Abstract
Background
In contrast to the rapid increase in thyroid cancer incidence, the mortality has remained low and stable over the last decades. In Ecuador, however, thyroid cancer mortality has increased. The objective of this study is to determine possible drivers of high rates of thyroid cancer mortality, through a cross-sectional analysis of all patients attending a thyroid cancer referral center in Ecuador.
Methods
From June 2014 to December 2017, a cross-sectional study was conducted at the Hospital de Especialidades Eugenio Espejo, a regional reference public hospital for endocrine neoplasia in adults in Quito, Ecuador. We identified the mechanism of detection, histopathology and treatment modalities from a patient interview and review of clinical records.
Results
Among 452 patients, 74.8% were young adults and 94.2% (426) were female. 13.7% had a family history of thyroid cancer, and patients’ median tumor size was 2 cm. The incidental finding was 54.2% whereas 45.8% was non-incidental. Thyroid cancer histology reported that 93.3% had papillary thyroid cancer (PTC), 2.7% follicular, 1.5% Hurtle cells, 1.6% medullary, 0.7% poor differentiated, and 0.2% anaplastic carcinoma. The mean MACIS (metastasis, age, completeness, invasion, and size) score was 4.95 (CI 4.15–5.95) with 76.2% of the thyroid cancer patients having MACIS score less than or equal to 6. The very low and low risk of recurrence was 18.1% (79) and 62% (271) respectively. An analysis of 319 patients with non-metastatic thyroid cancer showed that 10.7% (34) of patients had surgical complications. Moreover, around 62.5% (80 from 128 patients with thyroglobulin laboratory results) of TC patients had a stimulated-thyroglobulin value equal or higher than 2 ng/ml. Overall, a poor surgical outcome was present in 35.1% (112) patients. Out of 436 patients with differentiated thyroid carcinoma, 86% (375) received radioactive iodine.
Conclusion
Thyroid cancer histological characteristics and method of diagnosis are like those described in other reports without any evidence of the high frequency of aggressive thyroid cancer histology. However, we observed evidence of overtreatment and poor surgical outcomes that demand additional studies to understand their association with thyroid cancer mortality in Ecuador.
Keywords: Thyroid Cancer, Histopathology, Surgical, Outcome, Ecuador, Latin-America
Background
The incidence of thyroid cancer (TC) has increased over the last three decades in most countries around the globe [1]. In the United States, an analysis of the Surveillance, Epidemiology, and End Results (SEER) between 1975 and 2015 found that TC incidence has increased from 4.9 to 15 per 100,000 people [2]. Similar epidemiological changes have been observed in Central and South America. From 2008 to 2012, TC rates of incidence in these regions increased 8 to 12 times [3]. In Ecuador, the annual incidence fluctuated from 3 to 22 per 100,000 in the last 16 years, with women having higher rates of incidence than men [4].
Thyroid cancer overdiagnosis seems to be the most important driver of thyroid cancer diagnosis, although the contribution of other risk factors (e.g., obesity) to the rise in thyroid cancer incidence is currently being investigated. In contrast with the rapid increase in TC incidence [5–8], worldwide thyroid cancer mortality has remained low and stable over the last decades [9–11]. In Ecuador, however, thyroid cancer incidence and mortality have increased, and the Ecuadorian thyroid cancer mortality rate is one of the highest in the world [4, 9, 10]. The reason for the high thyroid cancer mortality in Ecuador is unknown.
Ideally, a large population-based study examining the thyroid cancer characteristics and treatment trends may help clarify the triggers of the rates of mortality in Ecuador. However, such a study design is not possible with the current TC data infrastructure in Ecuador. Instead, we conducted a cross-sectional analysis of all patients attending a thyroid cancer referral center in Ecuador to determine possible drivers of high rates of thyroid cancer mortality (type of thyroid cancer diagnosis and surgical outcome). This information might help gain insights into what factors could be contributing to thyroid cancer mortality.
Methods
Setting and participants
From June 2014 to December 2017, a cross-sectional study was conducted at the Hospital de Especialidades Eugenio Espejo (HEEE), a regional reference public hospital for endocrine neoplasia in adults in Quito, Ecuador. Ecuador is geographically divided into four major natural regions (Coast, Highland, Amazon, and Galapagos Islands). Due to HEEE being located within the Highland region, its patients come mostly from this area. All the patients who were seen for thyroid cancer at HEEE were included, except the patients who did not have the histopathology report. Patients who had initial management (including surgery) outside HEEE were also included.
Data collection and variables
Two sources of data were used to collect the variables of interest. First, a study coordinator interviewed eligible patients during their first postsurgical appointment at the endocrine clinic. During this process, the study coordinator captured: 1) demographic characteristics such as age, degree of education, region of residence (Coast, Highland, Amazon, or Galapagos Islands), age at diagnosis, and ethnicity; 2) family history of TC; 3) environmental risk factors; 4) methods of diagnosis (incidental or non-incidental findings). Second, study team members reviewed medical records of included patients to extract the following information: 1) thyroid gland functionality (euthyroid, hypothyroidism, or hyperthyroidism), thyroid ultrasound characteristics, and thyroid nodule fine-needle aspiration(FNA) cytologic results based on Bethesda System; 2) surgical characteristics such as type and extension of surgery; 3) thyroid gland histopathological features including tumor size, type, focality, minor or gross local invasion, and cervical lymph node involvement or distant metastases; 4) TC markers measured after thyroidectomy and before radioactive iodine therapy, including thyroid-stimulating hormone (TSH), stimulated thyroglobulin (sTg), inhibited thyroglobulin (iTg), and anti-thyroglobulin antibodies (aTg); 5) surgical characteristics such as type and extension of surgery, and complications (hypocalcemia < 6 months and > 6 months after procedure, recurrent laryngeal nerve injury); and finally 6) the radioactive iodine treatment, its doses, and scan results.
Data management
Baseline characteristics data were managed as follows: employment and education were classified according to the National Institute of Statistics and Census (INEC) from Ecuador [11], and thyroid surgery settings were grouped as tertiary (hospitals providing specialized TC management) and non-tertiary hospitals. Furthermore, patients were considered to have a family history of TC when first and second generation-degree relatives had the disease. Based on thyroid histopathologic features, patients were diagnosed as medullary or non-medullary TC, the latter being further classified as differentiated (papillary and follicular), poorly differentiated, undifferentiated (anaplastic), or squamous cell carcinoma [12]. The risk of recurrence in differentiated TC was calculated by using the American Thyroid Association (ATA) 2009 risk stratification system, which classifies patients’ risk of recurrence as low, intermediate, or high [13]. Due to the overwhelming increasing incidence of patients with papillary thyroid cancer (PTC) with an intrathyroidal tumor size of < 1 cm, a new category was included to the ATA risk of recurrence calculator: “very low risk” [14]. Furthermore, the risk of mortality in patients with PTC was estimated based on MACIS score (metastasis, age, completeness, invasion, and size) [15]. A cutoff of 6 was employed to group patients as either low (MACIS < 6) or high risk (MACIS ≥6) of mortality.
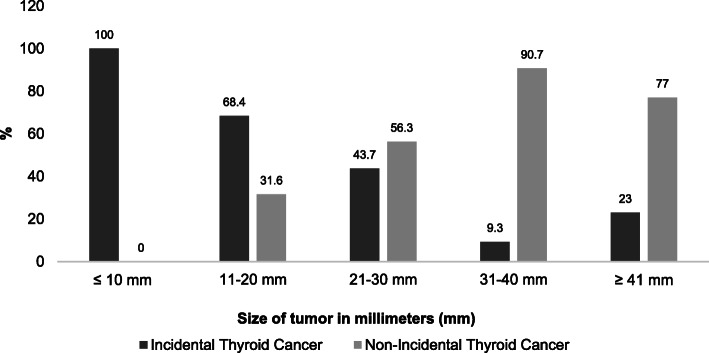
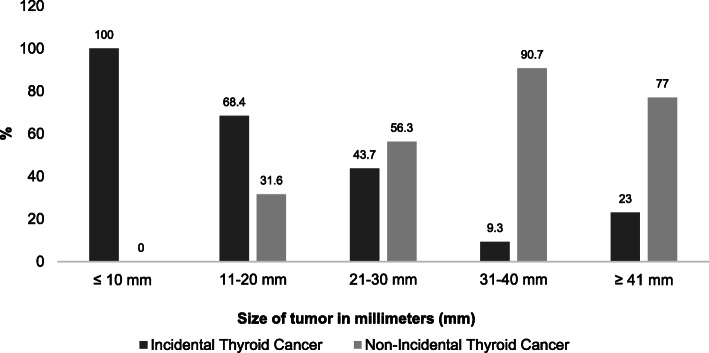
Thyroid cancer method of detection was divided in two groups: non-incidental diagnosis (when the TC was found in a symptomatic patient) and incidental diagnosis when a thyroid nodule harboring TC is found during the workup of non-nodular thyroid disease, or during an imaging test requested for reasons unrelated to a thyroid disorder or symptom (e.g., preventive ultrasound), or TC is found incidentally in the histological examination of the thyroid gland removed for a benign condition (Fig. 1) [16].
Fig. 1.
Tumor size by method of diagnosis
We classified the setting of the surgery as either tertiary hospital (HEEE and Hospital SOLCA) or non-tertiary hospital. Moreover, we evaluated the quality of thyroidectomy based on post-operative sTg levels (at least 6 weeks after the procedure) [17–19], and the frequency of surgical complications [20–22]. We considered that the quality of surgery was optimal when there were no post-surgical complications and when patients had a sTg ≤2 ng/dl, and poor when patients had at least one permanent surgical complication or post-operative sTg > 2 ng/dL. Given that surgical complications and post-operative sTg levels could be affected by the presence of metastatic disease, we limited the assessment of the quality of surgical outcomes to patients with non-metastatic differentiated TC undergoing initial thyroid surgery (total thyroidectomy and prophylactic central neck dissection). Before 2016, the criteria for using iodotherapy included the ATA 2009 guidelines; after 2016, the ATA 2015 guidelines were considered.
Statistical methods
For categorical variables, frequencies and percentages were reported. For numerical variables, we used mean and median with their corresponding standard deviation (SD) or interquartile ranges (IQR), as measurements of central tendency and dispersion. Normal distribution was determined by visual inspection and by using the Kolmogorov-Smirnov test. Our dependent variables used for exploratory analysis were incidental findings and quality of surgery, which are dichotomous variables. For our bivariate and multivariate analysis, we decided to use prevalence ratio (PR) instead of odds ratios (OR) because PR is easier to interpret and OR tend to overestimate the results [23]. To calculate this PR, we planned to use a generalized linear model (GLM) with the binomial family and the log link. However, convergence problems were found with some of the variables. Such issues are common [24, 25]. At the end we chose, from all possible solutions, to use Poisson as the family for the GLM with robust variance. For the multivariate analyses, we decided to include in the models for incidental findings and poor quality of surgery all variables in which p-value was less than 0.05 and those considered to be important by the investigators. The results are reported as PR and their respective 95% confidence intervals. Statistical analysis was performed with STATA [26].
Results
From 2014 to 2017, 452 TC patients were included, with 74.8% of the patients between the ages of 20 and 54 years old. The median tumor size of patients was 2 cm [IQ 1.2, 3.1]. Around 94.2% of TC patients were female and 13.7% had a family history of TC (Table 1). Thyroid cancer histology was: 93.3% had papillary thyroid cancer (PTC), 2.7% follicular, 1.5% Hurtle cells, 1.6% medullary, 0.7% poor differentiated, and 0.2% anaplastic. The mean MACIS score was 4.95 (IQ 4.15, 5.95) with 76.2% of the TCs having MACIS score equal or less than 6 (Table 2).
Table 1.
Characteristics of Thyroid Cancer Patients Before Thyroidectomy
| Variablea | Total (n = 452) n (%) | |
|---|---|---|
| Sex | ||
| Female | 426 | 94.2 |
| Males | 26 | 5.8 |
| Age at diagnosis (mean: 44.6, SD: 14.56) | ||
| < 20 years old | 17 | 3.8 |
| 20–34 years old | 106 | 23.5 |
| 35–44 years old | 104 | 23 |
| 45–54 years old | 128 | 28.3 |
| 55–64 years old | 53 | 11.7 |
| 65–74 years old | 26 | 5.8 |
| 75–84 years old | 16 | 3.5 |
| > 84 years old | 2 | 0.4 |
| Residence | ||
| Coast | 49 | 10.8 |
| Highland | 389 | 86.1 |
| Amazon | 14 | 3.1 |
| Galapagos | 0 | 0 |
| Employment | ||
| Domestic chores | 332 | 73.5 |
| Student | 20 | 4.4 |
| Labor | 100 | 22.1 |
| Education level | ||
| None | 22 | 4.9 |
| Elementary School | 267 | 59.1 |
| High school | 132 | 29.2 |
| College | 31 | 6.9 |
| Family history of thyroid cancer | ||
| Yes | 62 | 13.7 |
| No | 390 | 86.3 |
| BMI (n = 298) (mean: 28.75, SD: 5.53) | ||
| Normal | 76 | 25.5 |
| Overweight | 113 | 37.9 |
| Obesity | 109 | 36.6 |
| Self-reported exposure to (n = 63) | ||
| Radiation | 4 | 6.3 |
| Chemicals in agriculture | 59 | 93.7 |
| Cigarette Smoking (n = 393) | ||
| Yes | 17 | 4.3 |
| No | 376 | 95.7 |
| Thyroid function | ||
| Euthyroid | 372 | 82.3 |
| Hypothyroidism | 72 | 15.9 |
| Hyperthyroidism | 8 | 1.8 |
| Methods of detection | ||
| Non-incidental (palpable nodule) | 207 | 45.8 |
| Incidental | 245 | 54.2 |
| - Ultrasound | 229 | 93.5 |
| - Histology | 13 | 5.3 |
| - Unrelated test | 3 | 1.2 |
| Setting of thyroid surgery (n = 450) | ||
| Tertiary Hospital | 270 | 60 |
| Non- tertiary hospital | 180 | 40 |
| Size of tumor (n = 406) (median = 2 cm [IQ 1.2, 3.1]) | ||
| ≤ 1 cm | 89 | 21.9 |
| > 1 cm | 317 | 78.1 |
| Focality (n = 416) | ||
| Unifocal | 230 | 55.3 |
| Multifocal | 186 | 44.7 |
| Cervical Lymph nodes metastasis (n = 436) | ||
| Si | 211 | 48.4 |
| No | 225 | 51.6 |
| MACIS score (n = 408) (median = 4.95 cm [IQ 4.15, 5.95]) | ||
| ≤ 6 | 311 | 76.2 |
| > 6 | 97 | 23.8 |
| Histopathology, (n = 447) | ||
| Papillary | 417 | 93.3 |
| Follicular | 12 | 2.7 |
| Hurtle cells | 7 | 1.5 |
| Poor differentiated | 3 | 0.7 |
| Anaplastic | 1 | 0.2 |
| Medullary | 7 | 1.6 |
| Risk recurrence (n = 437) | ||
| Very low risk | 79 | 18.1 |
| Low risk | 271 | 62.0 |
| Indeterminate risk | 49 | 11.2 |
| High risk | 38 | 8.7 |
aAll variables without a specific number of patients were calculated from the whole population (n = 452). All the others show the number of people from which the variables were available
Table 2.
Factors associated with the prevalence of diagnosis
| Incidental | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Variables | No | Yes | PR (95% CI) | p | ||
| n = 206 | n = 246 | |||||
| Age, mean (SD) | 43.0 (16.1) | 46.3 (13.4) | 1.01 (1.00,1.01) | 0.037 | 1.01 (1.00, 1.02) | 0.033 |
| Sex, n (%) | ||||||
| Male | 14 (6.8) | 12 (4.9) | Reference | 0.433 | ||
| Female | 192 (93.2) | 234 (95.1) | 1.19 (0.78, 1.81) | |||
| Positive Family history n (%) | ||||||
| No | 179 (86.5) | 211 (86.1) | Reference | 0.914 | ||
| Yes | 28 (13.6) | 34 (13.8) | 1.01 (0.79, 1.29) | |||
| BMI, (n = 299) mean, (SD) |
n = 142 28.6 (5.7) |
n = 157 29.2 (5.8) |
1.00 (0.99, 1.02) | 0.630 | ||
| Tumor size in mm, (n = 406) mean, (SD) |
n = 177 35.7 (17.9) |
n = 229 22.3 (12.3) |
0.96 (0.95, 0.97) | 0.000 | 0.96 (0.94, 0.97) | 0.000 |
| Multifocal (n = 328), n (%) | ||||||
| Unifocal, n (%) | 103 (55.4) | 127 (55.2) | Reference | 0.974 | Reference | 0.000 |
| Multifocal, n (%) | 83 (44.6) | 103 (44.8) | 1.00 (0.84, 1.19) | 1.32 (1.13, 1.54) | ||
| Positive Cervical Lymph nodes, n (%) | ||||||
| No | 93 (46.3) | 132 (56.2) | Reference | 0.041 | Reference | 0.305 |
| Yes | 108 (53.7) | 103 (43.8) | 0.83 (0.70, 0.99) | 1.09 (0.93, 1.27) | ||
| MACIS score (n = 406), mean (SD) | 5.6 (1.6) | 4.9 (1.4) | 0.86 (0.79, 0.92) | 0.000 | 0.92 (0.81, 1.04) | 0.184 |
| Risk recurrence (n = 437) | ||||||
| Very low risk | 0 | 79 (33) | Referencea | |||
| Low risk | 147 (73.5) | 124 (52.3) | ||||
| Intermediate risk | 33 (16.5) | 16 (6.8) | 0.56 (0.37, 0.85) | 0.006 | 0.98 (0.67, 1.43) | 0.902 |
| High risk | 20 (10.0) | 18 (7.6) | 0.77 (0.54, 1.11) | 0.163 | 1.04 (0.82, 2.16) | 0.239 |
a For univariate and multivariate analysis low and very low risk categories were combined and taken as reference
Mechanism of detection
The methods of TC diagnosis were: 54.2% incidental (93.5% by ultrasound, 5.3% histology, and 1.2% unrelated test) and 45.8% non-incidental (palpable and symptomatic nodule) (Fig. 1). Furthermore, 100% of patients with microcarcinoma (≤10 mm) were incidental finding; the proportions further fell to 68.4, 43.7, 9.3, and 23% for the 11-20 mm, 21-30 mm, 31-40 mm and ≥ 41 mm groups, respectively (Fig. 2). The univariate analysis showed that age, tumor size, and MACIS score were associated with the mechanism of detection (Table 3).
Fig. 2.
Tumor size by method of diagnosis
Table 3.
Factors associated with poor optimal surgical outcomes
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| PR (95% CI) | P value | PR (95% CI) | P value | |
| Sex | ||||
| Male | Reference | 0.295 | ||
| Female | 0.74 (0.42, 1.30) | |||
| Age, mean (SD) | 1.00 (0.99, 1.01) | 0.684 | ||
| BMI | 1.02 (0.99, 1.05) | 0.191 | ||
| Setting of Surgery | ||||
| - Tertiary Hospital | Reference | 0.878 | Reference | 0.947 |
| - Non- tertiaty hospital | 0.98 (0.71, 1.32) | 1.01 (0.75, 1.37) | ||
| Tumor size (n = 306), mean (SD) | 1.01 (1.00, 1.02) | 0.001 | 1.01 (1.00, 1.02) | 0.182 |
| Tumor focality, n (%) | ||||
| - Unifocal | Reference | 0.425 | ||
| - Multifocal | 1.13 (0.84, 1.53) | |||
| Positive Central Cervical Lymph nodes metastasis, n (%) | ||||
| - No | Reference | 0.004 | Reference | 0.017 |
| - Yes | 1.54 (1.15, 2.06) | 1.45 (1.07, 1.97) | ||
| MACIS score | 1.14 (1.02, 1.28) | 0.017 | 1.08 (0.94, 1.24) | 0.285 |
| Histology variant | ||||
| - Non- aggresive | Reference | 0.651 | ||
| - Aggresive | 1.11 (0.70, 1.79) | |||
| Risk recurrence | ||||
| Very low risk | Referencea | |||
| Low risk | ||||
| Indeterminate risk | 0.95 (0.51, 1.77) | 0.861 | ||
| High risk | 0.95 (0.19, 4.73) | 0.946 | ||
a For univariate and multivariate analysis low and very low risk categories were combined and taken as reference
Treatment modalities
Surgical characteristics and outcomes
All patients were treated with total thyroidectomy. The operations were performed by the HEEE surgery team (7 surgeons) in 46% (205) of patients. Other patients had operations in other institutions and were transferred to the HEEE to continue the follow-up. The neck dissection was performed as follows: 46% (209) central compartment, 22% (97) lateral compartment, and 32% (146) did not have neck dissection. Of patients with central compartment neck dissection, 16% (34) had more than 5 positive lymph nodes removed. An analysis of 319 patients with non-metastatic differentiated thyroid carcinoma (DTC) showed that 10.7% (34) of patients had surgical complications, 7.8% (25) of patients developed permanent hypoparathyroidism, 2.2% (7) had recurrent laryngeal nerve injury, and 0.6% (2) showed spinal nerve injury. Moreover, around 61% (80 from 128 patients with thyroglobulin laboratory results) of TC patients had a sTg value equal or higher than 2 ng/ml. By using both surgical complications and sTg values, the percentage of patients who had a poor surgical outcome was 35%. The univariate analysis showed that the tumor size, MACIS score and the presence of metastatic cervical lymph nodes in the central compartment were associated with poor surgical outcome. However, in multivariate analysis, only metastatic cervical lymph nodes were associated with poor surgical outcome (PR = 1.45 [IC:1.07, 1.97]) (Table 4).
Table 4.
Radioactive Iodine and risk of recurrence
| Risk recurrence | Yes n = 375 (89.3%) | No n = 45 (10.7%) | PR (95% CI) | P value |
|---|---|---|---|---|
| Very low risk (micro PTC) n (%) | 49 (63.6) | 28 (36.4) | 0.2 (0.13, 0.35) | 0.001 |
| Low risk | 248 (95) | 13 (5) | ||
| Indeterminate risk | 43 (91.5) | 4 (8.5) | ||
| High risk | 35 (100) | 0 (0) |
Iodine therapy
Out of 436 patients with DTC, 86% (375) received RAI. The median dose of RAI was 100 mCi (IQR: 100–150) and the median lapse between surgery and RAI therapy was 4 months (IQR: 3–7 months). 95% of people with very low risk and low risk received RAI treatment (Table 5).
Table 5.
Radioactive Iodine Treatment in patients with thyroid cancer by their risk of recurrence
| Variables | Radioactive Iodine Treatment | |||
|---|---|---|---|---|
| Yes | No | PR (95% CI) | p value | |
| n = 375 (89.3%) | n = 45 (10.7%) | |||
| Risk of recurrence | ||||
| - Very low risk (micro PTC) n (%) | 49 (63.6) | 28 (36.4) | 0.2 (0.13, 0.35) | 0.001 |
| - Low risk | 248 (95) | 13 (5) | ||
| - Indeterminate risk | 43 (91.5) | 4 (8.5) | ||
| - High risk | 35 (100) | 0 (0) | ||
Discussion
We conducted a cross-sectional analysis of all TC patients receiving care at a regional reference hospital in Ecuador. This analysis revealed that 74.8% of TC patients were between 20 and 54 years old, and the majority was papillary thyroid cancer at low or very low risk of recurrence. Approximately half of these cases were found incidentally, and a quarter of TC patients had a poor surgical outcome. Despite being mostly low risk for cancer, all patients received total thyroidectomy, and the majority received RAI.
Although this sample only represents a small subset of all thyroid cancers in Ecuador, histological characteristics and methods of diagnosis are similar to the ones described in other reports [27–31]. We did not see an increased frequency of aggressive thyroid cancer histological findings that might explain the increase in thyroid cancer mortality in Ecuador. We observed that the majority of thyroid cancer cases were of low risk of recurrence and mortality. Moreover, we found that more than half of thyroid cancers were diagnosed incidentally, and the minority of patients presented with symptoms resembling findings in countries where thyroid cancer overdiagnosis drives increasing incident trends [32–35]. The driver of incidental thyroid cancer in this cohort was the used of neck ultrasound. This finding is consistent with other studies that shows that the use of thyroid ultrasound has increased at a rate of 20% per year from 2002 through 2013 in the United States [36, 37] and was associated with more thyroid cancer diagnosis, this cancer found by neck ultrasound was mostly of low risk. Although thyroid cancer histology and mode of presentation did not show any hint to explain thyroid cancer increased mortality, we found that there was evidence of overtreatment and poor surgical outcomes. One-third of patients had either surgical adverse events or a post-surgical Tg value that suggested residual benign or malignant thyroid tissue. Persistent thyroglobulin in our cohort may be the result of both insufficient surgical treatment of cervical lymph node metastases and a significant remnant of thyroid tissue in the glandular bed. The high frequencies of poor surgical outcomes suggest a lack of surgical thyroid cancer expertise [38–40]. In Ecuador, there are no residency programs dedicated to training surgeons about the treatment of TC. The few existing thyroid focused surgeons are insufficient in covering the rising demand for new patients with this tumor; therefore, before 2017 most of our patients underwent a thyroidectomy with a general surgeon and most of the patients underwent prophylactic central or lateral neck dissection (68%). In light of this, a retrospective study assessed the safety and efficiency of thyroid surgery, it found more hypocalcemia < 2.0 mmol/l (32.8 vs. 22.0%) and postoperative hemorrhage (5.6% vs. 1.9%) in surgeries performed by general surgeons [40]. These poor surgical outcomes might be higher among countries affected by higher rates of TC diagnosis that have limited thyroid surgical expertise [21] (e.g., Ecuador). Yet, most TC patients do not receive care or treatment in a reference hospital, and thus, they may be at higher risk of complications (e.g., hypocalcemia, recurrent laryngeal nerve injury, etc.) and perhaps unrecognized death due to thyroid cancer surgery.
Another driver of the increased thyroid cancer mortality in Ecuador, not assessed in this study, may be attribution bias. That is, patients with thyroid cancer who died, and the cause of death is attributed to thyroid cancer even if cancer was likely not the cause of death [41]. This misclassification bias exaggerates cancer-specific mortality. Morticians not familiar with thyroid cancer prognosis may be more willing to allocate cause of death to thyroid cancer when the chain of events leading to death is unclear or unknown. Moreover, we observed that the majority of thyroid cancer cases were of low or very low risk of recurrence, however, most of them received high doses of RAI therapy. In our study, it is not clear if the rise observed in the use of RAI is associated with diagnosis of higher-risk tumors or if clinicians continue to prescribe RAI due to a lack of knowledge or for thyroid remnant ablation. Although RAI use would not have a detrimental impact on thyroid cancer mortality, its use adds to the patient’s burden of treatment and risk of adverse events [42–44].
Limitations and strengths
This study has several limitations. This is not a population-based study; therefore, selection bias may influence our results. Furthermore, there were patients with missing data, lowering our sample size and confidence in the estimates. Information about the histopathological characteristics and post-surgical treatment were unavailable because not all patients began the treatment in HEEE and some of them came to this hospital after surgery or after radioactive therapy was performed. Moreover, in the interview of the patients, the question of family history was exposed to recall bias. Finally, we were not able to provide information about the outcomes for these patients as this data is currently being collected as data for a subsequent study. Despite these limitations, this study has several strengths. First, patient data was collected by extractors trained in thyroid cancer treatment and diagnosis using a well-designed extraction form. Second, our inclusion criteria and case finding process secured that all patients treated at the hospital were include for analysis. Finally, our team included a multidisplinary team of clinicians, thyroid cancer researched and epidemiologists who contributed to planning, execution, and dissemination of study’s results.
Conclusion
Considering the paucity of population-based cancer registries in Ecuador, this study provides additional information about the thyroid cancer diagnosis and treatment in a tertiary referral center in Ecuador. We observed that thyroid cancer histological characteristics and methods of diagnosis are like those described in other reports without any evidence of the high frequency of aggressive thyroid cancer histology. However, we observed evidence of overtreatment and poor surgical outcomes that demand additional studies to understand their association with thyroid cancer mortality in Ecuador. This study is significant because it shows the high rate of overtreatment for patients seen in a tertiary care center for thyroid cancer in Ecuador, particularly the high frequency of surgical adverse outcomes among patients with low-risk thyroid cancer, who may benefit from non-surgical options for their management. These results further invite to better understand the potential association of overtreatment and thyroid cancer mortality.
Acknowledgements
The authors thank the patients and their families who contributed to the completion of this analysis.
Abbreviations
- TC
Thyroid Cancer
- HEEE
Hospital de Especialidades Eugenio Espejo
- MACIS
Metastasis, age, completeness, invasion, and size
- DTC
Differentiated thyroid carcinoma
- RAI
Radioactive iodine
- SEER
Surveillance, Epidemiology, and End Results
- FNA
Fine-needle aspiration
- TSH
Thyroid-stimulating hormone
- sTg
Stimulated thyroglobulin
- iTg
Inhibited thyroglobulin
- aTg
Anti-thyroglobulin antibodies
- INEC
National Institute of Statistics and Census
- ATA
American Thyroid Association
- PTC
Papillary thyroid cancer
- SD
Standard deviation
- IQR
Interquartile ranges
- PR
Prevalence ratio
- OR
Odds ratios
- GLM
Generalized linear model
Authors’ contributions
PSP and JLS were fully responsible for the conceptualization, data collection and elaboration of the study and both participated in drafting the manuscript equally and are fully responsible for it. EL, GJ and CG contributed with the data collection (surgical information and pathological analysis) and the construction of figures and Tables. TL, TR, BA, CC, and OP contributed with the descriptive statistical analysis and the discussion section of the manuscript. EO-P and JPB added an important insight about the epidemiological point of view regarding rates of mortality and the overall analysis of thyroid cancer in Ecuador, respectively. Both critically reviewed the entire manuscript and produced several comments prior to the submission. All authors have read and approved the manuscript.
Funding
This work: design of the study and collection, analysis, interpretation of data, and writing, did not receive financial support of any kind except for the publication fee paid in full by Universidad de las Americas, Quito, Ecuador.
Availability of data and materials
Since data came from the medical records where sensitive information is collected, no database is publicly available. Nevertheless, anonymized information can be shared privately upon reasonable request at e.ortizprado@gmail.com or paosolis@stanford.edu.
Ethics approval and consent to participate
All data were collected from the patient’s medical records after obtaining written informed consent. The study was approved by the Hospital Eugenio Espejo review board. All data was anonymized, and all identifiable information and biological samples were stored according to the local guidelines.
Consent for publication
Written informed consent was obtained from every patient in the study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paola Solis-Pazmino and Jorge Salazar-Vega contributed equally to this work.
References
- 1.Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid Cancer. Thyroid. 2016;26:1541–1552. doi: 10.1089/thy.2016.0100. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJCK. SEER Cancer Statistics Review 1975–2014 National Cancer Institute SEER Cancer Statistics Review 1975–2014 National Cancer Institute. 2017. pp. 2012–2014. [Google Scholar]
- 3.Sierra MS, Soerjomataram I, Forman D. Thyroid cancer burden in central and South America. Cancer Epidemiol. 2016;44:S150–S157. doi: 10.1016/j.canep.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Salazar-Vega J, Ortiz-Prado E, Solis-Pazmino P, Gómez-Barreno L, Simbaña-Rivera K, Henriquez-Trujillo AR, et al. Thyroid Cancer in Ecuador, a 16 years population-based analysis (2001–2016) BMC Cancer. 2019;19:294. doi: 10.1186/s12885-019-5485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid Cancer incidence and mortality in the United States, 1974-2013. Jama. 2017;317:1338. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minelli G, Conti S, Manno V, Olivieri A, Ascoli V. The geographical pattern of thyroid Cancer mortality between 1980 and 2009 in Italy. Thyroid. 2013;23:1609–1618. doi: 10.1089/thy.2013.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keinan-Boker L, Silverman BG. Trends of thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med J. 2016;7:e0001. doi: 10.5041/RMMJ.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosário PW, Ward LS, Carvalho GA, Graf H, Maciel RMB, Maciel LMZ, et al. Thyroid nodules and differentiated thyroid cancer: update on the Brazilian consensus. Arq Bras Endocrinol Metabol. 2013;57:240–264. doi: 10.1590/S0004-27302013000400002. [DOI] [PubMed] [Google Scholar]
- 9.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 10.Enrique López G, Kempis Guerrero F, Segale Bajaña A, et al. Trends of Thyroid Cancer Mortality Rates in Ecuador. J Endocrinol Diab. 2018;5(5):1–6. 10.15226/2374-6890/5/5/001114.
- 11.INEC . Anuario de Estadisticas vitales: Nacimientos y Defunciones. 2013. [Google Scholar]
- 12.King-yin LA. Pathology of endocrine tumors update: World Health Organization new classification 2017—other thyroid tumors. AJSP Rev Reports. 2017;22:209–216. [Google Scholar]
- 13.Haugen BR. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: what is new and what has changed? Cancer. 2017;123:372–381. doi: 10.1002/cncr.30360. [DOI] [PubMed] [Google Scholar]
- 14.Pitoia F, Califano I, Vázquez A, Faure E, Gauna A, Orlandi A, et al. Consenso intersocietario∗ sobre tratamiento y seguimiento de pacientes con cáncer diferenciado de tiroides. Rev Argent Endocrinol Metab. 2014;51:85–118. [Google Scholar]
- 15.Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid Cancer (eighth edition): what changed and why? Thyroid. 2017;27:751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid Cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid. 2015;25:999–1007. doi: 10.1089/thy.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JI, Chung YJ, Cho BY, Chong S, Seok JW, Park SJ. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma. Surg (United States) 2013;153:828–835. doi: 10.1016/j.surg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Chang YW, Kim HS, Jung SP, Kim HY, Lee JB, Bae JW, et al. Pre-ablation stimulated thyroglobulin is a better predictor of recurrence in pathological N1a papillary thyroid carcinoma than the lymph node ratio. Int J Clin Oncol. 2016;21:862–868. doi: 10.1007/s10147-016-0956-2. [DOI] [PubMed] [Google Scholar]
- 19.Salvatori M, Raffaelli M, Castaldi P, Treglia G, Rufini V, Perotti G, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol. 2007;33:648–654. doi: 10.1016/j.ejso.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR. Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab. 2017;102:2543–2551. doi: 10.1210/jc.2017-00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam MA, Thomas S, Youngwirth L, Hyslop T, Reed SD, Scheri RP, et al. Is there a minimum number of thyroidectomies a surgeon should perform to optimize patient outcomes? Ann Surg. 2017;265:402–407. doi: 10.1097/SLA.0000000000001688. [DOI] [PubMed] [Google Scholar]
- 22.Liu JB, Sosa JA, Grogan RH, Liu Y, Cohen ME, Ko CY, et al. Variation of thyroidectomy-specific outcomes among hospitals and their association with risk adjustment and hospital performance. JAMA Surg. 2018;153:1–10. doi: 10.1001/jamasurg.2018.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:1–13. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espelt A, Marí-Dell M, Penelo E, Bosque-Prous M. Resumen Abstract Applied Prevalence Ratio estimation with different Regression models: An example from a cross-national study on substance use research Estimación de la Razón de Prevalencia con distintos modelos de Regresión: Ejemplo de un estudio interna. Adicciones. 2016;29:105–112. doi: 10.20882/adicciones.823. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho LMS, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica. 2008;42:992–998. doi: 10.1590/S0034-89102008000600003. [DOI] [PubMed] [Google Scholar]
- 26.Stata: Software for Statistics and Data Science. https://www.stata.com/. Accessed 9 Dec 2020.
- 27.Weeks KS, Kahl AR, Lynch CF, Charlton ME. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007–2014. Cancer. 2018:2007–14. 10.1002/cncr.31229. [DOI] [PMC free article] [PubMed]
- 28.Russo Picasso MF, Vicens J, Giuliani C, Jaén ADV, Cabezón C, Figari M, et al. Role of the mechanisms of detection in the increased risk of thyroid Cancer: a retrospective cohort study in an HMO in Buenos Aires. J Cancer Epidemiol. 2018;2018. [DOI] [PMC free article] [PubMed]
- 29.Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid Cancer detection by ultrasound among residents ages 18 years and younger in Fukushima. Japan Epidemiology. 2016;27:316–322. doi: 10.1097/EDE.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AL-A Y, AL-M B, AL-R O, Tunio MA, Islam T, AL-A M, et al. Impact of body mass index on survival outcome in patients with differentiated thyroid cancer. Brazilian J Otorhinolaryngol. 2017:25 http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=cctr&AN=CN-01371740http://sfxhosted.exlibrisgroup.com/mayo?sid=OVID:cctrdb&id=pmid:10.1016%2Fj.bjorl.2017.02.002&id=doi:&issn=&isbn=&volume=Date+of+Publication%3A+June+25&issue=&spage=&. [DOI] [PMC free article] [PubMed]
- 31.Estrada-Florez AP, Bohórquez ME, Sahasrabudhe R, Prieto R, Lott P, Duque CS, et al. Clinical features of Hispanic thyroid cancer cases and the role of known genetic variants on disease risk. Med (United States) 2016;95:1–7. doi: 10.1097/MD.0000000000004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janovsky CCPS, Bittencourt MS, Novais MAP d, Maciel RMB, Biscolla RPM. Thyroid cancer burden and economic impact on the Brazilian public health system. Arch Endocrinol Metab. 2018;62:537–544. doi: 10.20945/2359-3997000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massimino M, Evans DB, Podda M, Spinelli C, Collini P, Pizzi N, et al. Thyroid cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65(8). 10.1002/pbc.27025. [DOI] [PubMed]
- 34.Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid Cancer. Endocrinol Metab Clin N Am. 2019;48:23–35. doi: 10.1016/j.ecl.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Brito JP, Davies L. Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. 2014;21:405–408. doi: 10.1097/MED.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Maso LD, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8:468–470. doi: 10.1016/S2213-8587(20)30115-7. [DOI] [PubMed] [Google Scholar]
- 37.Haymart MR, Banerjee M, Reyes-Gastelum D, Caoili E, Norton EC. Thyroid ultrasound and the increase in diagnosis of low-risk thyroid Cancer. J Clin Endocrinol Metab. 2018;104:785–792. doi: 10.1210/jc.2018-01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavalheiro BG, Matos LL, Leite AKN, Kulcsar MAV, Cernea CR, Brandão LG. Surgical treatment for thyroid carcinoma: retrospective study with 811 patients in a Brazilian tertiary hospital. Arch Endocrinol Metab. 2016;60:472–478. doi: 10.1590/2359-3997000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doubleday A, Sippel RS. Surgical options for thyroid cancer and post-surgical management. Expert Rev Endocrinol Metab. 2018;13:137–148. doi: 10.1080/17446651.2018.1464910. [DOI] [PubMed] [Google Scholar]
- 40.Kohnen B, Schürmeyer C, Schürmeyer TH, Kress P. Surgery of benign thyroid disease by ENT/head and neck surgeons and general surgeons: 233 cases of vocal fold paralysis in 3509 patients. Eur Arch Oto-Rhino-Laryngology. 2018;275:2397–2402. doi: 10.1007/s00405-018-5077-2. [DOI] [PubMed] [Google Scholar]
- 41.Leite AKN, Cavalheiro BG, Kulcsar MA, Hoff A d O, Brandão LG, Cernea CR, et al. Deaths related to differentiated thyroid cancer: a rare but real event. Arch Endocrinol Metab. 2017;61:222–227. doi: 10.1590/2359-3997000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamartina L, Grani G, Durante C, Borget I, Filetti S, Schlumberger M. Follow-up of differentiated thyroid cancer - what should (and what should not) be done. Nat Rev Endocrinol. 2018;14:538–551. doi: 10.1038/s41574-018-0068-3. [DOI] [PubMed] [Google Scholar]
- 43.Metter D, Phillips WT, Walker RC, Blumhardt R. To use or not to use 131I in thyroid cancer. Clin Nucl Med. 2018;43:670–671. doi: 10.1097/RLU.0000000000002190. [DOI] [PubMed] [Google Scholar]
- 44.Michael Tuttle R, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of 131 I therapy in differentiated thyroid Cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Since data came from the medical records where sensitive information is collected, no database is publicly available. Nevertheless, anonymized information can be shared privately upon reasonable request at e.ortizprado@gmail.com or paosolis@stanford.edu.