Abstract
The progression of acute lung injury (ALI) is attributable to inflammation and oxidative stress. The cell-permeable itaconate analog 4-octyl itaconate (4-OI) provides protection against inflammatory responses and oxidative stress. However, whether 4-OI can protect against ALI remains poorly understood. The aim of this study was to explore the protective effects of 4-OI against LPS-induced ALI and the underlying mechanisms using hematoxylin and eosin (H&E) to observe lung morphology, ELISA and reverse transcription-quantitative PCR to measure the levels of IL-1β, TNF-α and IL-6 and western blotting to examine the levels of PI3K, Akt and NF-κB. The present study demonstrates that intraperitoneal administration of 4-OI (25 mg/kg) 2 h before lipopolysaccharide (LPS; 5 mg/kg) intratracheal injection significantly alleviated the lung tissue injury induced by LPS, reducing the production of proinflammatory cytokines and reactive oxygen species (ROS) in vivo. Furthermore, 4-OI and the antioxidant N-acetyl-L-cysteine markedly suppressed PI3K and Akt phosphorylation in LPS-treated RAW264.7 macrophage cells in vitro. Further study demonstrated that a pharmacological inhibitor of the phosphoinositide 3-kinase (PI3K)-Akt pathway, LY294002, inhibited the expression of NF-κB p65 in the nuclear fraction and decreased the production of inflammatory cytokines. Collectively, the experimental results of the present study provide evidence that 4-OI significantly decreased LPS-induced lung inflammation by suppressing ROS-mediated PI3K/Akt/NF-κB signaling pathways. These results suggest that 4-OI could be a valuable therapeutic drug in the treatment of ALI.
Keywords: 4-octyl itaconate, acute lung injury, inflammation, oxidative stress
Introduction
Acute lung injury (ALI) is a life-threatening disease characterized by increased vascular permeability and inflammation. Acute respiratory distress syndrome (ARDS) is a serious form of ALI that is associated with multiple organ failure and high mortality among patients in the intensive care unit (1). ARDS may be caused by sepsis, pneumonia, pancreatitis and trauma (2). Although pharmacological interventions and ventilatory management are used to treat ARDS, 40% of patients with ARDS die in hospitals (3). There are no effective pharmacological therapies for ALI. Therefore, it is urgent to identify valid therapeutic drugs for the improvement of ALI treatment.
Lipopolysaccharide (LPS) has often been used in some animal models of ALI to induce pulmonary inflammation (4). Membrane-bound Toll-like receptors (TLRs) have been demonstrated to play an important role in the innate immune system by recognizing specific damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) of invading microorganisms (5). TLR4 is widely expressed in various immune cells. When LPS binds to TLR4, it contributes to the activation of NF-κB, eventually leading to the production of proinflammatory cytokines (6-8). Many studies have shown that oxidative stress plays a major role in LPS-induced ALI. ROS are mainly generated by NADPH oxidase (Nox), which expands inflammation by activating downstream signal cascades (9,10). A body of evidence indicates that LPS can stimulate the production of ROS, which promotes diverse intracellular responses via the NF-κB pathway (11).
Itaconate, as a derivate of the tricarboxylic acid cycle, is derived from the decarboxylation of cis-aconitate mediated by immunoresponsive gene 1 in the mitochondrial matrix (12). It has been reported that itaconate has a direct antimicrobial effect by inhibiting isocitrate lyase (13). Itaconate markedly alleviated skin inflammation in a mouse model of psoriasis and decreased the production of proinflammatory mediators in LPS-treated macrophages (14,15). However, whether and how 4-OI plays a protective role in LPS-induced ALI remains largely unknown. In the present study, the protective effects of 4-OI against LPS-induced ALI were investigated, as well as the mechanisms associated with inflammation and oxidative stress in mice.
Materials and methods
Ethics statement
All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee of Ningxia Medical University [registration no. SCXK (Ning) 2018-0025], where experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, 8th edition (16).
Drugs and antibodies
4-OI was obtained from MedChemExpress. Polyclonal antibodies against phosphorylated (p)-NF-κB p65 (cat. no. 3033; 1:1,000), NF-κB p65 (cat. no. 8242; 1:1,000), phospho-PI3K (cat. no. 4228; 1:1,000), PI3K (cat. no. 4249; 1:1,000), phospho-Akt (cat. no. 4060; 1:1,000), Akt (cat. no. 4691; 1:1,000), lamin B (cat. no. 9087; 1:1,000) and β-actin (cat. no. 4970; 1:1,000), anti-rabbit IgG, and horseradish peroxidase (HRP)-linked antibody (cat. no. 7074; 1:10,000) were purchased from Cell Signaling Technology, Inc. N-acetyl-L-cysteine (NAC) and the PI3K inhibitor LY294002 were provided by Beyotime Institute of Biotechnology. LPS (Escherichia coli 055:B5) was purchased from Sigma-Aldrich (Merck KGaA).
Animals and treatment
Male C57BL/6 mice (20-25 g; 8 weeks old) were used in this study. Mice (n=15) were randomly assigned into three groups of five mice each: Control group, ALI group, and ALI + 4-OI group. According to a previous study (17), mice were anaesthetized with 2% sodium pentobarbital (80 mg/kg; Sigma-Aldrich; Merck KGaA) by intraperitoneal (i.p.) injection. Mice were then treated with an intratracheal (i.t.) injection of LPS (E. coli O111:B4; 5 mg/kg) in 50 µl saline. Mice in the ALI + 4-OI group received an i.p. injection of 4-OI (25 mg/kg/dose) in (2-hydroxypropyl)-β-cyclodextrin in phosphate-buffered saline (PBS) or vehicle control 2 h before i.t. injection of LPS, as previously described (18). Control animals received the same volume of vehicle (vehicle group). Lung tissue was harvested for subsequent experiments 12 h after LPS administration. Mice were anesthetized with inhaled isoflurane (1.5-4%) and euthanized by exsanguination through right ventricle aspiration, followed by cervical dislocation.
Histological examination
Mice were sacrificed 12 h after LPS administration. The left lungs of mice were harvested and fixed with 4% paraformaldehyde at 25˚C for 24 h. After fixation, lung tissue was embedded in paraffin and sectioned at 5-µm thickness. The sections were stained with hematoxylin at 25˚C for 10 min and eosin (H&E) at 25˚C for 3 min and visualized under a light microscope (magnification, x200). Morphological changes and injury were scored as follows: 0, no injury; 1, mild; 2, moderate; and 3, severe injury. This scoring system was based on the presence of exudates, hyperemia or congestion, infiltration of neutrophils, alveolar hemorrhage, presence of debris, and cellular hyperplasia (19).
Lung wet/dry (W/D) weight ratios
The lung W/D ratio was calculated as an indicator of pulmonary edema. The right upper lung of each mouse was excised for the determination of wet weight and then placed in an 80˚C oven for 4 days and reweighed to determine its dry weight.
Bronchoalveolar lavage fluid (BALF) acquisition and analysis
After the mice were sacrificed, the BALF was harvested by three i.t. injections of 0.5 ml cooled PBS. The harvested fluid was centrifuged for 10 min at 1500 x g at 4˚C. Total cell, neutrophil and macrophage counts were counted with a hemocytometer and Wright-Giemsa staining at 25˚C for 30 sec. The supernatant was collected for total protein content detection and cytokine examination.
RNA extraction and reverse transcription-quantitative (RT-q)PCR
Total RNA isolated from lung tissue using TRIzol® (Applied Biosystems; Thermo Fisher Scientific, Inc.) and reverse-transcribed with random hexamers and MultiScribe™ reverse transcriptase (Applied Biosystems; Thermo Fisher Scientific, Inc.) at 50˚C for 15 min and then at 85˚C for 5 sec. qPCR was performed using HiScript® II One Step qRT PCR kit (Vazyme Biotech, Co., Ltd.) on a deep-well Real-Time PCR Detection system (CFX96 Touch™; Bio-Rad Laboratories, Inc.) using the following thermocycling conditions: Initial denaturation at 95˚C for 5 min, followed by 35 cycles of denaturation at 94˚C for 60 sec, annealing at 58˚C for 60 sec and extension at 72˚C for 50 sec. The relative expression level of the target gene was examined using the comparative 2-∆∆Cq method (20). β-actin mRNA served as an internal control. The following mouse primers were used: Interleukin (IL)-1β forward, 5'-GGGCCTCAAAGGAAAGAATC-3' and reverse, 5'-TACCAGTTGGGGAACTCTGC-3'; tumor necrosis factor (TNF)-α forward, 5'-ACAGCAAGGGACTAGCCAGGAG-3' and reverse, 5'-GGAGTGCCTCTTCTGCCAGT-3'; IL-6 forward, 5'-CTGGGGATGTCTGTAGCTCA-3' and reverse, 5'-CTGTGAAGTCTCCTCTCCGG-3'; inducible nitric oxide synthase (iNOS) forward, 5'-TTTGTGCGAAGTGTCAGTGG-3' and reverse, 5'-AGAAACTTCGGAAGGGAGCA-3'; β-actin forward, 5'-GTGGACATCCGCAAAGAC-3' and reverse, 5'-AAAGGGTGTAACGCAACTA-3'.
Cell culture
RAW264.7 murine macrophage-like cells, purchased from the CBCAS (The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences), were cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% (v/v) fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37˚C. RAW264.7 cells were pretreated with 4-OI (125 µM), NAC (10 mM), the PI3K inhibitor LY294002 (25 µM) or vehicle control for 1 h and then stimulated with LPS (1 µg/ml) at 37˚C for 30 min to detect the phosphorylated levels of PI3K, AKT and p65 in LPS-treated macrophages and at 37˚C for 24 h to quantify the concentration of TNF-α, IL-1β, and IL-6 in macrophages. The dose and treatment duration of all drugs were selected according to previous studies (21,22).
Reactive oxygen species (ROS)-generation assay
After the mice were sacrificed, the BALF was harvested and lysed by Ammonium-Chloride-Potassium Lysis buffer (Beyotime Institute of Biotechnology). To detect ROS generation, the sedimented cells were resuspended in PBS. Briefly, the cells were incubated with 50 µM of DCFH-DA for 30 min at 37˚C in darkness. DCF fluorescence intensities were measured by flow cytometry. At the end of the treatment, the cells were incubated with 10 µM dichloro-dihydrofluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology) for 1 h at 37˚C in the dark. ROS generation in the cells was measured immediately using a fluorescence microscope (magnification, x200; Nikon Corporation). Myeloperoxidase (MPO) activity, malondialdehyde (MDA) content, superoxide dismutase (SOD) activity, and glutathione (GSH) content were also measured. The lung homogenate was dissolved in extraction buffer to detect the levels of MPO, MDA, SOD and GSH using commercially available assay kits (cat. nos. A044-1-1, A003-1-2, A001-3-2 and A005-1-2, respectively; Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's instructions.
Cytokine measurements
Cytokines in serum were measured using enzyme-linked immunosorbent assay (ELISA). Commercially available ELISA kits (IL-1β; cat. no. MLB00C; IL-6; cat. no. M6000B; TNF-α, cat. no. MTA00B; R&D Systems, Inc.) were used to determine the levels of IL-1β, TNF-α and IL-6, as previously described (23,24).
Extraction of nuclear and cytosolic proteins
To extract nuclear and cytosolic proteins, cells were washed and lysed with hypotonic buffer [20 mM Hepes (pH 8.0), 10 mM KCl, 1 mM EDTA, 1.5 mM MgCl2, 1 mM DTT, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF and 1% (v/v) protease inhibitor cocktail] on ice for 30 min. NP-40 (0.625%) was added to cell lysates. The lysates were collected and centrifuged at 13000 x g and 4˚C for 15 min. The supernatants served as the cytosolic extract. The nuclear pellets were resuspended in cold buffer for another 30 min and centrifuged at 15000 x g and 4˚C for 5 min, and the supernatants were collected as the nuclear extract.
Western blotting
Proteins were extracted with RIPA Lysis Buffer (Beyotime Institute of Biotechnology) from the cells with protein concentration determined using bicinchoninic acid protein assay. Equal amounts of protein (30 µg) were loaded onto 10% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes. The blots were blocked with 5% dry milk in Tris-buffered saline with 0.1% Tween-20 at 25˚C for 30 min and incubated with the appropriate primary antibody overnight at 4˚C. The blots were washed three times and then incubated with an HRP-conjugated secondary antibody at 25˚C for 1 h. Blots were then visualized using an enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.). Images were acquired using ImageQuant LAS 4000 mini (Cytvia).
Statistical analysis
All data are normally distributed and are expressed as the means ± SEM. Differences among multiple groups were analyzed by one-way ANOVA with Tukey's post hoc test. For the comparison of lung scoring, Kruskal-Wallis test followed by Dunn's multiple comparison test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
4-OI ameliorates lung tissue injury in mice with LPS-induced ALI
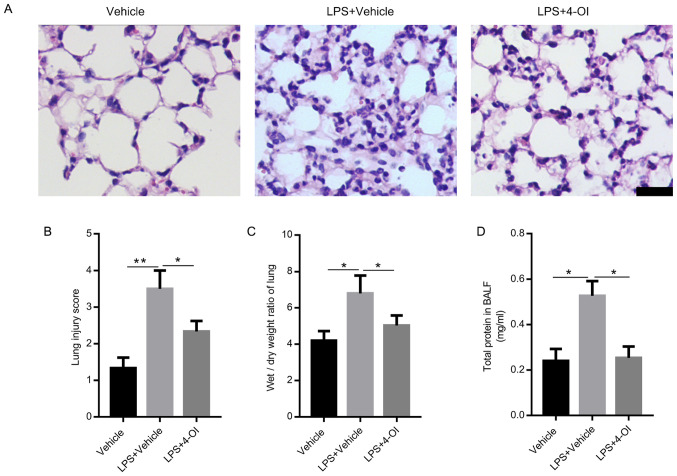
To confirm the role of 4-OI in the progression of LPS-induced ALI, cells were pretreated with a dose of 4-OI (25 mg/kg/dose; i.p.) before i.t. injection of LPS in mice. The results demonstrated that LPS led to severe inflammatory histological changes in lung tissues, including inflammatory cell infiltration, damage to the alveolar wall and pulmonary congestion, which were markedly reversed by 4-OI treatment (Fig. 1A). Moreover, compared with that in the LPS group, the lung injury score was decreased in the 4-OI treatment group (Fig. 1B). In addition, 4-OI treatment significantly decreased the W/D ratios (Fig. 1C) and the total protein concentration in BALF (Fig. 1D) compared with those in the LPS group, which are two indicators of pulmonary edema.
Figure 1.
4-OI alleviates lung injury induced by LPS in mice. Treatment of mice with 4-OI at doses of 25 mg/kg was administered intraperitoneally, and the control group received an equivalent volume of vehicle [(2-hydroxypropyl)-β-cyclodextrin in phosphate-buffered saline (PBS)] 2 h before saline or LPS injection (5 mg/kg, intratracheal). Twelve hours later, the mice were sacrificed. Hematoxylin and eosin staining (A, bar=100 µm) was used to detect the lung histopathological changes. (B) Lung injury scores were determined using four independent parameters, namely alveolar congestion, hemorrhage, leukocyte infiltration and alveolar wall thickness. The lung wet/dry ratio (C) and total protein in BALF (D) were measured to determine lung permeability (n=5). Data are expressed as the mean ± SEM. *P<0.05 and **P<0.01. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharides; BALF, bronchoalveolar lavage fluid.
4-OI suppresses inflammatory responses in LPS-induced ALI in mice
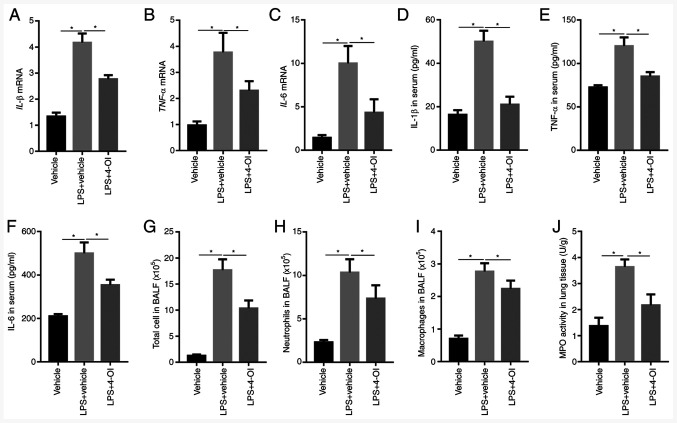
Subsequently, the effects of 4-OI on LPS-induced intrapulmonary inflammatory responses were determined in mice. The results showed that pretreatment with 4-OI decreased the gene expression and secretion of IL-1β, TNF-α and IL-6 in the lungs (Fig. 2A-F). Furthermore, pretreatment with 4-OI notably decreased the number of total cells, neutrophils and macrophages in BALF induced by LPS (Fig. 2G-I). In addition, pretreatment with 4-OI effectively decreased the LPS-induced increase in MPO activity, which is a marker of neutrophil infiltration in lung tissues (Fig. 2J). These results illustrated that 4-OI prevented LPS-induced ALI by inhibiting inflammatory responses.
Figure 2.
4-OI inhibits LPS-induced inflammatory responses in mice with acute lung injury. Treatment of mice with 4-OI at doses of 25 mg/kg was administered intraperitoneally, and the control group received an equivalent volume of vehicle [(2-hydroxypropyl)-β-cyclodextrin in phosphate-buffered saline (PBS)] 2 h before saline or LPS injection (5 mg/kg, intratracheal). Twelve hours later, IL-1β (A), TNF-α (B), and IL-6 (C) mRNA levels in the lungs were determined by reverse transcription-quantitative PCR (n=5). IL-1β (D), TNF-α (E), and IL-6 (F) protein contents in serum were determined by enzyme-linked immunosorbent assay (n=5). Twelve hours later, total cells (G), neutrophils (H) and macrophages (I) in BALF were assessed (n=5). MPO activity (J) in lung tissue was determined (n=5). Data are expressed as the mean ± SEM. *P<0.05. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharides; IL, interleukin; TNF, tumor necrosis factor; BALF, bronchoalveolar lavage fluid; MPO, myeloperoxidase.
4-OI decreases oxidative stress in mice with LPS-induced ALI
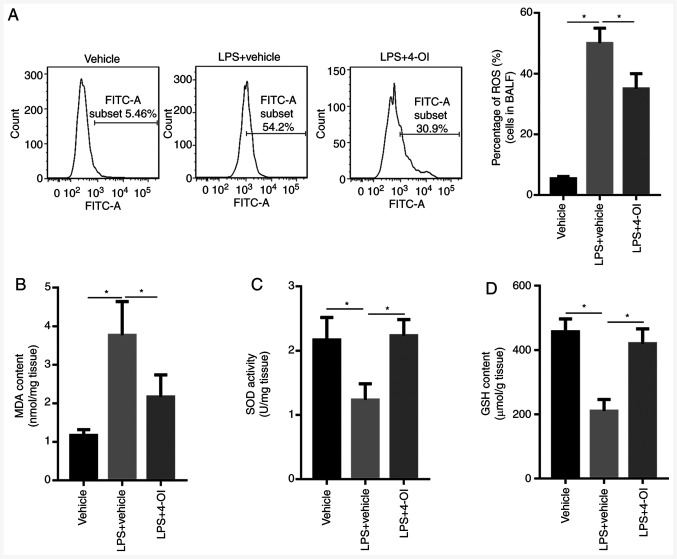
Given that oxidative stress plays an important role in ALI, whether 4-OI could decrease oxidative stress induced by LPS was determined. The results showed that LPS increased ROS generation in the BALF of ALI mice and MDA formation in the lung tissue of ALI mice, which were inhibited by 4-OI treatment (Fig. 3A and B). Since increased levels of SOD and GSH could inhibit oxidative stress, the levels of SOD and GSH were detected by ELISA. The results showed that LPS decreased the levels of SOD and GSH in the lung tissue of ALI mice, and this effect was significantly reversed by pretreatment with 4-OI (Fig. 3C and D). These results indicate that pretreatment with 4-OI could decrease oxidative stress in the lung tissue of ALI mice.
Figure 3.
4-OI inhibited oxidative stress in LPS-induced acute lung injury mice. Treatment of mice with 4-OI at doses of 25 mg/kg was administered intraperitoneally, and the control group received an equivalent volume of vehicle [(2-hydroxypropyl)-β-cyclodextrin in phosphate-buffered saline (PBS)] 2 h before saline or LPS injection (5 mg/kg, intratracheal). In total, 12 h later, ROS generation (A) in the BALF, MDA content (B), SOD activity (C) and GSH content (D) in lung tissue were determined (n=5). Data are expressed as the mean ± SEM. *P<0.05. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharides; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
4-OI decreases the production of inflammatory cytokines by inhibiting ROS-mediated PI3K/Akt/NF-κB activation
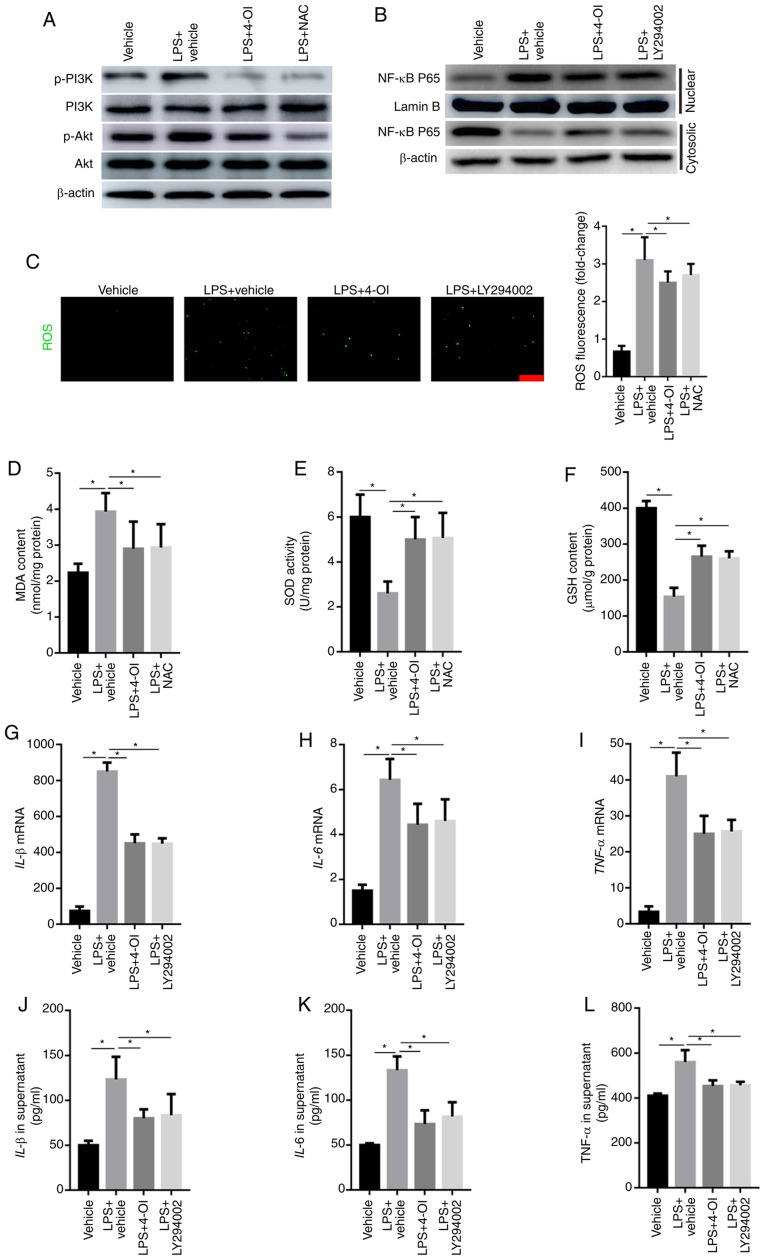
Growing evidence has demonstrated that ROS can activate the PI3K/Akt pathway, which further induces NF-κB translocation from the cytosol into the nucleus (21). Therefore, the phosphorylated levels of PI3K and Akt were detected by western blotting. The results illustrated that LPS increased the phosphorylated levels of PI3K and Akt, which were inhibited by the ROS scavenger NAC and 4-OI (Fig. 4A). To further demonstrate whether PI3K/Akt is involved in the activation of the NF-κB pathway in LPS-stimulated macrophages, the expression of NF-κB p65 in the nuclear and cytosolic fractions was detected by western blotting. The results illustrated that LY294002, a specific inhibitor of the PI3K/Akt pathway, significantly inhibited the LPS-induced increased level of NF-κB p65 in the nuclear fraction (Fig. 4B). Moreover, ROS generation and MDA formation were increased in LPS-treated RAW264.7 macrophage cells and were inhibited by 4-OI and NAC treatment (Fig. 4C and D). In addition, the levels of SOD and GSH were decreased in LPS-treated RAW264.7 macrophage cells, and this effect was significantly reversed by pretreatment with 4-OI and NAC (Fig. 4E and F). Furthermore, the production of TNF-α, IL-1β and IL-6 was also decreased by LY294002 (Fig. 4G-L). Collectively, these data demonstrate that 4-OI alleviated LPS-induced ALI by suppressing the ROS-mediated PI3K/Akt/NF-κB pathway.
Figure 4.
4-OI decreased the induction of inflammatory cytokines by inhibiting ROS-mediated PI3K/Akt/NF-κB activation in LPS-treated macrophages. RAW264.7 macrophage cells were pretreated with 4-OI (125 µM), NAC (10 mM), the PI3K inhibitor LY294002 (25 µM) or vehicle control for 1 h and then stimulated with LPS (1 µg/ml) for 30 min to detect the phosphorylated levels of PI3K and Akt and the expression of NF-κB p65 in the nuclear and cytosolic fractions in LPS-treated macrophages and for 24 h to quantify ROS production, MDA content, SOD activity and GSH content and the concentration of TNF-α, IL-1β, and IL-6 in macrophages. The phosphorylated levels of PI3K and Akt (A) and the expression of NF-κB p65 in the nuclear and cytosolic fractions (B) of LPS-treated macrophages were detected by western blotting. ROS generation (bar=100 µm) (C), MDA content (D), SOD activity (E) and GSH content (F) in RAW264.7 macrophage cells were determined. The expression of IL-1β (G), IL-6 (H) and TNF-α (I) in macrophages was measured by qPCR. IL-1β (J), IL-6 (K) and TNF-α (L) protein contents in the supernatant of RAW264.7 macrophage cells were determined. Data represent the means ± SEM of three independent experiments. *P<0.05. 4-OI, 4-octyl itaconate; LPS, lipopolysaccharides; NAC, N-acetyl-L-cysteine; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; IL, interleukin; TNF, tumor necrosis factor.
Discussion
Uncontrolled inflammatory responses and/or excessive oxidative stress are deemed to play an important role in the pathogenesis of ALI (25). The present study demonstrated that 4-OI alleviated LPS-induced ALI by inhibiting the inflammatory response and oxidative stress. 4-OI suppressed inflammatory cell infiltration and proinflammatory cytokine generation, decreased lung tissue structural damage, attenuated ROS generation and inhibited NF-κB activation in LPS-induced ALI mice in vivo. These results illustrate that 4-OI may be selected as an effective drug for the treatment of ALI in the future.
It has been reported that 4-OI attenuates hepatic I/R injury in vivo and protects hepatocytes from injury resulting from H/R in vitro (18). In addition, octyl itaconate (OI) markedly prolonged survival, improved body temperature regulation, decreased the clinical score, and reduced TNF and IL-1β levels in an LPS model of sepsis by activating the anti-inflammatory transcription factor Nrf2 (also known as NFE2L2) (15). Itaconate alkylates cysteine residues of KEAP1 protein, a central player in the antioxidant response, enabling Nrf2 to accumulate, migrate to the nucleus and further increase the expression of downstream genes with anti-inflammatory and antioxidant capacities (26). Moreover, OI ameliorated renal fibrosis by inhibiting NF-κB activation, decreasing the generation of ROS and suppressing autophagy (27). Whether 4-OI can protect against ALI remains unknown. The present study aimed to explore the protective effects of 4-OI against ALI in mice, and it was demonstrated that 4-OI could attenuate the inflammatory cell infiltration, NF-κB activation and oxidative stress induced by LPS. However, the exact mechanism needs to be further investigated. LPS binds to TLR4, which initiates two classic pathways. One pathway is toll/IL-1 receptor domain-containing adaptor including interferon-β-dependent, requiring the toll/IL-1 receptor (TIR) domain-containing adaptor protein. The other pathway is MyD88-dependent and promotes the translocation of NF-κB from the cytosol into the nucleus, leading to the release of proinflammatory cytokines (28). The excessive release of proinflammatory cytokines by activated macrophages aggravates tissue injury (29). In the present study, it was observed that treatment with 4-OI significantly decreased the LPS-induced expression of IL-1β, IL-6 and TNF-α in lung tissue.
Oxidative stress modulated by ROS plays an essential role in the progression of ALI (30). Under physiological conditions, ROS can help prevent pathological injury or noxious stimulation. However, excessive generation of ROS is thought to lead to cellular injury and oxidative stress (9). In the present study, it was found that treatment with 4-OI significantly decreased LPS-induced ROS generation and MDA formation and reversed the LPS-induced decrease in SOD and GSH levels in lung tissue.
The PI3K/Akt pathway plays an important role in cellular defense against inflammatory stimuli (31). Previous studies have also indicated that inhibition of the PI3K/Akt pathway attenuates LPS-induced ALI (32). Moreover, ROS lead to the activation of PI3K/Akt by inactivating the PTEN protein. Furthermore, PTEN inhibits the activation of NF-κB via the PI3K/Akt pathway (33). In addition, ROS, as secondary messengers, induce the nuclear translocation of NF-κB and the production of inflammatory cytokines (34). The present study demonstrated that 4-OI and NAC decreased the LPS-induced production of ROS, and the role of 4-OI in the PI3K/Akt pathway was further explored. In accordance with a previous study (21), the administration of NAC also suppressed the phosphorylated levels of PI3K and Akt in LPS-treated RAW264.7 macrophages. It has been reported that the PI3K and Akt pathways regulate LPS-induced NF-κB activation (35). Therefore, it was hypothesized that the PI3K/Akt pathway plays a pivotal role in ROS-mediated NF-κB activation. The results demonstrated that LY294002, a specific inhibitor of the PI3K/Akt pathway, markedly inhibited LPS-induced NF-κB activation. Moreover, LY294002 also decreased the levels of TNF-α, IL-1β and IL-6. These results demonstrated that 4-OI decreased the production of inflammatory cytokines by inhibiting the ROS-mediated PI3K/Akt/NF-κB pathway.
In conclusion, the present study confirmed that 4-OI exerts potential protective effects against LPS-induced lung injury in mice, possibly through inhibition of the ROS-mediated PI3K/Akt/NF-κB pathway.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Ningxia (grant no. NZ16137).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
SL and YX designed the study. YX and LZ performed all the experiments and analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experiments were approved by the Institutional Ethics Committee of Faculty of Ningxia Medical University (Yinchuan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shen Y, Cai G, Chen S, Hu C, Yan J. Fluid intake-related association between urine output and mortality in acute respiratory distress syndrome. Respir Res. 2020;21(24) doi: 10.1186/s12931-020-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.el-Ebiary M, Torres A, Fàbregas N, de la Bellacasa JP, González J, Ramirez J, del Baño D, Hernández C, Jiménez de Anta MT. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156:583–590. doi: 10.1164/ajrccm.156.2.9612023. [DOI] [PubMed] [Google Scholar]
- 4.Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kong L, Ge BX. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- 7.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badr G, Al-Sadoon MK, El-Toni AM, Daghestani M. Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFκB and ERK signaling. Lipids Health Dis. 2012;11(27) doi: 10.1186/1476-511X-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CS, Kim JJ, Lee SJ, Hwang JH, Lee CH, Lee MS, Jo EK. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J Immunol. 2013;190:6368–6377. doi: 10.4049/jimmunol.1202574. [DOI] [PubMed] [Google Scholar]
- 10.Hong HY, Jeon WK, Kim BC. Up-regulation of heme oxygenase-1 expression through the Rac1/NADPH oxidase/ROS/p38 signaling cascade mediates the anti-inflammatory effect of 15-deoxy-delta 12,14-prostaglandin J2 in murine macrophages. FEBS Lett. 2008;582:861–868. doi: 10.1016/j.febslet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Jiang K, Guo S, Yang C, Yang J, Chen Y, Shaukat A, Zhao G, Wu H, Deng G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int Immunopharmacol. 2018;64:140–150. doi: 10.1016/j.intimp.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Yu XH, Zhang DW, Zheng XL, Tang CK. Itaconate: An emerging determinant of inflammation in activated macrophages. Immunol Cell Biol. 2019;97:134–141. doi: 10.1111/imcb.12218. [DOI] [PubMed] [Google Scholar]
- 13.Rittenhouse JW, McFadden BA. Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Arch Biochem Biophys. 1974;163:79–86. doi: 10.1016/0003-9861(74)90456-1. [DOI] [PubMed] [Google Scholar]
- 14.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556:501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals. 8th edition. National Academies Press (US), Washington, DC, 2011. [Google Scholar]
- 17.Zhang Y, Xu T, Pan Z, Ge X, Sun C, Lu C, Chen H, Xiao Z, Zhang B, Dai Y, et al. Shikonin inhibits myeloid differentiation protein 2 to prevent LPS-induced acute lung injury. Br J Pharmacol. 2018;175:840–854. doi: 10.1111/bph.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi Z, Deng M, Scott MJ, Fu G, Loughran PA, Lei Z, Li S, Sun P, Yang C, Li W, et al. doi: 10.1002/hep.31147. IRG1/itaconate activates Nrf2 in hepatocytes to protect against liver ischemia-reperfusion injury. Hepatology: Jan 30, 2020 (Epub ahead of press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz M, Matsuda A, Yang WL, Jacob A, Wang P. Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J Immunol. 2012;189:393–402. doi: 10.4049/jimmunol.1200262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L, Yin Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int Immunopharmacol. 2012;12:278–287. doi: 10.1016/j.intimp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Peng F, Xie C, Wu W, Han X, Chen L. (E)-3-(3,4-Dimethoxyphenyl)-1-(5-hydroxy-2,2-dimethyl-2H-chromen-6-yl)prop-2-en-1-one ameliorates the collagen-arthritis via blocking ERK/JNK and NF-κB signaling pathway. Int Immunopharmacol. 2013;17:1125–1133. doi: 10.1016/j.intimp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Sun GY, Yang HH, Guan XX, Zhong WJ, Liu YP, Du MY, Luo XQ, Zhou Y, Guan CX. Vasoactive intestinal peptide overexpression mediated by lentivirus attenuates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation. Mol Immunol. 2018;97:8–15. doi: 10.1016/j.molimm.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Huang XT, Li C, Peng XP, Guo J, Yue SJ, Liu W, Zhao FY, Han JZ, Huang YH, Yang-Li , et al. An excessive increase in glutamate contributes to glucose-toxicity in β-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep. 2017;7(44120) doi: 10.1038/srep44120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang XT, Liu W, Zhou Y, Sun M, Yang HH, Zhang CY, Tang SY. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radic Biol Med. 2020;146:222–233. doi: 10.1016/j.freeradbiomed.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Tian F, Wang Z, He J, Zhang Z, Tan N. 4-Octyl itaconate protects against renal fibrosis via inhibiting TGF-β/Smad pathway, autophagy and reducing generation of reactive oxygen species. Eur J Pharmacol. 2020;873(172989) doi: 10.1016/j.ejphar.2020.172989. [DOI] [PubMed] [Google Scholar]
- 28.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S, Johnson CG, Sciurba J, Meng X, Stober VP, Liu C, Cyphert-Daly JM, Bulek K, Qian W, Solis A, et al. doi: 10.7554/eLife.50458. TLR5 participates in the TLR4 receptor complex and promotes MyD88-dependent signaling in environmental lung injury. Elife: Jan 28, 2020 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher AB, Dodia C, Chatterjee S, Feinstein SI. A peptide inhibitor of NADPH oxidase (NOX2) activation markedly decreases mouse lung injury and mortality following administration of lipopolysaccharide (LPS) Int J Mol Sci. 2019;20(2395) doi: 10.3390/ijms20102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, Han MJ, Kim DH. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol. 2013;708:21–29. doi: 10.1016/j.ejphar.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Zhao M, Li C, Shen F, Wang M, Jia N, Wang C. Naringenin ameliorates LPS-induced acute lung injury through its anti-oxidative and anti-inflammatory activity and by inhibition of the PI3K/AKT pathway. Exp Ther Med. 2017;14:2228–2234. doi: 10.3892/etm.2017.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Zhou A, Xu L, Zhang X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience. 2014;269:93–101. doi: 10.1016/j.neuroscience.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 34.Niu T, Tian Y, Wang G, Guo G, Tong Y, Shi Y. Inhibition of ROS-NF-κB-dependent autophagy enhances Hypocrellin A united LED red light-induced apoptosis in squamous carcinoma A431 cells. Cell Signal. 2020;69(109550) doi: 10.1016/j.cellsig.2020.109550. [DOI] [PubMed] [Google Scholar]
- 35.Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed Pharmacother. 2016;83:302–313. doi: 10.1016/j.biopha.2016.06.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.