Abstract
This opinion deals with the re‐evaluation of polydextrose (E 1200) when used as a food additive. The Panel followed the conceptual framework for the risk assessment of certain additives and considered that: adequate exposure estimates were available; the margin of safety (MOS)/margin of exposure (MOE) for arsenic was between 0.5‐14 and 8.5 for lead; the exhaustions of the tolerable weekly intake (TWI) for cadmium would be 165%, 10% for mercury, whereas the exhaustion of the tolerable daily intake (TDI) for nickel would be 9%; the absorption is limited and part of polydextrose is fermented in the large intestine into short‐chain fatty acids (SCFA); adequate toxicity data were available; there is no concern with respect to genotoxicity; no adverse effects were reported in subchronic studies in rats, dogs or monkeys nor in chronic or carcinogenicity studies in mice and rats at the highest doses tested of up 12,500 mg/kg body weight (bw) per day and 15,000 mg/kg bw per day, respectively; the nephrocalcinosis in dogs given high doses of polydextrose was considered to be a treatment‐related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment; no adverse effects were reported in reproductive or developmental toxicity studies in rats administered up to 10,000 mg polydextrose/kg bw per day, or in a developmental toxicity study in rabbits up to 1,818 mg/kg bw per day (the highest dose tested). Therefore, the Panel concluded that there is no need for numerical acceptable daily intake (ADI) for polydextrose (E 1200), and that there is no safety concern for the reported uses and use levels of polydextrose as a food additive. The Panel recommended that European Commission considers to lower the maximum limit for lead and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200), and to verify that polydextrose‐N as a food additive (E 1200) is no longer marketed in the EU.
Keywords: polydextrose, E 1200, polydextrose‐N, polydextrose‐A, food additive
Summary
The present opinion deals with the re‐evaluation of polydextrose (E 1200) when used as a food additive.
Polydextrose (E 1200) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.
In the EU, polydextrose (E 1200) has been evaluated by the Scientific Committee on Food (SCF) in 1990 (SCF, 1992), who allocated, an acceptable daily intake (ADI) ‘not specified’, and concluded also that the laxative effect should be considered, for the compound alone or when used in combination with other compounds having a similar effect (e.g. polyols), when considering appropriate levels for the use of polydextrose. Polydextrose (E 1200) was also evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1987 (JECFA, 1987a,b), who allocated an ADI ‘not specified’, based on the lack of adverse effects in the available toxicity studies.
According to the Commission Regulation (EU) 231/2012, polydextrose (E 1200) is a randomly bonded glucose polymer with some sorbitol end‐groups, and with citric or phosphoric acid residues attached to the polymer by mono‐ or diester bonds. The non‐neutralised product (polydextrose‐A) and the neutralised polydextrose (polydextrose‐N) are considered to fall within the specifications of the food additive (E 1200). According to the information from the interested party, polydextrose‐N is no longer marketed in the EU. Therefore, the Panel considered that it should be verified whether or not polydextrose‐N (E 1200) is still used as a food additive in the EU.
Based on the information provided by the interested party on elemental impurity limits, and the exposure estimation to the additive (non‐brand‐loyal scenario, P95, toddlers), the Panel calculated the potential exposure to the toxic elements from the use of polydextrose as a food additive. The Panel considered that there may be a need to lower the limit for lead and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200).
The Panel considered experimental data on the water solubility of four types of polydextrose used as the food additive (E 1200) and noted that the range of solubility observed (870–1,050 g/L) for these materials was substantially higher than the threshold of 33.3 g/L currently proposed in the EFSA ‘Draft Guidance on technical requirement for regulated food and feed product applications to established the presence of small particles including nanoparticles’, as a decision criterion to decide whether or not an additional assessment for the fraction of small particles is needed. Since this solubility criterion is met, the Panel considered that the risk assessment of polydextrose (E 1200) as a food additive does not require to be complemented with the nanospecific considerations according to the EFSA Guidance on Nanotechnology (EFSA Scientific Committee, 2018).
Several in vitro studies, in vivo studies in experimental animals and in humans investigated the absorption, distribution, metabolism and excretion of polydextrose. The metabolism of polydextrose is similar in rats and humans. In humans, the reported recovery of radioactivity was 33–50% in faeces, 15–36% in breath and 1.4 and 4% in the urine. Polydextrose is partially fermented in the large intestine into short‐chain fatty acids (SCFA).
Polydextrose (E 1200) (polydextrose‐A and/or polydextrose‐N) did not show a genotoxic potential in limited bacterial reverse mutation assays, did not induce chromosomal aberrations in vitro in human lymphocytes, and in vivo did not induce chromosomal aberrations in bone marrow and dominant lethal mutations in mice. The Panel noted that there are no structural alerts for genotoxicity. Overall, the Panel noted that the studies were not conducted according to the current guidelines and were limited in their protocols. However, in the absence of any structural alerts for genotoxicity, the Panel considered the results acceptable in the overall weight of evidence evaluation and considered that the available data do not indicate a genotoxic activity of polydextrose.
The subchronic studies in rats or monkeys with oral doses of polydextrose up to 10,000 mg/kg body weight (bw) per day or in dogs when the diet contained polydextrose‐A at 50% or 33% indicated no adverse effects on feed intake, clinical pathology, organ weights and histopathology. Decreases in body weight were reported in a 3‐month study in rats. Dogs receiving polydextrose in their diet for 135 days or 13 months developed nephrocalcinosis which was the result of hypercalcaemia, resulting from chronic watery diarrhoea. Monkeys receiving a high dose of polydextrose by gavage gained weight in a dose‐dependent manner and developed loose stool and/or diarrhoea.
Mice given 7,500 or 15,000 mg polydextrose‐A/kg bw per day in the diet for 18 months and rats given up to 5,000 mg polydextrose‐A/kg bw per day in their diet for 24 months revealed no effect in any of the parameters examined which could be ascribed to the feeding of polydextrose‐A. Studies in dogs showed that polydextrose‐N (containing up to 1.5% potassium), fed for 24 months in daily dietary levels of 10 or 20% or fed for 18 months in daily dietary levels of 50% induced a dose‐dependent osmotic watery diarrhoea which contributed to transient decreases in vascular fluid volume, electrolyte imbalance, enhanced renal reabsorption of sodium and calcium, a gradually developing hypercalcaemia and ultimately nephrocalcinosis. No carcinogenicity was observed in any of the studies. The Panel considered the nephrocalcinosis which developed in dogs given high doses of polydextrose, both in subchronic and chronic toxicity studies, as treatment‐related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment.
In a dietary three‐generation reproductive toxicity study in rats, no adverse effects were observed up to 10% polydextrose A in the diet (equivalent to 5,000 mg/kg bw per day, the highest dose tested). In a male and female fertility study, a prenatal developmental toxicity study and a peri‐ and postnatal toxicity study in rats, animals were dosed by gavage and no adverse effects were observed up to 10,000 mg polydextrose/kg bw per day (the highest dose tested). In a prenatal developmental toxicity study in rabbits, no maternal or developmental effects were observed up to the highest dose tested (1,818 mg polydextrose/kg bw per day).
The Panel agreed with the JECFA (1987a,b) and SCF (1992) evaluations concerning the laxative threshold for polydextrose (a mean laxative threshold of 90 g per person per day or 50 g as a single dose). The Panel considered that, in line with the conclusions by the SCF in 1990 (SCF, 1992), the laxative effect should be taken into account, for the compound alone or when used in combination with other compounds having a similar effect (e.g. polyols), when considering appropriate levels for the use of polydextrose.
Polydextrose (E 1200) is an authorised food additive in the EU at quantum satis (QS) in three food categories as set by Part E of Annex II to Regulation (EC) No 1333/2008. The use of polydextrose (E 1200) is also authorised according to Annex III, Part 1, 3 and 5 of Regulation (EC) No 1333/2008. Polydextrose (E 1200) is also authorised in the FC 17 Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children.
Polydextrose (E 1200) is used as a bulking agent and does not influence the organoleptic properties of the final food. For this reason, the Panel considered the non‐brand loyal scenario as the most appropriate scenario for risk characterisation. In this scenario, the exposure estimates ranged from 0.2 mg/kg bw per day in infants to 352 mg/kg bw per day in toddlers at the mean. At the 95th percentile, exposure ranged from 0 mg/kg bw per day in infants to 590 mg/kg bw per day in toddlers.
In all scenarios, all foods belonging to the food categories included in these scenarios were assumed to contain polydextrose (E 1200) at the reported use levels. Foods in which polydextrose (E 1200) is not authorised to be directly added but in which polydextrose (E 1200) can be present as carry‐over (according to Annex III) were taken into account when use levels were provided. In principle, the calculated exposure to the food additive E 1200 was considered to be overestimated based on the concentration data used and the methodology applied. However, not all uses of the food additive among those authorised in accordance with Annex III of Regulation (EC) No 1333/2008 may not necessarily be covered by the occurrence levels and therefore could not be taken into account in the estimated exposure. The Panel acknowledges that these incomplete data may have an influence on the direction of the uncertainty.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014) and given that:
adequate exposure estimates were available;
the margin of safety (MOS)/margin of exposure (MOE) for arsenic was low (0.5 and 14), and the exhaustions of the tolerable weekly intake (TWI) for cadmium would be 165%, whereas it would be 10% for mercury. The exhaustion of the tolerable daily intake (TDI) for nickel would amount to 9%. The MOS/MOE for lead was 8.5;
the absorption was limited and part of the polydextrose is fermented in the large intestine into SCFA;
adequate toxicity data were available;
there was no concern with respect to genotoxicity;
no adverse effects were reported in subchronic studies in rats or monkeys administered oral doses of polydextrose up to 10,000 mg/kg bw per day or in dogs given diet containing polydextrose‐A up to 12,500 mg/kg bw per day;
no adverse effects were reported in chronic or carcinogenicity studies up to 15,000 mg polydextrose‐A/kg bw per day in mice and 5,000 mg/kg bw per day in rats, the highest dose tested;
the nephrocalcinosis which developed in dogs given high doses of polydextrose, both in subchronic and chronic toxicity studies, was considered to be a treatment‐related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment;
no adverse effects were reported in reproductive or developmental toxicity studies in rats administered up to 5,000 mg polydextrose‐A/kg bw per day or 10,000 mg polydextrose/kg bw per day, respectively, or in a developmental toxicity study in rabbits up to 1,818 mg polydextrose/kg bw per day, the highest doses tested;
the Panel concluded that there is no need for numerical ADI for polydextrose (E 1200) (polydextrose‐A and polydextrose‐N), and that there is no safety concern for the reported uses and use levels of polydextrose as a food additive.
The Panel recommended that European Commission considers:
the need to lower the maximum limit for lead, and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200);
verifying that polydextrose‐N as a food additive (E 1200) is no longer marketed in the EU.
1. Introduction
The present opinion deals with the re‐evaluation of polydextrose (E 1200) when used as a food additive.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
Regulation (EC) No 1333/20081 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by EFSA.
For this purpose, a programme for the re‐evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20102. This Regulation also foresees that food additives are re‐evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re‐evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re‐evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU3 of 2001. The report “Food additives in Europe 20004” submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re‐evaluation. As colours were among the first additives to be evaluated, these food additives should be re‐evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re‐evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1.1.2. Terms of Reference
The Commission asks the European Food Safety Authority to re‐evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2. Information on existing authorisations and evaluations
Polydextrose (E 1200) is authorised as a food additive in the European Union (EU) in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20125.
In the EU, polydextrose (E 1200) has been evaluated by the Scientific Committee for Food (SCF) in 1990 (SCF, 1992). The SCF reported that large doses of polydextrose exerted a laxative effect with a mean laxative threshold of 90 g/day or 50 g as a single dose. The Committee allocated, based on the available data, an acceptable daily intake (ADI) not specified, and concluded also that the laxative effect should be taken into account, for the compound alone or when used in combination with other compounds having a similar effect (e.g. polyols), when considering appropriate levels for the use of polydextrose.
In 1993, the SCF at its 90th meeting (SCF, 1993) concluded that the data available on polydextrose (E 1200) were indicative of an energy value of 1–1.5 kcal/g, but that the data did not allow a precise value to be determined.
Polydextrose (E 1200) was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1980 (JECFA, 1980), who allocated an ADI of 0–70 mg/kg bw per day, based on the available toxicity studies (acute, subacute or chronic in three animal species) at doses equivalent of 10% of the diet. Polydextrose (E 1200) was re‐evaluated by JECFA in 1987 (JECFA, 1987a,b), who allocated an ADI ‘not specified’, based on the lack of adverse effects in the available toxicity studies which were considered in line with what would be normally required for an ADI to be set for a food additive.
Polydextrose (E 1200) has also been reviewed by the Nordic Council of Ministers (TemaNord, 2002), who concluded that ‘the toxicological data available included what normally would be required for an ADI to be set for a food additive’, and that ‘Polydextrose as defined by the specifications is covered by the toxicological evaluation’.
The EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel, 2011) provided a scientific opinion on a list of health claims in relation to polydextrose and changes in bowel function, changes in short‐chain fatty acid (SCFA) production and/or pH in the gastrointestinal (GI) tract, decreasing potentially pathogenic GI microorganisms and reduction of GI discomfort. On the basis of the data presented, the NDA Panel concluded that a cause and effect relationship has not been established between the consumption of polydextrose and changes in bowel function, a beneficial physiological effect related to changes in SCFA production and/or pH in the GI tract, the decreasing of potentially pathogenic GI microorganisms and the reduction of GI discomfort.
The EFSA NDA issued another scientific opinion in 2016 (EFSA NDA Panel, 2016) on the scientific substantiation of a health claim related to polydextrose and maintenance of normal defecation. The NDA Panel concluded that a cause and [beneficial] effect relationship has not been established between the consumption of polydextrose and maintenance of normal defecation.
2. Data and methodologies
2.1. Data
The Panel on Food Additives and Flavourings (FAF) was not provided with a newly submitted dossier. EFSA launched public calls for data6 , 7 to collect information from interested parties. The Panel based its assessment on information submitted to EFSA following the public calls for data, information from previous evaluations and additional available literature up to the date of the last Working Group (WG) meeting.8 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based however these were not always available to the Panel.
During the course of the assessment additional information was requested to the interested parties (Documentation provided to EFSA n. 5) and a technical hearing was held on 25 June 2020 during the 10 meeting of the FAF Panel Working Group on Specifications of food additives,9 followed by a further submission of data in response to the questions asked by the WG (Documentation provided to EFSA n. 7).
Food consumption data used to estimate the dietary exposure to polydextrose (E 1200) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database10).
The Mintel's Global New Products Database (GNPD) was used to verify the uses of polydextrose (E 1200) in food and beverage products and food supplements within the EU's market. The Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The FAF Panel assessed the safety of polydextrose (E 1200) as a food additive in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
When in animal studies, the test substance was administered in the feed or in drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake is calculated by the Panel using the relevant default values. In case of rodents, the values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b) are applied. In the case of other animal species, the default values by JECFA (2000) are used. In these cases, the dose was expressed as ‘equivalent to mg/kg bw per day’. If a concentration in feed or drinking water was reported and the dose in mg/kg bw per day was calculated (by the authors of the study report or the Panel) based on these reported concentrations and on reported consumption data for feed or drinking water, the dose was expressed as ‘equal to mg/kg bw per day’. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b).
Dietary exposure to polydextrose (E 1200) from its use as a food additive was estimated combining food consumption data available within the EFSA Comprehensive European Food Consumption Database with reported use levels submitted to EFSA following a call for data. Different scenarios were used to calculate exposure (see Section 3.4.1). Uncertainties on the exposure assessment were identified and discussed.
In the context of this re‐evaluation, the Panel followed the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EC) No 257/2010 (EFSA ANS Panel, 2014).
3. Assessment
3.1. Technical data
3.1.1. Identity of the food additive
According to the Commission Regulation (EU) 231/2012, polydextrose (E 1200) is a randomly bonded glucose polymer with some sorbitol end‐groups, and with citric or phosphoric acid residues attached to the polymer by mono or diester bonds. It is obtained by melting and condensation of the ingredients and consists of approximately 90 parts d‐glucose, 10 parts sorbitol and 1 part citric acid/or 0.1 part phosphoric acid. The 1,6‐glucosidic linkage predominates in the polymer, but other linkages are present. The product contains small quantities of free glucose, sorbitol, levoglucosan (1,6‐anhydro‐d‐glucose) and citric acid and may be neutralised with any food‐grade base and/or decolourised and deionised for further purification to obtain the neutralised polydextrose (polydextrose‐N). According to the above Regulation, polydextrose and polydextrose‐N are considered to fall within the specifications of the food additive E 1200. The products may also be partially hydrogenated with Raney nickel catalyst to reduce residual glucose. According to Commission Regulation (EU) No 231/2012, the synonym for the food additive E 1200 is modified polydextroses. Another synonym for the non‐neutralised polydextrose found in the literature is polydextrose‐A (Burdock and Flamm, 1999; Veena et al., 2016).
Polydextroses dissolve in water to give a clear, colourless to straw‐coloured solution: 10% solutions of polydextrose‐A and polydextrose‐N have pH values of 2.5–7.0 and 5.0–6.0, respectively.
Polydextrose (E 1200) exhibits a highly branched structure with an average degree of polymerisation (DP) of approximately 12 glucose units and a weight‐average molecular weight of 2,000 Da (Craig, 2001; Auerbach et al., 2007; Stowell, 2009). Because of the random bonding of glucose molecules, polydextrose exists in a wide range of molecular weights, up to approximately 20,000 Da. According to the interested party (Documentation provided to EFSA n. 6), the average molecular weight of the polydextrose manufactured and marketed is approximately 2,000 Da (± 10%), with the molecular weight substantially comprised between 250 and 18,000 Da, but predominantly not greater than 5,000 Da. During the polymerisation process, the targeted DP range is controlled by sorbitol acting as a chain terminator. Figure 1 shows a representative chemical structure of polydextrose.
Figure 1.
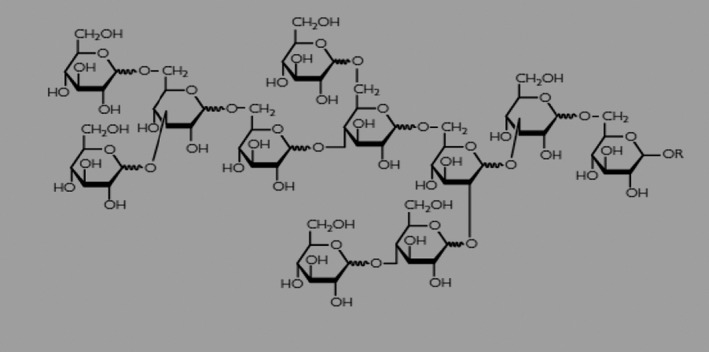
Representative polydextrose structure (redrawn from Documentation provided to EFSA n. 1). R stands for hydrogen, sorbitol or the continuation of polydextrose polymer
By carbon‐13 nuclear magnetic resonance (13C‐NMR) spectrometry, it was shown that all possible linkages with the glycosidic carbon of glucose were present in polydextrose (Auerbach et al., 2007): α‐ and β‐1,2, ‐1,3, ‐1,4 and ‐1,6, with the 1,6‐linkage predominating.
3.1.2. Specifications
The specifications for the food additive polydextrose (E 1200) as defined in the Commission Regulation (EU) No 231/2012 and by JECFA (2006) are listed in Table 1.
Table 1.
Specifications for polydextrose(s) (E 1200) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
| Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
|---|---|---|
| Synonyms | Modified polydextroses | Modified polydextroses; INS No 1200 |
| Definition | Randomly bonded glucose polymers with some sorbitol end‐groups, and with citric acid or phosphoric acid residues attached to the polymers by mono or diester bonds. They are obtained by melting and condensation of the ingredients and consist of approximately 90 parts d‐glucose, 10 parts sorbitol and 1 part citric acid and/or 0.1 part phosphoric acid. The 1,6‐glucosidic linkage predominates in the polymers but other linkages are present. The products contain small quantities of free glucose, sorbitol, levoglucosan (1,6‐anhydro‐d‐glucose) and citric acid and may be neutralised with any food‐grade base and/or decolourised and deionised for further purification. The products may also be partially hydrogenated with Raney nickel catalyst to reduce residual glucose. Polydextrose‐N is a neutralised polydextrose | Randomly bonded condensation polymers of glucose with some sorbitol end‐groups, and with citric acid or phosphoric acid residues attached to the polymers by mono or diester bonds. They are obtained by melting and condensation of the ingredients which consist of approximately 90 parts d‐glucose, 10 parts sorbitol and up to 1 part citric acid or 0.1 part phosphoric acid. The 1,6‐glucosidic linkage predominates in the polymers but other linkages are present. The products contain small quantities of free glucose, sorbitol, levoglucosan (1,6‐anhydro‐d‐glucose) and citric acid and may be neutralised with any food‐grade base and/or decolourised and deionised for further purification. The products may also be partially hydrogenated with Raney nickel catalyst to reduce residual glucose. Polydextrose‐N is a neutralised polydextrose |
| EINECS (EC) No: — | CAS No: 68424‐04‐4 | |
| Assay: content not less than 90 % of polymer on the ash‐free and anhydrous basis | Assay: not less than 90.0 % of polymer on the ash‐free and water‐free bases | |
| Description | White to light tan‐coloured solid. Polydextroses dissolve in water to give a clear, colourless to straw‐coloured solution | White to light tan‐coloured solid. Polydextroses dissolve in water to give clear, colourless to straw‐coloured solutions |
| Functional uses | — | Bulking agent, humectant, stabiliser, thickener |
| Identification | Test for sugar: passes test | Test for sugar: passes test(a) |
| Test for reducing sugar: passes test | Test for reducing sugar: passes test(a) | |
pH:
|
pH:(b)
|
|
| — | Solubility: very soluble in water | |
| — | Solubility in acetone: passes test(a) | |
| Purity | Water content: not more than 4.0% (Karl Fischer method) | Water: not more than 4.0% (Karl Fischer method) |
Sulfated ash:
|
Sulfated ash:
|
|
| Nickel: not more than 2 mg/kg for hydrogenated polydextroses | Nickel: not more than 2 mg/kg for hydrogenated polydextroses | |
| 1,6‐Anhydro‐d‐glucose: not more than 4.0% on the ash‐free and the dried basis | 1,6‐Anhydro‐d‐glucose: not more than 4.0% on the ash‐free and the dried basis(a) | |
| Glucose and sorbitol: not more than 6.0% combined on the ash‐free and the dried basis; glucose and sorbitol are determined separately | Glucose and sorbitol: not more than 6.0% combined on the ash‐free and the dried basis; glucose and sorbitol are determined separately(a) | |
| Molecular weight limit: negative test for polymers of molecular weight greater than 22,000 Da | Molecular weight limit: negative to test for polymer of molecular weight greater than 22,000 Da(a) | |
5‐Hydroxy‐methylfurfural:
|
5‐Hydroxy‐methylfurfural:(a)
|
|
| Lead: not more than 0.5 mg/kg | Lead: not more than 0.5 mg/kg |
The Panel noted that, according to the EU specifications for polydextrose (E 1200), impurities of the toxic elements nickel and lead are accepted up to concentrations of respectively 2 mg/kg (in hydrogenated polydextroses) and 0.5 mg/kg. Contamination at such levels could have a significant impact on the exposure to these metals, for which the exposure already are close to the health‐based guidance values or benchmark doses (lower confidence limits) established by EFSA (EFSA CONTAM Panel, 2010, 2020).
The Panel also noted that the EINECS (EC) identifier for polydextrose is missing in the EU Specifications. According to ECHA Inventory (online), EC List No 614‐467‐9 is linked to CAS No 68424‐04‐4.
The Panel further noted slight differences in the Definition of the EU Regulation compared with JECFA's, concerning the addition of citric and phosphoric acids to the condensation mixture (‘and 1 part citric acid and/or 0.1 part phosphoric acid’ vs ‘and up to 1 part citric acid or 0.1 part phosphoric acid’).
According to the information provided by the interested party (Documentation provided to EFSA n. 1), the polydextrose that was produced in the past could be acidic; a neutral form of polydextrose (polydextrose‐N) was then obtained by adding small amounts of potassium hydroxide or carbonate (Burdock and Flamm, 1999; Documentation provided to EFSA n. 1). The neutralised, light‐yellow product was available as a 70% aqueous solution. Improvements in the manufacturing process in later times have reduced the acid residues in the product and thereby eliminated the need for separate alkali neutralisation (see also Section 3.1.3). Consequently, as the manufacturing process has improved, according to the information from the interested party, polydextrose‐N is no longer marketed in the EU (Documentation provided to EFSA n. 1). Therefore, the Panel considered that it should be verified whether or not polydextrose‐N as a food additive (E 1200) is still used as a food additive in the EU.
The following elemental impurity limits were provided by the interested party (mg/kg): arsenic, < 1.0; lead, < 0.1; cadmium, < 1.0; mercury, < 0.1; nickel, < 2.0 (Documentation provided to EFSA n. 1). Based on these impurity limits (Documentation provided to EFSA n. 1), and the exposure estimation to the additive (non‐brand‐loyal scenario, P95, toddlers, see Section 3.4.1), the Panel calculated the potential exposure to the toxic elements from the use of polydextrose as a food additive. The resulting exposure calculations (see Annex G) to the toxic elements would result in MOS/MOE for arsenic between 0.5 and 14, and for lead of 8.5. The exhaustions of the TWI for cadmium would be 165%, and for mercury 10%. The exhaustion of the TDI for nickel would amount to 9%. The calculation of the Panel shows that the potential exposure to toxic elements, especially to arsenic and cadmium from the consumption of E 1200 could be substantial. The Panel considers that there may be a need to lower the limit for lead and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200).
Solubility and particle size
According to the information provided by the interested party (Documentation provided to EFSA n. 1), polydextrose is soluble in water at approximately 80 g/100 mL at 20°C.
An analysis report of the measurement of four different types of polydextrose (coarse grind, granular, fine powder and two powder) by laser diffraction (LD) was submitted (Documentation provided to EFSA n. 4). The Panel noted that LD is not a methodology appropriate to analyse for the presence of nanoparticles in this kind of polydisperse material (polydextrose), and therefore, the absence of nanoparticles based on LD analysis was not conclusive. A scanning electron microscope (SEM) analysis of the same samples was also provided (Documentation provided to EFSA n. 4). It was noted that the quality of the SEM images was poor and the magnification was only x200. Therefore, the images did not allow the detection of particles < 1 μm.
Following a further request from EFSA, an additional analysis by SEM of polydextrose (4 types) was submitted (Documentation provided to EFSA n. 5). The method used to analyse the samples of polydextrose was aligned with the requirements in ISO133322‐1. The report stated that ‘the particles are by nature irregular, protrusions that may be observed on some particles are considered as parts of the larger particle’. The Panel noted that smaller particles lying on the larger particles have been classified as part of those larger particles forming the ‘so‐called’ irregular protrusions. In addition, it was noted that the choice of magnification for SEM imaging, including the highest magnification of x200, does not allow to visualise particles in the nano range (1–100 nm) in the absence of specific data on the image width. Based on the data submitted, the Panel could not exclude the presence of nanosized particles in the analysed materials and requested additional data.
Upon a further request from EFSA, solubility results were obtained by testing (OECD TG 105 with some modifications) four types of polydextrose (coarse grind, granular, fine powder and two powder) (Documentation provided to EFSA n. 5). The Panel noted that for three of the tested materials, an increase of the concentration measured was observed from 24 h, 48 h up to 72 h. According to OECD Guideline TG 105, the whole test should have been repeated using longer equilibration times. Furthermore, according to the TG 105 protocol, the pH value for each test performed, should have been provided. This was not the case for the four samples and for each of the three duration tests performed. Further clarifications were requested and new solubility tests were submitted for the four types of polydextrose (Documentation provided to EFSA n. 7).
The Panel noted high variation between the results of the solubility tests performed at the different times (Documentation provided to EFSA n. 5 compared to n. 7). No measurement uncertainties that could explain these variations were reported. Based on the reported values and the variation between measurements (Documentation provided to EFSA n. 5 and 7), the Panel noted that the range of solubility varied from 87 to 105 g/100 mL (870–1,050 g/L). The Panel concluded that this solubility range is substantially higher than the value of 33.3 g/L proposed as a decision criterion to decide whether an additional assessment for the fraction of small particles is needed according to the Draft Guidance on technical requirement for regulated food and feed product applications to establish the presence of small particles including nanoparticles (Draft EFSA Guidance particle‐TR).11 In such a case, the draft EFSA Guidance particle‐TR does not require the measurement of number‐based distributions of the particle size of these four materials because solubility demonstrated that consumers will not be exposed to small particles.
Therefore, the EFSA Guidance on Nanotechnology (EFSA Scientific Committee, 2018) is not applicable and the risk assessment of polydextrose (E 1200) should be done following the Guidance on Food Additive (2012). A full physicochemical characterisation of the particles, including size measurement, was not deemed necessary.
3.1.3. Manufacturing process
As reported by the interested party (Documentation provided to EFSA n. 1), the first step in the manufacturing process of food grade polydextrose is a vacuum‐melt condensation. In this process, powdered, crystalline, and/or liquid glucose or glucose‐containing material (such as hydrolysed starch) is heated under vacuum at 150–160°C for about 20 min in the presence of a polyol such as sorbitol and with low levels of a catalytic acid chemical such as citric or phosphoric acid. Because of the low levels of catalyst used, minimal or no off‐flavours and little colour are formed during the course of the reaction. The product may be further purified using ion exchange, membrane filtration, carbon treatment or hydrogenation, or a combination of the aforesaid purification processes. According to Stowell (2009), a partially hydrogenated version of polydextrose, suited for high inclusion rates, for sugar‐free applications, and where Maillard reactions are not required, can also be manufactured. Polydextrose is provided in powdered form or as a 70% aqueous solution. The core polydextrose molecule remains the same in all qualities of polydextrose products; food grade polydextrose is manufactured according to current good manufacturing practice (cGMP).
3.1.4. Methods of analysis in food
Polydextrose is recognised as a soluble fibre in several countries and frequently used to increase the dietary fibre content of food. Until 2000, methods of the Association of Official Analytical Chemists (AOAC) for measuring total dietary fibre (TDF) in foods (e.g. Method 985.29) included an ethanol precipitation step in which polydextrose and similar carbohydrates were discarded and therefore not quantitated (Craig et al., 2000; Stowell, 2009). Therefore, Craig et al. (2000), and Craig (2001) developed a method to quantitate polydextrose in foods. The new method included hot water extraction, centrifugal ultrafiltration, hydrolysis with a multienzyme mixture (isoamylase, amyloglucosidase and fructanase), and high‐performance anion‐exchange chromatography with electrochemical detection (HPAEC–ED). Polydextrose was determined after removal of interfering food components (high molecular weight solubles). The method was subject to in‐house validation to test its ruggedness. Subsequently, the validation process was extended: eight collaborating laboratories assayed seven blind duplicate pairs of different foods for polydextrose content. The polydextrose level in the seven‐test sample pairs ranged from 2% to 95% (polydextrose itself was included as a test sample). The following foods were prepared with polydextrose mixed into the other ingredients and then baked, cooked or otherwise prepared: milk chocolate candy, iced tea, sugar cookie, grape jelly, soft jellied candy and powdered drink mix. Repeatability standard deviations ranged (rounded off) 3.9–9.0%; reproducibility standard deviations ranged 4.5–14.1%. The average recovery was 92.6% (81.1–102.8%). The method was adopted by AOAC International as AOAC Method 2000.11 (Craig, 2001; Documentation provided to EFSA n. 1).
3.1.5. Stability of the substance, and reaction and fate in food
The stability of polydextrose to processing and storage under several conditions was studied on model 5% w/w polydextrose solutions by Beer et al. (1991). Heat treatments at 70, 85 and 100°C were applied for up to 5 h on solutions in the pH range 3.0–6.0; samples were incubated in duplicates. After heat treatment, samples were stored at –20, 5, 20 and 40°C for up to 30 weeks. At regular intervals during storage, polydextrose was determined by high‐performance liquid chromatography (HPLC); the free glucose content was measured enzymatically (hexokinase/glucose‐6‐phosphate dehydrogenase method). Polydextrose remained stable to all treatments and storage conditions in the pH range 4.5–6.0. Under more acidic conditions (pH 3.0–4.0), an increase in free glucose and a decrease in polydextrose content were observed after heat treatments at 85 and 100°C and using storage temperatures of 20–40°C. These observations were confirmed by gel filtration where marked shifts in the molecular weight distribution of the polydextrose solutions were noticeable.
Stowell (2009) described the change in weight of a 70% w/w polydextrose solution over a wide range of temperatures. It was seen that over the range 20–160°C moisture was lost from the solution as would be expected and it was not until 260°C that gross changes began to occur. Similarly, polydextrose powder was heated over the same temperature range: the substance remained stable until approximately 300°C, when it began to melt and decompose. The predominant α‐1,6‐glycosidic linkages in polydextrose were acknowledged to be more than two to four times as resistant to hydrolysis than α‐1,2, α‐1,3 or α‐1,4 bonds, and in general, polydextrose appeared to be quite more stable at low pH (2.6), high temperature (100°C), and incubation time up to 5 h, than linear and regular polymers such as fructo‐oligosaccharides.
Results from 5 years stability studies for three grades of polydextrose powder, carried out at 25 and 37°C, were reported by the interested party (Documentation provided to EFSA n. 1). The results after 5 years showed that polydextrose degradation was between 0% and 3%. Additional analysis of powdered polydextrose stored for 39 months in typical warehouse conditions showed that the water specification was not exceeded within 3 years of storage in commercial packaging.
3.2. Authorised uses and use levels
Maximum levels of polydextrose (E 1200) have been defined in Part E of Annex II to Regulation (EC) No 1333/200812 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, polydextrose (E 1200) is an authorised food additive in the EU at quantum satis (QS) in three food categories as set by Part E of Annex II to Regulation (EC) No 1333/2008 displayed in Table 2.
Table 2.
MPLs of polydextrose (E 1200) in foods according to the Annex II to Regulation (EC) No 1333/2008
| Food category number | Food category name | E‐number/group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
|---|---|---|---|---|
| 11.4.2 | Table‐top sweeteners in powder form | E 1200 | Quantum satis | |
| 11.4.3 | Table‐top sweeteners in tablets | E 1200 | Quantum satis | |
| 14.2.1 | Beer and malt beverages | E 1200 | Only energy‐reduced and low‐alcohol beers | Quantum satis |
MPL: maximum permitted level.
The use of polydextrose (E 1200) is also authorised according to Annex III, Part 1, 3 and 5 of Regulation (EC) No 1333/2008:
Part 1: as a carrier in food additives with a maximum level of QS;
Part 3: as a food additive in food enzymes with a maximum level in the products (beverages or not) at QS;
Part 5, Section A: for use as a food additive in nutrients except nutrients intended to be used in foodstuffs for infants and young children with a maximum level in these products at QS.
3.3. Exposure data
3.3.1. Reported use levels or data on analytical levels of polydextrose (E 1200)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment, especially for those food additives for which no MPL is set and which are authorised according to QS.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re‐evaluation of approved food additives, EFSA issued a public call13 for occurrence data (usage level and/or concentration data) on polydextrose (E 1200). In response to this public call, industry provided information on the actual use levels of polydextrose (E 1200) in foods. No analytical data on the concentration of polydextrose (E 1200) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 208) of polydextrose (E 1200) in foods for 34 categories. Of these 34 food categories, only one use level was submitted for a food category in which the use of polydextrose (E 1200) is authorised according to Part E of Annex II of Regulation (EC) No 1333/2008 authorisation of polydextrose (E 1200), i.e. food category 11.4.2 Table‐Top Sweeteners in powder form. The remaining use levels were related to its authorised use according to the Annex III of Regulation (EC) No 1333/2008 (section 3.2).
Use levels were provided by DuPont Nutrition & Health (Documentation provided to EFSA n. 20), the Association of the European Self‐Medication Industry (AESGP) (Documentation provided to EFSA n. 21), FoodDrinkEurope (FDE) (Documentation provided to EFSA n. 22), Food Supplement Europe (FSE) (Documentation provided to EFSA n. 23), the International Chewing Gum Association (ICGA) (Documentation provided to EFSA n. 24), L'Alliance 7 (Documentation provided to EFSA n. 25), Specialised Nutrition Europe (SNE) (Documentation provided to EFSA n. 26) and Tate & Lyle (Documentation provided to EFSA n. 27).
The Panel noted that there was reference to two niche products, one use level for a particular chewing gum and one for a particular dietary food for special medical purposes. Both levels were used in the exposure assessment as no other data were available for either food category.
The Panel noted that some data providers (namely DuPont Nutrition & Health and Tate & Lyle) are not food industry users of polydextrose but food additive producers. Use levels reported by food additive producers are not considered at the same level as those provided by food industry. Food additive producers might recommend use levels to the food industry, but the final levels might ultimately be different. Therefore, unless food additive producers confirm that the recommended levels are used by food industry, they are not considered in the refined exposure scenario. Data from food additive producers will only be used in the maximum level exposure assessment scenario in case of QS authorisation when no data are available from food industry. In this way, the most complete exposure estimates are calculated. In the assessment of polydextrose (E 1200), the data from food additives producers were not used in the refined exposure scenario.
Appendix A provides data on the use levels of polydextrose (E 1200) in foods as reported by industry.
3.3.2. Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 3.4 million food and beverage products of which almost 1,300,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 member countries, Norway and UK presented in the Mintel's GNPD.14
For the purpose of this Scientific Opinion, the Mintel's GNPD15 was used for checking the labelling of food and beverages products and food supplements for polydextrose (E 1200) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, between January 2015 and October 2020, polydextrose (E 1200) was found to be labelled on 1,773 foods, including snack, cereal and energy bars, medicated confectionery, sweet biscuits/cookies and vitamins and dietary supplements.
Appendix B lists the percentage of the food products labelled with polydextrose (E 1200) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification in which at least one food was labelled with polydextrose (E 1200). The percentages ranged from less than 0.1% in many food subcategories (e.g. beer) to 7.8% in the Mintel's GNPD food subcategory ‘Snack/Cereal/Energy Bars’. Overall, polydextrose (E 1200) was found to be labelled on 0.7% of products within the GNPD considering the subcategories in which at least one food was labelled with polydextrose (E 1200).
3.3.3. Food consumption data used for exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a). Consumption surveys added in the Comprehensive database in 2020 were also taken into account in this assessment.16
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons may not be appropriate. Depending on the food category and the level of detail used for the exposure calculations, uncertainties could be introduced owing to possible subjects’ underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 40 different dietary surveys carried out in 23 European countries (Table 3).
Table 3.
Population groups considered for the exposure estimates of polydextrose (E 1200)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, UK |
| Toddlersa | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain, UK |
| Childrenb | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Portugal, Spain, Sweden, UK |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain, Sweden, UK |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden, UK |
| The elderlyb | From 65 years of age and older | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Spain, Sweden, UK |
The term ‘toddlers’ in the EFSA Comprehensive Database (EFSA, 2011a) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in the Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’ (EFSA, 2011a).
Consumption records were codified according to the FoodEx classification system (EFSA, 2011b). Nomenclature from the FoodEx classification system has been linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform exposure assessments. In practice, the FoodEx food codes were matched to the FCS food categories.
Food categories considered for the exposure assessment of polydextrose (E 1200)
The food categories for which use levels were reported were selected from the nomenclature of the EFSA Comprehensive Database (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011b).
Three food categories (i.e. 01.7.6 Cheese products, 02.3 Vegetable oil pan spray and 06.6 Batters) are not referenced in the EFSA Comprehensive Database and could therefore not be taken into account in the present estimate; this may have resulted in an underestimation of the exposure.
Data were also submitted for FC 13.2, 13.3 and 13.4. Food items belonging to these food categories, consumed by children, adolescents, adults and the elderly, may be very diverse and, in addition, the Comprehensive Database has only very limited information on their consumption. Therefore, eating occasions belonging to these food categories were reclassified under food categories in accordance to their main component. For this reason, the use levels available for food categories 13.2, 13.3 and 13.4 were not considered in the exposure assessment.
For the maximum level scenario, which considered recommended use level data as well as the actual use level data reported by food industry, a total of 28 food categories were taken into account, which can be found in Appendix C.
For the refined scenario, 21 food categories were not taken into account because no actual use level data were provided for these food categories (Appendix A). In the refined exposure scenario, seven food categories were taken into account, which can be found in Appendix C.
Use level data were also provided for FC 17, Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. Recommended use level data were provided by DuPont Nutrition and Health and Tate and Lyle (Documentation provided to EFSA n. 20 and 27, respectively). Actual use level data were provided by FSE and AESGP. The actual use levels were taken into account for the food supplement consumers only scenario.
No use levels were provided for two food categories in which polydextrose (E 1200) is authorised according to Part E of Annex II of Regulation (EC) No 1333/2008, i.e. 11.4.3 Table‐top sweeteners in tablets and 14.2.1 Beer and malt beverages. Table‐top sweeteners in tablets were attributed the level provided for Table‐top sweeteners in powder. Foods belonging to FC 14.2.1 Beer and malt beverages were not considered in any scenario.
3.4. Exposure estimates
3.4.1. Exposure to polydextrose (E 1200)
The Panel estimated the chronic dietary exposure to polydextrose (E 1200) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. Dietary exposure to polydextrose (E 1200) was calculated by multiplying concentrations of polydextrose (E 1200) per food category (Appendix C) with their respective consumption amount per kilogram body weight for each individual in the Comprehensive Database. The exposure per food category was subsequently added to derive an individual total exposure per day. These exposure estimates were averaged over the number of survey days, resulting in an individual average exposure per day for the survey period. Dietary surveys with only 1 day per subject were excluded as they are considered as not adequate to assess repeated exposure.
This was carried out for all individuals per survey and per population group, resulting in distributions of individual exposure per survey and population group (Table 3). On the basis of these distributions, the mean and 95th percentile of exposure were calculated per survey and per population group. The 95th percentile of exposure was only calculated for those population groups with a sufficiently large sample size (EFSA, 2011a). Therefore, in the present assessment, the 95th percentile of exposure for infants from France and Italy, for toddlers from Belgium and Italy and for adolescents from Estonia was not estimated.
Exposure assessment to polydextrose (E 1200) was carried out by the FAF Panel based on two types of concentration data: 1) maximum levels of recommended and actual use levels provided to EFSA (defined as the maximum level exposure assessment scenario); and 2) actual use levels (defined as the refined exposure assessment scenario). These two scenarios are discussed in detail below.
These scenarios do not consider the consumption of food supplements which is covered in an additional scenario detailed below (food supplements consumers only scenario).
There is a possible additional exposure from the use of polydextrose (E 1200) from its authorised uses in accordance with Annex III to Regulation (EC) No 1333/2008 as a carrier in food additives, an additive in food enzymes and food flavourings and also as a food additive in nutrients except nutrients intended to be used in foodstuffs for infants and young children with a maximum level in these products at QS. These uses could only be considered for the seven food categories in the refined exposure assessment scenario as no concentration data were available for the remaining food categories, for which only recommended use levels from food additive producers were provided.
Maximum level exposure assessment scenario
The regulatory maximum level exposure assessment scenario is based on the MPLs as set in Part E of Annex II to Regulation (EC) No 1333/2008. As polydextrose (E 1200) is authorised according to QS in all food categories, a ‘maximum level exposure assessment’ scenario was estimated based on the maximum reported use levels provided by industry (food industry and food additive producers), excluding exposure via food supplements, as described in the EFSA Conceptual framework (EFSA ANS Panel, 2014). This exposure scenario can consider only food categories for which these data were available to the Panel. This exposure assessment included authorised use of polydextrose (E 1200) according to both Annex II and III to Regulation (EC) No 1333/2008.
The Panel considers the exposure estimates derived following this scenario as the most conservative since it is assumed that the population will be exposed to the food additive present in food at the maximum reported use levels over a longer period of time.
Refined exposure assessment scenario
The refined exposure assessment scenario is based on use levels reported by food industry. This exposure scenario can consider only food categories for which these data were available to the Panel.
Appendix C summarises the concentration levels of polydextrose (E 1200) used in the refined exposure assessment scenario. Based on the available data set, the Panel calculated two refined exposure estimates based on two model populations:
-
The brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to polydextrose (E 1200) present at the maximum reported use level for one food category. This exposure estimate is calculated as follows:
-
‐
Combining food consumption with the maximum of the reported use levels for the main contributing food category at the individual level.
-
‐
Using the mean of the typical reported use levels for the remaining food categories.
-
‐
The non‐brand‐loyal consumer scenario: It was assumed that a consumer is exposed long‐term to polydextrose (E 1200) present at the mean reported use level. This exposure estimate is calculated using the mean of the typical reported use levels for all food categories.
‘Food supplement consumers only’ scenario
Polydextrose (E 1200) is authorised in the FC 17 Food supplements as defined in Directive 2002/46/EC excluding food supplements for infants and young children. As exposure via food supplements may deviate largely from that via food, and the number of food supplement users may be low depending on populations and surveys, an additional refined scenario was calculated in order to reflect additional exposure to food additives from food supplements compared to exposure to food additives excluding these sources. This additional scenario was estimated assuming that consumers of food supplements are exposed to polydextrose (E 1200) present at the maximum reported use levels in food supplements on a daily basis. For the remaining seven food categories, the mean of the typical reported use levels was used.
As FC 17 does not consider food supplements for infants and toddlers as defined in the legislation, exposure to polydextrose (E 1200) from food supplements was not estimated for these two population groups.
Dietary exposure to polydextrose (E 1200)
Table 4 summarises the estimated exposure to polydextrose (E 1200) from its use as a food additive in six population age groups (Table 3) according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix D.
Table 4.
Summary of dietary exposure to polydextrose (E 1200) from its use as a food additive in the maximum level exposure assessment scenario and in the refined exposure scenarios, in six population groups (minimum–maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks–11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Maximum level exposure assessment scenario | ||||||
| • Mean | 410‐3954 | 1190‐5185 | 1398‐3329 | 708‐1743 | 404‐971 | 309‐994 |
| • 95th percentile | 1073‐7873 | 2384‐8002 | 2411‐5733 | 1506‐3433 | 911‐2105 | 732‐2104 |
| Refined estimated exposure assessment scenario | ||||||
| Brand‐loyal scenario | ||||||
| • Mean | 0.2‐109 | 20‐670 | 111‐525 | 78‐327 | 35‐144 | 21‐101 |
| • 95th percentile | 0‐498 | 90‐1179 | 339‐1059 | 270‐708 | 124‐427 | 77‐264 |
| Non‐brand‐loyal scenario | ||||||
| • Mean | 0.2‐48 | 13‐352 | 70‐276 | 37‐163 | 17‐71 | 10‐48 |
| • 95th percentile | 0‐215 | 70‐590 | 196‐546 | 115‐364 | 59‐192 | 36‐117 |
| Food supplement consumers only scenario a | ||||||
| • Mean | – | – | 47‐275 | 36‐173 | 19‐67 | 11‐48 |
| • 95th percentile | – | – | 214‐548 | 118‐400 | 67‐181 | 49‐121 |
bw: body weight.
This scenario concerns consumers only, i.e. only individuals who have reported the consumption of food supplements on at least one day within the survey.
In the maximum level exposure assessment scenario, mean exposure to polydextrose (E 1200) from its use as a food additive ranged from 309 mg/kg bw per day in the elderly to 5,185 mg/kg bw per day in toddlers. The 95th percentile of exposure to polydextrose (E 1200) ranged from 732 mg/kg bw per day in the elderly to 8,002 mg/kg bw per day in toddlers.
In the refined estimated exposure scenario considering actual use levels provided for authorised use according to Annex II and III of Regulation (EC) No 1333/2008, mean exposure to polydextrose (E 1200) from its use as a food additive ranged from 0.2 mg kg bw per day in infants to 670 mg/kg bw per day in toddlers in the brand‐loyal scenario. The 95th percentile of exposure to polydextrose (E 1200) ranged from 0 mg/kg bw per day in infants to 1179 mg/kg bw per day in toddlers. In the non‐brand‐loyal scenario, mean exposure to polydextrose (E 1200) from its use as a food additive ranged from 0.2 mg/kg bw per day in infants to 352 mg/kg bw per day in toddlers. The 95th percentile of exposure to polydextrose (E 1200) ranged from 0 mg/kg bw per day in infants to 590 mg/kg bw per day in toddlers.
In the food supplement consumers only scenario, mean exposure to polydextrose (E 1200) from its use as a food additive in food supplements ranged from 11 mg/kg bw per day in the elderly to 275 mg/kg bw per day in children. The 95th percentile of exposure to polydextrose (E 1200) ranged from 49 mg/kg bw per day in the elderly to 548 mg/kg bw per day in children. Due to an insufficient sample size in certain surveys, the 95th percentile of exposure could not be calculated for children in Cyprus, Germany (VELS study), Italy and Portugal, for adolescents in Cyprus, Germany, Estonia, Italy, Latvia, Portugal and the United Kingdom, for adults in Cyprus, Croatia and Romania and for the elderly in Cyprus, Spain, Italy, Latvia and Slovenia.
Main food categories contributing to exposure for the general population
Main food categories contributing to exposure to polydextrose (E 1200) for the maximum level exposure assessment scenario
From the maximum level exposure assessment scenario, the main contributing food categories to the total mean exposure estimates were
breakfast cereals and processed cereal‐based foods and baby foods for infants and young children as defined by Directive 2006/125/EC for infants and toddlers;
fine bakery wares and flavoured drinks for all population groups except infants;
fruit and vegetable juices for children and adolescents, and
bread and rolls for adults and the elderly.
Main food categories contributing to exposure to polydextrose (E 1200) for the refined exposure assessment scenario
Important food categories that contributed to the exposure to polydextrose (E 1200) for the brand‐loyal scenario were fine bakery wares and flavoured drinks in all population groups. Additional food categories contributing significantly to the exposure in this scenario were edible ices for infants, toddlers, children and adolescents, and confectionary for children and adolescents. In the non‐brand‐loyal scenario, fine bakery wares and flavoured drinks contributed again largely to the exposure to polydextrose (E 1200) in all population groups. Edible ices contributed to the exposure for infants, toddlers, children and adolescents, and desserts for infants and the elderly.
Uncertainty analysis
Uncertainties in the exposure assessment of polydextrose (E 1200) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 5.
Table 5.
Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
| Sources of uncertainties | Directiona |
|---|---|
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/– |
| Methodology used to estimate high percentiles (95th) long‐term (chronic) exposure based on data from food consumption surveys covering only a few days | + |
| Correspondence of reported use levels and analytical data to the food items in the EFSA Comprehensive Food Consumption Database: uncertainties to which types of food the levels refer to | +/– |
| Uncertainty in possible national differences in use levels of food categories | +/– |
Concentration data:
|
+ |
| Food categories included in the exposure assessment: exclusion of food categories due to missing FoodEx linkage (n = 3 food categories) | – |
| Food categories included in the exposure assessment: no data for two food categories of which one was not considered in the exposure estimates | – |
| Foods which may contain the food additive according to Annex III to Regulation (EC) No 1333/2008 partially taken into account | – |
Maximum level exposure assessment scenario:
|
+ + |
Refined exposure assessment scenarios:
|
+/– |
+, uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
Polydextrose (E 1200) is authorised in three food categories according to Part E of Annex II to Regulation (EC) No 1333/2008 (Table 2); data were received for one of these FCs (FC 11.4.2 Table‐top sweeteners in powder, which level was attributed also to FC 11.4.3 Table‐top sweeteners in tablets). Polydextrose (E 1200) is also authorised according to Annex III and data were received from 31 different food categories.
The Panel also noted that information from the Mintel's GNPD (Appendix C) indicated that 59 food subcategories, categorised according to the Mintel's GNPD nomenclature, were labelled with polydextrose (E 1200). Data made available to EFSA covered most of these Mintel's subcategories, and represented around 80% of the food products labelled with polydextrose (E 1200) in the database. Furthermore, the percentage of foods per subcategory labelled to contain polydextrose (E 1200) was maximally about 7.8% (Appendix B), while in the assessment it was assumed that 100% of the foods belonging to an authorised food category contained the additive.
Polydextrose (E 1200) is used as a bulking agent and does not influence the organoleptic properties of the final food. For this reason, the Panel considered the non‐brand‐loyal scenario as the most appropriate scenario for risk characterisation.
Labelling of food additives from their uses according to Annex III to Regulation (EC) No 1333/2008 is not mandatory, therefore all possible uses of polydextrose (E 1200) may have not been considered. The Panel included the food categories which may contain polydextrose (E 1200) due to carry‐over (Annex III, Part 1, 3, 5) in the current exposure assessment when data were provided. Information from the Mintel's GNPD indicates that the majority of the food categories in which polydextrose (E 1200) is labelled are considered.
In principle, the calculated exposure to the food additive E 1200 was considered to be overestimated based on the concentration data used and the methodology applied. However, all uses of the food additive, among those authorised in accordance with Annex III of Regulation (EC) No 1333/2008, may not necessarily be covered by the occurrence levels and therefore could not be taken into account in the estimated exposure. The Panel acknowledges that these incomplete data may have an influence on the direction of the uncertainty.
3.5. Biological and toxicological data
This section summarises the biological and toxicological studies for which the original reports have been provided by the interested party (Documentation provided to EFSA n. 2). Most data were related to polydextrose‐A. Additional studies have been also identified in the open literature.
3.5.1. Absorption, distribution, metabolism and excretion
In vitro studies
In vitro digestion studies using mammalian enzymes
In vitro hydrolysis of polydextrose by small intestinal mucosal homogenates (Oku et al., 1991), jejunal mucosal scrapings (Ziese et al., 1995) or jejunal mucosal homogenates (Kruger et al., 1990) from rats demonstrated that glucose can be liberated from polydextrose. The yield of free glucose was approximately 6–8%. Another in vitro study demonstrated that pancreatic amylase is capable of cleaving the oligosaccharides in polydextrose (Richter et al., 1994).
In vitro small intestinal resistance of polydextrose was determined by using an enzyme‐HPLC method capable of analysing non‐digestible oligosaccharides with low molecular weights (that do not precipitate in 78% ethanol treatment) or by an artificial digestion using pancreatic α‐amylase and small intestinal brush‐boarder membrane vesicles prepared with the use of the rat small intestine (Kondo et al., 2017). The indigestible content of polydextrose in the small intestine measured with the use of the artificial digestion method was lower (67%) than that measured by the AOAC method 2001.03 (80%).
In vitro fermentation studies using human faecal bacteria
When faecal homogenates from eight human volunteers were fermented after addition of polydextrose the fermentation efficiency of polydextrose was estimated to an average of 24.8% based on the production of hydrogen gas (Solomons and Rosenthal, 1985).
The digestibility of several soluble carbohydrates was studied using human faeces as a source of faecal inoculum (Mazur et al., 1993). Polydextrose was reported to be slowly fermented and the gas production in this process was assessed as minimal by the authors.
The fermentation of polydextrose was studied in in vitro human colon model (Mäkivuokko et al., 2005; Mäkelainen et al., 2007, 2010). When the colon model was exposed to 1% or 2% polydextrose, the amount of degraded polydextrose increased in the vessels modelling proximal colon towards distal colon. The amount of intact polydextrose decreased from vessels modelling proximal colon towards distal colon.
In another study of microbial degradation of polydextrose in an in vitro model of human colon, it was demonstrated that after fermentation the relative abundance of non‐branched molecules was higher and of single‐branched molecules was lower in comparison to these in the intact polydextrose (Lahtinen et al., 2010). The form of glucose moieties (pyranose vs furanose) appeared important for the rate of microbial degradation.
Overall, in vitro studies of human colon indicated that less than 10% of polydextrose can be degraded to glucose.
In vivo studies
Several studies in rats were performed by Figdor and Rennhard (1981). The studies are summarised below.
Laboratory rats (N = 3/group, strain and sex not specified, body weight 125–200 g) received a single dose of [14C]polydextrose by gavage or by intravenous (i.v.) injection. The oral dose was 55 mg/kg bw of [14C]polydextrose (12.6 μCi) in water solution. The i.v. doses were 25 mg/kg bw (13.8 μCi) or 50 mg/kg bw (13.4 μCi). Exhaled CO2 was collected at hourly intervals for the first 13 h and afterwards in the interval 13–24 h (only in the i.v. part of the study). Urine and faeces were collected for up to 72 h post‐exposure. Following oral administration about 60% of administered radioactivity was recovered from faeces, 20% as exhaled 14CO2 and less than 2% in the urine. The majority of urinary excreted radioactivity was in the urea. No more than 0.24% of the oral dose secreted in the urine was the unchanged polydextrose. According to the authors, this indicated that polydextrose was not absorbed from the gut. Following i.v. administration, about 90% (from 80% to 104%) of administered radioactivity was excreted in urine, about 4.4% (from 0.5% to 10.5%) in the faeces and 1.16% (1.05% to 1.37%) as exhaled 14CO2.
Metabolism of polydextrose by intestinal microbiota was studied in male rats (body weight of 150 to 200 g, number/group and strain not informed) given by gavage 28 μCi of [14C]polydextrose and faeces were collected during the 24 h post‐treatment. One of these rats was killed 5 h after dosing and caecal content was collected. In the faeces, 2.5% of the dose of polydextrose was identified as volatile fatty acids (VFAs, acetic, n‐butyric and propionic acids). In the caecum, 13% of the administered was identified as VFAs. According to the authors, their study confirmed that polydextrose was fermented by microbiota to VFAs in the lower part of intestinal tract.
Metabolic fate of polydextrose was studied in rats after subchronic dietary exposure. Male rats (total number/group and strain not specified, no specific information on body weight at the start of the treatment) received a basal diet (controls) or this diet with polydextrose providing daily doses of 1 or 10 g/kg bw per day for 90 days. At the end of the treatment 2 rats from the control and two treated groups (body weight 460–650 g) received by gavage 14.7 mg of [14C]polydextrose (12.6 μCi) in water solution (total radioactivity per dose 36.7 μCi). Exhaled CO2 was collected at hourly intervals for the first 13 h and urine and faeces were collected at 24 h intervals for up to 72 h post‐exposure. The average percentage of the administered dose exhaled as 14CO2 was similar in all groups: 18.7%, 19.6% and 18.5% in the control, low‐ and high‐dose groups, respectively. The highest exhalation of 14CO2 was in the interval from 6 to 10 h post‐exposure. Urinary recovery of 14C was similar in all groups: on the average 1–2% of the administered dose. Faecal recovery in the control and low‐dose groups was similar, averaging approximately 56% and 52% of the dose, while it was 44% on the average in the high‐dose group. The lower value in this group as compared to control and low‐dose groups was ascribed by the authors to very soft consistence of the faeces, which affected the faeces collection. According to the authors, no induction of microbial metabolism was observed. The Panel noted that the repeated ingestion of polydextrose did not influence the pattern of excretion reported in studies using a single dose of the compound.
Male Wistar rats (N = 4, body weight 100–120 g) received either a control diet containing 100 g maize starch/kg diet, or a diet in which 1/3 of the maize starch was replaced by polydextrose for 7 days (Cooley and Livesey, 1987). Thereafter, all animals received by gavage 1.02 g of [14C]polydextrose (51.3 μCi), were placed in metabolism cages and kept on the diet in which half of maize starch was replaced by polydextrose. Exhaled CO2, urine and faeces were collected for 72 h. Less than 5% of the radiolabel was excreted in the urine, while 48% of radioactivity was detected in faeces. Approximately 33% of the administered radioactivity was found as exhaled 14CO2.
Male Wistar rats (N = 6) received by gavage a single dose of 300 mg polydextrose/kg bw with a phenol red marker (0.8% w/v) (Kruger et al., 1990). One hour after the administration the GI system was excised for analysis of polydextrose content in different GI parts. About 53% of the administered polydextrose was recovered in the small intestine. The relative absorption of polydextrose in the small intestine was calculated to 41% when relating polydextrose recovery to the amount of marker recovered. None of polydextrose was detected in large intestine.
In order to investigate the effect of non‐caecal fermentation of polydextrose intact or caecectomised germfree and conventional male Wistar rats (N = 8/group of non‐caecetomised rats, N = 2/group for caecectomised rats, 100–120 days old) were fed 1% polydextrose in a basal diet for 2 weeks (Juhr and Franke, 1992). Thereafter all rats received by gavage [14C]polydextrose (359 kBq, 17.9 kBq/mg), were placed in metabolic chambers for 30 h and expired air, faeces and urine were collected. In all four groups, excretion of the radiolabel from the administered dose varied from 54% to 81% in faeces and from 12% to 32% in the exhaled air. Urinary excretion accounted for 5% of the radiolabel. Germfree rats excreted ca. 20% more of the dose in the faeces compared to conventional rats. In germfree rats, there was no difference in exhaled radiolabel between the intact and caecectomised animals. Based on the difference in exhaled radiolabel between the intact and caecectomised conventional rat, the caecum was found to be an important site for microbial fermentation of polydextrose.
A commercial product polydextrose was analysed before and after intestinal degradation in rats (Radosta et al., 1992). Polydextrose contained about 32% oligomers with a degree of polymerisation of up to four. Analysis of undigested residue collected from different parts of the small intestine 1, 2 and 3 h after of a single dose of 1,200 mg/kg bw by gavage to rats (strain and number of animals not reported) was, according to the authors, ‘not very different’ from the starting material. According to the authors, the mixture of oligomer and polymer molecules is degraded, probably without special preference of any region.
The size and extend of intestinal breakdown of polydextrose was investigated in male Sprague–Dawley rats (Kondo et al., 2017). For this purpose, the ileorectostomised rats (5 weeks old at the start of the study, n = 6/group) were fed either a control diet with 5% cellulose or this control diet added 5% polydextrose. The supplementation of cellulose and polydextrose was done by replacing an equal amount of corn starch in the control diet. The feeding continued for 9 days followed by a 3‐day washout period, and a subsequent re‐division of the rats in two groups (n = 6/group) given 0.1% neomycin in drinking water to remove small intestinal microbiota and kept on either the control diet with 5% cellulose or this control diet added 5% polydextrose for 10 days. In addition, two groups of intact male rats (Sprague–Dawley, 8 weeks old at the start of the study, n = 6/group) were fed the same control and the polydextrose added diets for 4 weeks. Faeces from the ileorectostomised rats were collected in the last 3 days of each feeding period and on days 8‐10 from the intact rats. In ileorectostomised rats, the faecal content of polydextrose (1.61 g/3 days and 1.64 g/3 days) and the faecal recovery of sugars with a degree of polymerisation ≥ 3 (57.5% and 61.3%) were comparable regardless removal of microbiota by neomycin. In intact rats, the whole gut faecal content of polydextrose was 0.79 g/3 days and the faecal recovery of sugars with a degree of polymerisation ≥ 3 was 33.1%.
A study of fermentation of polydextrose in pigs (4 females and 6 castrated males/group of different races) fed either a control diet or this diet supplemented with 15 g polydextrose/animal twice daily for 21 days (Fava et al., 2007). The concentration of polydextrose was reported to decrease at even rate from approximately 53 mg/g dry matter. This according to the authors indicated an even fermentation of polydextrose through the intestinal tract and that polydextrose was still present in the distal colon.
Human studies
Healthy volunteers (4 men, no information on body weight or age) ingested 10 g daily of polydextrose in chocolate milk drink for 10 days. On day 8, the 10 g‐dose of polydextrose contained [14C]polydextrose (72 μCi) (Figdor and Bianchine, 1983). Expired air, urine and faeces were collected at intervals for the next 6 days. Percentages of administered radioactivity excreted were 15–22% in the breath, 1.4% in the urine and 50% in the faeces. About 48% of the administered dose was excreted within 48 h in the faeces. In the urine only 0.03% was accounted to be intact polydextrose while the remaining activity was accounted by urea and VFAs inferred to be produced from intestinal fermentation of polydextrose. According to the authors, the results indicated that metabolism in humans and in rats are similar.
Seven healthy men (age 27 ± 2 years) participated in a study divided in three periods: a control (CP) from the start to day 8, an ‘acute’ (PD1) from day 9 to day 16 and a ‘chronic’ (PD2) from day 17 to 38 (Achour et al., 1994). The daily dose of polydextrose was 30 g/person (10 g in a fruit juice three times per day at mealtimes). On days 13 and 35 [14C]polydextrose (20 μCi) was added to the breakfast dose. Collection of urine and faeces was done on days 5–8, 13–16 and 35–38. Expired air was collected on days 5, 13 and 35 at intervals up to 48 h. GI transit time was measured on days 5, 13 and 35. The percentage of radioactivity recovered from faeces was ca. 36%, from the breath about 30% and from urine about 4% in PD1 or PD2. GI transit time in PD1 (47 h ± 7) and in PD2 (46 h ± 10) were similar to that in the control period (44 h ± 6).
In addition, two unpublished polydextrose label distribution studies in man were reported in a review (Auerbach et al., 2007). The original reports of these two human studies were not available to the Panel. Therefore, only the information provided in the review (Auerbach et al., 2007) is presented below.
Three persons (sex, age, health status not reported) received daily 20 g polydextrose for 12 days followed by an oral bolus administration of a dose of a stable isotope 13C‐labelled polydextrose (no further details available) and collection of excreta for 48 h. 42% of the label was recovered in faeces, 36% in CO2 and 1.4 in urine (Mc Gaw, 1991; unpublished data reported in Auerbach et al., 2007).
Eight persons (sex, age, health status not reported) received daily 30 g polydextrose for 3 days followed by an oral bolus 50 μCi 14C‐labelled polydextrose (no further details available) and collection of faeces for 7 days. Thirty‐three per cent of the label was recovered in faeces (Pierce, 1991; unpublished data reported in Auerbach et al., 2007).
Overall, the metabolism of polydextrose is similar in rats and humans. In humans, the reported recovery of radioactivity was 33–50% in faeces, 15–36% in breath and 1.4% and 4% in the urine. Polydextrose is partially fermented in the large intestine into SCFA.
3.5.2. Acute toxicity
Acute oral toxicity of polydextrose (polydextrose‐A or polydextrose‐N) was studied in three species of laboratory animals (Burdock and Flamm, 1999). LD50 was reported to be above 18,000 mg/kg bw in rats, above 20,000 mg/kg bw in dogs and above 30,000 mg/kg bw in mice, these being the highest doses tested. At the highest doses tested, all animals had soft stools or diarrhoea shortly after administration but these effects were not present after 24 h.
3.5.3. Short‐term and subchronic toxicity
Rats
In a study investigating digestibility of digestion‐resistant dextrin derivatives (DRDDs) Sprague–Dawley rats (N = 6/group, males, body weight: 275–284 g) were fed a control diet or this diet added 50 g/kg (equivalent to 5.540 mg/kg bw per day) of either polydextrose (not further specified whether it is N or A type) or two other test compounds for 4 weeks (Kondo et al., 2017). Ingestion of polydextrose had no effect on body weight gain, serum cholesterol and triglycerides levels or absolute liver weight. Caecal tissue and content weights were statistically significantly higher, and caecal total content of short‐chain fatty acids and pH were statistically significantly lower than in the control group. The absolute weight of epididymal fat pad and the relative weight of perirenal and retroperitoneal fat were statistically significantly lower than in the control group. Faecal dry matter excretion was statistically significantly higher than in the control group.
Sprague–Dawley rats (Charles River CD, France) (N = 10/sex per group) received in the diet 0, 1,000, 2,000 or 10,000 mg polydextrose (Polydextrose‐A)/kg bw per day for 92 days (Document provided to EFSA n. 8). Body weight and feed intake were recorded weekly. Ophthalmoscopic, haematology, clinical chemistry examinations and urinalysis were conducted prior to the treatment, during the study period and before the termination. Organ weights were recorded at necropsy. Histological examination was performed on four males and six females from each group. Body weight gain of low‐dose males and females was 14% and 19% less than that of the controls. Also body weight gain of high‐dose females was 17% lower than that in the controls. No differences between the treated groups and the controls were recorded for all parameters investigated and by histological examination.
In a study investigating toxicity of corn starch fibre, Sprague–Dawley rats (N = 10/sex per group) received a basal diet, basal diet with corn starch added at three dose levels and polydextrose (not further specified whether it was N or A type, 93.9% purity) as a reference compound, approximately 10,000 mg/kg bw per day for a minimum 91 days (Crincoli et al., 2016a,b). The dose level of polydextrose corresponded to the highest dose of corn starch. Body weight and feed intake were recorded weekly. Ophthalmoscopic examination was performed before the start and at the end of the treatment. Haematology and clinical chemistry examinations were performed on day 14, 45 and before the termination. Urinalysis was done on samples collected 24 h before the termination. All clinical pathology parameters and post‐mortem gross and microscopic findings indicated no differences among the groups given polydextrose, corn starch or the basal diet.
Dogs
Male beagle dogs (age: approximately 10 months) received daily feed without (N = 4, controls) or with polydextrose‐A at a level of 50% of the dry weight of the entire daily feed ration (N = 6) for 97 days (Document provided to EFSA n. 9). Body weights were recorded daily for the first 3 days and at weekly intervals thereafter. Water intake was monitored on days 1–3 and 6–9. Haematology, clinical chemistry examinations and urinalysis were conducted twice prior to the treatment, on several occasions during the study period and before the termination. Necropsies were performed on all dogs, organ weights recorded and tissue samples were taken for histological examination. All dogs survived to the end of the study. Body weight loss was recorded in all dogs on polydextrose containing diet (0.6–2.2 kg) and in three control dogs (0.5–1.8 kg). Dogs from the test group had loose, occasionally watery stools 3–6 h after feeding, and 2–2.5 times higher water intake compared with controls. Their urinary sodium excretion was statistically significantly lower than that of controls on days 43 and 71. Their urinary calcium excretion, although within the normal ranges, was greater than in controls (statistical significance not reported). Other clinical pathology parameters and clinical appearance, weights of liver, kidneys or testes and results of gross and histological examinations were not different between the control and polydextrose fed dogs.
Male beagle dogs (N = 4/group, age not informed) received daily feed without (controls) or with modified polydextrose‐N (potassium salt) at a level of 50% of the dry weight of the entire daily feed ration for 135 days (equivalent to 12,500 mg/kg bw per day) (Document provided to EFSA n. 10). This experiment was conducted to elucidate whether the nephrocalcinosis which developed in dogs exposed to high levels of polydextrose‐N (potassium salt) is the result of hypercalcaemia in combination with a potassium‐induced chronic watery diarrhoea. From day 136 to the termination on day 195, polydextrose ‐N was changed for modified polydextrose‐A (the acidic fusible potassium free polydextrose) in the diet. Clinical appearance was checked twice daily. Body weights were recorded daily until day 126 and weekly thereafter. Daily water intake was measured on day 9, days 13–17 and 174–177. Haematology, clinical chemistry examinations and urinalysis (with emphasis on careful monitoring of electrolytes) were conducted on several occasions. At termination, all dogs were subjected to necropsy, and tissue samples were taken for microscopic examination. All dogs survived until termination. All controls and one dog from the test group lost body weight during the study (range: 0.5–1.9 kg bw). The remaining three dogs from the test group gained between 0.2 and 1 kg bw during the study. The dogs kept on feed with polydextrose‐N for 135 day had two to eight times higher water intake than the controls and they had daily watery diarrhoea within 2–3 h after feeding. Their stool became less watery on the polydextrose‐A containing feed. The author attributed the reduction in diarrhoea to the absence of potassium in polydextrose‐A. Feeding either polydextrose‐N or polydextrose‐A had no influence on haematological parameters. Several clinical chemistry parameters of dogs ingesting polydextrose‐N were different from controls. Parameters demonstrating a rather consistent statistically significant increase in serum levels during the study were: calcium, transient postprandial potassium and mean sodium levels (although the differences in individual values were small and values were well within the normal ranges). Furthermore, irregularly increased serum levels were recorded for glucose and lactic dehydrogenase. Urinary sodium excretion during feeding of polydextrose‐N was lower than in controls (statistical significance not reported). Most of the above differences subsided on the feed with polydextrose‐A. Serum calcium levels decreased and urinary calcium and sodium concentrations increased after polydextrose‐N was changed with polydextrose‐A. Microscopical examination revealed calcium nephropathy in two of four treated dogs. According to the authors, feeding of dogs with polydextrose‐N at 50% dry matter of the diet caused a syndrome of gradually developing hypercalcaemia, which led to development of calcium nephropathy. Substitution of polydextrose‐N with polydextrose‐A reduced the diarrhoea and enabled the clinical chemistry parameters of dogs which were not yet clinically hypercalcaemic to return to normal. The Panel considered this finding on nephrocalcinosis at the high dose, as treatment‐related, but probably also due to the presence of high level of potassium in polydextrose‐N leading to hypercalcaemia. The Panel noted that this study was designed to support the hypothesis that the renal lesions observed in dogs resulted from hypercalcaemia induced by polydextrose‐N.
Fifteen male and 15 female beagle dogs (Marshall Farms), 9 months of age, were divided into three groups of 5 animals/sex per group and received 0, 16.7% or 33% polydextrose‐A in their diet (equal to 4,000 mg or 8,000 mg polydextrose‐A/kg bw per day, respectively) for 13 months (Documentation provided to EFSA, n. 11). All animals were observed at least twice daily, and body weights were determined weekly. Ophthalmology was performed prior to dosing, at 6 and 12 months and at termination of the study. Electrocardiography, blood pressure, heart and respiratory rate, and rectal temperature were measured from all animals twice prior to the start of the study, during the first week and at 3‐month interval, thereafter. Haematology, clinical chemistry and urinalysis were conducted prior to the start of the study and on days 2 (urinalysis only), 15, 29, 58, 85, 113, 141, 169, 204, 232, 260, 288, 330, 351, 379 and 407. All dogs were necropsied on days 414–416. Gross examination was conducted, and the weight of the kidneys, liver and testis were recorded. The following organs and tissues were collected for histopathological examination: kidneys, liver, heart, aorta, lungs, spleen, thyroid, adrenal, pituitary, gall bladder, thymus, mesenteric lymph nodes, pancreas, mandibular salivary glands, urinary bladder, mammary glands, skin, oesophagus, stomach, small and large intestines, testis, epididymides, prostate gland, ovaries, uterus, brain, spinal cord, eyes, sternum, femur with bone marrow and all grossly visible lesions.
No mortalities occurred during the study. Dogs receiving 33% polydextrose‐A in their diet had soft and occasionally watery stools within 3 h after feeding, at 6 h after feeding the stools were more watery. Occasionally dogs of the high dose group exhibited diarrhoea and were taken off the polydextrose‐A diet for a few days until stools were normal again. Dogs at 16.7% polydextrose‐A had loose stools, which were only occasionally watery. The high‐dose dogs drank about 1.5 times more water than the controls. ECG, blood pressure, heart rate, ophthalmology and haematology showed no treatment‐related changes. Urinary volume and pH were unaffected by polydextrose‐A treatment. Mean urinary calcium excretion of polydextrose‐fed female dogs at both dose levels was consistently greater than that of controls. Two high‐dose male dogs, which showed high serum calcium levels and low specific gravity of their urine, demonstrated macroscopic focal areas of pale discolouration of the renal cortex with occasional linear white streaks through the cortex and medulla. Microscopically, nephrocalcinosis was diagnosed in these dogs. Kidneys from all other animals were essentially normal. Histopathological examination of all other organs and tissues was unremarkable among all groups. The Panel considered that the nephrocalcinosis in the two males of the high‐dose group related to the high calcium levels in serum and urine was associated with to the very high level of polydextrose in the diet which resulted in a general salt imbalance.
Monkeys
Rhesus monkeys (N = 2/sex per group) received by gavage 1,000, 2,000 or 10,000 mg/kg bw per day polydextrose‐N, 7 days/week for 91 days (Documentation provided to EFSA, n. 12). The high dose was administered as two doses of 5,000 mg/kg bw twice daily. The controls received distilled water (the vehicle). Body weight was recorded weekly. Electrocardiography, ophthalmoscopic, haematology, clinical chemistry examinations and urinalysis were performed twice before the start of exposure and on three occasions (on day 29, 57 and 87) during the treatment. At necropsy several organs were weighed, and samples were taken for microscopy. One low‐dose male was sacrificed on day 43 due to reasons not related to the treatment (positive reaction to tuberculin). All other animals survived until the scheduled termination. Monkeys from all polydextrose‐N groups gained more weight than the controls. The body weight gain appeared to be dose related. Loose stools were rarely observed in low‐ and mid‐dose animals. In high‐dose animals, diarrhoea was seen daily throughout the treatment period. In this group, a decrease in serum calcium levels to the lower range was recorded and at necropsy a moderate dilatation of colon was observed. Histological examination revealed focal accumulation of macrophages containing haemosiderin in colonic mucosa. The Panel considered these alterations as a response to the laxative effect of the high dose of polydextrose.
Overall, the subchronic studies in rats or monkeys with oral doses of polydextrose up to 10,000 mg/kg bw per day or in dogs when the diet contained polydextrose‐A at 50% or 33% indicated no adverse effects on feed intake, clinical pathology, organ weights and histopathology. Decreases in body weight were reported in a 3‐month study in rats. Dogs receiving 50% polydextrose‐N (containing potassium salt) in their diet for 135 days developed nephrocalcinosis which was the result of hypercalcaemia in combination with a potassium‐induced chronic watery diarrhoea. In a 13‐month feeding study with polydextrose‐A (potassium free) in beagle dogs, 2 high‐dose males developed nephrocalcinosis, which indicated a general salt imbalance, resulting from excessive loss of water. Therefore, the Panel considered the nephrocalcinosis which developed in dogs given high doses of polydextrose as treatment‐related, but secondary effect to diarrhoea, and hence not relevant for the risk assessment. Monkeys receiving a high dose of polydextrose by gavage gained weight in a dose‐dependent manner and developed loose stool and/or diarrhoea.
3.5.4. Genotoxicity
Original study reports were not made available, but the detailed description of unpublished study reports on the genotoxicity testing in vitro and in vivo of two forms of polydextrose; an acidic form (polydextrose‐A) and a neutralised potassium salt (polydextrose‐N) were provided to the Panel by the interested party (Documents provided to EFSA n. 3) and are summarised below.
In vitro
The bacterial mutagenicity of two forms of polydextrose (reported as type A and type N) was tested in a spot test and a plate incorporation assay with Salmonella Typhimurium strains. The spot test was performed with strains C207, C340, C3076, D3052, G46, TA1535, TA1536, TA1537, TA1538 and TA1978. No mutagenic activity was observed with any of the strains. The plate incorporation assay was performed at a single concentration of 10 mg/plate without metabolic activation with strains TA1536, TA1537, TA1538 and CA340. Polydextrose (type N) was in strain TA1536 tested at a single concentration of 20 mg/plate. Neither of the two forms of polydextrose was positive. Both forms of polydextrose were tested in the mouse host‐mediated assay with S. Typhimurium strains G46, C207, C3076, C340 and TA1534. Neither of the two forms of polydextrose was mutagenic.
In vitro clastogenicity of polydextrose‐A and polydextrose‐N was tested with the chromosomal aberration assay in human lymphocytes. The lymphocytes were exposed to 500 and 1,000 μg/mL polydextrose‐A or polydextrose‐N for 24 h. No increase in chromosomal damage was observed in polydextrose exposed lymphocytes.
In vivo
The in vivo clastogenicity studies were performed with CD‐1 male mice (6–8 weeks old, 30–40 g; 5 mice per group) that were orally administered 2 g/kg bw polydextrose‐A or polydextrose‐N. The mice were sacrificed at 6‐, 12‐, 24‐, 48‐ or 72‐h post‐treatment. For prolonged exposure, the mice were administered 1 g/kg bw per day polydextrose‐A or polydextrose‐N for 7 days and sacrificed 24 h after the last dose. Three hours prior to sacrifice, each animal received colchicine (1 mg/kg bw, intraperitoneal (i.p.)). Chromosomal aberrations were determined in bone marrow. Polydextrose‐A and polydextrose‐N did not induce chromosomal aberrations.
Polydextrose‐N was tested in a dominant lethal assay in mice using a subacute dosing regimen in 8‐week‐old male CD‐1 mice. Male mice were administered orally a solution of polydextrose‐N in distilled water at a level of 1.0 g/kg bw per day for 7 days. Both, the control and treated groups contained 15 male mice, which were caged with three virgin females each on the 7th day of dosing. These females were replaced at 7‐day intervals for an 8‐week period. All females were autopsied 11 days after removal from the mating cages. The results showed that polydextrose at the applied exposure conditions did not induce dominant lethality.
In summary, polydextrose (E 1200) (polydextrose‐A and/or polydextrose‐N) did not show a genotoxic potential in limited bacterial reverse mutation assays, did not induce chromosomal aberrations in vitro in human lymphocytes and in vivo did not induce chromosomal aberrations in bone marrow and dominant lethal mutations in mice. The Panel noted that there are no structural alerts for genotoxicity.
Overall, the Panel noted that the studies were not conducted according to the current guidelines and were limited in their protocols. However, in the absence of any structural alerts for genotoxicity, the Panel considered the results acceptable in the overall weight of evidence evaluation and considered that the available data do not indicate a genotoxic activity of polydextrose.
3.5.5. Chronic toxicity and carcinogenicity
Mice
Mice CD‐1 mice (50/sex per group; Charles River, France) were treated from weaning for 18 months with 5% or 10% polydextrose‐A in a commercial powdered diet (equivalent to 7,500 or 15,000 mg polydextrose‐A/kg bw per day) (Documents provided to EFSA n. 13). A reference control group received the same diet with 10% sucrose and the control group received the basal diet only. Body weights were recorded weekly. Food consumption was determined regularly during each month until day 365 and once every 8 weeks, thereafter. Clinical observations were recorded weekly. Ophthalmology was performed on controls, sucrose and 10% polydextrose‐A groups on days 0, 365 and 548 and on all surviving animals. Blood samples for haematology (only white blood cells and differential counts) and clinical chemistry (only glucose, urea, aspartase aminotransferase, alkaline phosphatase and Ca) were taken from all moribund mice and all surviving animals at necropsy. At necropsy body weight and weight of liver, kidneys and testis were determined. Further tissue samples (no details given) were collected for histopathology, in addition to any grossly observed tumour and any gross lesions found at necropsy.
No treatment‐related changes were observed in clinical symptoms, survival, body weight gain, food consumption, ophthalmology, terminal body weights, organ weights, gross findings at necropsy, or incidences of benign and malignant tumours. No significant variations were noted in any of the haematological and clinical chemistry parameters determined.
The Panel considered 10% polydextrose‐A (equivalent to 15,000 mg polydextrose‐A/kg bw per day), the highest dose tested, as the no‐observable‐adverse‐effect level (NOAEL).
Rats
Sprague–Dawley rats (Charles River, France), 50 males and 50 females per group, obtained from the F0 generation of a three‐generation study (reported in Section 3.5.6) treated for about 100 days (prior to mating until weaning of the F1) were treated with 0, 5 or 10% polydextrose‐A or 10% sucrose (as reference control) in the diet from birth for up to 775 days (equivalent to 0, 2,500 or 5,000 mg polydextrose‐A/kg bw per day) (Document provided to EFSA n. 14). Body weights were recorded weekly, food consumption once a week during the first 3 months and once a month, thereafter. Clinical observations were recorded weekly. Ophthalmology was performed on days 21, 365, 548 and 730 on all surviving animals. Haematology (haemoglobin, white blood cell, red blood cell, differential count and bone marrow smears) and clinical chemistry (glucose, urea, proteins, aspartate aminotransferase alanine aminotransferase alkaline phosphatase, cholesterol, Na, K, Ca and triglycerides) was determined in all groups, but limited to 20 rats/sex at day 40, 10/sex at day 391, 10/sex at days 391 and 560 and all animals at necropsy. At necropsy, body weights and weights of liver, kidneys and testis were recorded. Samples for histopathology included brain, heart, mesenteric lymph nodes, spleen, stomach, duodenum, ileum, colon, pancreas, liver pituitary, thyroid, adrenals, lungs, kidneys, urinary bladder, ovaries, testis, epididymides, mammary gland, bone marrow (femur), uterus, prostate, salivary glands, sternum and any gross lesion, including potential tumours.
No treatment‐related changes were recorded in general health of the rats. The only treatment‐related symptom was soft and dark faeces in most rats treated with polydextrose‐A. Treatment‐related changes did not occur in mortality, growth, food intake, ophthalmology, clinical chemistry, haematology, gross observations at necropsy and organ weights. Histopathological examination did not reveal an increase in benign and malignant tumours in the polydextrose‐A‐treated groups. The authors concluded that administration of 5% and 10% polydextrose‐A in the diet for 24 months to a generation of rats born from treated parents, revealed no effect in any of the parameters examined which could be ascribed to the feeding of polydextrose‐A.
The Panel considered 10% polydextrose‐A (equivalent to 5,000 mg/kg bw per day), the highest dose tested, as the NOAEL.
Dogs
Six dogs (CERM‐RIOM, France) per sex per group were given an untreated diet or either 50% polydextrose‐N (equivalent to 12,500 mg/kg bw per day) or 20% sucrose (as reference control) in their diet (Document provided to EFSA n. 15). The animals were 14–15 months old at the start of the study and maintained on their respective diets for 18 months, followed by 6 months withdrawal. Body weights were determined weekly and clinical signs were recorded daily. Electrocardiography was performed on all dogs at pre‐treatment and month 12, 18 and 24. Ophthalmology was performed at 0, 6, 12, 18 and 24 months on all animals. Haematology determinations (haemoglobin, white blood cell, red blood cell, platelet count, PCV, differential count, plasma fibrinogen and clotting time) were conducted at the beginning of the study and at months 3, 6, 12, 15, 18, 21 and 24. Bone marrow smears (femur) were taken from all animals at necropsy. Clinical chemistry (Na, K, Cl, Ca, cholesterol, triglycerides, glucose, urea, aspartate aminotransferase alanine aminotransferase alkaline phosphatase and total proteins) was analysed at 0, 3, 6, 9, 12, 15, 18, 21 and 24 months. At necropsy, body weight and weight or heart, liver, kidneys, testis and adrenals were determined.
Polydextrose‐N=treated dogs showed no consistent weight changes during the 18 months of treatment, whereas controls gained weight steadily during the first year and weighed 3–4 kg more than the treated dogs from week 50 to 82. After polydextrose‐N withdrawal, the treatment groups gained weight steadily but did not reach the control values. All polydextrose‐N‐treated dogs, except two females, exhibited some degree of anorexia and reduced appetite. After polydextrose‐N withdrawal at 18 months, no further anorexia was observed. All polydextrose‐N dogs had daily diarrhoea in the morning after the first dietary administration, which continued during the whole exposure period. This condition stopped within 3 days after compound withdrawal. Ophthalmologic observations demonstrated no treatment‐related changes. Clinical chemistry observations were within the normal range, except plasma calcium levels, which were raised in 9 out of 12 polydextrose‐N‐treated dogs throughout the study until 18 months and returned to normal after exposure to polydextrose‐N stopped. Haematology parameters were generally unremarkable. Kidney weights were reduced in the polydextrose‐N‐treated dogs. The Panel considered the finding on nephrocalcinosis due to the high serum calcium levels resulting from the loss of water due to the very high level of polydextrose‐N in the diet.
Six dogs (CERM‐RIOM, France) per sex per group were given 10% or 20% polydextrose‐N in the diet (equal to 2,000 or 4,000 mg/kg bw per day) (Documentation provided to EFSA n. 15). A reference control group received 10% sucrose in the diet (equal to 4,000 mg/kg bw per day) and the control group was maintained on the basal diet only. The dogs were 11–14 months old at the start of the study and received their respective diets for 24 months, with an interim sacrifice of 2 dogs per group at month 12. Body weights were determined weekly and clinical symptoms daily. Electrocardiography was performed on all survivors at the end of the study and ophthalmology was performed at 0, 6, 12, 18 and 24 months on all animals. Haematology (haemoglobin, white blood cell, red blood cell, Ht, platelet count, differential count, plasma fibrinogen, partial thromboplastin time and prothrombin time) and clinical chemistry (Na, K, Cl, Ca, cholesterol, triglycerides, glucose, urea, aspartate aminotransferase alanine aminotransferase, alkaline phosphatase, protein, bilirubin and albumin) determinations were made at the beginning of the study and at months 1, 3, 4, 6, 18 and 24. In addition, bone marrow smears (femur) were taken from all animals at necropsy. Urinalysis was performed at the end of the study and consisted of osmolality, glucose, ketone bodies, urobilin, gamma glutamyl transferase, total protein, blood and microscopy of sediment. At necropsy, weights of heart, spleen, liver, kidneys, testis and adrenals were determined. Organs were collected and histology performed on 40 organs and tissues and on any grossly observed lesion.
Treatment of the dogs with polydextrose‐N did not cause effects on body weight, behaviour, electrocardiogram, ophthalmology, urinalysis or organ weights. All animals given 10% or 20% polydextrose‐N exhibited mild to moderate diarrhoea during the study. This was not seen in control animals. A progressive fall in haemoglobin, red blood cell and haematocrit was observed in three out of four males of the 20% polydextrose‐N group leading to anaemia at month 24. One female of this group showed progressive anaemia. Platelet counts, white blood cell, fibrinogen and prothrombin time did not show treatment‐related changes. The plasma calcium values were increased in the males of the 20% polydextrose‐N group throughout the study, and in females of the 20% polydextrose‐N group and males of the 10% polydextrose‐N group at month 24 only. When comparisons were made among the groups, kidney pathology (mineralisation within the collecting tubules in the renal papilla), plasma calcium and blood urea, the animals of the 10% and 20% polydextrose‐N group showed treatment‐related changes of these parameters, indicative for nephrocalcinosis. The Panel considered the finding on nephrocalcinosis to be a consequence of the increased serum calcium level resulting from the loss of water.
Overall, mice given 7,500 or 15,000 mg polydextrose‐A/kg bw per day in the diet for 18 months and rats given up to 5,000 mg polydextrose‐A/kg bw per day in their diet for 24 months revealed no effect in any of the parameters examined which could be ascribed to the feeding of polydextrose‐A. Studies in dogs showed that polydextrose‐N (containing up to 1.5% potassium), fed for 24 months in daily dietary levels of 10% or 20%, or fed for 18 months in daily dietary levels of 50% induced a dose‐dependent osmotic watery diarrhoea which contributed to transient decreases in vascular fluid volume, electrolyte imbalance, enhanced renal reabsorption of sodium and calcium, a gradually developing hypercalcaemia and ultimately nephrocalcinosis. The Panel considered the nephrocalcinosis observed in dogs at very high levels, which was caused by an imbalance in the homeostasis as such treatment‐ related, but not relevant for the risk assessment. No carcinogenicity was observed in any of the studies.
3.5.6. Reproductive and developmental toxicity
Reproductive toxicity studies
In a dietary three‐generation study in rats (CD‐COBS® albino rats, 20 animals/sex per group in each generation), polydextrose A (powdered form) was administered at constant concentrations of 0, 5 or 10% in the diet (equivalent to 0, 2,500 or 5,000 mg/kg bw per day) (Documentation provided to EFSA n. 16). Most of the animals of the polydextrose groups showed dark and soft faeces after eating polydextrose for a few days. Furthermore, the general health of the rats was not affected. No statistically significant differences were observed in body weights. Copulation rate, mating behaviour, pregnancy rate and gestation length appeared to be normal. The number of live pups at birth of the test groups and the lactation index were comparable to the control group. On day 1 after birth and before culling of litters to 10, the mean body weights of male and female pups were slightly higher than that of controls in any generation. At weaning the male and female pup weights were similar to the controls.
In a fertility study, male rats (Documentation provided to EFSA n. 17) (CD‐COBS® albino rats, 15 males/group) were dosed by gavage with 0, 1,000, 2,000 or 4,000 mg modified polydextrose/animal (equivalent to 0, 2,500, 5,000 or 10,000 mg/kg bw per day)17 during 79 days before mating with untreated females (2 females per male). The high‐dose males had soft faeces which persisted until the end of the study. No significant differences were observed in body weight. Pregnancy rate of the females and the gestation length was similar between the control and the test groups. No treatment‐related differences were observed in litter size, number of pups born alive, viability of the pups during lactation, growth of the pups and the number of abnormal pups.
In a female fertility study, rats (Documentation provided to EFSA n. 17) (CD‐COBS® albino rats, 30 females/group) were dosed by gavage with 0, 1,000, 2,000 or 4,000 mg modified polydextrose/animal (equivalent to 0, 2,500, 5,000 or 10,000 mg/kg bw per day)20 day during 2 weeks before mating with untreated males. Females were dosed up to GD 13. Part of the females were sacrificed on gestation day 13 and another part were allowed to litter and raise their pups until weaning. No clinical signs were observed. No significant differences were observed in body weight premating or during gestation. In the dams sacrificed on GD 13, pregnancy rate, number of corpora lutea, implantation sites and viable fetuses were comparable in all groups. Furthermore, of the group females which were allowed to litter the pregnancy rate of the females and the gestation length was similar between the control and the test groups. No treatment‐related differences were observed in litter size, number of pups born alive, viability of the pups during lactation, growth of the pups and the number of abnormal pups.
Developmental studies
Rats
In a prenatal developmental toxicity study female rats (Documentation provided to EFSA n. 18) (CD‐COBS® albino rats, 20 mated females/group) were dosed by gavage with 0, 1,000, 2,000 or 4,000 mg modified polydextrose/animal (equivalent to 0, 2,500, 5,000 or 10,000 mg/kg bw per day)20 from GD 6 to 15. During the study, clinical signs were recorded. Body weight of the dams was recorded at regular intervals. On GD 20, the animals were sacrificed and the number of pregnant females, number of corpora lutea, number of live and dead fetuses, fetal weight and sex were recorded. The fetuses were examined for external abnormalities and half of the fetuses were examined for skeletal of visceral findings. No treatment‐related maternal or developmental effects were observed.
In a peri‐ and postnatal toxicity study female rats (Documentation provided to EFSA n. 19) (CD‐COBS® albino rats, 20 mated females/group) were dosed by gavage with 0, 1,000, 2,000 or 4,000 mg modified polydextrose/animal (equivalent to 0, 2,500, 5,000 or 10,000 mg/kg bw per day)20 from GD 15 until the end of lactation. During the study clinical signs were recorded. Body weight of the dams was recorded at regular intervals. Pregnancy rate and length of gestation was comparable in the test and control groups. No treatment‐related differences were observed in the number viable pups at birth or during lactation. Apart from a slightly lower growth rate (not significant, approx. 5%) in the high‐dose group, the growth of the pups was comparable between the groups and the abnormalities observed were not considered to be treatment‐related.
Rabbits
In a prenatal developmental toxicity study, female New Zealand White rabbits (Documentation provided to EFSA n. 18) (species not specified, 15 inseminated females/group) were dosed by gavage with 0, 3,000, 6,000 or 12,000 mg polydextrose/animal (equivalent to 0, 454, 909 or 1,818 mg/kg bw per day)18 from GD 7 to 18. In the control, low‐, mid‐ or high‐dose group, 3, 4, 0 or 1 does died during the study. According to the authors, the deaths were related to stress. At necropsy, 4, 9, 11 or 10 does were pregnant per group for the control, low‐ mid‐ or high‐dose group. An increase in water consumption was observed in the polydextrose treated groups (not measured quantitatively); no other treatment‐related clinical signs were recorded. Body weight of the dams recorded at regular intervals did not show treatment‐related effects. On GD 28, the does were sacrificed the number of implantations and corpora lutea, number of live and dead fetuses, fetal and placental weight, the weight of the amniotic fluid and sex were recorded. The fetuses were examined for external abnormalities and half of the fetuses were examined for skeletal and the other half for visceral findings. No adverse treatment‐related developmental effects were observed.
Overall, in a dietary three‐generation reproductive toxicity study in rats, no adverse effects were observed up to 10% polydextrose A in the diet (equivalent to 5,000 mg/kg bw per day, the highest dose tested). In a male and female fertility study, a prenatal developmental toxicity study and a peri‐ and postnatal toxicity study in rats, animals were dosed by gavage and no adverse effects were observed up to 10,000 mg polydextrose/kg bw per day (the highest dose tested). In a prenatal developmental toxicity study in rabbits, no maternal or developmental effects were observed up to the highest dose tested (1,818 mg polydextrose/kg bw per day).
3.5.7. Hypersensitivity, allergenicity and food intolerance
Polydextrose is considered as a potentially immune stimulatory agent, most likely through influencing the microbiota. Fava et al. (2007) administered daily 30 g polydextrose to pigs (4 females and 6 castrated males/group of different breeds) for 21 days and measured IgA levels. No difference was found between the exposure and control group in distal small intestine, caecum, proximal colon, middle colon and distal colon, which indicates that polydextrose had no immune‐stimulatory effects in this model. In contrast, in Wistar rats fed a diet containing 2% polydextrose, an increase in the secretion of IgA in the caecum has been reported (Peuranen et al., 2004). The different results may be explained by the difference in the gut microbiota between pigs and rats. No adverse immunological findings were reported.
3.5.8. Other studies
Study in juvenile piglets
One‐day old piglets (N = 13/group; from personal communication with one of the authors: treatments were assigned at random without regard to sex) received either a cow's milk‐based formula or this formula adjusted to contain 1.7, 4.3, 8.5 or 17 g polydextrose‐A/L for 18 days (equal to 0, 990, 2,260, 4,480 or 8,350 mg/kg bw per day) (Herfel et al., 2009). Growth rate, formula intake, stool consistency, behaviour score, blood chemistry and haematology, relative organ weights (liver, kidney, spleen, gallbladder, heart, brain, left eye, lungs, pancreas, caecum and colon, tissue morphology (i.e. liver, kidney and pancreas), thickness of the muscularis externa of the caecum and colon, and pancreas biochemistry (protein, amylase and DNA) did not differ among formula‐fed pigs. Polydextrose had no adverse effect at the highest tested level of 17.0 g/L, equivalent to a dose of 8.35 g/kg of body weight per day.
Herfel and colleagues studied in the same piglets the potential prebiotic activity of polydextrose (Herfel et al., 2011, personal communication with one of the authors). The Panel considered that this study was not relevant for safety assessment.
Effect of polydextrose on microbiota
A study of effect of polydextrose on intestinal microbiota in pigs (4 females and 6 castrated males/group of different races) fed either a control diet or this diet supplemented with 15 g polydextrose/animal twice daily for 21 days demonstrated that the supplementation with polydextrose did not change the composition of microbial community but increased abundance of intestinal microbes in the distal small intestine as compared to the control group (Fava et al., 2007).
The effect of ingestion of polydextrose on the faecal microbiota was studied in human volunteers (16 females and 15 males, average age 33 years, average body mass index (BMI) of 24.1 ± 2.8 kg/m2) in a double blind, randomised, placebo‐controlled, cross‐over feeding study (Costabile et al., 2012). The first group (n = 16) received 8 g polydextrose/day for 3 weeks followed by a 3‐week washout period and thereafter by 3‐week daily consumption of 8 g of maltodextrose which served as placebo. The second group (n = 15) received first placebo for 3 weeks and after a 3‐week washout period polydextrose for 3 weeks. The authors reported that polydextrose was slowly degraded in the colon. Polydextrose consumption was associated with a significant increase in butyrate producing bacteria Ruminococcus intestinalis and bacteria of Clostridium cluster I, II and IV and a significant decrease in the faecal Lactobacillus–Enterococcus group as compared to the pretreatment period. Furthermore, less abdominal discomfort and a trend for less hard and more formed stools) were reported during polydextrose consumption compared to placebo.
Effect of polydextrose on intestinal enzyme activity in in vitro systems
The activities of maltase and sucrase were studied in mucosa from small intestine harvested from rats (Sprague–Dawley, males, n = 10/group) after 6 weeks on diet added 5% of either polydextrose, or other soluble fibre or cellulose (Choi et al., 1998). Maltase activity of polydextrose fed rats was similar to that from other experimental groups while activity of sucrase was lower. Rats fed polydextrose had soft faeces through the entire feeding period.
The activities of maltase, sucrase and alkaline phosphatase in intestinal mucosal scrapings from rats (Wistar, males, 4 weeks old at the start of the study) receiving a control diet or this diet with added 5% polydextrose for 2 or 4 weeks were not significantly different (Bamba et al., 1993).
The activities of maltase, isomaltase and sucrase measured in the small intestinal mucosal homogenates from rats (Sprague–Dawley, males, n = 5) in the presence or absence of 10% polydextrose solution were comparable (Oku et al., 1991). This indicated, according to the authors, that polydextrose did not have any inhibitory effect on activities of disaccharidases such as maltase, sucrase and isomaltase in the small intestinal mucosa.
In vitro studies on gas production due to fermentation of polydextrose with human faecal bacteria
Several studies investigated in vitro gas production due to fermentation of different carbohydrates and polydextrose using human faecal inoculum from healthy human volunteers. These studies reported that polydextrose produced gas later and at a lower rate than other carbohydrates (Hernot et al., 2009; Vester Boler et al., 2009) or that the gas production from this anaerobic fermentation was at the middle level compared to that from fermentation of other tested carbohydrates (Wang and Gibson, 1993; Ghoddusi et al., 2007; Beards et al., 2010).
Human studies on fermentation of polydextrose by microbiota
In a cross‐over study, healthy volunteers (15 women and 12 men, age: 19–45 years) received single doses of 15 g of either polydextrose, glucose or lactulose in 100 mL of aqueous solution. The wash up period between the treatments was 72 h (Solomons and Rosenthal, 1985). Glucose served as a negative control for hydrogen production after its ingestion. Lactulose served as a positive control as it is a fermentable and hydrogen‐producing carbohydrate, when fermented by microorganisms. According to the authors, polydextrose did not increase breath H2, when measured at 30 min intervals up to 5 and 10 h after ingestion (the data were not shown in the paper) and this indicated a slow rate of fermentation of polydextrose in comparison to lactulose. In the same paper, no increase in blood glucose was detected in the healthy volunteers after ingestion of 25 g of polydextrose contrary to those volunteers, who ingested the same dose of glucose.
Breath hydrogen production in vivo and in vitro (using human faecal microorganisms) following ingestion of a 42 g of chocolate added 10.9 g polydextrose or other sugar alcohols was investigated in 10 human volunteers (2 men, age: 30 and 32 years; 8 women, age 25–46 years; BMI 20–30 kg/m2) using a substrate factorial design with test compounds and test compound mixtures (Livesey et al., 1993). Breath hydrogen was measured for 8 h post‐ingestion. Increase in production of breath hydrogen was approximately 11%/g for polydextrose and 112%/g and 73%/g for lactitol and isomalt, respectively. In vitro fermentation of polydextrose resulted in low hydrogen production.
Healthy volunteers (3 women and 2 men, age: 22‐48 years) received a single dose of 7 g polydextrose in a beverage (Kondo and Nakae, 1996). Breath H2 and CH4 production was increased only very slightly as compared to that from the test persons who ingested either lactulose, dietary fibre or galacto‐oligosaccharide. Flatus was common, diarrhoea was observed on three occasions with polydextrose, lactulose and soybean oligosaccharide in different subjects. The description of the study design was unclear and the reporting of the results was very limited. Therefore, due to these limitations, the Panel could not draw any conclusion from this study.
Nine healthy women (age: ca. 22 years, BMI 20.1 ± 2.4 kg/m2) received on a single occasion 5 g polydextrose in 120 ml of soy flavoured soup (Oku and Nakamura, 2014). Breath hydrogen was measured post‐exposure every hour up to 8 h, every 2 h between 8 and 12 h, after 20 h and after 24 h. Breath hydrogen excretion started to increase 2–3 h post‐ingestion and reached a peak 5 h post‐ingestion. Thereafter, it decreased gradually until the end of experiment (24 h post‐ingestion). The curve of excretion of breath hydrogen after ingestion of polydextrose was flat compared to the curves for fructooligosaccharide, resistant maltodextrin or partially hydrolysed guar gum.
The effect of ingestion of 56.7 g polydextrose in the diet on hydrogen concentration in exhaled air was studied in overweight 9 men and 9 women (age: 20–50 years) (Konings et al., 2014). Hydrogen concentration was increased over 24 h after consumption of polydextrose diet compared to that after consumption of a control diet without polydextrose.
Human studies on gastrointestinal tolerance
Non‐digestable carbohydrates are known to provoke GI distress (flatulence, loose stool or osmotic diarrhoea). GI effects of polydextrose have been studied in the past and the studies were reviewed by JECFA (1987a,b) (n = 7) and the SCF (1990). The original reports of the studies reviewed by both committees were not available to the Panel, who based its evaluation on the descriptions in the JECFA monograph on polydextrose (REF) and in a review by Flood et al. (2004), which additionally reviewed other studies. Altogether there were nine human studies evaluating GI symptoms using endpoints related to laxation (e.g. increased stool weight and water content, decreased GI transit time, loose stools, bloating and distention, borborygmus, abdominal discomfort, flatus, diarrhoea). One of these studies was performed with children from 2‐3 years up to 13‐16 years old, another one with type 2 diabetic patients, and seven in healthy volunteers. Except for the study with type 2 diabetic subjects in which a single dose (50 g/person) was used, other studies applied repeated doses. The dose levels tested varied from 4 g/person three times daily to 50 g/person three times daily. The duration was from 10 days in the shortest and 8 weeks (2 months) in the longest study. The results of the studies demonstrated inter‐individual difference in the tolerance. Some human subjects manifested mild diarrhoea at 35 g/person (2/57 male subjects, Knirsh, 1974 as cited in Flood et al., 2004) or watery stool at 40 g/person (Scrimshaw and Young, 1971 as cited in Flood et al., 2004). Some participants experienced only an increase in flatus at 58 g/person (the highest dose tested, Beer et al. 1989 as cited in Flood et al., 2004). Maximum tolerated dose in healthy volunteers varied from 50 to 130 g/person/day (Raphan, 1975 as cited in Flood et al., 2004). Circa half of children 2–3 years old experienced one or more episodes of diarrhoea over a 4‐week test period after consumption of daily doses of 15 g/person (6/11, Bunde, 1975 as cited in Flood et al., 2004). In type 2 diabetic subjects, diarrhoea was reported at a single dose of 50 g/person (MacMahon, 1974 as cited in Flood et al., 2004). Based on these studies JECFA (1987a,b) concluded that the threshold for laxation was at an intake of about 90 g/person per day or a single dose of 50 g/person. The Panel noted that GI symptoms in adults and children were observed at the same daily dose of 1.3 g/kg bw, a single dose causing GI symptoms in adults was 0.7 g/kg bw (EFSA Scientific Committee, 2012a,b).
Flood et al. (2004) reviewed three additional studies considered as relevant for assessment of laxative effect of polydextrose; two of them (Tomlin and Read, 1988; Zhong et al., 2000) were available to the Panel while the information on the third one (Nakagawa et al., 1990 as cited in Flood et al., 2004) was taken from the review as this study was in Japanese. Twelve male healthy volunteers ingested twice daily for 10 days 100‐mL beverage containing 15 g polydextrose (30 g/day) (study 1) or (15 g/day). The stool was softened by the dose of 30 g (Tomlin and Read, 1988). The soft stool was reported in a 4‐week study with 22 adolescent women who received doses of 5, 7 or 10 g polydextrose in a beverage (Nakagawa et al., 1990 as cited in Flood et al., 2004). When 120 persons (66 man and 54 women) received daily doses of 4, 8 or 12 g polydextrose in 100 mL water for 28 days none of the participants reported abdominal distension or cramps, or diarrhoea (Zhong et al., 2000).
Furthermore, Flood et al. (2004) included three studies designed to evaluate other endpoints than laxation but which also provided information on symptoms related to laxative effect or stool production. These studies were available to the Panel. Ten grams of polydextrose ingested three times daily (total 30 g/person per day) with meals for 30 days increased faecal weight of all participants (7 healthy male volunteers) without provoking adverse GI symptoms (Achour et al., 1994; see also section 3.5.1). Stool water content was not changed but faecal weight was increased and faecal pH was decreased in 8 healthy volunteers (6 males and 2 females), who received daily 15 g polydextrose/person along with a high cholesterol diet for 2 weeks (Endo et al. 1991). In the last study, 5 g polydextrose three times daily in 10 mL solution after meals (total 15 g/person per day) during 2 months was associated with soft stool or diarrhoea in 56% of participants (n = 61 healthy volunteers, 25 men and 36 women) during the first month and in 53% during the second month. Two participants dropped out from the study because of diarrhoea during the first month of the study (Saku et al., 1991). The Panel noted that the diarrhoea was not defined by authors and there was no detailed description of the reported laxative effect.
Overall, based on the information from the JECFA monograph and other studies published after the JECFA evaluation of polydextrose, the Panel agreed with the JECFA (1987a,b) and SCF (1990) evaluations concerning the laxative threshold for polydextrose (a mean laxative threshold of 90 g per person per day or 50 g as a single dose). At the same time, the Panel noted variation in susceptibility to a laxative effect of polydextrose among participants of the studies and between the studies. The Panel noted the recommendation of the SCF (21st report, opinion expressed in 1988) that ‘while considering appropriate levels for the use of polydextrose the laxative effect should be taken into account for the substance alone or in combination with other substances having similar osmotic action (e.g. polyols)’.
Studies on energy (caloric) value of polydextrose
Rat and human studies described in section 3.5.1 provided information on the energy value of polydextrose.
Metabolic studies in rats reported energy value of polydextrose of approximately 1 kcal/g or about 25% the value of glucose (Figdor and Rennhard, 1981). The caloric utilisation of polydextrose was calculated from the quantity of 14CO2 obtained from rats after administration of labelled polydextrose.
The energy resulting from the metabolism of polydextrose was calculated to 7.8 kJ/g (corresponding to 1.9 kcal/g) in the radiochemical balance study and to 12.1 kJ/g (corresponding to 2.9 kcal/g) in the energy balance study in rats (Cooley and Livesey, 1987).
A study in rats investigating caloric value of polydextrose reported physiological energy value of polydextrose of at least 2 kcal/g (Kruger et al., 1990). This estimation was based on consideration that polydextrose was partly available as a carbohydrate digested and absorbed from the small intestine, which provided at least 1.6 kcal/g (a digestible energy), and that the unabsorbed part underwent microbial fermentation in large intestine to SCFA, which are also a source of energy.
The energy utilisation for polydextrose in rats was estimated to 27.4% (corresponding to 1.1 kcal/g) (Juhr and Franke, 1992).
Finally, a study in rats reported energy value of polydextrose to be 0.8 kcal/g (Ranhotra et al., 1993).
Human studies reported energy value of polydextrose to be 1 kcal/g (Figdor and Bianchine, 1983), 1.3 kcal/g (McGaw, 1991), 1 or 1.5 kcal/g (Achour et al., 1994).
The Panel noted that the differences in the energy value of polydextrose in rats (from 0.8 to 2.9 kcal/g) and humans (from 1 to 1.5 kcal/g) originated from different methods used in different studies.
Studies of effect of polydextrose on blood glucose in type 2 diabetics
Two human studies measuring this end point were mentioned in a review by Auerbach et al. (2007). In one study 50 g polydextrose as a bolus had no effect on blood glucose in two men and eight women with type 2 diabetes (Mc Mahon, 1978, unpublished report as cited in Auerbach et al., 2007, not available to the Panel). In the other study, which was available to the Panel, 50 g polydextrose as a bolus had a small effect on blood glucose but not on insulin kinetics in six type 2 diabetics (Bachmann, 1982).
Additional studies
The Panel is also aware of published studies on effects of polydextrose on production of SCFA (e.g. Probert et al., 2004; Hengst et al., 2009; Hernot et al., 2009; Mäkeläinen et al., 2010b; Vester Boler et al., 2011, as cited in Röytiö and Ouwehand, 2014, table 3; Yamamoto et al., 2020) and on effects of polydextrose on intestinal microbiota/microbiome in humans and animals (e.g. Peuranen et al., 2004; Kimura et al., 2004; Hengst et al., 2009; Mäkeläinen et al., 2010a; Vester Boler et al., 2011, as cited in Röytiö and Ouwehand, 2014, table 3; Hibberd et al., 2019; Verkhnyatskaya et al., 2019; Creswell et al., 2020; Wang et al., 2019). The results of these studies are considered by the Panel as not directly applicable to the risk assessment of polydextrose and therefore the studies are not discussed in this opinion.
3.6. Discussion
Polydextrose (E 1200) is authorised as a food additive in the EU in accordance with Annex II and Annex III to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.
According to the Commission Regulation (EU) 231/2012, polydextrose (E 1200) is a randomly bonded glucose polymer with some sorbitol end‐groups, and with citric or phosphoric acid residues attached to the polymer by mono‐ or diester bonds. It is obtained by melting and condensation of the ingredients and consist of approximately 90 parts d‐glucose, 10 parts sorbitol and 1 part citric acid/or 0.1‐part phosphoric acid. The 1,6‐glucosidic linkage predominates in the polymer, but other linkages are present.
Because of the random bonding of glucose molecules, polydextrose exists in a wide range of molecular weights, up to approximately 20,000 Da. According to the interested party (Documentation provided to EFSA n. 6), the average molecular weight of the polydextrose manufactured and marketed by the company is approximately 2,000 Da (± 10%), with the molecular weight substantially comprised between 250 and 18,000 Da, but predominantly not greater than 5,000 Da.
According to the information provided by the interested party (Documentation provided to EFSA n. 1), the polydextrose that was produced in the past could be acidic (polydextrose‐A); a neutral form of polydextrose (polydextrose‐N) was then obtained by neutralisation with a food‐grade base such as potassium hydroxide. In the Commission Regulation (EU) 231/2012, polydextrose and Polydextrose‐N are considered to fall within the specifications of the food additive E 1200. Subsequently, as the manufacturing process has improved, according to the information from the interested party, polydextrose‐N is no longer marketed in the EU (Documentation provided to EFSA n. 1). Therefore, the Panel considered that it should be verified whether or not polydextrose‐N (E 1200) is still used as a food additive in the EU.
Based on the information provided by the interested party on elemental impurity limits (Documentation provided to EFSA n. 1), and the exposure estimation to the additive, the Panel calculated the potential exposure to the toxic elements from the use of polydextrose as a food additive as listed in Section 3.1.2.
The resulting exposure calculations to the toxic elements would result in MOS/MOE for arsenic between 0.5 and 14, and for lead of 8.5. The exhaustions of the TWI for cadmium would be 165%, and for mercury 10%. The exhaustion of the TDI for nickel would amount to 9%.
The calculation of the Panel indicates that the potential exposure to arsenic and cadmium from the consumption of E 1200 as a food additive could be substantial. The Panel noted that the MOS/MOE for arsenic is low. For arsenic, the reference point is based on carcinogenicity for which the MOS/MOE should be at least 10,000 (EFSA, 2005). The TWI of cadmium is derived from a meta‐analysis on data reflecting renal tubular damage.
The exposure estimations demonstrate that there may be a need to introduce limits for arsenic, cadmium and mercury in the EU specifications.
The Panel considered experimental data on the water solubility of four types of polydextrose used as the food additive E 1200 and noted that the range of solubility observed (870–1,050 g/L) for these materials was substantially higher than the threshold of 33.3 g/L currently proposed in the EFSA ‘Draft Guidance on technical requirement for regulated food and feed product applications to established the presence of small particles including nanoparticles’,11 as a decision criterion to decide whether or not an additional assessment for the fraction of small particles is needed. Since this solubility criterion is met, the Panel considered that the risk assessment of polydextrose (E 1200) as a food additive does not require to be complemented with the nanospecific considerations according to the EFSA Guidance on Nanotechnology (EFSA Scientific Committee, 2018).
Several in vitro studies, in vivo studies in experimental animals and in humans investigated the absorption, distribution, metabolism and excretion of polydextrose. The metabolism of polydextrose is similar in rats and humans. In humans, the reported recovery of radioactivity was 33–50% in faeces, 15–36% in breath and 1.4% and 4% in the urine. Polydextrose is partially fermented in the large intestine into SCFA.
Polydextrose (E 1200) (polydextrose‐A and/or polydextrose‐N) did not show a genotoxic potential in limited bacterial reverse mutation assays, did not induce chromosomal aberrations in vitro in human lymphocytes, and in vivo did not induce chromosomal aberrations in bone marrow and dominant lethal mutations in mice. The Panel noted that there are no structural alerts for genotoxicity. Overall, the Panel noted that the studies were not conducted according to the current guidelines and were limited in their protocols. However, in the absence of any structural alerts for genotoxicity, the Panel considered the results acceptable in the overall weight of evidence evaluation and considered that the available data do not indicate a genotoxic activity of polydextrose.
The subchronic studies in rats or monkeys with oral doses of polydextrose up to 10,000 mg/kg bw per day or in dogs when the diet contained polydextrose‐A at 50 or 33% indicated no adverse effects on feed intake, clinical pathology, organ weights and histopathology. Decreases in body weight were reported in a 3‐month study in rats. Dogs receiving polydextrose in their diet for 135 days or 13 months developed nephrocalcinosis which was the result of hypercalcaemia, resulting from chronic watery diarrhoea. Monkeys receiving a high dose of polydextrose by gavage gained weight in a dose‐dependent manner and developed loose stool and/or diarrhoea.
Mice given 7,500 or 15,000 mg polydextrose‐A/kg bw per day in the diet for 18 months and rats given up to 5,000 mg polydextrose‐A/kg bw per day in their diet for 24 months revealed no effect in any of the parameters examined which could be ascribed to the feeding of polydextrose‐A. Studies in dogs showed that polydextrose‐N (containing up to 1.5% potassium), fed for 24 months in daily dietary levels of 10 or 20% or fed for 18 months in daily dietary levels of 50% induced a dose‐dependent osmotic watery diarrhoea which contributed to transient decreases in vascular fluid volume, electrolyte imbalance, enhanced renal reabsorption of sodium and calcium, a gradually developing hypercalcaemia and ultimately nephrocalcinosis. No carcinogenicity was observed in any of the studies. The Panel considered the nephrocalcinosis which developed in dogs given high doses of polydextrose, both in subchronic and chronic toxicity studies, as treatment‐related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment.
In a dietary three‐generation reproductive toxicity study in rats, no adverse effects were observed up to 10% polydextrose A in the diet (equivalent to 5,000 mg/kg bw per day, the highest dose tested). In a male and female fertility study, a prenatal developmental toxicity study and a peri‐ and postnatal toxicity study in rats, animals were dosed by gavage and no adverse effects were observed up to 10,000 mg polydextrose/kg bw per day (the highest dose tested). In a prenatal developmental toxicity study in rabbits, no maternal or developmental effects were observed up to the highest dose tested (1,818 mg polydextrose/kg bw per day).
Overall, based on the information from the JECFA monograph and other studies published after the JECFA evaluation of polydextrose, the Panel agreed with the JECFA (1987a,b) and SCF (1992) evaluations concerning the laxative threshold for polydextrose (a mean laxative threshold of 90 g per person per day or 50 g as a single dose). At the same time, the Panel noted variation in susceptibility to a laxative effect of polydextrose among participants of the studies and between the studies. The Panel considered that, in line with the conclusions by the SCF in 1990 (SCF, 1992), the laxative effect should be taken into account, for the compound alone or when used in combination with other compounds having a similar effect (e.g. polyols), when considering appropriate levels for the use of polydextrose.
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014), the Panel considered that sufficient toxicity data were available in animals showing no adverse effects at highest doses tested up to 15,000 mg polydextrose‐A/kg bw per day in mice and 5,000 mg/kg bw per day in rats. The Panel considered the nephrocalcinosis observed in dogs given polydextrose‐N up to 12,500 mg/kg bw per day as treatment‐related, but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment. Therefore, the Panel considered that there is no need to allocate a numerical ADI for polydextrose (E 1200).
Dietary exposure to polydextrose (E 1200) from its use as a food additive was calculated according to different exposure scenarios based on reported use levels as described in Section 3.4. Food categories for which use levels were provided according to Annex III of regulation (EC) No 1333/2008 were also considered in these exposure scenarios.
Exposure estimates in the maximum level exposure assessment scenario were not considered reliable as most of them were based on maximum recommended use levels reported by the food additives industry. In reality, the food industry uses levels that are lower than the recommended ones (Section 3.3.1 and Appendix A).
Polydextrose (E 1200) is used as a bulking agent and does not influence the organoleptic properties of the final food. For this reason, the Panel considered the non‐brand loyal scenario as the most appropriate scenario for risk characterisation. In this scenario, the exposure estimates ranged from 0.2 mg/kg bw per day in infants to 352 mg/kg bw per day in toddlers at the mean. At the 95th percentile, exposure ranged from 0 mg/kg bw per day in infants to 590 mg/kg bw per day in toddlers.
In all scenarios, all foods belonging to the food categories included in these scenarios were assumed to contain polydextrose (E 1200) at the reported use levels. Foods in which polydextrose (E 1200) is not authorised to be directly added but in which polydextrose (E 1200) can be present as carry‐over (according to Annex III) were taken into account when use levels were provided.
In principle, the calculated exposure to the food additive E 1200 was considered to be overestimated based on the concentration data used and the methodology applied. However, not all uses of the food additive among those authorised in accordance with Annex III of Regulation (EC) No 1333/2008 may not necessarily be covered by the occurrence levels and therefore could not be taken into account in the estimated exposure. The Panel acknowledges that these incomplete data may have an influence on the direction of the uncertainty.
The Panel noted that the dietary exposure estimates are based on information provided on the reported use levels of polydextrose (E 1200). If actual practice changes, these estimates may no longer be representative and should be updated.
Regulation (EC) No 1333/2008 contain dimethyl polysiloxane (E 900) as a food additive at the MPL
4. Conclusions
According to the conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010 (EFSA ANS Panel, 2014) and given that:
adequate exposure estimates were available;
the MOS/MOE for arsenic was low (0.5 and 14), and the exhaustions of the TWI for cadmium would be 165%, whereas it would be 10% for mercury. The exhaustion of the TDI for nickel would amount to 9%. The MOS/MOE for lead was 8.5;
the absorption was limited and part of the polydextrose is fermented in the large intestine into SCFAs;
adequate toxicity data were available;
there was no concern with respect to genotoxicity;
no adverse effects were reported in subchronic studies in rats or monkeys administered oral doses of polydextrose up to 10,000 mg/kg bw per day or in dogs given diet containing polydextrose‐A up to 12,500 mg/kg bw per day;
no adverse effects were reported in chronic or carcinogenicity studies up to 15,000 mg polydextrose‐A/kg bw per day in mice and 5,000 mg/kg bw per day in rats, the highest dose tested;
the nephrocalcinosis which developed in dogs given high doses of polydextrose, both in subchronic and chronic toxicity studies, was considered to be a treatment‐related but a secondary effect related to diarrhoea, and hence not relevant for the risk assessment;
no adverse effects were reported in reproductive or developmental toxicity studies in rats administered up to 5,000 mg polydextrose‐A/kg bw per day or 10,000 mg polydextrose/kg bw per day, respectively, or in a developmental toxicity study in rabbits up to 1,818 mg polydextrose/kg bw per day, the highest doses tested;
the Panel concluded that there is no need for numerical ADI for polydextrose (E 1200) (polydextrose‐A and polydextrose‐N), and that there is no safety concern for the reported uses and use levels of polydextrose as a food additive.
5. Recommendations
The Panel recommended that European Commission considers:
the need to lower the maximum limit for lead, and to introduce limits for arsenic, cadmium and mercury in the EU specifications for polydextrose (E 1200);
verifying that polydextrose‐N as a food additive (E 1200) is no longer marketed in the EU.
Documentation provided to EFSA
Dupont, December 2017. Reply to the call for technical and toxicological data on miscellaneous food additives to be re‐evaluated under the Regulation (EU) No 257/2010 (2017). Technical data on Polydextrose (E 1200): sections 1 and 3, Annex 1‐3.
Dupont, December 2017. Reply to the call for technical and toxicological data on miscellaneous food additives to be re‐evaluated under the Regulation (EU) No 257/2010 (2017). Toxicological data on Polydextrose (E 1200): sections 2 and 3.
Dupont, November 2019. Communication: Reply to the letter “Additional information request on Toxicological data on Polydextrose (E 1200)”.
Dupont, December 2019. Communication: Reply to the letter “Additional information request on Technical data on Polydextrose (E 1200)”.
Dupont, April 2020. Communication: Reply to the letter “Additional information request on Technical data on Polydextrose (E 1200)”.
Dupont, July 2020. Communication: Reply to the letter “Additional information request on Technical data on Polydextrose (E 1200)”.
Dupont, October 2020. Communication: Reply to the letter “Additional information request on Technical data on Polydextrose (E 1200)”.
Pfizer Inc, 1973. Three months oral dose study in rats with CP‐31, 081. Submitted by Dupont, November 2019.
Pfizer Inc, 1978. 90 day feeding study in Beagle dogs with CP‐31, 081, Polydextrose (PD) type A (50% of dry weight of the diet) low caloric food ingredient Protocol 77‐129-07. Submitted by Dupont, November 2019.
Pfizer Inc, 1978. A six‐month feeding study with CP‐31, 081, Polydextrose (PD) type N followed by type A) in Beagle dogs (50% of dry weight of the diet) low caloric food ingredient Protocol 77‐129-06. Submitted by Dupont, November 2019.
Pfizer Inc, 1979. A thirteen‐month feeding study with CP‐31, 081, Polydextrose (PD type A) in Beagle dogs (33% and 16.7% dry weight of the diet) low caloric food ingredient Protocol 77‐129-08. Submitted by Dupont, November 2019.
Pfizer Inc, 1973. A three‐month feeding study in monkeys with CP‐31, 081. Submitted by Dupont, November 2019.
Pfizer Inc, 1975. 18‐month carcinogenicity study with MPD (Polydextrose type A). Submitted by Dupont, November 2019.
Pfizer Inc, 1975. 24‐month carcinogenicity study in rats with Polydextrose A (MPD). Submitted by Dupont, November 2019.
Pfizer Inc, 1975. a) Report on a 24‐month toxicity study in Beagle dogs with MPD (10% and 20% of diet) (Polydextrose type N) and sucrose (20% of diet) Protocol 73‐009. b) Report on a 2‐year study in dogs with modified polydextrose (MPD) administered at 50% of diet‐ MPD Type N. Protocol 130. Submitted by Dupont, November 2019.
Pfizer Inc, 1975. Three‐generation study in rats. Submitted by Dupont, November 2019.
Pfizer Inc, 1975. Action of MPD (polydextrose type A) on fertility and general reproductive performance in the rat (Type segment I of the FDA Guidelines). Submitted by Dupont, November 2019.
Pfizer Inc, 1973. Study of the action of MPD (modified polydextrose) on pregnancy and foeatal development in rats and rabbits (Type FDA, Segment II). Submitted by Dupont, November 2019.
Pfizer Inc, 1974. Study of the action of MPD (modified polydextrose) on the perinatal and postnatal development of the rats (Type FDA, Segment III). Submitted by Dupont, November 2019.
Dupont Nutrition & Health, 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 23 November 2017.
Association of the European Self‐Medication Industry (AESGP), 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 16 November 2017.
FoodDrinkEurope (FDE), 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
Food Supplement Europe (FSE), 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
The International Chewing Gum Association (ICGA), 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
L'Alliance 7, 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 30 November 2017.
Specialised Nutrition Europe (SNE), 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 8 November 2017.
Tate & Lyle, 2017. Data on use levels of polydextrose (E 1200) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (2017). Submitted to EFSA on 29 November 2017.
Abbreviations
- ADI
acceptable daily intake
- AESGP
Association of the European Self‐Medication Industry
- AOAC
Association of Official Analytical Chemists
- BMI
body mass index
- bw
body weight
- 13C‐NMR
carbon‐13 nuclear magnetic resonance
- CAS
Chemical Abstract Service
- cGMMP
current good manufacturing practice
- DP
degree of polymerisation
- ECHA
European Chemicals Agency
- EINECS
European Inventory of Existing Commercial chemical Substances
- F1
first‐generation pups
- F2
second‐generation pups
- FAF
Food Additives and Flavourings
- FCS
food categorisation system
- FDA
US Food and Drug Administration
- FDE
FoodDrinkEurope
- FSE
Food Supplement Europe
- GD
gestation days
- GI
gastrointestinal
- GNPD
Global New Products Database
- HPAEC–ED
high‐performance anion‐exchange chromatography with electrochemical detection
- HPLC
high‐performance liquid chromatography
- ICGA
International Chewing Gum Association
- i.v.
intravenous
- i.p.
intraperitoneal
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LD
laser diffraction
- LD50
lethal dose, 50%, i.e. dose that causes death among 50% of treated animals
- MOE
margin of exposure
- MOS
margin of safety
- MPL
maximum permitted level
- NOAEL
no‐observable‐adverse‐effect level
- OECD
Organisation for Economic Co‐operation and Development
- P1
first‐generation adults
- P2
second‐generation adults
- P3
third‐generation adults
- QS
quantum satis
- SCF
Scientific Committee for Food
- SCFA
short‐chain fatty acid
- SEM
scanning electron microscope
- SNE
Specialised Nutrition Europe (SNE)
- TemaNord
Nordic Council of Ministers
- TDI
tolerable daily intake
- TDF
total dietary fibre
- TWI
tolerable weekly intake
- VFA
volatile fatty acid
- WG
Working Group
- WHO
World Health Organization
Appendix A – Summary of reported use levels (mg/kg or mg/L as appropriate) of polydextrose (E 1200) provided by industry
Appendix B – Number and percentage of food products labelled with polydextrose (E 1200) out of the total number of food products present in the Mintel's GNPD per food subcategory between 2015 and 2020
Appendix C – Concentration levels of polydextrose (E 1200) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Appendix D – Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Appendix E – Main food categories contributing to exposure to polydextrose (E 1200) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Appendix F – Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the food supplement consumers only scenario (mg/kg bw per day)
1.
Appendices Appendix A – Summary of reported use levels (mg/kg or mg/L as appropriate) of polydextrose (E 1200) provided by industry, Appendix B – Number and percentage of food products labelled with polydextrose (E 1200) out of the total number of food products present in the Mintel's GNPD per food subcategory between 2015 and 2020, Appendix C – Concentration levels of polydextrose (E 1200) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate), Appendix D – Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day), Appendix E – Main food categories contributing to exposure to polydextrose (E 1200) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)–F can be found in the online version of this output (‘Supporting information’ section)
Appendix G – Exposure calculations for the toxic elements from the use of polydextrose as a food additive
1.
Based on the information provided by the interested party on elemental impurity limits (Documentation provided to EFSA n. 1), and the exposure estimation for the additive itself, the Panel calculated the potential exposure to the toxic elements from the use of polydextrose as a food additive as listed in Section 3.1.2.
The following elemental impurity limits were provided by the interested party (mg/kg): arsenic, < 1.0; lead, < 0.1; cadmium, < 1.0; mercury, < 0.1; nickel, < 2.0 (Documentation provided to EFSA n. 1). The potential exposure to these toxic elements from the use of the food additives E 1200 can be calculated by assuming contamination of the additive may be up to the impurity limits provided by the business operator and then by calculation pro‐rata to the estimates of exposure to the food additive itself.
With regard to the dietary exposure to the food additive, the maximum P95 exposure of the non‐brand‐loyal exposure scenario (590 mg/kg bw per day for toddlers, see Table 4) is used as the most realistic estimate.
The above‐mentioned impurity limits combined with the estimated intake of polydextrose (E 1200) could result in an exposure which can be compared with the following reference points or health‐based guidance values for the five elements; a BMDL01 of 0.3–8 μg/kg bw per day for arsenic (EFSA CONTAM Panel, 2009a,b), a BMDL01 of 0.5 μg/kg bw per day for lead (EFSA CONTAM Panel, 2010), a TWI of 2.5 μg/kg bw for cadmium (EFSA CONTAM Panel 2011), a TWI of 4 μg/kg bw for mercury (EFSA CONTAM Panel, 2012a,b,c) and a TDI of 13 μg/kg bw for nickel (EFSA CONTAM Panel, 2020).
For arsenic, the reference point is based on carcinogenicity for which the MOS/MOE should be at least 10,000 (EFSA, 2005). For lead, the reference point is based on a study demonstrating perturbation of intellectual development in children with the critical response size of 1 point reduction in IQ. The TWI of cadmium is derived from a meta‐analysis of data reflecting renal tubular damage. The health‐based guidance value for inorganic mercury is derived from kidney weight changes in male rats as the pivotal effect. The TDI for nickel is based on an increased incidence of post‐implantation loss in rats which was identified as the critical effect for the risk characterization of chronic oral exposure.
The resulting exposure calculations to the toxic elements would result in MOS/MOE for arsenic between 0.5 and 14, and for lead of 8.5. The exhaustion of the TWI for cadmium would be 165%, and for mercury 10%. The exhaustion of the TDI for nickel would amount to 9%. The calculations by the Panel shows that the potential exposure to toxic elements from the consumption of E 1200 could be substantial. In particular, the Panel noted that the calculated MOS/MOE for arsenic is low.
As noted above in the Uncertainty Analysis, overall, the uncertainties identified resulted in an overestimation of the exposure to polydextrose (E 1200) as a food additive if the data provided covers all uses. Also, assumption of contamination of the additive always at the impurity limit value is a worse‐case assumption. Lastly, the exposure to nickel would only occur for the additive that is partially hydrogenated with Raney nickel catalyst (see Table 1) and this type of E 1200 will be only a fraction of the total polydextrose E 1200 used as a food additive.
Supporting information
Summary of reported use levels (mg/kg or mg/L as appropriate) of polydextrose (E 1200) provided by industry
Number and percentage of food products labelled with polydextrose (E 1200) out of the total number of food products present in the Mintel’s GNPD per food subcategory between 2015 and 2020
Concentration levels of polydextrose (E 1200) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to polydextrose (E 1200) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the food supplement consumers only scenario (mg/kg bw per day)
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler P, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen DH, Wölfle D, Wright M, Boon P, Crebelli R, Di Domenico A, Filipič M, Mortensen A, Woutersen R, Van Loveren H, Giarola A, Lodi F, Rincon AM, Tard A and Frutos Fernandez MJ, 2021. Scientific Opinion on the re‐evaluation of polydextrose (E 1200) as a food additive. EFSA Journal 2021;19(1):6363, 46 pp. 10.2903/j.efsa.2021.6363
Requestor: European Commission
Question number: EFSA‐Q‐2011‐00583
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Melania Manco, Wim Mennes, Sabina Passamonti, Peter Moldeus, Romina Shah, Dina Hendrika Waalkens‐Berendsen, Detlef Wölfle, Matthew Wright and Maged Younes.
Acknowledgements: The Panel wishes to thank Brian Flynn for the support provided to this scientific output. The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 25 November 2020
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re‐evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
COM(2001) 542 final.
Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Call for Call for technical and toxicological data on miscellaneous food additives to be re‐evaluated under the Regulation (EU) No 257/2010. Published: 11 August 2017. Available online: https://www.efsa.europa.eu/en/consultations/call/170811
Call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6). Available from: https://www.efsa.europa.eu/en/data/call/170223
13/11/2020.
Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
Missing Cyprus, Luxembourg and Malta.
Available online: http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
These dose levels correspond to 5, 10 or 20% of the daily food consumption. Therefore, the dose in mg/kg bw per day could be calculated using the EFSA default factor.
These dose levels correspond to 1.5, 3 or 6% of the daily food consumption of a pregnant rabbit (200 g). Therefore, the dose in mg/kg bw per day could be calculated using the default factor described in JECFA (2000).
References
- Achour L, Flourie B, Briet F, Pellier P, Marteau P and Rambaud JC, 1994. Gastrointestinal effects and energy value of polydextrose in healthy nonobese men. American Journal of Clinical Nutrition, 59, 1362–1368. [DOI] [PubMed] [Google Scholar]
- Auerbach MH, Craig SAS, Howlett JF and Hayes KC, 2007. Caloric availability of polydextrose. Nutrition Reviews, 65, 544–549. [DOI] [PubMed] [Google Scholar]
- Bachmann W, Haslbeck M and Mehnert H, 1982. Untersuchungen zur diatetischen Verwendung von polydextrose bei diabetikern. Akt. Endokr Stoffw 3.
- Bamba T, Fuse K, Chun W and Hosoda S, 1993. Polydextrose and activities of brush‐border membrane enzymes of small‐intestine in rats and glucose‐absorption in humans. Nutrition, 9, 233–236. [PubMed] [Google Scholar]
- Beards E, Tuohy K and Gibson G, 2010. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe, 16, 420–425. [DOI] [PubMed] [Google Scholar]
- Beer M, Arrigoni E, Uhlmann D, Wechsler D and Amado R, 1991. Stability of polydextrose solutions to heat‐treatment and storage under acidic conditions. Lebensm. Wiss u Technol., 24, 245–251. [Google Scholar]
- Burdock GA and Flamm WG, 1999. A review of the studies of the safety of polydextrose in food. Food and Chemical Toxicology, 37, 233–264. [DOI] [PubMed] [Google Scholar]
- Choi YS, Cho SH, Kim HJ and Lee HJ, 1998. Effects of soluble dietary fibers on lipid metabolism and activities of intestinal disaccharidases in rats. Journal of Nutritional Science and Vitaminology, 44, 591–600. [DOI] [PubMed] [Google Scholar]
- Cooley S and Livesey G, 1987. The metabolizable energy value of polydextrose in a mixed diet fed to rats. British Journal of Nutrition, 2, 235–243. [DOI] [PubMed] [Google Scholar]
- Costabile A, Fava F, Röytiö H, Forssten SD, Olli K, Klievink J, Rowland IR, Ouwehand AC, Rastall RA, Gibson GR and Walton GE, 2012. Impact of polydextrose on the faecal microbiota: a double‐blind, crossover, placebo‐controlled feeding study in healthy human subjects. British Journal of Nutrition, 108, 471–481. [DOI] [PubMed] [Google Scholar]
- Craig SAS, Holden JF and Khaled MY, 2000. Determination of Polydextrose as Dietary Fiber in Foods Journal of AOAC INTERNATIONAL, 83, 1006–1012. 10.1093/jaoac/83.4.1006 [DOI] [PubMed] [Google Scholar]
- Craig SAS, 2001. Polydextrose: analysis and physiological benefits In: McCleary B. and Prosky L. (eds.). Advanced Dietary Fibre Technology. [Google Scholar]
- Creswell R, Tan J, Leff JW, Brooks B, Mahowald MA, Thieroff‐Ekerdt R and Gerber GK, 2020. High‐resolution temporal profiling of the human gut microbiome reveals consistent and cascading alterations in response to dietary glycans. Genome Medicine, 12, 59 10.1186/s13073-020-00758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crincoli CM, Nikiforov AI, Rihner MO, Lambert EA, Greeley MA, Godsey J, Eapen AK and svan de Ligt JLG, 2016a. A 90‐day oral (dietary) toxicity and mass balance study of corn starch fiber in Sprague Dawley rats. Food and Chemical Toxicology, 97, 57–69. [DOI] [PubMed] [Google Scholar]
- Crincoli CM, Garcia‐Campayo V, Rihner MO, Nikiforov AI, Liska D and van de Ligt JLG, 2016b. Evaluation of the gastrointestinal tolerability of corn starch fiber, a novel dietary fiber, in two independent randomized, double‐blind, crossover studies in healthy men and women. International Journal of Food Sciences and Nutrition, 67, 844–885. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to arsenic as undesirable substance in animal feed. EFSA Journal 2005;3(3):180, 35 pp. https://doi.org/10.2903./j.efsa.2005.180 [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. 10.2903/j.efsa.2014.3697 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009a. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009b. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011. Scientific Opinion on tolerable weekly intake for cadmium. EFSA Journal 2011;9(2):1975, 19 pp. 10.2903/j.efsa.2011.1975 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on lead dietary exposure in the European population. EFSA Journal 2012;10(7):2831, 59 pp. 10.2903/j.efsa.2012.2831 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012c. Scientific Opinion on cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 59 pp. 10.2903/j.efsa.2012.2551 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenbo om LR, Leblanc J‐C,Nebbia CS, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Guérin T, Massanyi P,Van Loveren H, Baert K, Gergelova P and Nielse n E, 2020. Scientific Opinion on the update of the risk assessment of nickel in food and drinking water. EFSA Journal 2020;18(11):6268, 101 pp. 10.2903/j.efsa.2020.6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011. Scientific Opinion on the substantiation of health claims related to polydextrose and changes in bowel function (ID 784), changes in short chain fatty acid (SCFA) production and/or pH in the gastro‐intestinal tract (ID 784), decreasing potentially pathogenic gastro‐intestinal microorganisms (ID 785) and reduction of gastro‐intestinal discomfort (ID 784) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(6):2256. [18 pp.]. 10.2903/j.efsa.2011.2256. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Scientific opinion on polydextrose and maintenance of normal defecation: evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA Journal 2016;14(5):4480,12 pp. 10.2903/j.efsa.2016.4480 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA Journal 2012;10(3):2578, 5 pp. 10.2903/j.efsa.2012.2578 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018. The principles and methods behind EFSA's Guidance on the Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Kumemura M, Nakamura K, Fujisawa T, Suzuki K, Benno Y and Mitsuoka T, 1991. Bifidobacteria Microflora, 10, 53–64. [Google Scholar]
- Fava F, Mäkivuokko H, Siljander‐Rasi H, Putaala H, Tiihonen K, Stowell J, Tuohy K, Gibson G and Rautonen N, 2007. Effect of polydextrose and intestinal microbes and immune functions in pigs. British Journal of Nutrition, 98, 123. [DOI] [PubMed] [Google Scholar]
- Figdor SK and Bianchine JR, 1983. Caloric utilization and disposition of [14C]polydextrose in man. Journal of Agriculture and Food Chemistry, 31, 389–393. [DOI] [PubMed] [Google Scholar]
- Figdor SK and Rennhard HH, 1981. Caloric utilization and disposition of [14C]polydextrose in the rat. Journal of Agriculture and Food Chemistry, 29, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Flood MT, Auerbach MH and Craig SA, 2004. A review of the clinical toleration studies of polydextrose in food. Food and Chemical Toxicology, 42, 1531–1542. [DOI] [PubMed] [Google Scholar]
- Ghoddusi HB, Grandison MA, Grandison AS and Tuohy KM, 2007. In vitro study on gas generation and prebiotic effects of some carbohydrates and their mixtures. Anaerobe, 13, 193–199. [DOI] [PubMed] [Google Scholar]
- Hengst C, Ptok S, Roessler A, Fechner A and Jahreis G, 2009. Effects of polydextrose supplementation on different faecal parameters in healthy volunteers. International journal of food sciences and nutrition. 60(Suppl 5), 96–105. [DOI] [PubMed] [Google Scholar]
- Herfel TM, Jacobi SK, Lin X, Walker DC, Jouni ZE and Odle J, 2009. Safety evaluation of polydextrose in infant formula using a suckling piglet model. Food and Chemical Toxicology, 47, 1530–1537. [DOI] [PubMed] [Google Scholar]
- Herfel TM, Jacobi SK, Lin X, Fellner V, Walker DC, Jouni ZE and Odle J, 2011. Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. Journal of Nutrition, 141, 2139–2145. [DOI] [PubMed] [Google Scholar]
- Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS and Fahey GC, 2009. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. Journal of Agricultural and Food Chemistry, 57, 1354–1361. [DOI] [PubMed] [Google Scholar]
- Hibberd AA, Yde CC, Ziegler ML, Honoré AH, Saarinen MT, Lahtinen S, Stahl B, Jensen HM and Stenman LK, 2019. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes, 10, 121–135. 10.3920/BM2018.0028 [DOI] [PubMed] [Google Scholar]
- JECFA , 1980. Evaluation of certain food additives (24th report). WHO technical report series, n. 653. Available online: http://www.inchem.org/documents/jecfa/jecmono/v16je18.htm
- JECFA , 1987a. Evaluation of certain food additives (25th report). WHO technical report series, n. 669. Available online: https://apps.who.int/iris/bitstream/handle/10665/39108/WHO_TRS_759.pdf;jsessionid=6021FBBDA18E9C84C6C48F09D7101D3F?sequence=1
- JECFA , 1987b. Evaluation of certain food additives and contaminants. Thirty‐first Report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization Technical Report Series 759, Geneva. Available online: http://apps.who.int/iris/bitstream/10665/39108/1/WHO_TRS_759.pdf [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
- JECFA (Join FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. Combined compendium of food additive specifications. All specifications monographs from the 1st to the 65th meeting (1965‐2005). Volume 4. Available online: http://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-314.pdf
- Juhr NC and Franke J, 1992. A method for estimating the available energy of incompletely digested carbohydrates in rats. Journal of Nutrition, 122, 1425–1433. [DOI] [PubMed] [Google Scholar]
- Kondo T and Nakae Y, 1996. Breath hydrogen and methane excretion produced by commercial beverages containing dietary fiber. J of Gastrolenterology, 31, 654–658. [DOI] [PubMed] [Google Scholar]
- Kondo T, Handa K, Genda T, Hino S, Hamaguchi N and Morita T, 2017. Digestion‐resistant dextrin derivatives are moderately digested in the small intestine and contribute more to energy production than predicted from large‐bowel fermentation in rats. Journal of Nutrition, 147, 330–336. [DOI] [PubMed] [Google Scholar]
- Konings E, Schoffelen PF and Stegen J, 2014. Effect of polydextrose and soluble maize fibre on energy metabolism, metabolic profile and appetite control in overweight men and women. Br J Nutr., 111, 111–121. [DOI] [PubMed] [Google Scholar]
- Kruger D, Zieze T and Grossklaus R, 1990. Caloric availability of polydextrose in rats. Akt. Ernahr. Med., 15. [Google Scholar]
- Lahtinen SJ, Knoblock K, Drakoularakou A, Jacob M, Stowell J, Gibson GR and Ouwehand AC, 2010. Effect of molecule branching and glycosidic linkage on the degradation of polydextrose by gut microbiota. Bioscience, Biotechnology, and Biochemistry, 74, 100251‐1-6. [DOI] [PubMed] [Google Scholar]
- Livesey G, Johnson IT, Gee JM, Smith T, Lee WE, Hillan KA, Meyer J and Turner SC, 1993. Determination of sugar alcohol and polydextrose absorption in humans by the breath hydrogen (H2) technique – the stoichiometry of hydrogen‐production and the interaction between carbohydrates assessed In vivo and In vitro. European Journal of Clinical Nutrition, 47, 419–430. [PubMed] [Google Scholar]
- Mäkelainen HS, Mäkivuokko HA, Salminen SJ, Rautonen NE and Ouwehand AC, 2007. The effects of polydextrose and xylitol on microbial community and activity in a 4‐stage colon simulator. Journal of Food Science, 72, M153–M159. [DOI] [PubMed] [Google Scholar]
- Makelainen H, Saarinen M, Stowell J, Rautonen N and Ouwehand AC, 2010. Xylo‐oligosaccharides and lactitol promote the growth of Bifidobacterium lactis and Lactobacillus species in pure cultures. Beneficial Microbes, 1, 139–148. [DOI] [PubMed] [Google Scholar]
- Mäkivuokko H, Nurmi J, Nurminen P, Stowell J and Rautonen N, 2005. In vitro effects on polydextrose by colonic bacteria and Caco‐2 cell cyclooxygenase gene expression. Nutrition and Cancer, 52, 94. [DOI] [PubMed] [Google Scholar]
- Mazur AW, Mohlenkamp MJ, Hiler G, Wilkins TD and Van Tassell RL, 1993. Digestibility of selected carbohydrates by anaerobic bacteria. Journal of Agricultural and Food Chemistry, 41, 1925–1930. [Google Scholar]
- Oku T and Nakamura S, 2014. Evaluation of the relative available energy of several dietary fiber preparations using breath hydrogen evolution in healthy humans. Journal of Nutritional Science and Vitaminology, 60, 246–254. [DOI] [PubMed] [Google Scholar]
- Oku T, Fujii Y and Okamatsu H, 1991. Polydextrose as dietary fiber: hydrolysis by digestive enzyme and lts effect on gastrointestinal transit time in rats. J. Clin. Biochem. Nutr., 11, 40. [Google Scholar]
- Peuranen S, Tiihonen K, Apajalahti J, Kettunen A, Saarinen M and Rautonen N, 2004. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. British Journal of Nutrition, 91, 905–914. [DOI] [PubMed] [Google Scholar]
- Probert HM, Apajalahti JHA, Rautonen N, Stowell J and Gibson GR, 2004. Polydextrose, lactitol, and fructo‐oligosaccharide fermentation by colonic bacteria in a three‐stage continuous culture system. Applied and Environmental Microbiology, 70, 4505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radosta S, Boczek P and Grossklaus R, 1992. Composition of polydextrose® before and after intestinal degradation in rats. Starch, 44, 150–153. [Google Scholar]
- Ranhotra J, Gelroth A and Glaser BK, 1993. Usable energy value of selected bulking agents G. S. Journal of Food Science, 58, 1176–1178. [Google Scholar]
- Richter M, Grossklaus R and Boczek P, 1994. Enzymatic degradation of polydextrose and of a high molecular polydextrose fraction. Starke, 46, 27–32. [Google Scholar]
- Röytiö H and Ouwehand AC, 2014. The fermentation of polydextrose in the large intestine and its beneficial effects. Beneficial microbes, 5, 305–313. [DOI] [PubMed] [Google Scholar]
- Saku K, Yoshinaga K, Okura Y, Ying H, Harada R and Arakawa K, 1991. Effects of polydextrose on serum lipids, lipoproteins, and apolipoproteins in healthy subjects. Clinical therapeutics, 13, 254‐258. [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food), 1990. Reports of the Scientific Committee for foods 26th series. EU Commission. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_reports_26.pdf [Google Scholar]
- SCF (Scientific Committee for Food), 1992. 26th series. Opinion expressed in 1990. Food Science and Techniques, 1992.
- SCF (Scientific Committee for Food), 1993. 31st series. Nutrient and energy intakes for the European Community. Opinion expressed on 11 December 1992.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001. Available online: http://ec.europa.eu/food/fs/sc/scf/out98_en.pdf
- Solomons NW and Rosenthal A, 1985. Intestinal metabolism of a random bonded polyglucose bulking agent in humans: in vitro and in vivo studies of hydrogen evolution. Journal of Laboratory and Clinical Medicine, 105, 585–592. [PubMed] [Google Scholar]
- Stowell JD, 2009. Polydextrose In: Cho SS. (ed.). Fiber Ingredients: Food Applications and Health Benefits. Taylor & Francis; pp. 173–201. [Google Scholar]
- Stuart AS, Holden JC and Khaled MY, 2000. Determination of polydextrose as dietary fiber in foods. Journal of AOAC International, 83. [PubMed] [Google Scholar]
- TemaNord , 2002. Food Additives in Europe 2000 ‐ Status of safety assessments of food additives presently permitted in the EU.
- Tomlin J and Read NW, 1988. A comparative study of the effects on colon function caused by feeding ispaghula husk and polydextrose. Alimentary pharmacology & therapeutics, 2, 513–519. [DOI] [PubMed] [Google Scholar]
- Veena N, Surendra Nath B and Arora S, 2016. Polydextrose as a functional ingredient and its food applications: a review. Indian J Dairy Sci, 69. [Google Scholar]
- Verkhnyatskaya S, Ferrari M, de Vos P and Walvoort MTC, 2019. Shaping the Infant Microbiome With Non‐digestible Carbohydrates. Frontiers in Microbiology, 10, 343. https://doi.org/10.3389/fmicb.2019.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester Boler BM, Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS and Fahey J, 2009. Carbohydrates blended with polydextrose lower gas production and short‐chain fatty acid production in an in vitro system. Nutrition Research, 29, 631–639. [DOI] [PubMed] [Google Scholar]
- Vester Boler BM, Rossoni Serao MC, Bauer LL, Staeger MA, Boileau TW, Swanson KS and Fahey GC, 2011. Digestive physiological outcomes related to polydextrose and soluble maize fibre consumption by healthy adult men. British Journal of Nutrition, 106, 1864–1871. [DOI] [PubMed] [Google Scholar]
- Wang X and Gibson GR, 1993. Effects of the in‐vitro fermentation of oligofructose and inulin by bacteria growing in the human large‐intestine. Journal of Applied Bacteriology, 75, 373–380. [DOI] [PubMed] [Google Scholar]
- Wang HS, Ren PF, Mang L, Shen N, Chen JR and Zhang YW, 2019. In vitro fermentation of novel microwave‐synthesized non‐digestible oligosaccharides and their impact on the composition and metabolites of human gut microbiota. Journal of Functional Foods, 55, 156–166. [Google Scholar]
- Yamamoto Y, Morozumi T, Takahashi T, Saruta J, To M, Sakaguchi W, Shimizu T, Kubota N and Tsukinoki K, 2020. 2020 Faster Short‐Chain Fatty Acid Absorption from the Cecum Following Polydextrose Ingestion Increases the Salivary Immunoglobulin A Flow Rate in Rats. Nutrients, 12, 1745 10.3390/nu12061745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Luo BY, Xiang MJ, Liu HW, Zhai ZK and Wang TS, 2000. Studies on the effects of polydextrose intake on physiologic functions in Chinese people. American Journal of Clinical Nutrition, 72, 1503–1509. [DOI] [PubMed] [Google Scholar]
- Ziese T, Kruger D and Grossklaus R, 1995. In vitro and in vivo inhibition of small intestinal digestion of sugar substitutes by acarbose. Aktuelle Ernahrungsmedizin, 20, 229–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of reported use levels (mg/kg or mg/L as appropriate) of polydextrose (E 1200) provided by industry
Number and percentage of food products labelled with polydextrose (E 1200) out of the total number of food products present in the Mintel’s GNPD per food subcategory between 2015 and 2020
Concentration levels of polydextrose (E 1200) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to polydextrose (E 1200) using the maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Summary of total estimated exposure of polydextrose (E 1200) from their use as food additives for the food supplement consumers only scenario (mg/kg bw per day)


