Abstract
Low-dose computed tomography (LDCT), an accepted U.S. screening tool for early lung cancer detection, is not widely-used in Puerto Rico. We investigated knowledge and attitudes about LDCT in focus groups of primary care physicians (PCP) and individuals at high-risk for lung cancer (HRI) in Puerto Rico. Transcribed/translated audio-recorded discussions were analyzed with the constant comparison method. Both groups had limited knowledge about LDCT and concerns regarding insurance coverage. Most HRIs had never had a provider recommend LDCT and believed that having symptoms was necessary to obtain LDCT screening. Perceived barriers included fears about results and the procedure; a perceived benefit was having early detection and possibly being cured. Few PCPs had ever recommended LDCT to a patient, with those who had basing their decision on symptoms/smoking history but having challenges with insurance. More education on LDCT is needed among HRIs, and U.S. Preventive Services Task Force guidelines should be widely distributed to encourage physician recommendations.
Keywords: Computed tomography, early lung cancer detection, focus groups, Hispanics, Puerto Rico
Cancer is the leading cause of death in Puerto Rico (PR),1 with lung cancer having the highest mortality rates. In 2016, 706 new cases of lung cancer were diagnosed in PR and 528 individuals died from the disease.2 It is well known that smoking is the main risk factor for the development of lung cancer.3 When all lung cancer cases are considered, 80% to 90% are attributable to smoking.4 Although the age-adjusted prevalence of tobacco use has diminished in PR, falling from 14.7% in 2005 to 10.9% in 2016,5 lung cancer persists as the second- and third-leading cause of cancer death in men and women, respectively.6 In addition, lung cancer survival rates among Hispanics generally in the U.S. have not improved compared with rates shown in non-Hispanic Whites.7 Resulting premature cancer death, loss of productivity, and years of potential life lost impose a great burden on PR’s economy.
In 2013, the U.S. Preventive Service Task Force (USPSTF) issued a recommendation for the use of low-dose computed tomography (LDCT) for the early detection of lung cancer in high-risk individuals (HRIs). This was followed in 2015 by coverage of the test by the U.S. Centers for Medicare and Medicaid Services. However, LDCT is still not commonly used during lung cancer screening in the mainland United States or in other countries.8-11 Although there are a number of reasons why use of LDCT is still uncommon, findings from the U.S. mainland point primarily to a lack of knowledge among both primary care providers (PCPs) and HRIs.8-11 To date, there are no data regarding whether similar or distinct barriers to LDCT exist in PR, particularly given observed differences in health care between the mainland and PR.
The history of Puerto Rico provides a context to understanding its health care delivery system. Despite the granting of U.S. citizenship to Puerto Ricans in 1917,12 the health care system in PR continues to demonstrate marked discrepancies compared with the U.S. mainland.13 Nearly half of the PR population (49%) is covered by Medicaid,14 and, in contrast to the mainland U.S., funds are limited to a pre-determined amount, regardless of actual levels of need or cost (i.e., capped allotment). The health service model manages care through a per capita incentive model. Therefore, it imposes a financial cost for the services provided by other providers to the beneficiaries subscribed to their primary center, causing delayed or restricted receipt of required clinical procedures.15 The current PR debt crisis16 has also caused uncertainly related to government reimbursements for health care expenses, as well as insecurity and mistrust among patients and health care providers. Together, these historical and current economic circumstances in PR have contributed to important health disparities in comparison with the United States.
In addition to the challenges related to the economy and health care system, Hispanic populations have particular cancer screening behaviors that likely play a role in terms of their preventive care. Fatalism, low health literacy, poverty, spiritual well-being, and familism (supportive family relationships characterized by the prioritization of family over self)17 have been identified as relevant to influencing screening behaviors.18-21 Lung cancer screening reduces mortality in all populations of those at high risk of lung cancer.22 However, a national survey of medical directors of Federally Qualified Health Centers, who provide care for the underserved, showed they face significant economic and resource challenges in offering lung cancer screening.23 Coupled with the challenges faced by the institutions, medically underserved populations are less likely to receive referrals for cancer prevention screening tests as they are also less likely to receive routine primary care.24
Seventy-two of 78 municipalities in PR are designated as medically underserved areas. Of these, 40 municipalities experience a shortage of primary care providers.25 Although the unique health care challenges and barriers to cancer screening in U.S. populations have been reported, it is largely unknown whether these same observations are generalizable to PR populations. Therefore, we conducted a qualitative study to examine perspectives among PCPs and HRIs in Puerto Rico regarding knowledge and attitudes to LDCT screening for lung cancer. Qualitative methods allow for rich data to understand better both structural and cognitive barriers that may exist in the offer of lung cancer screening. One goal of this study was to show existing media products, such as news coverage and promotional advertising for lung cancer screening, and to see the physician’s response to these examples. Focus groups allowed us to show the examples and to receive feedback on their relevance and effectiveness for this particular group and whether or not such media would affect decision-making about the offer of lung cancer screening to their at-risk patients.26, 27 For this, we used a design similar to one previously conducted with HRIs and PCPs in Florida.11 Our aim was to identify the unique barriers in PR compared with the U.S. regarding LDCT screening for lung cancer.
Methods
This study was reviewed and approved by the Institutional Review Board (FWA00000345) of the Ponce Medical School Foundation (IRB approval no. 161626-MR). Written informed consent was obtained from study participants.
Participants.
The focus group discussions were conducted with HRI and PCP community members in PR. The inclusion criteria for HRIs corresponded with the USPSTF criteria for lung cancer screening, which included participants being aged 55 to 80 years old, having a 30-pack (minimum) per year smoking history, and being a current or former smoker who quit within the past 15 years.28 The participants were also required to be able to read and speak Spanish. Potential participants were excluded if they were currently receiving cancer treatment or if they had been previously screened for lung cancer with LDCT.
The HRI participants received a 30-dollar gift card and a meal for their participation in the focus group. Eligible PCPs were licensed to practice medicine in the Commonwealth of Puerto Rico and provided health care for at least 50 individuals (aged 55 or older) per year. They received 100 dollars and a meal for their participation in the focus group discussions.
Recruitment procedures for the HRIs included visits to PCP offices, flyers in medical facilities, notices on social media, newspaper advertisements, and radio interviews. Other efforts to recruit HRIs included: visits to senior centers and residential centers in southern PR, and referrals from a hospital in northern PR (Caguas) and community leaders in the Ponce area. Interested participants called the study phone number and were screened for eligibility; if eligible they were scheduled for an in-person focus group. Recruitment of PCPs included visits to PCP offices, phone calls, and emails to physician liaison groups, as well as visits to hospitals in the north, south, and west areas of PR (Caguas, Ponce, Juana Díaz, and Mayaguez).
Focus group procedures.
All focus groups were moderated by the lead author, a clinical psychologist trained in conducting focus groups. We performed six HRI focus group and five PCP focus group discussions. Each focus group included between three and 11 participants and lasted from 60 to 90 minutes.
HRI focus groups.
The HRI focus groups were conducted in two phases. During the first phase, the participants were asked about their perceptions regarding cancer screening in general and their specific knowledge of lung cancer screening. After they viewed two videos about lung cancer screening with LDCT, participants were asked to discuss the perceived benefits and barriers of testing as well as future intentions for screening (see Figure 1 for the HRI focus group guide).
Fig. 1.
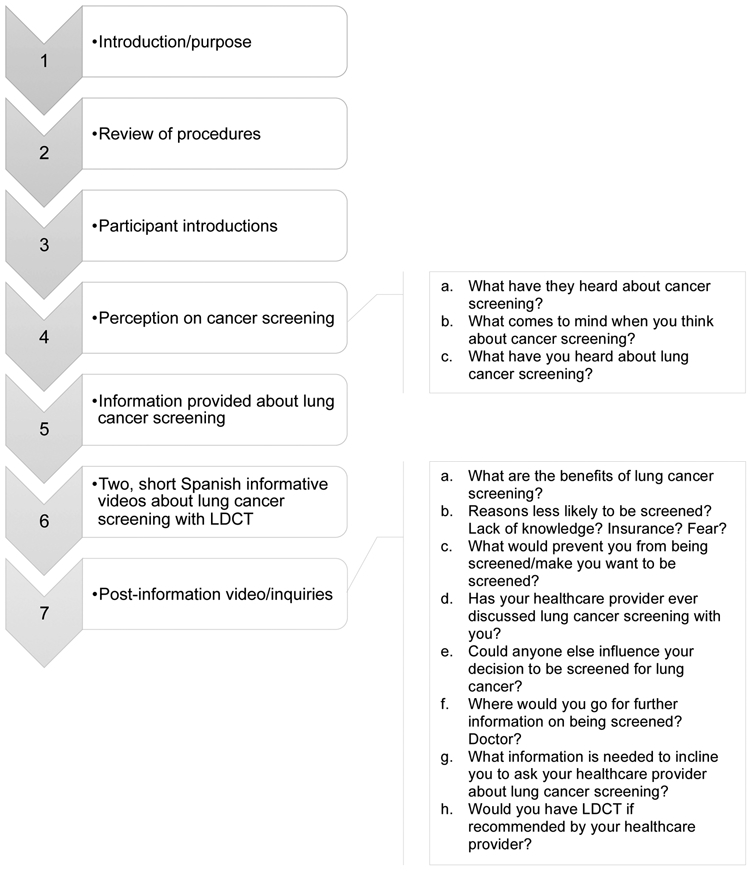
High risk individuals focus group guide.
PCP focus groups.
The PCP focus group discussions consisted of two phases. The first phase assessed overall practice patterns regarding lung cancer screening referrals, knowledge of guidelines, and concerns/barriers related to making referrals for screening LDCTs. During the second phase, participants viewed a PowerPoint presentation with current evidence (clinical, epidemiological, and technological) about lung cancer screening to facilitate discussions about LDCT screening. The PCP focus groups were also asked to provide insights about ideas for promotional efforts and sample educational messages that could be directed toward other PCPs (see Figure 2 for PCP focus group guide).
Fig. 2.
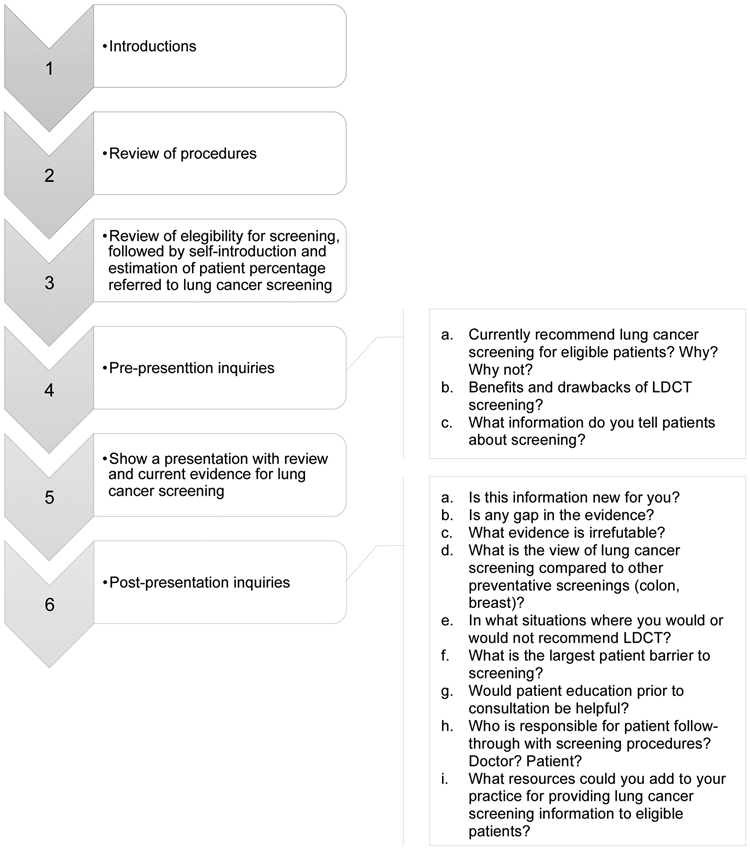
Primary care physicians focus group guide.
Data analyses.
All focus groups were audio-recorded, and verbatim transcripts were created for content analyses using the constant comparison method. The transcripts were then translated from Spanish to English by a professional translation company that employs certified translators who use a forward and backward translation process. The results were then evaluated by two bilingual members of the research team (MR and AV) for fidelity of the translation. Results from the focus group transcripts were analyzed using a combination of initial hand coding for the first round of analysis and the establishment of the codebook. Data were analyzed using a combination of content analysis and the constant comparison method.29 The study codebook consisted of a priori codes, derived from existing literature, and emergent codes, which were identified as the analysis progressed. In accordance with best practices30 and our prior work,11 two members of the study team coded each transcript independently, and the coded transcripts were compared to ensure reliability. Thematic saturation was assessed and achieved.
Results
High-risk individuals.
Characteristics of HRIs (N = 37) are displayed in Table 1. The majority of participants were female (20, 54.1%), had Medicare insurance (23, 62.2%), reported being current smokers (20, 54.1%), and began smoking before 15 years of age (19, 51.4%).
Table 1.
Demographics of High-Risk Individuals (N = 37)
| Total (%) | |
|---|---|
| Sex | |
| Male | 17 (45.9) |
| Female | 20 (54.1) |
| Age | |
| 55-60 years | 10 (27.0) |
| 61-70 years | 17 (45.9) |
| 71-80 years | 10 (27.0) |
| Race | |
| White | 24 (64.9) |
| Black or African American | 6 (16.2) |
| Other | 3 (8.1) |
| Prefer not to answer | 4 (10.8) |
| Ethnicity | |
| Hispanic/Latino | 35 (94.6) |
| Prefer not to answer | 2 (5.4) |
| Marital status | |
| Single | 4 (10.8) |
| Living with partner/Domestic relationship | 2 (5.4) |
| Married | 17 (45.9) |
| Separated/Divorced | 10 (27.0) |
| Widowed | 4 (10.8) |
| Insurance type | |
| Medicare | 23 (62.2) |
| Medicaid | 6 (16.2) |
| Private | 7 (18.9) |
| Uninsured | 1 (2.7) |
| Highest grade level completed | |
| Less than 9th grade | 2 (5.4) |
| Some high school/High school graduate | 17 (45.9) |
| Some college/Technical school/Associate degree | 12 (32.4) |
| Baccalaureate/Doctoral degree | 6 (16.2) |
| Age began smoking | |
| ≤ 15 years | 19 (51.4) |
| 16-21 years | 15 (40.5) |
| ≥ 22 years | 3 (8.1) |
| Current smoker | |
| Yes | 20 (54.1) |
| Cigarettes per day | |
| ≤ 10 | 4 (11.4) |
| 11-20 | 19 (51.4) |
| ≥ 21 | 12 (32.4) |
| Missing | 2 (5.4) |
| Times attempted to quit last year | |
| Do not smoke | 17 (45.9) |
| < 5 | 17 (45.9) |
| ≥ 5 | 3 (8.1) |
| Other smokers in household | |
| Yes | 11 (29.7) |
At first, before they saw the videos, in response to the question about what came to mind when thinking about cancer screening, most HRIs professed that they were aware of cancer screening tests, such as mammograms, but only a few participants had heard of LDCT for lung cancer screening. Furthermore, most HRIs associated cancer with symptoms and fear and anxiety: "Sometimes when you feel something and start feeling ill, you think, could it be cancer? You live with the fear of having it.”
Although few HRIs had heard of LDCT for lung cancer, upon learning of its existence, most were pleased to know that such a test now existed:
It [lung cancer] is the least talked about [cancer]. They always talk about colon or breast [cancer], but they never talk or tell you about what you can do … No one has ever sent me to get, or has done, a test for lung cancer to see whether I have anything.
However, most HRIs also expressed concern that the opportunities were ubiquitous for the development of cancer and that cancer is often “hidden” and undetectable:
Lung cancer doesn’t come only from cigarettes. And certainly there are a bunch of other things that, in fact, are in our own houses—mixing up “bombs” to clean the toilets and the bathrooms, exposing ourselves to all that stuff.
I think that it is not only the cigarettes that cause damage, because you are exposed to a lot of things in the environment that cause damage.
The reality is that the disease is silent and does not hurt and does not give you any signs of pain.
Upon learning that LDCT was available to those at high risk, most HRIs reported that they would partake in this screening if their doctor recommended it:
When they tell me, “You have to do this,” give me an appointment as quickly as possible, and I’ll go.
My doctor needs to tell me why I should do it, [tell me] what there is in my history that leads him to recommend that I get the test. But first, my doctor has to tell me.
One HRI participant reported he would not ask his doctor for lung cancer screening – the reason: he did not want to know; he did not wish to suffer from knowing he has cancer.
They say you have cancer; you suffer more mentally, physically, and then you start thinking about … chemotherapy … They discover cancer now, and then you die within three days, just thinking of that.
No, because then they'll tell me I have cancer and I'll die.
When asked what barriers might affect their ability to pursue screening, most were concerned about knowledge and financial barriers:
I don’t know what the process is for the detection [of lung cancer].
My physician has never told me about it.
The economic factor [is a barrier] because if you do not even have enough [money] to get medication, imagine a treatment. It isn’t easy; it is expensive.
Most participants noted that concerns with medical insurance (Medicaid, Medicare, and private health insurance), including lack of insurance, would prevent them from getting screened:
Medical insurance plans do not approve procedures that they [the doctors] order. You must wait a week for an answer, only to be told no.
We go back to the same thing: the health plans don’t tell you when to do it [screen for lung cancer] … they tell you to do a prostate exam, get a urinalysis, etc., but never a lung screening—no plan does.
About one-fourth of participants said that the fear of results and the fear of the actual procedure could inhibit them from getting screened:
The fear of knowing the truth, that you are sick or that you have little time left, that is the fear that you have.
If it is an enclosed machine, I cannot do it. I have suffered from claustrophobia ever since I was young. And that [machine] provoked two months of panic attacks.
When asked about what motivational factors were important for making a decision to get screened, the majority cited their families, peace of mind, and the possibility of early detection leading to a cure:
… At my age, I would get all the tests done because I want to see my grandchildren grow.
I would say the tranquility, even though I still smoke, but if you know that you don’t have it, well, you have a chance to quit.
Well, we didn’t know that with a CT scan you could see whether or not you had cancer … and as we have just been oriented, I feel motivated.
Many participants also stated that having symptoms would also serve as a motivator: "Pain, your chest feeling tight, or the symptoms that you feel … feeling as though you lack air and you can’t breathe well.”
When asked what additional information would be needed to make a screening decision, the participants noted that they needed to know whether their insurance would cover the tests, what additional costs they would incur, and what treatments would be involved if they had an abnormal screen, as well as the efficacy of those treatments. Most participants found it difficult to accept that individuals might do well to participate in a lung cancer screening program even in the absence of symptoms. Further, several were focused on the perception that lung cancer was caused by many other things beyond smoking.
If I touch my neck and feel a lump, if I feel something strange in my body, my breathing, my lungs, well then [I ask to get screened].
Yes, only cigarettes are associated with lung cancer, but what about the environment?
Right now, people talk only about cigarettes, but there are many things … the barbeque, people do a lot of barbecuing. That also causes cancer.
In discussions about communication preferences for educational materials to help individuals learn more about lung cancer screening, most participants wanted personal stories, related in a serious way, from those who had undergone the procedure:
A real-life story that presents all the components, a history.
A story is more entertaining.
Something serious, not to cause fear, but with authority.
In addition, most participants wanted a physician to transmit any educational information.
Primary care providers.
Characteristics of PCPs (N = 30) are displayed in Table 2. The majority of participants were male (24, 80.0%), graduated between 2010-2015 (26, 53.3%), and had a primary practice in a teaching hospital (63.3%) in a rural area (19, 53.4%).
Table 2.
Demographics of Primary Care Providers (N = 30)
| Total (%) | |
|---|---|
| Sex | |
| Male | 24 (80.0) |
| Female | 6 (20.0) |
| Age | |
| 25-34 years | 14 (46.7) |
| 35-44 years | 8 (26.7) |
| 45-54 years | 4 (13.3) |
| 55-64 years | 4 (13.3) |
| Race | |
| White | 12 (40.0) |
| Black or African American | 3 (10.0) |
| Other | 12 (40.0) |
| Prefer not to answer | 3 (10.0) |
| Ethnicity | |
| Hispanic/Latino | 30 (100.0) |
| Year of professional school graduation | |
| 2010-2015 | 16 (53.3) |
| 2000-2009 | 7 (23.3) |
| 1990-1999 | 4 (13.3) |
| 1980-1989 | 3 (10.0) |
| Average number of age 55+ patients per week | |
| 0-25 | 7 (23.3) |
| 26-50 | 10 (33.3) |
| 51-75 | 8 (26.7) |
| 75-100 | 3 (10.0) |
| > 100 | 2 (6.7) |
| Primary practice location | |
| Private practice | 10 (33.3) |
| Teaching hospital | 19 (63.3) |
| Community-based | 1 (3.3) |
| Practice setting | |
| Rural | 19 (51.4) |
| Urban | 15 (40.4) |
| Suburban | 6 (16.2) |
| Number of physicians in practice | |
| 1 | 5 (16.7) |
| 2-5 | 4 (13.3) |
| 6-15 | 2 (6.7) |
| 16-49 | 5 (16.7) |
| 50-99 | 6 (20.0) |
| 100+ | 8 (26.7) |
When asked how many patients they have talked to about lung cancer screening, most PCPs stated that they have discussed it with only few eligible patients. Those who had recommended screening did so based on symptomatology, smoking history, and other risk factors. Furthermore, most PCPs reported that the first choice for screening was chest radiography, which would only then be followed by an LDCT if positive:
Yes, if they are within a determined age and if they have had a history of, you have to determine the number of years, of continuous smoking, well … If we think that a patient has been smoking for a long time, this person might benefit from screening.
The family history also serves as an important flag when you come right down to it. The patient is a smoker and obviously, this is sad, but the problem is already there, the patient who has arrived has lost weight, has some symptoms. When you verify the history of being a smoker, that’s when you realize that, really, it isn’t early detection.
Symptoms and the risk factor of having smoked … that is what motivated me to do it [recommend screening]: the x-ray and then, later, the CT scan.
Of the reasons provided for not recommending screening, the most frequently mentioned were the lack of insurance coverage (all types of insurance) and the need to focus on acute conditions:
Before this came out [the LDCT], we would only do an x-ray image. Then, we started trying to do the CT scan, but the difficulties are important, because it is the medical insurance that requires us to do a chest x-ray image first.
One of the biggest challenges with regard to the CT scan is convincing the insurance plan to cover [it]; usually it is denied, but those with a diagnosis are approved.
Yes, I have patients who are eligible [for screening], and I have not referred them because they have come to the office for acute causes and not for annual preventive-care tests.
Although most of the PCPs acknowledged that LDCT screening would increase opportunities for receiving timely treatment, as well as for reducing medical costs, most agreed that lack of insurance coverage (all types of insurance) is the primary barrier:
As is evident, a decrease in mortality, an increase in the patient’s quality of life. In terms of early detection, [if you find] a small lesion that is located in an area that can be accessed surgically, and that qualifies for surgery, you remove it and that patient is practically cured; obviously, we decrease treatment costs and complications.
Well, to look for lung cancer before the patient has any symptoms—or to find them at an early stage—makes it possible to offer treatment that will heal them.
… But we also have limitations regarding the medical coverage and the insurance companies.
Yes, perhaps there would be an economic impact [not having the money to cover the cost] if the insurance plan won’t cover the study; rather, it would be inconvenient for the patient and would possibly limit his ability to do any kind of screening study.
A few participants also expressed concerns regarding radiation exposure and how the possibility of a false positive would result in anxiety for patients:
As is the case with any kind of screening test, there are always going to be false positives, which could have a negative impact on the well-being of the patient.
False positives … you find things that might not [be cancer], but if you share the idea to the patient, they get it into their heads that they have something …
When PCPs were asked about the information that they provide to patients about lung cancer screening, several participants noted they discussed risk factors for lung cancer, provided smoking-cessation counseling, and discussed the risks and benefits of screening. Furthermore, a few stated that they directed patients to the Internet as a source of additional information:
… The patient must understand that smoking is equal to cancer, unfortunately.
I usually go by looking at the risk factors the patient has; it’s more like educating the patient by explaining, look you have a genetic predisposition for this persistent condition that runs in your family plus you have these habits that are not favorable for you … that’s how you orient them as we do the smoking-cessation counseling.
Communicate with the patient and explain the things we are doing [screening], why we are doing them, and the benefits that the patient might have as a result of having done them.
When PCPs received evidence about lung cancer in Puerto Rico and the benefits of LDCT, most were unaware whether insurance companies in Puerto Rico would cover LDCT for lung cancer screening:
Well I gave the wrong information because I told them that Medicare did not cover it, but I—Now we are educated.
I, at least, didn’t know that they—the insurance plans—cover it [screening] and will approve it. So why is there so much resistance [on the part of the local insurance companies]?
But basically, the guide [for recommending screening] is for smokers, because of the frequency, but there are other types of patient who also merit it [screening].
Most participants reported that they do not refer patients for LDCT for lung cancer screening, although they did refer their patients for other types of cancer screening tests (e.g., mammography and colonoscopy). The primary cited reasons for non-referral were lack of knowledge about the existence of LDCT testing and their view that insurance companies tended not to reimburse for the procedure:
… for colon [screening], but they [the patients] tend to have more awareness [of colon screening], because it is better promoted, which influences patient compliance in terms of their getting screened. That’s one issue. Another is that … there is also an aspect of medical ignorance in terms of what the best practices [for lung cancer screening] are and how often it should be done.
Economically speaking, the health system, as such, wants to be more cost effective. Because breast cancer is frequent in women, as is prostate cancer in men, they [health practitioners] are more aggressive with those types of screening. But not for lung [cancer]. I would say that, up to now—I’m saying that in daily practice there has not been any demand for a metric for lung cancer [screening].
I believe there is still that resistance on lung cancer. Lung cancer hasn’t received the publicity that it should be so that the people will become aware and do the screening. It isn’t where it should be.
The PCPs expressed a need for educational resources, such as informative handouts and continuing medical education, to discuss lung cancer screening effectively with their high-risk patients and to disseminate information about LDCT guidelines to other PCPs:
And the patient who reads [this kind of education material], then goes to the office and says, “Doctor, I read this out there, and I am a candidate.”
… The best way is to contact those institutions that provide medical education and [convince them to] make it part of their curriculum.
When participants were asked to discuss situations in which they would not recommend LDCT for lung cancer screening, most PCPs reported that they would not recommend screening to a patient who had a short life expectancy. A few mentioned other reasons, including the individual’s previous history of cancer, not being a good candidate for treatment (due to factors such as being terminally ill or at an advanced stage of Alzheimer disease), and not being eligible: "… [When a patient] has a severe condition for which the survival time is short, you will not increase that time by finding another chronic condition that is also very severe.”
When asked about patient barriers, those most frequently mentioned by PCPs included the patient’s reluctance to quit smoking, fear of results or requiring treatment, and insurance barriers:
I remember someone who told me, “Oh but there are a lot of people who smoke and don’t get cancer.”
“I have to die of something.” They also answer that.
… People do not want to be screened because they suspect that something will be found; they don’t want to live with the stress that they might have cancer because it’ll make them depressed or something and they don’t want to undergo the treatment … Everyone has some family member who underwent chemotherapy and they know that it’s horrible.
… This [getting screened] requires authorization and approval, and the process is difficult for the patient, which might be the one thing that proves to be a barrier to their getting screened.
Finally, most participants agreed that ensuring that screening tests take place is the responsibility of both the health care provider and the patient and that the decision-making process that leads to the screening must be shared by both. A few noted that electronic medical records have been part of a useful strategy to follow-up on a given patient and determine whether the patient had been screened:
It is shared because the duty of the doctor is to guide them; however, we can’t make them [comply]. They are the ones who decide.
You can get to a certain point, but you can’t force anyone to do the test. Your responsibility is to send them, to educate the patient.
Discussion
Our results illustrate that, although screening guidelines that include LDCT and insurance coverage for it have been available for over two years, this test is not commonly used for lung cancer screening in PR. Similar to our previous findings in a study conducted in Florida, the key barriers reported by both PCPs and HRIs were lack of knowledge and the financial costs for patients.8 However, we also found that insurance coverage stood out significantly as a barrier in PR. Our participants agreed that, despite other screening practices that are highly promoted for other types of cancer (e.g., breast and prostate), lung cancer screening is rarely recommended. Most of the PCPs were unaware of insurance coverage and argued that lung cancer screening was not part of the insurance metrics (referred to as a star rating from U.S. Centers for Medicare and Medicaid Services31) and therefore was not required by PR insurance companies, unlike other cancer screening tests. In addition, the few participating PCPs who had recommended LDCT for lung cancer screening mentioned that they had difficulties getting reimbursed by insurers. Insurance coverage often required practitioners to prescribe a chest radiography first and then the LDCT scan (if the radiography was positive). These findings underline the distinctive challenge that PCPs in PR have in working with insurance companies to cover LDCT for lung cancer screening. Furthermore, because the U.S. Preventative Services Task Force guidelines do not recommend screening for symptomatic patients,28 a PCP who perceives that symptoms are required for insurance coverage would subsequently cause a financial burden for the patient. Misunderstandings among health care and insurance providers are significant barriers for the use of LDCT for lung cancer screening.
Another key barrier identified in lung cancer screening was cost. Nearly half of PR individuals live in poverty32 and thus are unable to pay out-of-pocket costs, or they depend exclusively on government-provided medical services. Negative cancer health statistics in Puerto Rico are greater compared with the United States due to limited access in PR to what is now the standard of care for HRIs.13, 14
We also found that HRI participants had challenges accepting that lung cancer screening did not require the presence of symptoms for them to participate. Many HRIs asserted that their PCPs usually ordered other standardized screening tests (e.g., mammography and colonoscopy) but never had ordered lung cancer screening, despite knowledge of their smoking history. In line with these HRI perceptions, many PCPs believed that symptoms needed to be present in the patient before the LDCT test could be ordered.
The participating HRIs also reported being afraid of receiving (negative) test results and—that being the case—the subsequent possibility of having to undergo cancer treatment. This barrier has been previously described in association with other cancer screening efforts in Puerto Rican populations.18, 20, 33 Similar to prior studies, our participants stated that a primary facilitator of cancer screening is trust in their physicians, with the subsequent expectation that their physician would recommend LDCT screening if it were needed.34, 35 However, we found that there was a lack of knowledge about qualifying and insurance guidelines regarding lung cancer screening in both the PCP and HRI participants. Increased awareness of clinical guidelines, counseling, and shared decision-making visits are needed to increase utilization of LDCT for early detection.36, 37
Several resources were identified by PCPs as potential channels to promote early detection of lung cancer through screening. First, PCPs noted the benefits of continuous medical education on lung cancer screening guidelines through workshops or conferences. Medical associations in Puerto Rico were also mentioned as a source to disseminate these guidelines. Most of the participating HRIs perceived their PCPs as the most knowledgeable individuals and preferred to receive education about LDCT screening from them. Our findings suggest that, so far, information on these guidelines has been limited regarding its dissemination. Another key finding in our study was that HRIs reported preferring to receive educational materials regarding lung cancer screening through personal stories. This finding is consistent with prior studies on cancer health-related communication preferences among Hispanic populations.38, 39
Limitations and conclusions.
Although our qualitative design limits the generalization of our presented results to PR populations who live in mainland U.S., our study unveiled a number of barriers regarding LDCT screening in Puerto Rico. In addition to not being knowledgeable about either LDCT screening or insurance coverage, both HRIs and PCPs were unfamiliar with the criterion for LDCT screening and falsely believed that the presence of symptoms was required if a patient was to participate in screening. The few PCPs who attempted to order screening also noted that they struggled to convince medical insurance companies to reimburse them. Several of the unique barriers identified can potentially be reduced by offering educational opportunities to HRIs and to PCPs. Opportunities are needed to disseminate screening guidelines and information regarding LDCT effectiveness for the early detection of lung cancer. Clarifying the current policy regarding health insurance company requirements for screening would also help to promote screening. The development of targeted educational materials for Hispanic populations would help PCPs and HRIs increase awareness about LDCT screening and encourage communication between physicians and patients and facilitate the process of shared decision-making, thus reducing lung cancer mortality rates in Puerto Rico. Therefore, the findings of this study can be translated into recommendations to inform future promotion strategies and educational messages to increase knowledge about LDCT and to attract a diverse cohort of HRIs to lung cancer screening.
Acknowledgements
We thank Mrs. Jeanette Calderón and Mrs. Velmarie Hernández for assisting with participant recruitment and study promotion, respectively. We also thank Ruthmarie Hernández and Jean Ruiz for assisting with the data collection of the study. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U54CA163068 and U54CA163071, and by the National Institute on Minority Health and Health Disparities under award numbers S21MD001830, R25MD007607, and U54MD007587.
The research presented in this paper is that of the authors and does not reflect the official policy of the NIH. No financial disclosures were reported by the authors.
Abbreviations
- LDCT
low-dose computed tomography
- PCP
primary care physician
- HRI
high-risk individual
- PR
Puerto Rico
- USPSTF
US Preventive Service Task Force
References
- 1.Sánchez EM, Morales JJ, Machín S, et al. Informe de la salud en Puerto Rico, 2015. San Juan, Puerto Rico: Departamento de Salud, 2015. Available at http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Publicaciones/Informe%20de%20la%20Salud%20en%20Puerto%20Rico%202015_FINAL.pdf. [Google Scholar]
- 2.Registro Central de Cáncer de Puerto Rico. Tasas y mapas, 2019. Puerto Rico: Departamento de Salud, 2019. Available at http://www.rcpr.org/Datos-de-C%C3%A1ncer/Tasas-y-Mapas. [Google Scholar]
- 3.National Cancer Institute. Lung cancer—Health professional version. Bethesda, Maryland: National Institute of Health, 2018. Available at https://www.cancer.gov/types/lung/hp. [Google Scholar]
- 4.Division of Cancer Prevention and Control. What are the risk factors for lung cancer? Atlanta, Georgia: Center for Disease Control and Prevention, 2018. Available at https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm. [Google Scholar]
- 5.National Center for Chronic Disease Prevention and Health Promotion DoPH. BRFSS prevalence & trends data: current smoker status. Atlanta, Georgia: Centers for Disease Control and Prevention, 2016. Available at https://nccd.cdc.gov/BRFSSPrevalence/rdPage.aspx?rdReport=DPH_BRFSS.ExploreByTopic&irbLocationType=StatesAndMMSA&islClass=CLASS17&islTopic=TOPIC15&islYear=2016&rdRnd=44178. [Google Scholar]
- 6.Zavala-Zegarra D, Tortolero-Luna G, Torres-Cintrón C, et al. Cancer en Puerto Rico: 2008-2012; incidencia y mortalidad. San Juan, PR: Puerto Rico Central Cancer Registry, 2015. Available at: http://www.rcpr.org/Portals/0/Informe%202008-2012.pdf. [Google Scholar]
- 7.Pulte D, Redaniel MT, Brenner H, et al. Changes in survival by ethnicity of patients with cancer between 1992-1996 and 2002-2006: is the discrepancy decreasing? Ann Oncol. 2012. March 6;23(9):2428–34. [DOI] [PubMed] [Google Scholar]
- 8.Abbasi A, Siddiqi R, Owais A, et al. Prevalence and barriers to lung cancer screening in Karachi, Pakistan: A Cross-sectional survey of smokers and physicians. Cureus. 2017. May 15;9(5):e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman RM, Sussman AL, Getrich CM, et al. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev Chronic Dis. 2015. July 9;12:E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JA, Petty WJ, Tooze JA, et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015. April;24(4):664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons VN, Gray JE, Schabath MB, et al. High-risk community and primary care providers knowledge about and barriers to low-dose computed topography lung cancer screening. Lung Cancer. 2017. April;106:42–9. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto D, Jones CAL. Ethical issues in cross-cultural psychology In: Mertens DM & Ginsberg PE, eds. The handbook of social research ethics. Thousand Oaks: Sage Publications, 2009. [Google Scholar]
- 13.Rodríguez-Vilá O, Nuti SV, Krumholz HM. Healthcare disparities affecting americans in the US territories: a century-old dilemma. Am J Med. 2017. February;130(2):e39–e42. [DOI] [PubMed] [Google Scholar]
- 14.The Kaiser Commission on Medicaid and the Uninsured 8 questions and answers about Puerto Rico. San Francisco, California: The Henry J. Kaiser Family Foundation, 2016. Available at https://www.kff.org/disparities-policy/fact-sheet/8-questions-and-answers-about-puerto-rico/. [Google Scholar]
- 15.Colon D, Vazquez M, Figueroa R. Perfil de los sistemas de salud Puerto Rico. Washington, DC: Organización Panamericana de la Salud/Organización Mundial de la Salud, 2007. Available at http://paho.org/hq/dmdocuments/2010/Perfil_Sistema_Salud-Puerto_Rico_2007.pdf. [Google Scholar]
- 16.Wilson B PROMESA: a summary of the Puerto Rico Oversight, Management, and Economic Stability Act. New York: Mondaq, 2016. Available at: http://www.mondaq.com/unitedstates/x/530862/Fiscal+Monetary+Policy/PROMESA+A+Summary+Of+The+Puerto+Rico. [Google Scholar]
- 17.Campos B, Ullman JB, Aguilera A, et al. Familism and psychological health: the intervening role of closeness and social support. Cultur Divers Ethnic Minor Psychol. 2014. April;20(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraído-Lanza AE, Viladrich A, Flórez KR, et al. Commentary: fatalismo reconsidered: a cautionary note for health-related research and practice with Latino populations. Ethn Dis. 2007. Winter;17(1):153–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey VM, Patel H, Sandhu S, et al. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer Control. 2014. October 1;21(4):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shelton RC, Jandorf L, Ellison J, et al. The influence of sociocultural factors on colonoscopy and FOBT screening adherence among low-income Hispanics. J Health Care Poor Underserved. 2011. August;22(3):925–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terán L, Baezconde-Garbanati L, Márquez M, et al. On-time mammography screening with a focus on Latinas with low income: a proposed cultural model. Anticancer Res. 2007. Nov-Dec;27(6C):4325–38. [PubMed] [Google Scholar]
- 22.Su CT, Bhargava A, Shah CD, et al. Screening patterns and mortality differences in patients with lung cancer at an urban underserved community. Clin Lung Cancer. 2018. September 17;19(5):e767–e773. [DOI] [PubMed] [Google Scholar]
- 23.Zeliadt SB, Hoffman RM, Birkby G, et al. Challenges implementing lung cancer screening in Federally Qualified Health Centers. Am J Prev Med. 2018. April;54(4):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Cancer disparities. Bethesda, Maryland: National Institute of Health, 2019. Available at https://www.cancer.gov/about-cancer/understanding/disparities. [Google Scholar]
- 25.Health Resources & Services Administration. Data by geography: Puerto Rico. Bethesda, Maryland: Department of Health and Human Services, 2019. Available at https://data.hrsa.gov/hdw/Tools/DataByGeographyResults.aspx?geoTyp=State&geoCd=72. [Google Scholar]
- 26.Newcomer KE, Hatry HP, Wholey JS. Focus group interviewing In: Newcomer KE, Hatry HP, Wholey JS, eds. Handbook of practical program evaluation. New Jersey: John Wiley & Sons, Inc, 2015. [Google Scholar]
- 27.Koskela T, Sandström S, Mäkinen J, et al. User perspectives on an electronic decision-support tool performing comprehensive medication reviews - a focus group study with physicians and nurses. BMC Med Inform Decis Mak. 2016. January 22;16(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013. September 17;159(6):411–20. [DOI] [PubMed] [Google Scholar]
- 29.Patton M Qualitative evaluation and research methods. Beverly Hills, CA: Sage, 1990. [Google Scholar]
- 30.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013. September 18;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triple-S ADVANTAGE. Clasificación de las estrellas de Medicare. San Juan, Puerto Rico: Triple-S Group, 2017. Available at https://advantage.grupotriples.com/clasificacion-de-las-estrellas-de-medicare/. [Google Scholar]
- 32.U.S. Census Bureau. QuickFacts: Puerto Rico, 2017. Washington, DC: US Census Bureau, 2019. Available at https://www.census.gov/quickfacts/fact/table/pr/IPE120217#viewtop. [Google Scholar]
- 33.Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012. September;77(3):526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Brenner AT, Ratanawongsa N, et al. Patient trust in physician influences colorectal cancer screening in low-income patients. Am J Prev Med. 2014. October;47(4):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pucheril D, Dalela D, Sammon J, et al. The influence of physician recommendation on prostate-specific antigen screening. Urol Oncol. 2015. October;33(10):424.e1–7. [DOI] [PubMed] [Google Scholar]
- 36.Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017. March;151(3):572–8. [DOI] [PubMed] [Google Scholar]
- 37.Raz DJ, Wu GX, Consunji M, et al. The effect of primary care physician knowledge of lung cancer screening guidelines on perceptions and utilization of low-dose computed tomography. Clin Lung Cancer. 2018. January;19(1):51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn GP, McIntyre J, Gonzalez LE, et al. Improving awareness of cancer clinical trials among Hispanic patients and families: audience segmentation decisions for a media intervention. J Health Commun. 2013;18(9):1131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn GP, McIntyre J, Vadaparampil ST. Preferences for hereditary breast and ovarian cancer information among Mexican, Cuban and Puerto Rican women at risk. Public Health Genomics. 2011. July;14(4–5):248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


