Abstract
Background
In the EMPA-REG OUTCOME trial (Empagliflozin Cardiovascular Outcome Event Trial) treatment with the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin significantly reduced heart failure hospitalization (HHF) in patients with type 2 diabetes mellitus (T2D) and established cardiovascular disease. The early separation of the HHF event curves within the first 3 months of the trial suggest that immediate hemodynamic effects may play a role. However, hitherto no data exist on early effects of SGLT2 inhibitors on hemodynamic parameters and cardiac function. Thus, this study examined early and delayed effects of empagliflozin treatment on hemodynamic parameters including systemic vascular resistance index, cardiac index, and stroke volume index, as well as echocardiographic measures of cardiac function.
Methods
In this placebo-controlled, randomized, double blind, exploratory study patients with T2D were randomized to empagliflozin 10 mg or placebo for a period of 3 months. Hemodynamic and echocardiographic parameters were assessed after 1 day, 3 days and 3 months of treatment.
Results
Baseline characteristics were not different in the empagliflozin (n = 22) and placebo (n = 20) group. Empagliflozin led to a significant increase in urinary glucose excretion (baseline: 7.3 ± 22.7 g/24 h; day 1: 48.4 ± 34.7 g/24 h; p < 0.001) as well as urinary volume (1740 ± 601 mL/24 h to 2112 ± 837 mL/24 h; p = 0.011) already after one day compared to placebo. Treatment with empagliflozin had no effect on the primary endpoint of systemic vascular resistance index, nor on cardiac index, stroke volume index or pulse rate at any time point. In addition, echocardiography showed no difference in left ventricular systolic function as assessed by left ventricular ejections fraction and strain analysis. However, empagliflozin significantly improved left ventricular filling pressure as assessed by a reduction of early mitral inflow velocity relative to early diastolic left ventricular relaxation (E/eʹ) which became significant at day 1 of treatment (baseline: 9.2 ± 2.6; day 1: 8.5 ± 2.2; p = 0.005) and remained apparent throughout the study. This was primarily attributable to reduced early mitral inflow velocity E (baseline: 0.8 ± 0.2 m/s; day 1: 0.73 ± 0.2 m/sec; p = 0.003).
Conclusions
Empagliflozin treatment of patients with T2D has no significant effect on hemodynamic parameters after 1 or 3 days, nor after 3 months, but leads to rapid and sustained significant improvement of diastolic function.
Trial registration EudraCT Number: 2016-000172-19; date of registration: 2017-02-20 (clinicaltrialregister.eu)
Keywords: SGLT2 inhibitors, Diabetes, Diastolic function, Hemodynamic parameters
Background
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are glucose-lowering drugs currently used to treat patients with type 2 diabetes mellitus (T2D). These agents act by inhibiting SGLT2 in the proximal tubule of the kidney with a subsequent increase in urinary glucose excretion thus lowering blood glucose levels. Several placebo-controlled cardiovascular outcome trials (CVOTs) with SGLT2 inhibitors (EMPA-REG OUTCOME with empagliflozin [1], the CANVAS program [2] and CREDENCE [3] with canaglifozin, DECLARE with dapagliflozin [4], VERTIS with Ertugliflozin [5]) demonstrated a reduction in CV events as well as a reduction in hospitalisation for heart failure (HHF) in patients with T2D and atherosclerotic CV disease (ASCVD), multiple CV risk factors, or diabetic nephropathy. Moreover, the favourable effects of SGLT2 inhibitors on HHF and CV death in these trials were present in patients with or without HF at baseline [6], suggesting that these agents could prevent the development of HF in patients with T2D. In addition, data from the DAPA-HF [7] and the EMPEROR reduced trial [8] suggest that SGLT2 inhibitors may reduce HF related endpoints and CV death even independent of the presence of diabetes.The underlying mechanisms of these beneficial effects of SGLT2 inhibitors on HF-related events remain unclear but changes in blood pressure, blood glucose, or body weight are unlikely to solely explain the observed results. The early separation of HHF event curves in the CVOTs suggested SGLT2 inhibition to provide immediate effects on volume status and/or modulation of hemodynamic parameters potentially mediated by early diuretic effects [9–13]. Therefore, we conducted a prospective, placebo-controlled, double blind, randomized, exploratory pilot study in patients with T2D to assess the effect of empagliflozin on urinary volume, left ventricular filling pressure and function in addition to hemodynamic parameters after 1 day, 3 days and 3 months of treatment.
Methods
Study population and study design
In this single center, prospective, placebo-controlled, double blind, randomized, 2-arm parallel, interventional and exploratory pilot study 44 patients with T2D were randomized into 2 groups. The randomisation list was computer generated using a permuted block randomisation with block size of 4. The sequence generation method and the block size was concealed from the investigators. An independent pharmacist labelled the study medications according to the randomisation list. Study participants received empagliflozin 10 mg or placebo for a period of 3 months in addition to their concomitant medication. Non-invasive hemodynamic measurement, transthoracic echocardiography, blood pressure, blood- and urine-chemistry were performed at baseline (day 0), day 1, day 3 and after 3 months. Participants were recruited from the Department of Internal Medicine I at University Hospital Aachen, RWTH Aachen University, Germany. Inclusion criteria were as follows: type 2 diabetes, HbA1c ≥ 6.5% and age ≥ 18 years. Exclusion criteria were type 1 diabetes, uncontrolled hypertension, age ≥ 85 years, pregnancy, renal impairment (eGFR < 30 mL/min/1.73 m2), liver disease (serum levels of AST, ALT or AP more than three times the upper limit of normal), uncontrolled thyroid disease, endocrinopathies like Graves’ disease, akromegaly, Cushings’ disease, secondary hypertension due to renal artery stenosis, pheochromocytoma or hyperaldosteronism, hypertensive retinopathy or encephalopathy, acute coronary syndrome, stroke or transient ischemic attack in last 6 weeks prior to randomization. The study protocol was approved by the local ethic committee and all subjects gave written informed consent. The trial was registered: EudraCT Number: 2016-000172-19.
Laboratory measurement
Serum chemistry including haematology, lipid profile, glucose metabolism, eGFR (CKD-EPI formula), cystatin C, NT-proBNP, aldosterone were performed at every visit of the clinical trial. We collected 24 h urine at baseline, day 1, day 3 and after 3 months to measure renal excretion of glucose and sodium.
Hemodynamics
We used ClearSight System® (Edwards Lifesciences, Irvine, USA) as a validated [14] non-invasive tool to explore effects of empagliflozin on hemodynamic parameters including cardiac index (CI), stroke volume index (SVI), heart rate (HR), and systemic vascular resistance index (SVRI) at baseline, day 1, day 3 and after 3 months. ClearSight System® uses finger arterial pressure measurement based on the volume clamp method in combination with Physiocal calibration. Dividing the systolic area of the time integral of the pressure curve above the diastolic pressure by the estimated arterial impedance gives a beat-to-beat stroke volume which is multiplied with the heart rate to reach cardiac output, as has been described previously [14].
Transthoracic echocardiography
Transthoracic and Doppler echocardiography were performed by technicians blinded to clinical information and treatment assignment with commercially available ultrasound systems (GE Healthcare, Chicago, USA). Standardized echocardiographic measurements were obtained in accordance with the guidelines of the EACI (European Association of Cardiovascular Imaging) and ASE (American Society of Echocardiography). Left ventricular systolic function (EF) was measured in 4 chamber and 2 chamber views by Simpson’s Biplane Method. Additionally we performed myocardial deformation analysis of the left ventricle to assess peak global longitudinal strain (GLS) of the endocardial layer by speckle-tracking echocardiography in 4 chamber, 2 chamber and apical 3 chamber views. For diastolic function we determined early (E) and late (A) diastolic mitral inflow velocities, deceleration time (DT), septal early diastolic mitral annular tissue velocity (septal eʹ) and lateral early diastolic mitral annular tissue velocity (lateral eʹ) by mitral pulse wave Doppler and tissue Doppler. We calculated E/e' ratio and E/A ratio by dividing E peak by average eʹ calculated from septal eʹ and lateral eʹ respectively E peak by A. Additionally we performed myocardial deformation imaging as determined by 2D and 3D parameter global strain rate. Images were stored digitally for subsequent offline analysis. Interpretation of the echocardiograms was performed by two independent blinded investigators. Interobserver variability of the key echocardiographic endpoints E and eʹ was 0.8 for E and 0.77 for eʹ.
Endpoints
The study was powered for primary study outcome of empagliflozin on systemic vascular resistance index (SVRI) in comparison to placebo after 1 day, 3 days and 3 months of treatment. Secondary endpoints included changes in the following parameters after 1 day, 3 days and 3 months: cardiac index (CI), stroke volume index (SVI), blood pressure, sodium excretion in 24 h urine collection, body weight, heart rate, serum levels of NT-proBNP, cystatin C, glucose, HbA1c and aldosterone.
Further secondary analysis included changes in left ventricular systolic function as determined by EF and GLS, and in left ventricular diastolic function as determined by standardized parameters.
Statistical analysis
The sample size calculation was conducted based on a repeated measure analysis of variance of the primary endpoint including baseline and 3 repeated measures, 2 treatment levels, and a treatment-by-time interaction tested using an F-Test. A mean difference of zero at baseline and constant differences over time were assumed, and a standard deviation of 930 dynes s cm−5 m−2 was used based on sample standard deviation in previous work [15]. The correlation structure was assumed to follow compound symmetry with correlation of 0.3. A significance level of 5% and a power of 80% were chosen. Based on these assumptions, a total of 42 patients allows a detection of a minimal difference of 800 dynes s cm−5 m−2 in SVRI.
Descriptive statistics of baseline characteristics were calculated as relative (%) and absolute frequencies for categorical variables. Quantitative variables were described as means and standard deviations, in case of non-normally distributed data, as median with 1st and 3rd quartiles. Data distributions were visualized using box-plots.
Outcome variables were analysed using linear mixed models with fixed effects for treatment, visits (day 1, day 3 and 3 months) and baseline measurement of the variable. For the primary endpoint analysis, randomisation blocks were also included as fixed effect. The random part of the models consisted of intercepts grouped by individuals. Restricted maximum likelihood estimation was used. For NT-proBNP the log transformed variable was used in the analyses. Treatment effects were estimated at each visit along with Wald type 95% confidence intervals. For the primary endpoint the null hypothesis that all treatment-visit interactions are zero was tested against the alternative that at least one of them is not zero using an F test. Kenward–Roger approximation of the degrees of freedom was used. As additional analyses, correlation between changes from baseline to 3 months were calculated for selected variables using the Pearson correlation coefficient, and changes from baseline were compared between treatment groups separately at each visit. Results were not adjusted for multiple comparison.
Results
Baseline characteristics
From May 2017 to January 2019 a total of 44 patients underwent randomization. Data analysis was performed on 42 patients with 2 patients in the empagliflozin group being excluded because of protocol violations (concomitant intake of SGLT2 inhibitors at baseline and throughout study). No difference in baseline characteristics were observed between empagliflozin and placebo treated patients. Mean age of study participants was 62 ± 6.8 years, 81% were male, with a mean glycated hemoglobin of 7.7 ± 1.1%, a mean BMI of 31.3 ± 4.6 kg/m2, a mean eGFR of 83 ± 19 mL/min/1.73 m2, a history of CVD in 71%, and presence of chronic heart failure in 43% of all patients. Patients had a baseline blood pressure of 135/81 mmHg (SD 16.9/13.2) and a mean LDL cholesterol of 99 ± 36.9 mg/dL. Baseline medication was not different in both groups including anti-diabetic drugs, RAAS-inhibition, beta blockers and statins (Table 1).
Table 1.
Baseline characteristics of the study population
| Placebo (N = 22) | Empagliflozin (N = 20) | p | |
|---|---|---|---|
| Age—years | 61.2 ± 7.9 | 62.8 ± 5.4 | 0.466 |
| Male—no. (%) | 18 (81.8) | 16 (80) | 0.881 |
| BMI—kg/m2 | 31.2 ± 4.0 | 31.4 ± 5.3 | 0.905 |
| Systolic blood pressure—mmHg | 136 ± 18 | 135 ± 16 | 0.934 |
| Diastolic blood pressure—mmHg | 81 ± 14 | 82 ± 13 | 0.964 |
| Heart rate—bpm | 69 ± 15 | 71 ± 12 | 0.587 |
| Type 2 diabetes | |||
| Glycated hemoglobin—% | 7.9 ± 1.3 | 7.5 ± 0.9 | 0.228 |
| Diabetes duration—years | 9 (6–18) | 10 (4–14) | 0.523 |
| Insulin treated—no. (%) | 8 (36) | 11 (55) | 0.475 |
| Metformin—no. (%) | 18 (82) | 13 (65) | 0.410 |
| DPP-4 inhibitors—no. (%) | 6 (27) | 8 (40) | 0.552 |
| Others—no. (%) | 1 (5) | 3 (15) | 0.308 |
| History of CVD—no. (%) | |||
| Coronary heart disease | 15 (68.2) | 15 (75) | 0.625 |
| Myocardial infarction | 10 (45.5) | 5 (25) | 0.205 |
| CABG | 4 (18.2) | 4 (20) | 0.881 |
| PCI | 12 (54.5) | 10 (50) | 0.768 |
| Peripheral artery disease | 2 (9.1) | 4 (20) | 0.313 |
| Chronic heart failure—no. (%) | 11 (50) | 7 (35) | 0.327 |
| Medication—no. (%) | |||
| Antiplatelets | 16 (73) | 11 (55) | 0.975 |
| Oral anticoagulants | 5 (23) | 6 (30) | 0.914 |
| Diuretics | 10 (45) | 10 (50) | 0.549 |
| Statins | 15 (68) | 15 (75) | 0.455 |
| Calcium channel blockers | 5 (23) | 4 (20) | 0.637 |
| Beta blockers | 16 (73) | 16 (80) | 0.345 |
| RAAS inhibitors | 20 (91) | 15 (75) | 0.115 |
| e GFR—mL/min/1.73 m2 | 88 ± 16 | 77 ± 21 | 0.076 |
| Total cholesterol—mg/dL | 155 ± 39 | 169 ± 41 | 0.257 |
| LDL-C—mg/dL | 95 ± 38 | 103 ± 36 | 0.522 |
| HDL-C—mg/dL | 44 ± 9 | 43 ± 9 | 0.530 |
| Triglycerides—mg/dL | 156 ± 71 | 245 ± 150 | 0.023 |
Values are mean ± SD for normally distributed data and median and interquartile range for non-normally distributed data, or no. (%); p-values for continuous variables were calculated using t test, the p-value for diabetes duration was assessed by Kruskal–Wallis test; p-values for categorical variables were calculated using chi-squared test; p-values ≤ 0.05 were categorized as statistically significant
BMI body mass index, CABG coronary artery bypass graft, CVD cardiovascular disease, eGFR estimated glomerular filtration rate, HDL-C high density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, PCI percutaneous coronary intervention, RAAS renin–angiotensin–aldosterone system
Effect of empaglifozin on hemodynamic parameters
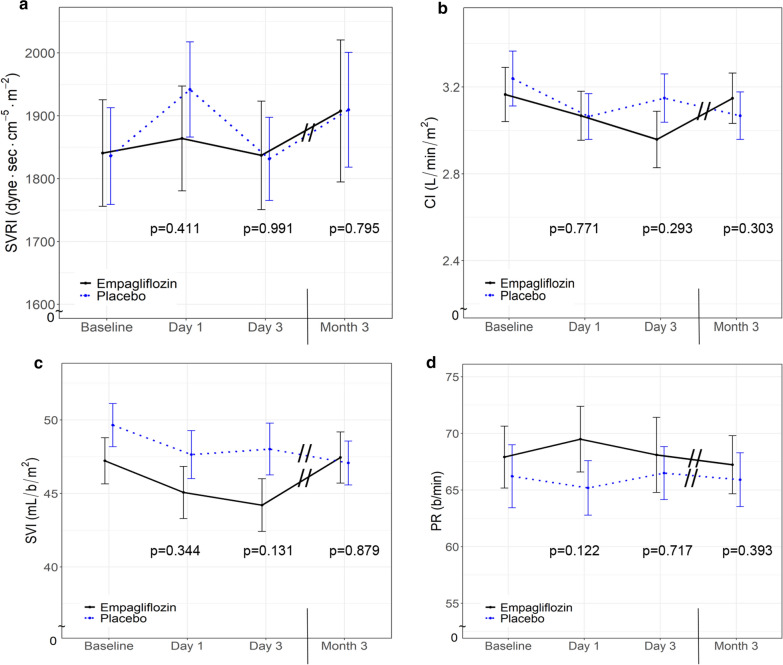
Systemic vascular resistance index (SVRI) and cardiac index (CI) were assessed as primary outcomes by non-invasive pulse wave contour analysis (ClearSight System®) with no significant difference between empagliflozin and placebo treated patients at any time point (Fig. 1a, b, Table 2). No treatment dependent difference in left ventricular stroke volume index (SVI) or pulse rate (PR) was observed (Fig. 1c, d, Table 2). Over time, blood pressure was reduced in empagliflozin-treated participants but the effect did not reach statistical significance (Table 2).
Fig. 1.
Hemodynamic parameters. Systemic vascular resistance index (SVRI) (a), cardiac index (CI) (b), stroke volume index (SVI) (c), and heart rate (HR) (d) in patients with type 2 diabetes treated with empagliflozin (n = 20; black line) or placebo (n = 22; blue dotted line). Data are shown as mean ± standard error at baseline, after 1 day, 3 days, and 3 months. p-values are calculated from Wald tests for the intervention effect at each visit
Table 2.
Comparison of laboratory values, 24 h urine, body weight, hemodynamics, blood pressure and echocardiography during the study
| Baseline | Day 1 | Day 3 | Month 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Empagliflozin | p | Placebo | Empagliflozin | p | Placebo | Empagliflozin | p | Placebo | Empagliflozin | p | |
| Laboratory | ||||||||||||
| Glucose—mg/dL | 175 ± 52 | 181 ± 75 | 0.742 | 171 ± 48 | 152 ± 38 | 0.049 | 166 ± 52 | 146 ± 35 | 0.037 | 156 ± 52 | 151 ± 42 | 0.754 |
| HbA1c—% | 7.9 ± 1.3 | 7.5 ± 0.9 | 0.228 | 7.9 ± 1.3 | 7.4 ± 0.9 | 0.722 | 7.9 ± 1.2 | 7.4 ± 0.9 | 0.690 | 7.8 ± 1.5 | 7.1 ± 0.7 | 0.595 |
| Total cholesterol—mg/dL | 155 ± 39 | 169 ± 41 | 0.257 | 155 ± 37 | 174 ± 43 | 0.178 | 152 ± 40 | 168 ± 39 | 0.824 | 152 ± 42 | 185 ± 48 | 0.001 |
| LDL-C—mg/dL | 95 ± 38 | 103 ± 36 | 0.522 | 94 ± 36 | 102 ± 36 | 0.915 | 93 ± 39 | 102 ± 40 | 0.477 | 89 ± 39 | 112 ± 47 | < 0.001 |
| HDL-C—mg/dL | 44 ± 9 | 43 ± 9 | 0.530 | 44 ± 9 | 42 ± 10 | 0.947 | 44 ± 9 | 43 ± 9 | 0.900 | 46 ± 11 | 46 ± 10 | 0.937 |
| eGFR—mL/min/1.73 m2 | 88 ± 16 | 77 ± 21 | 0.076 | 85 ± 16 | 70 ± 19 | 0.014 | 85 ± 17 | 70 ± 21 | 0.039 | 85 ± 16 | 68 ± 20 | 0.108 |
| Cystatin C—mg/L | 1.0 ± 0.2 | 1.2 ± 0.4 | 0.148 | 1.0 ± 0.2 | 1.3 ± 0.4 | < 0.001 | 1.0 ± 0.2 | 1.3 ± 0.4 | 0.001 | 1.0 ± 0.2 | 1.3 ± 0.4 | < 0.001 |
| NT-proBNP—pg/mL | 166 (73–238) | 239 (91–463) | 0.481 | 168 (67–252) | 192 (63–385) | 0.224 | 147 (58–226) | 173 (57–402) | 0.408 | 158 (42–262) | 133 (32–500) | 0.723 |
| Aldosterone—pg/mL | 83 ± 33 | 104 ± 65 | 0.213 | 88 ± 32 | 111 ± 67 | 0.825 | 96 ± 50 | 108 ± 59 | 0.635 | 108 ± 71 | 137 ± 104 | 0.522 |
| 24 h urine | ||||||||||||
| Urinary volume—mL/24 h | 1788 ± 756 | 1740 ± 601 | 0.829 | 1626 ± 681 | 2112 ± 837 | 0.011 | 2007 ± 913 | 2111 ± 758 | 0.429 | 1664 ± 594 | 2319 ± 873 | 0.001 |
| Glucose excretion—g/24 h | 10.9 ± 22.7 | 7.3 ± 22.7 | 0.617 | 6.9 ± 14.1 | 48.4 ± 34.7 | < 0.001 | 7.5 ± 14.5 | 65.7 ± 43.3 | < 0.001 | 10.2 ± 18.7 | 67.6 ± 50.9 | < 0.001 |
| Sodium excretion—mmol/24 h | 196 ± 84 | 164 ± 88 | 0.255 | 181 ± 76 | 185 ± 111 | 0.223 | 203 ± 107 | 181 ± 126 | 0.970 | 175 ± 55 | 201 ± 145 | 0.054 |
| Electrolyte-free water clearance—mL/24 h | − 15 ± 721 | 166 ± 830 | 0.467 | − 124 ± 654 | 417 ± 802 | 0.011 | 90 ± 874 | 461 ± 551 | 0.070 | − 91 ± 597 | 380 ± 765 | 0.013 |
| Body weight—kg | 94.0 ± 14.0 | 95.8 ± 17.3 | 0.724 | 94.1 ± 14.1 | 94.9 ± 17.0 | 0.044 | 94.0 ± 14.1 | 94.5 ± 17.2 | 0.007 | 93.7 ± 14.3 | 96.0 ± 19.6 | 0.059 |
| Hemodynamics | ||||||||||||
| CI—L/min/m2 | 3.2 ± 0.6 | 3.2 ± 0.6 | 0.682 | 3.1 ± 0.5 | 3.1 ± 0.5 | 0.771 | 3.1 ± 0.5 | 3.0 ± 0.6 | 0.293 | 3.1 ± 0.5 | 3.1 ± 0.5 | 0.303 |
| SVI—mL/b/m2 | 50 ± 7 | 47 ± 7 | 0.266 | 48 ± 8 | 45 ± 8 | 0.344 | 48 ± 8 | 44 ± 8 | 0.131 | 47 ± 7 | 47 ± 7 | 0.879 |
| SVRI—dyne*s*cm−5*m−2 | 1836 ± 361 | 1841 ± 379 | 0.967 | 1942 ± 355 | 1864 ± 373 | 0.411 | 1831 ± 310 | 1837 ± 376 | 0.991 | 1909 ± 428 | 1908 ± 451 | 0.795 |
| HR—bpm | 66 ± 13 | 68 ± 12 | 0.665 | 65 ± 11 | 69 ± 13 | 0.122 | 66 ± 11 | 68 ± 14 | 0.717 | 66 ± 11 | 67 ± 10 | 0.393 |
| Blood pressure | ||||||||||||
| Systolic—mmHg | 136 ± 18 | 135 ± 16 | 0.934 | 134 ± 16 | 128 ± 16 | 0.279 | 133 ± 19 | 125 ± 16 | 0.115 | 132 ± 20 | 128 ± 15 | 0.318 |
| Diastolic—mmHg | 81 ± 14 | 82 ± 13 | 0.964 | 80 ± 13 | 80 ± 11 | 0.876 | 80 ± 12 | 80 ± 9 | 0.925 | 82 ± 11 | 79 ± 11 | 0.197 |
| Echocardiography | ||||||||||||
| LV-EF—% | 48 ± 6.8 | 51 ± 5.0 | 0.183 | 48 ± 6.2 | 51 ± 4.6 | 0.852 | 48 ± 6.1 | 51 ± 4.7 | 0.333 | 48 ± 6.4 | 51 ± 4.4 | 0.375 |
| LVEDD—mm | 50 ± 5 | 49 ± 5 | 0.365 | 50 ± 6 | 49 ± 5 | 0.864 | 49 ± 5 | 48 ± 5 | 0.449 | 50 ± 6 | 48 ± 6 | 0.994 |
| LVESD—mm | 36 ± 8 | 34 ± 6 | 0.386 | 36 ± 9 | 35 ± 5 | 0.595 | 35 ± 8 | 34 ± 5 | 0.187 | 37 ± 8 | 33 ± 6 | 0.284 |
| IVSd—mm | 10 ± 2 | 10 ± 1 | 0.940 | 10 ± 2 | 11 ± 2 | 0.764 | 10 ± 2 | 11 ± 2 | 0.630 | 10 ± 2 | 10 ± 1 | 0.497 |
| LV mass index—g/m2 | 91 ± 21 | 86 ± 19 | 0.474 | 94 ± 24 | 87 ± 19 | 0.448 | 90 ± 21 | 84 ± 19 | 0.701 | 89 ± 23 | 84 ± 17 | 0.807 |
| LA area—cm2 | 20 ± 4.2 | 20 ± 3.7 | 0.484 | 20 ± 5.3 | 18 ± 4.3 | 0.082 | 20 ± 5.7 | 19 ± 4.8 | 0.691 | 20 ± 4.1 | 18 ± 4.0 | 0.503 |
| LA volume index—mL/m2 | 31 ± 11 | 28 ± 9 | 0.414 | 30 ± 13 | 26 ± 10 | 0.300 | 30 ± 13 | 28 ± 10 | 0.840 | 31 ± 12 | 26 ± 9 | 0.239 |
| E—m/s | 0.78 ± 0.14 | 0.80 ± 0.20 | 0.686 | 0.80 ± 0.12 | 0.73 ± 0.20 | 0.003 | 0.78 ± 0.11 | 0.72 ± 0.18 | 0.005 | 0.78 ± 0.13 | 0.72 ± 0.21 | 0.001 |
| A—m/s | 0.73 ± 0.17 | 0.82 ± 0.17 | 0.095 | 0.74 ± 0.20 | 0.81 ± 0.15 | 0.786 | 0.75 ± 0.19 | 0.80 ± 0.15 | 0.550 | 0.73 ± 0.20 | 0.83 ± 0.15 | 0.927 |
| E/A | 1.18 ± 0.53 | 0.97 ± 0.22 | 0.121 | 1.20 ± 0.55 | 0.88 ± 0.20 | 0.042 | 1.16 ± 0.58 | 0.89 ± 0.23 | 0.181 | 1.21 ± 0.52 | 0.85 ± 0.23 | 0.038 |
| eʹ septal—cm/s | 7.2 ± 1.7 | 7.5 ± 1.9 | 0.603 | 6.6 ± 2.0 | 7.8 ± 2.1 | 0.077 | 7.2 ± 1.6 | 7.5 ± 2.0 | 0.785 | 7.2 ± 1.6 | 7.8 ± 2.1 | 0.470 |
| eʹ lateral—cm/s | 9.8 ± 2.0 | 10.4 ± 1.8 | 0.319 | 9.6 ± 2.4 | 10.1 ± 2.4 | 0.970 | 9.5 ± 1.9 | 9.8 ± 2.3 | 0.840 | 9.3 ± 2.0 | 10.4 ± 2.9 | 0.262 |
| eʹ mean | 8.5 ± 1.5 | 8.9 ± 1.6 | 0.361 | 8.1 ± 1.8 | 8.9 ± 2.1 | 0.352 | 8.4 ± 1.3 | 8.7 ± 2.0 | 0.968 | 8.3 ± 1.5 | 9.1 ± 2.3 | 0.280 |
| E/eʹ mean | 9.3 ± 2.2 | 9.2 ± 2.6 | 0.898 | 10.1 ± 1.4 | 8.5 ± 2.2 | 0.005 | 9.5 ± 1.5 | 8.5 ± 2.4 | 0.079 | 9.7 ± 1.9 | 8.3 ± 2.9 | 0.004 |
| DT—msec | 198 ± 54 | 206 ± 45 | 0.610 | 189 ± 45 | 212 ± 35 | 0.234 | 202 ± 54 | 215 ± 43 | 0.624 | 196 ± 59 | 218 ± 59 | 0.261 |
| RVSP—mmHg + CVP | 28 ± 6 | 29 ± 4 | 0.515 | 26 ± 5 | 26 ± 6 | 0.827 | 25 ± 4 | 29 ± 10 | 0.261 | 27 ± 8 | 26 ± 11 | 0.632 |
| GLS (endocardial layer) | − 17 ± 5.3 | − 19 ± 4.1 | 0.107 | − 17 ± 4.8 | − 19 ± 3.4 | 0.877 | − 17 ± 4.4 | − 19 ± 3.7 | 0.735 | − 17 ± 4.6 | − 19 ± 2.7 | 0.608 |
Values are mean ± SD for normally distributed data and median and interquartile range for non-normally distributed data; p-values at baseline were calculated using t test, the p-value for NT-proBNP was assessed by Kruskal–Wallis test; p-values for the intervention effect at day 1, day 3 and month 3 were calculated using the Wald method, eʹ mean is the mean of eʹ septal and eʹ lateral; p-values ≤ 0.05 were categorized as statistically significant
bpm beats per min, CI cardiac index, CVP central venous pressure, DT deceleration time, eGFR estimated glomerular filtration rate, GLS global longitudinal strain, HDL-C high density lipoprotein cholesterol, HR heart rate, IVSd interventricular septum in diastole, LA left atrial, LDL-C low density lipoprotein cholesterol, LV left ventricular, LVEDD left ventricular enddiastolic diameter, LV-EF left ventricular ejection fraction, LVESD left ventricular endsystolic diameter, RVSP right ventricular systolic pressure, SVI stroke volume index, SVRI systemic vascular resistance index
Effect of empagliflozin on metabolic parameters and renal function
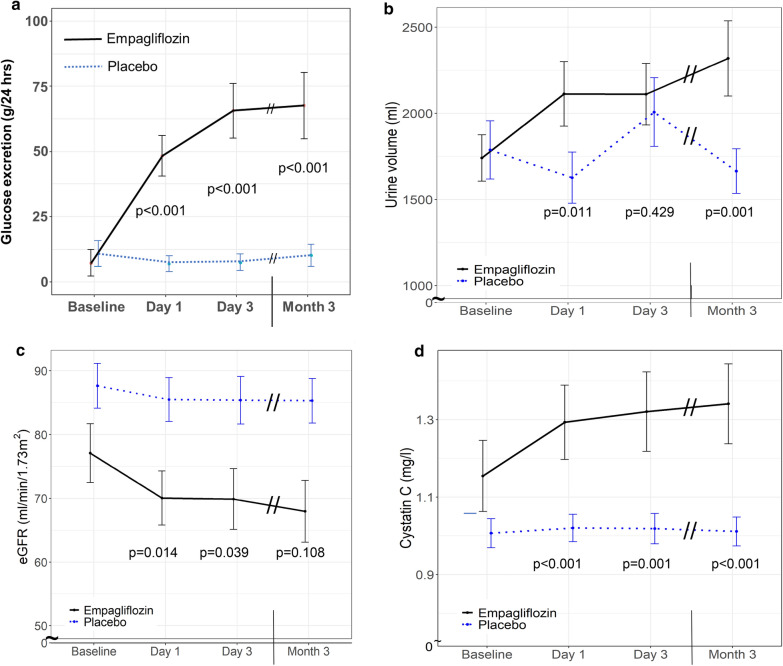
As expected, empagliflozin treatment significantly increased urinary glucose excretion already after one day from 7.3 ± 22.7 g/24 h to 48.4 ± 34.7 g/24 h (p < 0.001) (Fig. 2a and Table 2) which led to an early decrease of fasting blood glucose levels from 181 ± 75 mg/dL to 152 ± 38 mg/dL (p = 0.049) (Table 2). Urinary volume significantly expanded in parallel with glucosuria after day 1 from 1740 ± 601 mL/24 h to 2112 ± 837 mL/24 h (p = 0.011) and remained significantly increased after 3 months of treatment (2319 ± 873 mL/24 h; p = 0.001) compared to placebo (Fig. 2b and Table 2). Body weight decreased at day 1 and day 3 of empagliflozin treatment, which was however not sustained after 3 months (Table 2). We did not find a significant correlation between body weight reduction and urinary volume excretion.
Fig. 2.
Metabolic parameters and renal function. Urinary glucose excretion (a), 24 h urinary volume (b), eGFR (c), and plasma cystatin C levels (d) in patients with type 2 diabetes treated with empagliflozin (n = 20; black line) or placebo (n = 22; blue dotted line). Data are shown as mean ± standard error at baseline, after 1 day, 3 days, and 3 months. p-values are calculated from Wald tests for the intervention effect at each visit
Consistent with the initiation of renal tubule-glomerular feedback, empagliflozin significantly decreased eGFR from 77 ± 21 mL/min/1.73 m2 at baseline to 70 ± 19 mL/min/1.73 m2 (p = 0.014) after 1 day of treatment (Fig. 2c and Table 2) and increased serum cystatin C compared to placebo (Fig. 2d and Table 2). 24 h urinary sodium excretion increased in empagliflozin-treated patients without reaching statistical significance (Table 2). In addition, empagliflozin increased electrolyte-free water clearance from 166 ± 830 mL/24 h at baseline to 417 ± 802 mL/24 h after 1 day (p = 0.011), an effect that was sustained over the 3 month study period (Table 2).
Effect of empagliflozin on echocardiographic parameters
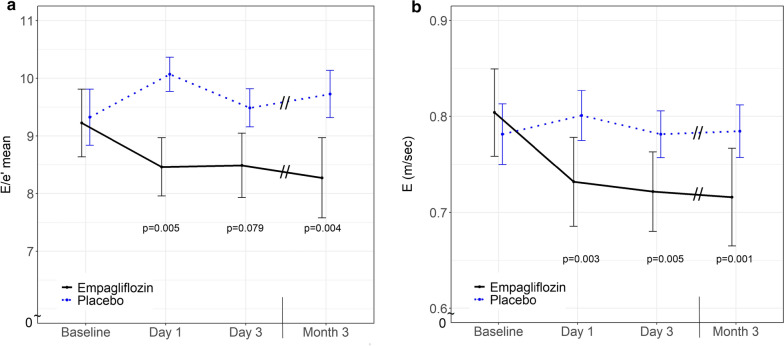
Empaglifozin did not affect left ventricular systolic function as indicated by unchanged left ventricular EF and GLS values (Table 2). However, empagliflozin significantly improved left ventricular diastolic function as assessed by early mitral inflow velocity relative to early diastolic left ventricular relaxation (E/eʹ) which became significant at day 1 of treatment (baseline: 9.2 ± 2.6; day 1: 8.5 ± 2.2; p = 0.005) and remained apparent throughout the study (Fig. 3a, Table 2). Moreover, empagliflozin treatment significantly reduced early mitral inflow velocity (E) (baseline: 0.80 ± 0.20 m/s; day 1: 0.73 ± 0.20 m/s; p = 0.003) (Fig. 3b), but no differences were observed for early diastolic left ventricular relaxation (eʹ) (Table 2). Further analyses did not detect significant treatment dependent effects on left ventricular mass index, atrial volume index (Table 2), NT-proBNP or aldosterone, levels between groups during the 3 months treatment period (Table 2).
Fig. 3.
Left ventricular diastolic function. Early mitral inflow velocity relative to early diastolic left ventricular relaxation (E/eʹ) in patients with type 2 diabetes treated with empagliflozin (n = 20; black line) or placebo (n = 22; blue dotted line). Data are shown as mean ± standard error at baseline, after 1 day, 3 days, and 3 months. p-values are calculated from Wald tests for the intervention effect at each visit
Changes in E/eʹ did not correlate with changes in urinary volume, urinary glucose or sodium excretion, left ventricular mass index or electrolyte-free water clearance (Additional file 1: Figure S1).
Safety
A similar proportion of patients experienced adverse events in the empagliflozin and placebo arm of the study while more patients receiving empagliflozin experienced serious adverse events (Table 3). Genital infections and events consistent with volume depletion were reported more often from patients with empagliflozin (Table 3).
Table 3.
Safety and adverse events
| Placebo (N = 22) | Empagliflozin (N = 20) | |
|---|---|---|
| Adverse events | 10 | 5 |
| Serious adverse events | 4 | 6 |
| Hypoglycemic events | 2 | 4 |
| Death | 0 | 0 |
| Adverse event leading to discontinuation of a study drug | 0 | 1 |
| Acute renale failure | 0 | 0 |
| Event consistent with volume depletion | 0 | 3 |
| Thromboembolic event | 0 | 1 |
| Diabetic ketoacidosis | 0 | 0 |
| Urinary tract infection | 0 | 1 |
| Genital infection | 0 | 4 |
| Bone fracture | 0 | 0 |
Discussion
In this randomized, placebo-controlled, double-blind study in patients with T2D and prevalent ASCVD or high CV risk, resembling the populations studied in CVOTs with SGLT2 inhibitors, empagliflozin had no significant effect on hemodynamic parameters including systemic vascular resistance index, cardiac index, stroke volume indexor pulse rate after 1 or 3 days of treatment nor after 3 months. These data suggest that the early reduction in HF hospitalization seen in EMPA-REG OUTCOME [1], the CANVAS program [2], CREDENCE [16],DECLARE [4] and VERTIS [5] is unlikely to be caused by changes in these parameters. However, we found a rapid improvement in left ventricular filling pressure as shown by a reduction of early mitral inflow velocity relative to early diastolic left ventricular relaxation (E/eʹ) as a main measure of diastolic function, an effect already significant after one day of treatment and sustained until the end of the study. This was attributable to reduced early diastolic transmitral inflow (E), most likely a consequence of persistently increased diuresis induced by empagliflozin being apparent throughout the whole study period, whereas no difference was observed for early diastolic left ventricular relaxation.
The lack of a hemodynamic response to SGLT2 inhibition seen here differs from the response to classic diuretic drugs like loop diuretics. Acutely, loop diuretics increase urine excretion by reducing intravasal volume with apparent hemoconcentration and the diuretic-induced preload reduction impairs cardiac output with a compensatory increase in pulse rate and systemic vascular resistance [17]. In contrast, SGLT2 inhibition in our study rapidly expanded urinary volume excretion already after one day—along with an increase in electrolyte-free water clearance—which did not effect cardiac index, systemic vascular resistance nor pulse rate. Furthermore, treatment with empagliflozin did not decrease serum sodium levels as a common side effect of loop diuretics—with hyponatraemia being a powerful predictor of mortality in patients with heart failure [18]. So, it has been suggested that SGLT2 inhibition more efficiently reduces interstitial relative to intravasal volume in comparison to loop diuretics [19], which maybe supported by increased electrolyte free water clearance upon empagliflozin treatment in our study. Early effects of empagliflozin on body fluid content was further suggested by significant reduction of body weight at day 1 and 3 of treatment, which was however not sustained at the 3 month time point despite ongoing diuretic efficacy. Consistently Schork et al. reported rapid loss of extracellular water by SGLT2 inhibition using bioimpedance spectroscopy [20], which was not anymore apparent after 3 months of treatment. This suggests adaptive mechanisms of fluid regulation to compensate for the ongoing loss of urinary volume at later time points. Furthermore this might indicate additional mechanisms to be of relevance for the sustained reduction of heart failure events in respective CVOTs [1–5]. Importantly, SGLT2 inhibition has recently been found to reduce heart failure events to a similar extend in patients with and without diabetes demonstrating broad therapeutic efficacy of the drug class in HFrEF [7, 8].
The main—albeit exploratory—finding of our study, the early and sustained improvement of left ventricular filling pressure as indicated by E/eʹ in empagliflozin treated patients might provide important information to better understand the early beneficial effects on HF hospitalization seen in SGLT2 inhibitor outcome trials. Patient with T2D are at risk for diastolic dysfunction, resulting from increased left ventricular fibrosis, stiffness, and wall thickness as predisposing factors for heart failure with preserved ejection fraction. Consequential increase of left ventricular filling pressure causes augmentation of E/eʹ (early transmitral inflow velocity / early diastolic mitral annular tissue velocity) as an established echocardiographic parameter of diastolic dysfunction.
Given that impaired diastolic function is a crucial pathophysiological feature of HF, mainly subclinical HFpEF,—often present long before HF becomes clinical apparent—our data bolster the hypothesis that SGLT2 inhibitors could prevent the development of HF by improving left ventricular filling pressure in patients with T2D. An observatory study of 37 patients with T2D demonstrated a reduction in E/eʹ, which was also attributable to reduced early transmitral inflow velocity (E) and combined with a decrease in systolic blood pressure and LVMI after 3 months of treatment with canagliflozin [21]. In contrast, Soga et al. observed a decrease in E/eʹ unrelated to changes of blood pressure in 58 T2D patients with HF treated for 6 months with dapagliflozin under non-randomised conditions. In this study the improvement in diastolic function was independent of E, but attributable to reduced early diastolic left ventricular relaxation and paralleled by a reduction in LVMI [22]. Furthermore Higashikawa et al. reported Tofogliflozin to improve E/eʹ in 42 elderly patients with diabetes [23].
Our randomized, placebo-controlled study extends the understanding of SGLT2 inhibitors’ effect on diastolic function by demonstrating time dependent effects of empagliflozin on left ventricular filling pressure being apparent already after 1 day of treatment. This might be attributable to empagliflozin dependent osmotic diuresis with electrolyte free water excretion leading to cardiac preload reduction as suggested by reduced early mitral inflow velocity E. Still, modulation of E/eʹ did not correlate with changes in urinary volume, urinary glucose or sodium excretion, left ventricular mass index, electrolyte-free water clearance. Additional studies using larger populations will be required to clarify the relevance of volume unloading by SGLT2 inhibition for diastolic function. While observational studies suggest SGLT2 inhibition to improve outcome in patients with HFpEF, this is currently evaluated in large clinical trials (ClinicalTrials.gov Identifier: NCT03619213) [24, 25].
This study has certain limitations. First, hemodynamic parameters were assessed by non-invasive pulse contour analysis (ClearSight System®). However, this technique has extensively been validated against invasive hemodynamic measurements and is an established method in clinical practice [14, 26, 27]. Second, we did not measure other hemodynamic parameters such as pulse pressure, central arterial blood pressure, markers of arterial stiffness that have been shown to be affected by empagliflozin treatment for 6 weeks [28]. Third, the immediate improvement in diastolic function, shown by an early reduction of E/eʹ upon empagliflozin treatment, is an exploratory finding in a limited number of patients, and warrants confirmation in a larger study with changes in diastolic function defined as primary outcomes. Still, the present study was randomized, blinded and placebo-controlled, and changes in cardiac function assessed by echocardiography were predefined exploratory endpoints. Finally, improved diastolic function by empagliflozin treatment was not associated with reduced left ventricular mass index or reduced left atrial volume index nor RVSP after 3 months in our study, while others have found SGLT2 inhibition to reduce left ventricular mass [29]. Additional studies using larger populations will be required to investigate effect of SGLT2 inhibition on structural changes of the left ventricle.
Conclusion
Taken together, our data suggest that empagliflozin treatment of patients with T2D and ASCVD/high CV risk leads to an immediate volume unloading and a rapid and sustained improvement of left ventricular filling pressure. These mechanisms could contribute to the early beneficial effects of SGLT2 inhibitors on HF hospitalisation seen in various SGLT2 inhibitor CVOTs.
Supplementary information
Additional file 1: Figure S1. Change of E/e’ for each single patient treated with empagliflozin (n=20; black line) or placebo (n=22; blue dotted line) after 1 day, 3 days, and 3 months.
Acknowledgements
The authors gratefully acknowledge the expert technical assistance of Gabriele Heuer, Hedwig Reichardt, Zakiya Coenen-Basmadjie, Mareike Wienands and continuous support by Prof. Müller-Wieland, employees of the University Hospital Aachen, Department of Internal Medicine I.
Authors’ contributions
MR and KT researched data and wrote the manuscript. NUKH researched data. JM performed laboratory analyses. AK analyzed the data. AS, EA and MB contributed to the discussion. NM and ML reviewed and edited the manuscript. All authors have read and approved the manuscript as submitted.
Funding
Open Access funding enabled and organized by Projekt DEAL. This investigator-initiated trial was supported by a research grant provided by Boehringer Ingelheim Pharma GmbH & Co. KG. NM and ML are funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – TRR 219 – Project-ID 322900939 (DFG, TTR 219, M-03, M-05). MB funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – TRR 219 – Project-ID 322900939 (DFG, TTR 219, M-02, S-01).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
This study was approved by ethics committee of University Hospital Aachen (reference number: EK 250/16). All subjects gave written informed consent.
Consent for publication
Not applicable.
Competing interests
MR, KT, NUKH, AS and JM report no potential conflict of interest, EA did receive personal fees from AstraZeneca und Novartis and receive research grants from Bayer und Novartis, MB served as a consultant and gave talks for Abbott, Amgen, Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Sqibb, Cytokinetics, Medtronic, Novartis, Servier; NM has received support for clinical trial leadership from Boehringer Ingelheim, Novo Nordisk, served as a consultant to Boehringer Ingelheim, Merck, Novo Nordisk, AstraZeneca, BMS, received grant support from Boehringer Ingelheim, Merck, Novo Nordisk, and served as a speaker for Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and Astra Zeneca. NM declines all personal compensation from pharma or device companies. ML received grants and personal fees from Boehringer Ingelheim, MSD and Novo Nordisk, personal fees from Amgen, Sanofi, Astra Zeneca, Bayer and Lilly.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matthias Rau and Kirsten Thiele contributed equally to this work
Contributor Information
Nikolaus Marx, Email: nmarx@ukaachen.de.
Michael Lehrke, Email: nmarx@ukaachen.de.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01175-5.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Biomath D, Devins T, Johansen O, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. NEnglJMed. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Investigators D-T. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK, Investigators VC. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 6.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2018 doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D-HT and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Investigators EM-RT Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 9.Zhang N, Feng B, Ma X, Sun K, Xu G, Zhou Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019;18:107. doi: 10.1186/s12933-019-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45. doi: 10.1186/s12933-019-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016;37:3192–3200. doi: 10.1093/eurheartj/ehw110. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Marx N. SGLT2 inhibitors: the future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia. 2018;61:2134–2139. doi: 10.1007/s00125-018-4678-z. [DOI] [PubMed] [Google Scholar]
- 14.Broch O, Renner J, Gruenewald M, Meybohm P, Schottler J, Caliebe A, Steinfath M, Malbrain M, Bein B. A comparison of the Nexfin(R) and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012;67:377–383. doi: 10.1111/j.1365-2044.2011.07018.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamzaoui O, Monnet X, Richard C, Osman D, Chemla D, Teboul JL. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med. 2008;36:434–440. doi: 10.1097/01.CCM.OB013E318161FEC4. [DOI] [PubMed] [Google Scholar]
- 16.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 17.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 18.Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, Poppe KK, Guazzi M, Macin SM, Komajda M, Doughty RN, Investigators M. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis(dagger): Meta-Analysis Global Group in Chronic heart failure (MAGGIC) Eur J Heart Fail. 2012;14:1139–1146. doi: 10.1093/eurjhf/hfs099. [DOI] [PubMed] [Google Scholar]
- 19.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 20.Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, Haring HU, Stefan N, Fritsche A, Artunc F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18:46. doi: 10.1186/s12933-019-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17:73. doi: 10.1186/s12933-018-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:132. doi: 10.1186/s12933-018-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashikawa T, Ito T, Mizuno T, Ishigami K, Kohori M, Mae K, Usuda D, Takagi S, Sangen R, Saito A, Iguchi M, Kasamaki Y, Fukuda A, Kanda T, Okuro M. Effects of tofogliflozin on cardiac function in elderly patients with diabetes mellitus. J Clin Med Res. 2020;12:165–171. doi: 10.14740/jocmr4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, Committees EM-PT and Investigators Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019;21:1279–1287. doi: 10.1002/ejhf.1596. [DOI] [PubMed] [Google Scholar]
- 25.Sezai A, Sekino H, Unosawa S, Taoka M, Osaka S, Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. doi: 10.1186/s12933-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecconi M, Monge Garcia MI, Gracia Romero M, Mellinghoff J, Caliandro F, Grounds RM, Rhodes A. The use of pulse pressure variation and stroke volume variation in spontaneously breathing patients to assess dynamic arterial elastance and to predict arterial pressure response to fluid administration. Anesth Analg. 2015;120:76–84. doi: 10.1213/ANE.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 27.Pouwels S, Lascaris B, Nienhuijs SW, Bouwman AR, Buise MP. Short-term changes in cardiovascular hemodynamics in response to bariatric surgery and weight loss using the Nexfin(R) non-invasive continuous monitoring device: a pilot study. Obes Surg. 2017;27:1835–1841. doi: 10.1007/s11695-017-2564-2. [DOI] [PubMed] [Google Scholar]
- 28.Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, Schmieder RE. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–1169. doi: 10.1161/CIRCULATIONAHA.117.029529. [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al-Omran M, Gilbert RE, Bhatt DL, Leiter LA, Juni P, Zinman B, Connelly KA. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Change of E/e’ for each single patient treated with empagliflozin (n=20; black line) or placebo (n=22; blue dotted line) after 1 day, 3 days, and 3 months.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.