood was detected in < 10% of each An. coustani and An. squamosus/cydippis, but in 25.6% (20/78) and 11.6% (8/69) of An. gambiae s.l. and An. funestus s.l., respectively (Fig. 2). Just over 5.4% (26/480) of all mosquito abdomens successfully assayed were positive for more than one type of blood meal, indicating multiple feedings (Table 1).
Fig. 2.
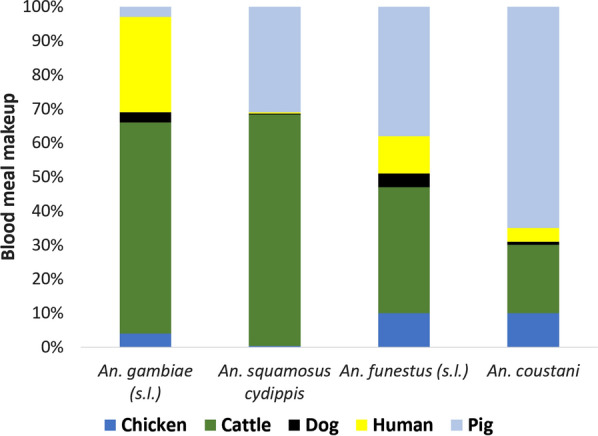
Blood meal analysis of Anopheles spp. in seven surveyed villages in southeastern Madagascar reveals high levels of zoophily in An. gambiae s.l. (n = 82), An. funestus s.l. (n = 71), An. coustani (n = 127) and An. squamosus/ cydippis (n = 214)
Table 1.
Detection of multiple blood meals from different Anopheles spp. (n = 26) suggests potential plasticity of host choice in the Ifanadiana region of Madagascar
| Human | Cattle | Chicken | Dog | Pig | Cattle + Pig | Cattle + Human | Chicken + Human | Dog + Human | Pig + Human | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| An. gambiae s.l. | 19 | 44 | 2 | 1 | 11 | 0 | 0 | 0 | 1 | 0 | 1a |
| An. funestus s.l. | 8 | 23 | 7 | 2 | 26 | 3 | 0 | 0 | 0 | 1 | 1b |
| An. coustani | 5 | 25 | 9 | 2 | 78 | 0 | 0 | 1 | 0 | 1 | 3a |
| An. squamosus/ cydippis | 0 | 136 | 1 | 0 | 58 | 12 | 1 | 0 | 0 | 0 | 1c |
Other combinations: aChicken + Pig bDog + Pig cCattle + Dog
Of the 113 blood meals identified in mosquitoes trapped indoors, 8 (7.1%) were taken from human hosts while 105 (92.9%) were taken from livestock hosts. Human hosts accounted for 14 (31.1%) of the 45 blood meals identified in mosquitoes trapped near villages or rice fields and 14 (4.4%) of the 420 blood meals identified in mosquitoes trapped near livestock pens. Livestock hosts accounted for the remaining 31 (68.9%) blood meals identified in mosquitoes trapped near villages or rice fields, and the remaining 406 (95.6%) blood meals identified in mosquitoes trapped near livestock pens. Seven additional blood meals taken from livestock hosts were identified in mosquitoes trapped in forested sites or fallow fields (Table 2).
Table 2.
Numbers and percentages of human vs. livestock blood meals at each trapping location type reveal many human blood meals identified in mosquitoes trapped outdoors and many livestock blood meals detected in mosquitoes trapped indoors
| Collection location | # of human blood meals | % of human blood meals | # of livestock blood meals | % of livestock blood meals |
|---|---|---|---|---|
| Indoors (n = 113) | 8 | 22.2 | 105 | 23.4 |
| Village/ rice field (n = 45) | 14 | 38.9 | 31 | 6.9 |
| Livestock pen (n = 320) | 14 | 38.9 | 306 | 68.1 |
| Other sites (n = 7) | 0 | 0 | 7 | 1.6 |
| Total (n = 485) | 36 | 100 | 449 | 100 |
Plasmodium spp. infection
Only An. gambiae s.l. (n = 83) and An. funestus s.l. (n = 71) mosquitoes tested positive for P. falciparum, with a single infected specimen of each species detected. Plasmodium vivax 210 was detected in An. gambiae s.l., An. squamosus/cydippis (n = 216) and An. coustani (n = 127) mosquitoes, with An. coustani demonstrating the highest sporozoite rate (> 3%). Plasmodium vivax 247 was found in An. gambiae s.l., An. funestus s.l., An. squamosus/cydippis and An. coustani as well as a number of unidentified Anopheles spp. mosquitoes (n = 75). The sporozoite rate of P. vivax 247 among Anopheles spp. ranged from 4–16 mosquitoes. Coinfections between different Plasmodium strains were observed in a single An. gambiae s.l. (Table 3).
Table 3.
Detection of P. falciparum, P. vivax 210 and P. vivax 247 in captured Anopheles spp. reveals high prevalence of infection with human malaria parasites, and mixed infections with P. falciparum and P.vivax 210 in An. gambiae s.l.
| Species | P. falciparum + | P. vivax 210 + | P. vivax 247 + | Mixed Plasmodium spp. infections |
|---|---|---|---|---|
| An. gambiae s.l. (n = 83) | 1 (1.2%) | 1 (1.2%) | 6 (7.2%) | 1 (1.2%) |
| An. squamosus/cydippus (n = 216) | 0 | 1 (<1%) | 16 (7.4%) | 0 |
| An. funestus s.l. ( n = 71) | 1 (1.4%) | 0 | 5 (7.0%) | 0 |
| An. coustani (n = 127) | 0 | 4 (3.2%) | 4 (3.2%) | 0 |
| Anopheles spp. (n = 75) | 0 | 0 | 5 (6.6%) | 0 |
| Totals (n = 572) | 2 (<1%) | 6 (1%) | 36 (6.3%) | 1 (<1%) |
Discussion
Vector species
This study found diverse communities of Anopheles species at study sites, including An. gambiae (s.l.), An. funestus (s.l.), as well as An. squamosus/ cydippis and An. coustani. The majority of visibly blood fed mosquitoes identified were species typically considered to be secondary vectors, particularly An. coustani and An. squamosus/cydippis. This is in line with other recent reports of Anopheles spp. in the Madagascar [39, 40]. Anopheles coustani, which comprised nearly 22% of blood fed mosquitoes, has been indicated as a new malaria vector of importance in Madagascar [41], while the role of An. squamosus/cydippis (over 37% of blood fed mosquitoes) in malaria transmission has long been suspected but less well understood [39, 41–44]. There were also relatively high numbers of common vector species identified, namely An. gambiae s.l. and An. funestus s.l., which comprised over 14% and over 12% of blood fed mosquitoes, respectively. The species complexes were unable to be further analysed because of inconclusive PCR results. The only regional vector species of note not collected was Anopheles mascarensis [45]. Several specimens listed as unknown Anopheles spp. were morphologically similar to species not previously detected in Madagascar, and may have been incorrectly identified. Future taxonomic work on Anopheles mosquitoes in this region in Madagascar may shed more light on the region’s true morphological and molecular diversity.
Blood meal diversity
These findings suggest overwhelmingly zoophagic behavior on the part of all Anopheles species collected, including the traditionally anthropophagic species An. gambiae s.l. and An. funestus s.l. The high level of livestock and animal blood feeding demonstrated by the Anopheles spp. captured outdoors here is in line with published descriptions of Anopheles host choice in Madagascar [39, 45]. As with other studies in the region, a high proportion of An. gambiae s.l. and An. funestus s.l. blood meals came from cattle (Fig. 2). In addition, almost as many Anopheles, most notably specimens of An. funestus s.l., and An. coustani had fed on pigs (Fig. 2). Tedrow et al. also observed high numbers of pig blood meals among Malagasy Anopheles captured outdoors in December, 2017, although they observed a marked switch to human blood meals in April of the following year [39].
The detection of multiple blood meals, including mixed human-livestock blood meals (n = 5), indicates some degree of plasticity in host choice among both primary and traditionally secondary Anopheles vectors in this region of Madagascar. This behavioural plasticity is further supported by mosquitoes with livestock blood meals indoors and mosquitoes with human blood meals near livestock pens. Previous studies of vector behaviour in the region suggest moderate to high levels of exophagy and exophily among many vector species [40, 41, 46], but these results are the first to indicate high levels of plasticity in feeding among these species. This plasticity may be in part due to heterogeneities in host availability or implementation of insecticide-based and other preventive measures in this region, as has been observed in other locations [18, 47]. This behavioural plasticity combined with high levels of zoophagy among all collected species points to a unique ecology among the Anopheles vectors of this region, one which requires new and integrative vector management strategies for effective malaria prevention.
Despite differences in trapping and molecular methods, the results presented here show predominant blood feeding on livestock by malaria vectors in Madagascar are consistent with Tedrow et al. [36]. While there were multiple blood meals detected within the same mosquito, using ELISA, there were far fewer mixed bloodmeals detected than observed by Tedrow et al. who used the BLOODART method- a PCR followed by multiplex bead assay, which may be more sensitive in detecting host DNA than direct ELISA [39]. In this study, odour-baited CDC light traps were used to target host-seeking mosquitoes. Trapping methods such as the QUEST method used by Tedrow et al. are passive and do not target host-seeking mosquitoes, but rather target outdoor resting mosquitoes which have already taken a blood meal [39], and therefore may better capture the extent to which multi-species feeding occurs among these vectors. The screening of all collected mosquitoes, i.e. inclusion of non-visibly blood fed mosquitoes, may yield more complete results. To monitor malaria control efforts, direct ELISA for human or bovine blood meal detection is commonly used to determine the human blood index (HBI) and bovine blood index (BBI) in a cost-effective way.
The majority of blood fed mosquitoes sampled, both those that had taken human and non-human blood meals, were collected from traps set outside of human habitations. In fact, over 50% of all blood fed mosquitoes collected in this study were trapped near livestock pens, away from human habitation. This suggests that Malagasy Anopheles mosquitoes may be particularly exophagic, a trend perhaps amplified by the successes of IRS and ITN strategies, which target endophilic mosquitoes. Host availability can influence feeding behaviour [18, 19], and these mosquitoes may be exhibiting plastic feeding behaviour, feeding largely on livestock, but switching to humans when available. Livestock and human densities may be important in interpreting blood meal composition in this context. These exophagic mosquitoes may be the key to understanding residual malaria transmission in Madagascar.
Plasmodium spp. infection
Plasmodium falciparum, P. vivax 210 and P. vivax 247 (two different genotypes of P.vivax) were detected, with a higher than expected sporozoite rates of P. vivax. While the total prevalence of P. falciparum across all Anopheles specimens (0.35%; n = 572, n positive = 2) was fairly low, the higher prevalence of P. vivax 210 (1%; n = 572, n positive = 6) and P. vivax 247 (6.2%, n = 572, n positive = 36) may indicate that these parasites are being widely circulated in the region. These findings are contrary to current reports of malaria incidence in Madagascar, which state that P. vivax accounts for only 4% of the country’s human malaria burden [26]. One explanation for this may be that most P. vivax infections in Madagascar are either subclinical or unreported. Plasmodium vivax cases may also be commonly misdiagnosed as P. falciparum infection, due to the clinical similarities of infection and use of methods for diagnosis, such as rapid detection tests (RDTs) which only yield results of P. falciparum, non-P. falciparum, and mixed Plasmodium infection. Another potential explanation for the high prevalence detected in mosquitoes is that false positive sporozoite detection for P. falciparum and P. vivax by csELISA has been shown to be associated with bovine and swine blood meals [48]. Given the prevalence of bovine and swine blood meal in this dataset, it is possible that cross-reactive factors from the blood meals may influence our findings. To minimize the potential for false positives in this study, mosquitoes positive for P. vivax 247 were boiled, which has been shown to decrease cross-reactivity between blood proteins and P. falciparum [36], although its impact on P. vivax false positivity remains unresolved. Experimental laboratory studies using molecular strategies may elucidate this issue.
The status of P. vivax in Madagascar is unique. Over 80% of the population is thought to be Duffy-negative, meaning they express neither the Fya nor Fyb antigens that allow infection by P. vivax [49]. However, in Madagascar, P. vivax clinical malaria is commonly observed among Duffy-negative individuals [50]. The high prevalence of P. vivax observed in Anopheles mosquitoes in this study further suggests the importance of considering P. vivax in malaria control programmes in southeastern Madagascar. In this study, P. vivax was detected in highly zoophilic and exophagic secondary vectors, such as An. squamosus/cydippis. While An. squamosus/cydippis has been suspected as a secondary vector of P. falciparum [42–44], this is the first documented evidence of their potential role in the transmission of P. vivax. Further investigation into the role of An. squamosus/cydippis and other secondary vectors in the transmission of P. vivax is needed.
Limitations
These results provide evidence of zoophagy among Anopheles spp. malaria vectors in southeastern Madagascar; however, there are a few limitations to this work. For example, the collection method used here relied on CO2 as an attractant bait, while human-baited methods such as human landing catches (HLCs) or human baited light traps may have attracted more human biting vectors. Similarly, previous studies have found proximity to livestock pens to be a significant predictor of number of Anopheles spp. collected, therefore trapping near these sites may have influenced collection numbers. Additionally, PCR species identification results within the An. gambiae complex and An. funestus group were inconclusive, perhaps due to sample degradation. More accurate species identification may have revealed typically zoophagic species, such as Anopheles arabiensis. Further work characterizing species may provide additional insight into species specific trends in feeding behaviour. Due to cultural sensitivities and privacy around livestock ownership, accurate ratios of livestock to humans in these study sites, which may influence opportunistic feeding patterns, could not be determined.
Zooprophylaxis and cattle in Madagascar
While there is still much debate about the potential of zooprophylaxis for malaria control [51–53], endectocide-aided zooprophylaxis, combined with IRS and ITNs, has proven to be effective in reducing residual malaria transmission by zoophilic vector species, especially in areas where these species make up a significant portion of the overall Anopheles spp. [51, 54–59]. Placement of livestock in pens away from housing and activity centers may draw behaviorally plastic feeders away from humans [57, 60]. Ingestion of endectocides during blood feeding from livestock can significantly reduce mosquito life-span and survival, thus reducing vector populations and lowering parity rates and transmission probability [55, 61–64]. This reduction in vector lifespan to a number of days below the extrinsic incubation period (EIP) of malaria disrupts the parasite’s transmission cycle, decreasing human cases of malaria [65]. This has mainly been studied in areas where An. arabiensis is the dominant vector species [56, 60, 66–68], but has the potential to be applied to any system with highly zoophagic, exophagic vectors [51, 54, 69, 70], providing a One Health solution to residual malaria transmission.
In Madagascar, despite overall reductions in malaria in recent decades [26], new approaches are necessary to combat the spread of malaria. The high level of zoophily described here and in other literature [39, 45] suggests potential exists to affect malaria transmission through some form of zooprophylaxis. The population of Madagascar primarily consists of rural, agrarian societies where cattle are significant [71]. The local cattle, known as zebu, are an extremely important part of Malagasy culture and society [71, 72], and may be used as insurance for periods of crop failures [73]. Furthermore, recent social and political instability has resulted in the rise of organized criminal gangs specialized in stealing zebu, leading to a change in traditional grazing regimes and movement of livestock from pastures to pens closer to human habitation [74]. Experimental studies have shown that the primary vector of malaria in the Central Highlands, An. funestus, preferred to seek out human odours to calf odors, while the primary vector of malaria at lower altitudes, An. gambiae, preferred calf odours [75]. Furthermore, another study indicated that traps near livestock pens were significantly more likely to capture Anopheles mosquitoes than those far from livestock pens [76]. This high level of zoophily among these and other Malagasy Anopheles species may grow in response to the ever-increasing population of livestock in Madagascar, as well as to recent changes in grazing regimes. The zoophily observed here combined with large numbers of cattle and swine in southeastern Madagascar living in close proximity to communities, may make this location an ideal intervention site for the application of zooprophylaxis using endectocides to treat livestock [27, 48, 76]. This would simultaneously function as a veterinary health preventive measure for helminth infections and lead to healthier livestock while controlling malaria vector populations.
Conclusion
Understanding local vector species composition, host choice and sporozoite rates allows for improved malaria control efforts, tailored to the transmission dynamics of the locality. There were large numbers of Anopheles species typically considered to be secondary vectors of malaria identified, as well as evidence of Plasmodium spp. sporozoites among both primary and secondary vector species and high levels of zoophagy among all species of malaria vectors collected in our study area in southeastern Madagascar. These findings, combined with evidence of mixed human-livestock blood feeding in this region, suggest potential for the use of tools such as endectocide-aided zooprophylaxis, to complement current prevention efforts. Also, a high sporozoite rate of P. vivax was found in assayed Anopheles spp., which contradicts the low reported cases of P. vivax in humans in Madagascar. This discrepancy between observed sporozoite rates and reported human cases may in part be due to high Duffy negativity in Malagasy populations and a high number of asymptomatic individuals not seeking treatment and therefore continuously contributing P. vivax into the ecosystem. Future vector surveillance in Madagascar is suggested to include surveillance of P. vivax. Finally, malaria prevention efforts in southeastern Madagascar are suggested to explore the use of tools such as endectocide-aided zooprophylaxis to supplement other efforts in this region.
Acknowledgements
We are grateful for field and logistical support from Madagascar Institute Conservation Environment Tropical (MICET), and Centre ValBio field station and staff. We appreciate field support from Patricia C. Wright, Eileen Larney, Razafindraibe Faustin Jean Guy, Rakotonjatovo Justin, and Wes Mason. We are grateful to Seth Irish, Bill Hawley, Barbara Marston for their feedback and suggestions on how to improve the manuscript. Additional thanks to Ray Beach and two anonymous reviewers for strengthening this manuscript with their reviews.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- BLOODART
Bloodmeal detection assay for regional transmission
- CS
Circumsporozoite
- DNA
Deoxyribonucelic acid
- ELISA
Enzyme-linked immunosorbent assay
- ITN
Insecticide treated bed nets
- IRS
Indoor residual spraying
- PCR
Polymerase chain reaction
- QUEST
Quadrant enabled screen trap
Authors’ contributions
MF, AS, ED, SZ conceived the study and participated in its design and coordination. BR and SZ conducted the field work and collected samples. MF and AS conducted laboratory analysis and analysed all molecular results. MF and BM wrote the manuscript and created maps and figures. BM, MF, AS, ED, and SZ participated in data analysis and interpretation. All authors read and approved the final manuscript.
Funding
This work was supported by a USDA NIFA Young Investigator Research Award, and the School of Forestry and Wildlife Sciences and the Office of the Vice President for Research (OVPR) at Auburn University. Laboratory support was provided by the Center for Disease Control and Prevention Malaria Research and Reference Reagent and Resource Center (MR4).
Availability of data and materials
All data generated are included in this manuscript and supplementary files.
Ethics approval and consent to participate
This research was conducted in Madagascar under national permit #120/16/MEEF/SG/DGF/DAPT/SCBT, and samples were imported under USDA import permit #107234.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Micaela Finney, Benjamin A. McKenzie contributed equally
References
- 1.WHO. This year’s World malaria report at a glance. Geneva, World Health Organization, 2018. http://www.who.int/malaria/media/world-malaria-report-2018/en/. Accessed 18 Jul 2019.
- 2.WHO Global Malaria Programme. A framework for malaria elimination. Geneva, World Health Organization. http://apps.who.int/iris/bitstream/10665/254761/1/9789241511988-eng.pdf. Accessed 30 2019.
- 3.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo Uganda. Malar J. 2019;18:445. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 5.Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HCJ, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sovi A, Govoétchan R, Ossé R, Koukpo CZ, Salako AS, Syme T, et al. Resistance status of Anopheles gambiae s.l. to insecticides following the 2011 mass distribution campaign of long-lasting insecticidal nets (LLINs) in the Plateau Department, south-eastern Benin. Malar J. 2011;2020(19):26. doi: 10.1186/s12936-020-3116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail BA, Kafy HT, Sulieman JE, Subramaniam K, Thomas B, Mnzava A, et al. Temporal and spatial trends in insecticide resistance in Anopheles arabiensis in Sudan: outcomes from an evaluation of implications of insecticide resistance for malaria vector control. Parasit Vectors. 2018;11:122. doi: 10.1186/s13071-018-2732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J. 2016;15:146. doi: 10.1186/s12936-016-1201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Moyes CL, Athinya DK, Seethaler T, Battle KE, Sinka M, Hadi MP, et al. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc Natl Acad Sci USA. 2020;117:22042–22050. doi: 10.1073/pnas.2006781117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matiya DJ, Philbert AB, Kidima W, Matowo JJ. Dynamics and monitoring of insecticide resistance in malaria vectors across mainland Tanzania from 1997 to 2017: a systematic review. Malar J. 2019;18:102. doi: 10.1186/s12936-019-2738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira CP, Lyra SP, Azevedo F, Greenhalgh D, Massad E. Modelling the impact of the long-term use of insecticide-treated bed nets on Anopheles mosquito biting time. Malar J. 2017;16:373. doi: 10.1186/s12936-017-2014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomsen EK, Koimbu G, Pulford J, Jamea-Maiasa S, Ura Y, Keven JB, et al. Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J Infect Dis. 2017;215:790. doi: 10.1093/infdis/jix071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards HM, Sriwichai P, Kirabittir K, Prachumsri J, Chavez IF, Hii J. Transmission risk beyond the village: entomological and human factors contributing to residual malaria transmission in an area approaching malaria elimination on the Thailand-Myanmar border. Malar J. 2019;18:221. doi: 10.1186/s12936-019-2852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356. doi: 10.1186/1475-2875-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orsborne J, Mohammed AR, Jeffries CL, Kristan M, Afrane YA, Walker T, et al. Evidence of extrinsic factors dominating intrinsic blood host preferences of major African malaria vectors. Sci Rep. 2020;10:741. doi: 10.1038/s41598-020-57732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsborne J, Furuya-Kanamori L, Jeffries CL, Kristan M, Mohammed AR, Afrane YA, et al. Using the human blood index to investigate host biting plasticity: a systematic review and meta-regression of the three major African malaria vectors. Malar J. 2018;17:479. doi: 10.1186/s12936-018-2632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar J. 2015;14:35. doi: 10.1186/s12936-015-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maharaj R, Seocharan I, Qwabe B, Mkhabela M, Kissoon S, Lakan V. Decadal epidemiology of malaria in KwaZulu-Natal, a province in South Africa targeting elimination. Malar J. 2019;18:368. doi: 10.1186/s12936-019-3001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paaijmans KP, Huijben S. Taking the ‘I’ out of LLINs: using insecticides in vector control tools other than long-lasting nets to fight malaria. Malar J. 2020;19:73. doi: 10.1186/s12936-020-3151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreaux P, Barreaux AMG, Sternberg ED, Suh E, Waite JL, Whitehead SA, et al. Priorities for broadening the malaria vector control tool kit. Trends Parasitol. 2017;33:763–774. doi: 10.1016/j.pt.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madagascar Malaria Fact Sheet. Fact Sheet: Madagascar. U.S. Agency for International Development. https://www.usaid.gov/madagascar/fact-sheets/madagascar-malaria-fact-sheet. Accessed 31 Jul 2019.
- 27.Kesteman T, Rafalimanantsoa SA, Razafimandimby H, Rasamimanana HH, Raharimanga V, Ramarosandratana B, et al. Multiple causes of an unexpected malaria outbreak in a high-transmission area in Madagascar. Malar J. 2016;15:57. doi: 10.1186/s12936-016-1113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhulst NO, Andriessen R, Groenhagen U, Bukovinszkine-Kiss G, Schultz S, Takken W, et al. Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS ONE. 2010;5:e15829. doi: 10.1371/journal.pone.0015829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Pub South African Institute Med Research. https://www.cabdirect.org/cabdirect/abstract/19872042481. Accessed 2 Aug 2019.
- 30.Grjebine A. Faune de Madagascar. XXII. Insectes Diptères Culicidae Anophelinae. Paris, Centre National de la Recherche Scientifique, Office de la Recherche Scientifique et Technique Outre-Mer; 1966.
- 31.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 32.Walker ED, Thibault AR, Thelen AP, Bullard BA, Huang J, Odiere MR, et al. Identification of field caught Anopheles gambiae s.s. and Anopheles arabiensis by TaqMan single nucleotide polymorphism genotyping. Malar J. 2007;6:23. doi: 10.1186/1475-2875-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D, Gargan TP, Koech DK. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J Med Entomol. 1988;25:9–16. doi: 10.1093/jmedent/25.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 35.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. doi: 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirtz R, Sattabongkot J, Hall T, Burkot T, Rosenberg M. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax- VK247 sporozoites. J Med Entomol. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- 37.Malaria Research and Reference Reagent Resource Center (MR4). Methods in Anopheles Research Laboratory Manual. 2015. https://www.beiresources.org/Portals/2/VectorResources/2016%20Methods%20in%20Anopheles%20Research%20full%20manual.pdf. Accessed 30 July 2020.
- 38.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tedrow RE, Rakotomanga T, Nepomichene T, Howes RE, Ratovonjato J, Ratsimbasoa AC, et al. Anopheles mosquito surveillance in Madagascar reveals multiple blood feeding behavior and Plasmodium infection. PLoS Negl Trop Dis. 2019;13:e0007176. doi: 10.1371/journal.pntd.0007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrianaivolambo L, Domarle O, Randrianarivelojosia M, Ratovonjato J, Le Goff G, Talman A, et al. Anthropophilic mosquitoes and malaria transmission in the eastern foothills of the central highlands of Madagascar. Acta Trop. 2010;116:240–245. doi: 10.1016/j.actatropica.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Nepomichene TNJJ, Tata E, Boyer S. Malaria case in Madagascar, probable implication of a new vector Anopheles coustani. Malar J. 2015;14:475. doi: 10.1186/s12936-015-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillies MT. The role of secondary vectors of malaria in North-East Tanganyika. Trans R Soc Trop Med Hyg. 1964;58:154–158. doi: 10.1016/0035-9203(64)90004-5. [DOI] [PubMed] [Google Scholar]
- 43.Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha Zambia. Vector-Borne Zoonotic Dis. 2010;11:1173–1179. doi: 10.1089/vbz.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson JC, Simubali L, Mbambara S, Musonda M, Mweetwa S, Mudenda T, et al. Detection of Plasmodium falciparum Infection in Anopheles squamosus (Diptera: Culicidae) in an area targeted for malaria elimination. Southern Zambia J Med Entomol. 2016;53:1482–1487. doi: 10.1093/jme/tjw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duchemin J-B, Tsy J-MLP, Rabarison P, Roux J, Coluzzi M, Costantini C. Zoophily of Anopheles arabiensis and An gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol. 2001;15:50–57. doi: 10.1046/j.1365-2915.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 46.Fontenille D, Lepers JP, Campbell GH, Coluzzi M, Rakotoarivony I, Coulanges P. Malaria transmission and vector biology in Manarintsoa, High Plateaux of Madagascar. Am J Trop Med Hyg. 1990;43:107–115. doi: 10.4269/ajtmh.1990.43.107. [DOI] [PubMed] [Google Scholar]
- 47.Lefèvre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med Hyg. 2009;81:1023–1029. doi: 10.4269/ajtmh.2009.09-0124. [DOI] [PubMed] [Google Scholar]
- 48.Somboon P, Morakote N, Koottathep S, Trisanarom U. Detection of sporozoites of Plasmodium vivax and Plasmodium falciparum in mosquitoes by ELISA: false positivity associated with bovine and swine blood. Trans R Soc Trop Med Hyg. 1993;87:322–324. doi: 10.1016/0035-9203(93)90148-J. [DOI] [PubMed] [Google Scholar]
- 49.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menard D, Chan ER, Benedet C, Ratsimbasoa A, Kim S, Chim P, et al. Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis. 2013;7:e2489. doi: 10.1371/journal.pntd.0002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J. 2015;14:313. doi: 10.1186/s12936-015-0822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bøgh C, Clarke SE, Pinder M, Sanyang F, Lindsay SW. Effect of passive zooprophylaxis on malaria transmission in the Gambia. J Med Entomol. 2001;38:822–828. doi: 10.1603/0022-2585-38.6.822. [DOI] [PubMed] [Google Scholar]
- 53.Hasyim H, Dhimal M, Bauer J, Montag D, Groneberg DA, Kuch U, et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17:302. doi: 10.1186/s12936-018-2447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pooda HS, Rayaisse JB, de Sale Hien DF, Lefèvre T, Yerbanga SR, Bengaly Z, et al. Administration of ivermectin to peridomestic cattle: a promising approach to target the residual transmission of human malaria. Malar J. 2015;14:496. doi: 10.1186/s12936-015-1001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foy BD, Kobylinski KC, da Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asale A, Duchateau L, Devleesschauwer B, Huisman G, Yewhalaw D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): a systematic review. Infect Dis Poverty. 2017;6:160. doi: 10.1186/s40249-017-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwashita H, Dida GO, Sonye GO, Sunahara T, Futami K, Njenga SM, et al. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit Vectors. 2014;7:52. doi: 10.1186/1756-3305-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franco AO, Gomes MGM, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: a One Health approach. PLoS ONE. 2014;9:e101699. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Njoroge MM, Tirados I, Lindsay SW, Vale GA, Torr SJ, Fillinger U. Exploring the potential of using cattle for malaria vector surveillance and control: a pilot study in western Kenya. Parasit Vectors. 2017;10:18. doi: 10.1186/s13071-016-1957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tirados I, Gibson G, Young S, Torr SJ. Are herders protected by their herds? An experimental analysis of zooprophylaxis against the malaria vector Anopheles arabiensis. Malar J. 2011;10:68. doi: 10.1186/1475-2875-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaccour CJ, Rabinovich NR. Oral, slow-release ivermectin: biting back at malaria vectors. Trends Parasitol. 2017;33:156–158. doi: 10.1016/j.pt.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Burrows J, Slater H, Macintyre F, Rees S, Thomas A, Okumu F, et al. A discovery and development roadmap for new endectocidal transmission-blocking agents in malaria. Malar J. 2018;17:462. doi: 10.1186/s12936-018-2598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann Trop Med Parasit. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- 65.Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013;12:153. doi: 10.1186/1475-2875-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poché RM, Burruss D, Polyakova L, Poché DM, Garlapati RB. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar J. 2015;14:351. doi: 10.1186/s12936-015-0883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaccour CJ, Ngha’bi K, Abizanda G, Irigoyen Barrio A, Aldaz A, Okumu F, et al. Targeting cattle for malaria elimination: marked reduction of Anopheles arabiensis survival for over six months using a slow-release ivermectin implant formulation. Parasit Vectors. 2018;11:287. doi: 10.1186/s13071-018-2872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasay CJ, Yakob L, Meredith HR, Stewart R, Mills PC, Dekkers MH, et al. Treatment of pigs with endectocides as a complementary tool for combating malaria transmission by Anopheles farauti (s.s.) in Papua New Guinea. Parasit Vectors. 2019;12:124. doi: 10.1186/s13071-019-3392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yakob L. Endectocide-treated cattle for malaria control: a coupled entomological-epidemiological model. Parasite Epidemiol Control. 2016;1:2–9. doi: 10.1016/j.parepi.2015.12.001. [DOI] [Google Scholar]
- 71.Fauroux E, Fiéloux M, Joelson G, Rabedimy JF, Rabibisoa P, Ramiandrisoa C, et al. Le boeuf dans la vie économique et sociale d’un village Vezo : les nouveaux pâturages forestiers de la région de Salary (Sud-Ouest de Madagascar). ORSTOM. http://agris.fao.org/agris-search/search.do?recordID=AV20120164408. Accessed 31 Mar 2020.
- 72.Feldt T, Neudert R, Fust P, Schlecht E. Reproductive and economic performance of local livestock in southwestern Madagascar: Potentials and constraints of a highly extensive system. Agr Sys. 2016;149:54–64. doi: 10.1016/j.agsy.2016.08.007. [DOI] [Google Scholar]
- 73.Hänke H, Barkmann J. Insurance function of livestock, Farmers coping capacity with crop failure in southwestern Madagascar. World Dev. 2017;96:264–275. doi: 10.1016/j.worlddev.2017.03.011. [DOI] [Google Scholar]
- 74.Goetter JF. The cattle raiders leave us no choice: New transhumance in the Mahafaly Plateau region in Madagascar. Madagascar Conservation & Development. https://www.ajol.info/index.php/mcd/article/view/137591. Accessed 31 Mar 2020.
- 75.Zohdy S, Derfus K, Andrianjafy MT, Wright PC, Gillespie TR. Field evaluation of synthetic lure (3-methyl-1-butanol) when compared to non odor-baited control in capturing Anopheles mosquitoes in varying land-use sites in Madagascar. Parasit Vectors. 2015;8:145. doi: 10.1186/s13071-015-0729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zohdy S, Derfus K, Headrick EG, Andrianjafy MT, Wright PC, Gillespie TR. Small-scale land-use variability affects Anopheles spp. distribution and concomitant Plasmodium infection in humans and mosquito vectors in southeastern Madagascar. Malar J. 2016;15:114. doi: 10.1186/s12936-016-1164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated are included in this manuscript and supplementary files.