Abstract
OBJECTIVES
Parents and caregivers of children with medical complexity (CMC) manage complex medication regimens (CMRs) at home. Parental understanding of CMRs is critical to safe medication administration. Regarding CMR administration, we 1) described the population of CMC receiving CMRs; 2) assessed parental perceived confidence and understanding; and 3) evaluated parental demonstrated understanding.
METHODS
Cross-sectional clinic-based assessment of knowledge and understanding of CMC using CMRs who received primary care in a large pediatric complex care clinic. CMRs were identified by the receipt of ≥1 of the following: 1) ≥10 concurrent medications; 2) ≥1 high-risk medication; or 3) ≥1 extemporaneously compounded medication. Parents reported their perceived confidence and understanding of CMRs, and then demonstrated understanding through 3 medication-related tasks.
RESULTS
Of 156 CMCs, most were <10 years of age (63.5%), white (75%), had neurologic impairment (76.9%), and used a median of 8 medications (IQR, 5–10). Parents were female (76.9%) with a mean age of 38.8 ± 11.5 years, white (69.9%), spoke English (94.2%), and had some college education (82.1%). On 11 confidence and understanding statements, most parents reported a high perceived level of understanding and confidence, with combined agreement or strong agreement ranging between 81.2% and 98.7%. Only 73.1% correctly identified medications taken for specified conditions, 40.4% reported complete dosing parameters, and 54.8% correctly measured 2 different medication doses. Significant differences existed between parental perceived understanding versus the 3 demonstrated tasks (all p < 0.05).
CONCLUSIONS
Substantial opportunities exist to improve medication safety and efficacy in the outpatient, in-home setting including improved medication-specific education and medication-related supports.
Keywords: ambulatory care, caregivers, child, comprehension, medication errors, parents, polypharmacy
Introduction
Parents and caregivers (subsequently referred to as parents) of children with medical complexity (CMC) manage complex medication regimens (CMRs). CMRs may consist of numerous, complex, and high-risk medications. Literature supports that CMC can be regularly prescribed ≥5 scheduled medications, with parents administering up to 35 daily doses, often via alternative routes of administration requiring dosage form manipulation.1 In outpatient, in-home settings, the administration and monitoring of medications are performed most frequently by parents, rather than trained health care professionals. Although some CMC have available home nursing resources, parents have the primary responsibility in the care of CMC, including accurate administration of CMRs. Correct administration and adherence require knowledge and understanding of CMRs, including indications and medication-specific information (e.g., dosage form, instructions, expected outcomes). However, gaps in understanding of medication instructions across all literacy levels have been identified; in a study evaluating comprehension of after-visit instructions after primary care visits, parents were only able to accurately name 71% of the medications and describe administration instructions 37% of the time.2
While CMRs and polypharmacy are often medically necessary for CMC, the concomitant use of multiple medications increases the potential risk of administration errors, interactions, adverse events, adherence issues, and parental misunderstanding, which may affect patient safety.3 For example, certain high-risk medications (e.g., clonidine and non-stimulant psychotropic medications) have been reported in the literature to be associated with major safety events such as inadvertent dosing errors resulting in significant toxicity.4,5 Two recent systematic reviews6,7 argued for expanded research in children, aimed at interventions targeted at reducing medication errors and improving adherence.
Parental knowledge of and skills related to CMRs are critical to ensuring safety. Thus, the primary objectives of this study were to 1) describe the population of children with CMC receiving CMRs, including the types of medications used; 2) assess parents' perceived confidence related to CMRs; 3) assess parents' understanding of CMRs; and 4) compare differences in parental perceived confidence and understanding versus demonstrated understanding and knowledge of CMRs.
Materials and Methods
Study Design. This was a single-center cross-sectional study of CMC using CMRs who received primary care in a large pediatric complex care clinic serving >4000 patients. The study was approved by the Colorado Multiple Institutional Review Board, an administrative body that protects the rights and welfare of human research subjects participating in research activities conducted under the auspices of the University of Colorado and its affiliates.
Identification of Subjects. Subjects between the ages of 0 and 21 years with CMC and CMRs who could speak and understand English were consecutively enrolled between November 1, 2015, and May 31, 2016. Children with medical complexity were identified by using the Pediatric Medical Complexity Algorithm and further categorized by neurological impairment status and AHRQ's (Agency for Healthcare Research and Quality's) chronic condition indicator.8–10 CMRs were identified by meeting ≥1 of the following criteria: 1) ≥10 concurrent scheduled medications (polypharmacy); 2) receipt of ≥1 high-risk medication; and/or 3) receipt of ≥1 extemporaneously compounded medication. For this study, high-risk medications were defined as scheduled, long-term opioids; anticoagulants; anti-epileptic agents; stimulant medications; psychotropic medications; agents to treat tone/spasticity; or sleep aids.11,12 These medications were chosen owing to their association with adverse drug reactions and owing to CMC use patterns. Parents were approached for study participation during routine, in-person clinic visits.
Consent, Enrollment, and Survey Administration. After consent and enrollment, demographic, diagnoses, and medication regimen–specific data were manually extracted from the electronic health record. Parents then completed an in-person survey adapted from similar previously published surveys and accounting for key pharmacotherapy aspects required for complete understanding, adherence, and outcomes (for full survey, see Supplemental Table 1).13–16 The survey included demographic questions as well as 5-point Likert scale questions about perceived understanding/knowledge and confidence pertaining to CMRs. Study data were managed with REDCap electronic data capture tools.17
Parental Understanding. Parents demonstrated knowledge of CMRs through assessment of 3 tasks. First, a live, in-clinic and observed assessment of the parent's ability to accurately draw up 2 doses of liquid medication, using oral syringes. Accuracy was defined strictly as no errors in measurement, including no air bubbles, in order to account for all medication types. Because parent medication measurement accuracy may have different implications based on a specific medication's therapeutic window, we defined accuracy by using the strictest definition. To isolate measuring technique irrespective of specific medications, parents performed this assessment using water.
The second assessment of parental understanding was correct identification of medications prescribed for 2 patient-specific complex conditions identified by the principal investigator. Selected conditions were those of highest clinical relevance. Owing to the medical complexity of the patient population, the correct recall and identification of medications associated with 2 patient-specific complex conditions served as a proxy measure to represent knowledge of all patient-specific conditions and associated medications.
The third and final assessment of demonstrated parental understanding was parents' recall and description of complete medication instructions for 1 patient-specific complex medication identified by the principal investigator, including indication, formulation (e.g., tablet/capsule versus liquid, and milligram strength or concentration), dose, frequency, and route of administration.13,15 The selected medication was chosen on the basis of clinical relevance. Complete accuracy required correct identification of all parameters. For both the patient-specific indications and the complex medication regimen, parents were asked to recall this information without any written information in front of them.
Statistical Analysis. Descriptive statistics were calculated for patient demographics and for survey responses. Five-point Likert survey responses were collapsed into binary responses, with Strongly Agree or Agree indicating a positive response. The McNemar test for paired proportions was used to compare parental perceived versus demonstrated understanding. Confounding variables (e.g., socioeconomic status, highest level of education, presence of home nursing) were accounted for in a multivariable logistic regression in order to describe the relationship between parental characteristics and demonstrated understanding, adjusting for the type of CMR. Only survey items with <5% missing data, unless noted otherwise, were analyzed. Owing to extremely low usage of intravenous and intramuscular medications in our subjects, only data pertaining to enteral or inhaled medications were analyzed. A significance level of 0.05 was used. Data were analyzed by using Stata 16.0 (Stata Corp, College Station, TX).
Results
Of 480 eligible CMCs identified during the study period, 32.5% (n = 156) were consented for study participation and completed all study procedures (Figure 1). The in-clinic demonstration of measurement of medications was completed by 86.5% of parents.
Figure 1.
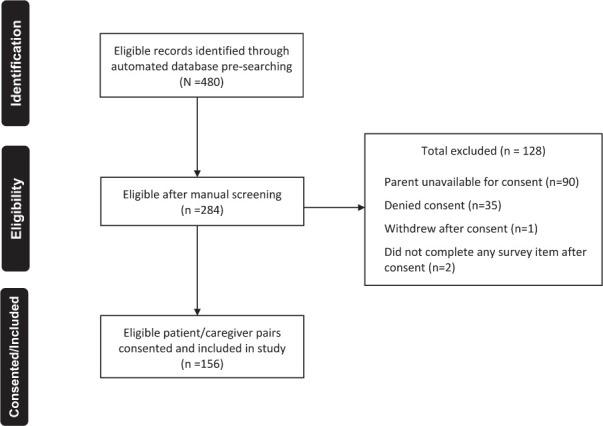
Screening, eligibility, and study inclusion.
Demographics and Clinical Characteristics of Study Population. Most patients were <10 years of age (63.5%), white (75%), had neurological impairment (76.9%), and used a median of 8 medications (IQR, 5–10) (Table 1). Of these children, 23.1% met the polypharmacy enrollment criteria, 95.5% met the high-risk medication criteria, and 35.9% met the compounded medication criteria; 41.7% of children met 2 enrollment criteria and 6.4% of children met all 3 criteria. The most common primary diagnoses included cerebral palsy, unspecified (11.5%); extreme prematurity (i.e., gestational age 24 weeks) (7.6%); and chromosomal abnormality, unspecified (6.4%) (Supplemental Table 2). Many of the study subjects had medical comorbidities; among all recorded diagnoses, comorbidities occurring with >5% frequency were disorders of psychological development (11.1%), generalized epilepsy (10.1%), and sleep disorders (5.1%) (Supplemental Table 3).
Table 1.
Demographic, Clinical, and Medication-Related Characteristics of Children With Medical Complexity and Complex Medication Regimens (N = 156)
| Characteristic | Result |
|---|---|
| Age, n (%), yr | |
| <1 | 7 (4.5) |
| 1–4 | 39 (25) |
| 5–9 | 53 (34) |
| 10–12 | 23 (14.5) |
| 13–18 | 34 (22) |
| Sex, n (%) | |
| Male | 78 (50) |
| Female | 78 (50) |
| Race, n (%) | |
| Caucasian | 117 (75) |
| African American | 9 (5.8) |
| Hispanic/Latino | 2 (1.3) |
| Asian | 2 (1.3) |
| Hawaiian/Pacific Islander | 1 (0.6) |
| American Indian/Alaska Native | 3 (1.9) |
| Other/unknown | 22 (14.1) |
| Complex chronic condition, n (%) | 156 (100) |
| Neurological impairment, n (%) | 120 (77) |
| Number of medications in all patients, median (IQR) | 8 (5–10) |
| ≥10 Medications | |
| Patients receiving, % | 23.1 |
| Number of medications, median (IQR) | 11 (10–13) |
| ≥1 High-risk medication | |
| Patients receiving, % | 95.5 |
| Number of high risk medications, median (IQR) | 3 (2–4) |
| ≥1 Compounded medication | |
| Patients receiving, % | 35.9 |
| Number of compounded medications, median (IQR) | 1 (1–2) |
| Receipt of Home Nursing, n (%) | 79 (50.6) |
| Medications Administered by Family at Home, % | |
| 0–24 | 9.0 |
| 25–49 | 4.5 |
| 50–74 | 13.5 |
| 75–100 | 70.5 |
| Unknown | 2.5 |
Most parents were female (76.9%) with a mean age of 38.8 ± 11.5 years, white (69.9%), had a biological relationship to the child (82.1%), spoke English (94.2%), and had at least some college education (82.1%) (Table 2).
Table 2.
Demographic Characteristics of Parents of Children With Medical Complexity and Complex Medication Regimens
| Parameter | Overall (N = 156) |
|---|---|
| Age, mean ± SD, yr | 38.8 ± 11.5 |
| Sex, n (%) | |
| Male | 21 (13.5) |
| Female | 120 (76.9) |
| Not specified | 15 (9.6) |
| Relationship to child, n (%) | |
| Biological mother | 112 (71.8) |
| Biological father | 16 (10.3) |
| Step, foster, or adoptive mother | 8 (5.1) |
| Step, foster, or adoptive father | 5 (3.2) |
| Other | 15 (9.6) |
| Race, n (%) | |
| Caucasian | 109 (69.9) |
| African American | 6 (3.9) |
| Hispanic/Latino | 27 (17.3) |
| Asian | 4 (2.6) |
| Hawaiian/Pacific Islander | 1 (0.6) |
| American Indian/Alaska Native | 2 (1.3) |
| Other/unknown | 7 (4.5) |
| Language spoken in home, n (%) | |
| English | 147 (94.2) |
| Spanish | 4 (2.6) |
| Other | 5 (3.2) |
| Highest level of education, n (%) | |
| ≤ 8th grade | 3 (1.9) |
| Some high school | 8 (5.1) |
| High-school graduate | 14 (9) |
| Some college | 69 (44.2) |
| College graduate | 34 (21.8) |
| More than college education | 25 (16) |
| Other | 3 (1.9) |
| Annual income, n (%) | |
| <$15,000 | 14 (9) |
| $15,000–$30,000 | 18 (11.5) |
| $30,001–$60,000 | 50 (32.1) |
| $60,000–$100,000 | 28 (18.0) |
| >$100,000 | 33 (21.2) |
| Other | 13 (8.3) |
Most Frequent Medications Used. A total of 1175 medications were prescribed. The most frequent medications included vitamins (14.5%), antiepileptic drugs (13.6%), and psycholeptic (e.g., antipsychotics, anxiolytics, hypnotics, and sedatives) drugs (8.9%) (Table 3). A full list of medications used by CMC, including non-prescription medications, is described in Supplemental Table 4. The most frequently prescribed medications differed by the type of enrollment criteria (e.g., polypharmacy, high-risk medications, or compounded medications); however, vitamins and antiepileptic drugs were commonly used amongst all 3 groups. Of the 97 unique high-risk prescription medications used, the top 3 were levetiracetam (10.3%), baclofen (6.7%), and clonidine (5.2%). Of the 27 unique compounded medications used, the top 3 were lansoprazole (17.1%), baclofen (11.8%), and zinc (7.9%). A list of all compounded medications used by CMC is described in Supplemental Table 5.
Table 3.
Top 10 * Most Common Medication Classes Comprising the Overall, Polypharmacy, High-Risk, and Compounded Groups
| Medication Group† | % |
|---|---|
| Overall study population (N = 1175 total medications) | |
| Vitamins | 14.5 |
| Antiepileptic drugs | 13.6 |
| Psycholeptic drugs‡ | 8.9 |
| Drugs for acid-related disorders | 7.4 |
| Drugs for constipation | 6.7 |
| Antihistamines for systemic use | 5.5 |
| Psychoanaleptics§ | 4.0 |
| Nasal preparations | 3.9 |
| Analgesic drugs | 2.7 |
| Muscle relaxants | 2.7 |
| Polypharmacy group (n = 413 total medications) | |
| Vitamins | 13.6 |
| Antiepileptic drugs | 11.1 |
| Psycholeptic drugs‡ | 8.7 |
| Drugs for constipation | 8.0 |
| Drugs for obstructive airway diseases | 6.8 |
| Antihistamines for systemic use | 6.3 |
| Drugs for acid-related disorders | 6.1 |
| Nasal preparations | 4.4 |
| Psychoanaleptics§ | 3.9 |
| Topical corticosteroids | 2.7 |
| High-risk medication group (n = 1127 total medications) | |
| Antiepileptic drugs | 13.9 |
| Vitamins | 13.9 |
| Psycholeptic drugs‡ | 9.1 |
| Drugs for acid-related disorders | 7.4 |
| Drugs for constipation | 6.6 |
| Drugs for obstructive airway diseases | 5.5 |
| Antihistamines for systemic use | 5.2 |
| Psychoanaleptics§ | 4.0 |
| Nasal preparations | 3.8 |
| Muscle relaxants | 2.8 |
| Compounded medication group (n = 395 total medications) | |
| Vitamins | 14.4 |
| Antiepileptic drugs | 12.2 |
| Drugs for acid-related disorders | 8.4 |
| Drugs for constipation | 6.1 |
| Psycholeptic drugs‡ | 5.8 |
| Drugs for obstructive airway diseases | 5.1 |
| Nasal preparations | 5.1 |
| Antihistamines for systemic use | 4.3 |
| Diuretic drugs | 3.5 |
| Muscle relaxants | 3.3 |
* Only the top 10 most common medication classes are reported; therefore, reported percentages may not add up to 100%.
† Using the Anatomic Therapeutic Chemical (ATC) classification system Level 2 subgroups.
‡ The psycholeptic category includes the antipsychotics, anxiolytics, hypnotics, and sedatives.
§ The psychoanaleptic category includes the antidepressants, psychostimulants, nootropics, antidementia drugs, and combinations with psycholeptics.
Perceived Parental Confidence and Understanding. On 11 confidence and understanding statements, parents reported a high perceived level of understanding and confidence, with combined agreement or strong agreement ranging between 81.2% and 98.7% (Table 4). Parents perceived a high level of understanding of handling of missed doses (90.1%) and when to call the physician (95.4%) but were less clear about potential side effects (81.2%) or how to handle adverse effects (83.2%). Parents reported confidence in their abilities to administer enteral medications (>90% for each statement).
Table 4.
Parental Response to Perceived Understanding and Confidence Survey Questions
| % Positive Response* | % Negative Response† | |
|---|---|---|
| Understanding items | ||
| I know if/when to call my physician regarding my child’s medications | 95.4 | 4.6 |
| I understand what to do if my child misses a dose of a medication | 90.1 | 9.9 |
| I am satisfied with the explanations I was given regarding my child’s medications | 88.9 | 11.1 |
| I understand what to do if my child experiences a side effect to a medication | 83.2 | 16.8 |
| I understand the possible side effects of my child’s medications | 81.2 | 18.8 |
| Confidence items | ||
| I am confident in my abilities to accurately measure liquid medications | 98.7 | 1.3 |
| I am confident in my abilities to correctly administer all of the medications my child is taking | 97.4 | 2.6 |
| I am confident I understand why my child was prescribed each of the medications | 95.5 | 4.5 |
| I am confident in administering partial tablets or crushed tablets to my child | 92.9 | 7.1 |
| If my provider said it was possible, I would be willing to stop 1 or more of the regular medications my child is taking | 86.5 | 13.5 |
| I feel confident that the number of medications my child is taking is needed | 86.5 | 13.5 |
* Strongly Agree or Agree.
† Neutral, Disagree, or Strongly Disagree.
Demonstrated Parental Understanding. Despite a high perceived understanding and confidence related to CMRs, when asked to identify the medications taken for the specified complex problem, 78.1% and 85.4% of parents correctly reported for condition 1 and condition 2, respectively. Only 73.1% were able to correctly report for both conditions. Regarding dosing parameters for high-risk medications, most parents accurately identified from memory the indication for 1 high-risk medication (94.9%), but many fewer correctly identified the route (84.5%), frequency (80%), dose (69.2%), and formulation (45.9%). Only 40.4% were able to report complete dosing parameters. For the demonstrated measurement of a liquid medication dose, 66.4% were able to accurately measure a 4-mL dose, and 63.9% measured a dose of 0.35 mL accurately. Only 54.8% were able to correctly measure both doses. Regression analyses revealed no significant relationships between parental characteristics and the ability to successfully complete any demonstrated understanding task.
Parental Perceived Versus Demonstrated Understanding. For each of the 3 measured domains of demonstrated understanding, significant differences existed between parental perceived versus demonstrated understanding (McNemar test, all p < 0.05) (Figure 2). For example, although 97.4% of parents reported confidence in accurately measuring liquid medications, 54.8% correctly measured 2 different doses of medications (p < 0.05).
Figure 2.
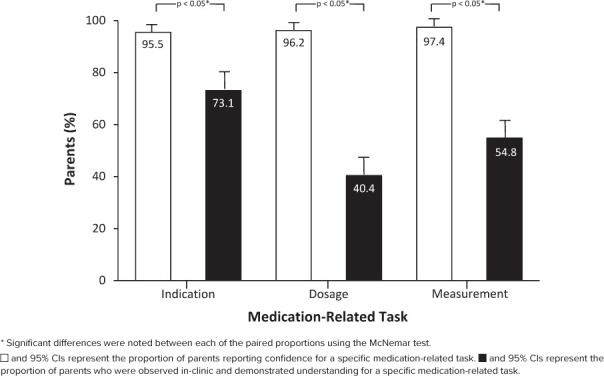
Comparison of parental perceived versus demonstrated understanding.
Discussion
In this study of parental understanding of CMRs used by CMC, most parents reported high confidence in understanding of CMRs, but substantially fewer parents demonstrated actual understanding of CMRs. The study population was composed of younger CMC, many of whom had polypharmacy and exposure to high-risk and compounded medications. Most of their medications—many of which included CNS-active medications—were administered at home by parents. Parents tended to be in their late 30s and with some college education. Parents had some difficulty correctly identifying which medications were indicated for certain conditions. Parents struggled with identification of dosing parameters for high-risk medications and were particularly challenged by accurate measurement of medications.
While this study represents the first published report comparing parental perceived versus demonstrated confidence and knowledge pertaining to CMRs for CMC, our findings are consistent with existing literature examining the broader population. Parent liquid dosing errors have been consistently reported across all health literacy and language groups.18–21 Our study similarly found substantial parent errors with liquid measurements when using oral dosing syringes even without having to first interpret a medication label, identify the current oral dosing syringe size, and then draw up doses. Unlike previously published studies, we also evaluated parents' ability to correctly identify medications for a given complex condition, as well as identify dosing parameters associated with a high-risk medication. Our study identified gaps in parental understanding of CMRs for CMC, which highlight the critical need for improved medication education and support available to parents. Such efforts represent an important target for mitigating potential medication-related errors in the population of CMC. In light of this, 3 aspects of our study findings deserve further discussion.
First, in order to intervene, we need to be able to identify CMC at the highest risk of experiencing medication-related issues. Although we used an automated medication screening process in the electronic health record, approximately 40% of potential subjects did not have a CMR upon manual review. An accurate, robust, validated tool focused on medication regimen complexity (e.g., number and type of medications, number of distinct daily doses), degree of required dosage form manipulation, health literacy, and spoken language is urgently needed to improve medication use and safety in CMC. Our data indicate that CMC with CMRs take a variety of prescription and over-the-counter medications, but also a wide range of vitamins and minerals; efficient identification of at-risk patients may need to focus on specific medications rather than polypharmacy counts. Future work focused on the identification of the most impactful medications on patient safety will be important to help with patient risk stratification. Scoring systems for the stratification of children by medical complexity and identification of resources needed (e.g., care coordination) have been reported.8,22–24 The development of a clinical pharmacy priority score has been described in a family medicine setting.25 However, to our knowledge, a validated, robust medication regimen acuity scoring tool for the identification of at-risk patients and their families for adverse drug events, including parental understanding, has not yet been developed. The tool should also account for transitions of care across various health care settings as well as between pediatric and adult care, where medication-related problems or points of confusion may be more likely to occur.26–29
The substantial gaps we observed between parental confidence and understanding reinforces the need for improved medication-related education. Standardized education tools to support correct at-home medication administration are needed. A recent article written by a group of experienced parents of CMC notes the benefit of in-person, teach-back sessions as well as visual and written instructions to improve understanding.30 Such standardized tools should be used universally to ensure understanding in the pediatric population. For particularly high-risk patients, a specialized pediatric clinical pharmacist, incorporated into the multidisciplinary care team, may be well positioned to provide one-on-one counseling, particularly as it relates to CMRs. The success of the pharmacists' roles on improving medication understanding, beliefs, and adherence has been previously reported in the adult population and in a variety of specialty care settings.31–33 These models should be considered and trialed in the pediatric population, especially in the complex care setting. Specialized pediatric pharmacists, incorporated into clinic visits, can provide an initial evaluation of parental understanding, provide baseline education and ongoing assessments and educational updates at subsequent clinic visits and/or through telehealth visits. This type of recurring assessment and education is particularly important when new high-risk medications are started or when dosing changes occur. Changes in understanding should be systematically tracked through integration of a universal and standardized approach to medication education in this vulnerable population. Additionally, assessments and educational interventions need to be developed and validated in multiple languages and cultural contexts in order to have the broadest impact.
Finally, we measured 3 discrete tasks in clinic, but it may additionally be important to understand what happens in vivo in the home setting, especially since our findings indicate most medications are administered by parents without home nursing supervision. We measured 3 aspects of importance for medication safety, but there are almost certainly additional factors related to safe medication administration. Neither this study nor previous reports account for administration errors after measurement. For example, if the concentration of an extemporaneously compounded medication changes and the family measures the previous volume rather than following new instructions on the bottle, significant dosing errors can occur. While national efforts to standardize medication concentrations are underway, a high level of variability of compounded concentrations and pediatric-specific dosage forms currently exists, which may increase the chance for errors.34–36 A lack of precision, especially for narrow therapeutic index medications, could result in significant adverse drug events. Furthermore, medications are commonly administered via alternative routes, such as through enteral feeding tubes. Finally, even if medications are perfectly administered in the in-home environment, the monitoring of medication efficacy and safety relies heavily on parental knowledge and attention. Parents must be well informed about what desired effects and side effects should be expected with CMRs to appropriately monitor medication response.
Our findings must be interpreted in the context of several limitations. First, this study was conducted at a single center and the findings may not be generalizable to all CMCs who use CMRs. The study site, however, is one of the largest outpatient complex care programs in the country and a member of the Children and Youth with Special Healthcare Needs National Research Network (www.cyshcnet.org). Secondly, inclusion was limited to those who could understand and speak English, and most parents were white with a higher level of education. Similar assessments in non-English speakers, and a lower income, lower education, minority, underserved population may broaden applicability. Third, we provided conservative estimates of accuracy (i.e., no errors in measurement) to account for high-risk medications with narrow therapeutic windows by using a strict definition of accuracy. For medications with a wide therapeutic window, our definition of accuracy may overestimate the impact of parent measurement on clinical outcomes. Caregivers were also not allowed to use notes during the observation. If notes had been allowed, it remains possible that our results would be altered. The strict definition of accuracy and the inability to use notes was used to account for the worst-case scenario. Next, the in-clinic demonstrated measurement of medications was completed by only 86.5% of parents. It remains possible that our results may have been altered with a more complete response rate. Next, although we measured demonstrated parental understanding, using standardized tasks, the study design did not assess parental interpretation and understanding of medication labels or successful at-home administration of medications. In their specific home environment, parents might have been able to correctly demonstrate understanding of their child's specific CMR, as opposed to the in-clinic study tasks. Furthermore, this study evaluated parental understanding but did not evaluate other members of the child's care-team (e.g., home nursing), whose presence could have reduced the potential risk of medication-related errors. To reduce the in-clinic study burden, we selected certain complex indications and medications as a proxy of understanding of the entire regimen. This may not be representative of the parent's understanding as a whole.
These limitations notwithstanding, substantial opportunities exist to improve medication safety and efficacy in the outpatient, in-home setting including a systematic approach to identification of patients most at risk, improved education, and assessment of in-home medication use. Care in this population will remain difficult to optimize without a systemwide focus on addressing these critical gaps. With a unique focus on and understanding of medication complexity and nuance in this population, pediatric clinical pharmacists may be well positioned to participate in the development and implementation of interventions.37,38 For example, clinical pharmacy telemedicine interventions in the ambulatory setting have been shown to positively impact outcomes.39–41 Moving forward, pediatric clinical pharmacists may be able to engage in telehealth initiatives to better evaluate, understand, and intervene on ambulatory and in-home medication-related issues. Another potential approach is the implementation of required clinical pharmacist-led educational program in-clinic at the point of prescribing with a teach-back “check off” prior to the parent picking up the medication at the pharmacy.
Conclusions
A substantial gap exists between parent-reported perceived knowledge and actual demonstrated understanding of CMRs, which could impact patient safety. The development, implementation, and evaluation of educational supports and assessment of parental understanding both within clinic visits as well as in the home setting are needed and may directly benefit parents of CMC who must administer CMRs at home. Ultimately, enabling parents to continue to provide safe and effective medication administration at home may improve the ability for CMC to continue to thrive in the outpatient environment.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr Lucas Orth for his comprehensive review and editorial support provided during the preparation of this manuscript.
ABBREVIATIONS
- AHRQ
Agency for Healthcare Research and Quality
- CMC
children with medical complexity
- CMR
complex medication regimen
- CNS
central nervous system
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
This study was supported by the Children's Hospital Colorado Clinical and Operational Effectiveness and Patient Safety (COEPS) Small Grants Program as well as the Colorado Clinical and Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780). Dr Feinstein was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award No. K23HD091295. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by the NIH or the US Government.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the appropriate committees at our institution. All patients and/or parents/caregiver(s) provided written informed consent and/or assent (as applicable) at enrollment.
Supplemental Material
DOI: 10.5863/1551-6776-26.1.62.S1
DOI: 10.5863/1551-6776-26.1.62.S2
DOI: 10.5863/1551-6776-26.1.62.S3
DOI: 10.5863/1551-6776-26.1.62.S4
DOI: 10.5863/1551-6776-26.1.62.S5
References
- 1.Feinstein JA, Feudtner C, Valuck RJ, Kempe A. The depth, duration, and degree of outpatient pediatric polypharmacy in Colorado fee-for-service Medicaid patients. Pharmacoepidemiol Drug Saf. 2015;24(10):1049–1057. doi: 10.1002/pds.3843. [DOI] [PubMed] [Google Scholar]
- 2.Bayldon BW, Glusman M, Fortuna NM et al. Exploring caregiver understanding of medications immediately after a pediatric primary care visit. Patient Educ Couns. 2013;91(2):255–260. doi: 10.1016/j.pec.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Pearson GS. Use of polypharmacy with children and adolescents. J Child Adolesc Psychiatr Nurs. 2013;26(2):158–159. doi: 10.1111/jcap.12034. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi M, Seifert S, Kunkel S et al. Toxicity from a clonidine suspension. J Med Toxicol. 2009;5(3):130–133. doi: 10.1007/BF03161223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XI, Schuette P, Burckart GJ et al. A comparison of pediatric and adult safety studies for antipsychotic and antidepressant drugs submitted to the United States Food and Drug Administration. J Pediatr. 2019;208:236–242 e233. doi: 10.1016/j.jpeds.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinke ML, Bundy DG, Velasquez CA et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134(2):338–360. doi: 10.1542/peds.2013-3531. [DOI] [PubMed] [Google Scholar]
- 7.McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics. 2013;132(4):730–740. doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon TD, Cawthon ML, Stanford S et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647–e1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry JG, Poduri A, Bonkowsky JL et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. doi: 10.1371/journal.pmed.1001158. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healthcare Cost and Utilization Project - Chronic Condition Indicator (CCI) for ICD-9-CM. Web site. Accessed May 3, 2020. https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp.
- 11.Cohen E, Kuo DZ, Agrawal R et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein JA, Hall M, Antoon JW et al. Chronic medication use in children insured by Medicaid: a multistate retrospective cohort study. Pediatrics. 2019;143(4) doi: 10.1542/peds.2018-3397. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuyan B, Sancar M, Izzettin FV. Assessment of medication knowledge and adherence among patients under oral chronic medication treatment in community pharmacy settings. Pharmacoepidemiol Drug Saf. 2013;22(2):209–214. doi: 10.1002/pds.3275. [DOI] [PubMed] [Google Scholar]
- 14.Okuyan B, Sancar M, Izzettin FV, Morisky DE. Erratum to and corrections on the article entitled “Assessment of medication knowledge and adherence among patients under oral chronic medication treatment in community pharmacy settings”. Pharmacoepidemiol Drug Saf. 2013;22(2):218–220. doi: 10.1002/pds.3394. [DOI] [PubMed] [Google Scholar]
- 15.Reeve E, Wiese MD, Hendrix I et al. People's attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61(9):1508–1514. doi: 10.1111/jgs.12418. [DOI] [PubMed] [Google Scholar]
- 16.ASHP guidelines on pharmacist-conducted patient education and counseling. Am J Health Syst Pharm. 1997;54(4):431–434. doi: 10.1093/ajhp/54.4.431. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin HS, Dreyer BP, van Schaick L et al. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adolesc Med. 2008;162(9):814–822. doi: 10.1001/archpedi.162.9.814. [DOI] [PubMed] [Google Scholar]
- 19.Yin HS, Dreyer BP, Ugboaja DC et al. Unit of measurement used and parent medication dosing errors. Pediatrics. 2014;134(2):e354–e361. doi: 10.1542/peds.2014-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin HS, Mendelsohn AL, Wolf MS et al. Parents' medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164(2):181–186. doi: 10.1001/archpediatrics.2009.269. [DOI] [PubMed] [Google Scholar]
- 21.Yin HS, Parker RM, Sanders LM et al. Liquid medication errors and dosing tools: a randomized controlled experiment. Pediatrics. 2016;138(4):e20160357. doi: 10.1542/peds.2016-0357. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon TD, Cawthon ML, Popalisky J, et al. Center of Excellence on Quality of Care Measures for Children with Complex Needs Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) Version 2.0. Hosp Pediatr. 2017;7(7):373–377. doi: 10.1542/hpeds.2016-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon TD, Haaland W, Hawley K et al. Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) Version 3.0. Acad Pediatr. 2018;18(5):577–580. doi: 10.1016/j.acap.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry JG, Hall M, Cohen E et al. Ways to identify children with medical complexity and the importance of why. J Pediatr. 2015;167(2):229–237. doi: 10.1016/j.jpeds.2015.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vande Griend JP, Saseen JJ, Bislip D et al. Prioritization of patients for comprehensive medication review by a clinical pharmacist in family medicine. J Am Board Fam Med. 2015;28(3):418–424. doi: 10.3122/jabfm.2015.03.140303. [DOI] [PubMed] [Google Scholar]
- 26.Leyenaar JK, O'Brien ER, Leslie LK et al. Families' priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. doi: 10.1542/peds.2016-1581. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breneol S, Belliveau J, Cassidy C, Curran JA. Strategies to support transitions from hospital to home for children with medical complexity: a scoping review. Int J Nurs Stud. 2017;72:91–104. doi: 10.1016/j.ijnurstu.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Porepa M, Hoffman A, Fellin M, Kublick L. Children with medical complexities: addressing the gaps in respite care during transition from paediatrics to adult health care in Ontario. Paediatr Child Health. 2017;22(7):369–371. doi: 10.1093/pch/pxx142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leyenaar JK, Rizzo PA, Khodyakov D et al. Importance and feasibility of transitional care for children with medical complexity: results of a multistakeholder Delphi process. Acad Pediatr. 2018;18(1):94–101. doi: 10.1016/j.acap.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allshouse C, Comeau M, Rodgers R, Wells N. Families of children with medical complexity: a view from the front lines. Pediatrics. 2018;141(suppl 3):S195–S201. doi: 10.1542/peds.2017-1284D. [DOI] [PubMed] [Google Scholar]
- 31.Birand N, Bosnak AS, Diker O et al. The role of the pharmacist in improving medication beliefs and adherence in cancer patients. J Oncol Pharm Pract. 2019;25(8):1916–1926. doi: 10.1177/1078155219831377. [DOI] [PubMed] [Google Scholar]
- 32.Peasah SK, Granitz K, Vu M, Jacob B. Effectiveness of a student pharmacist-led telephone follow-up intervention to improve hemoglobin A1C in diabetic patients. J Pharm Pract. 2019;33(6):832–837. doi: 10.1177/0897190019857409. [DOI] [PubMed] [Google Scholar]
- 33.Hawley CE, Triantafylidis LK, Paik JM. The missing piece: clinical pharmacists enhancing the interprofessional nephrology clinic model. J Am Pharm Assoc (2003) 2019;59(5):727–735. doi: 10.1016/j.japh.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batchelor HK, Marriott JF. Formulations for children: problems and solutions. Br J Clin Pharmacol. 2015;79(3):405–418. doi: 10.1111/bcp.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134(2):361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- 36.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59(6):674–676. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eiland LS, Benner K, Gumpper KF et al. ASHP-PPAG guidelines for providing pediatric pharmacy services in hospitals and health systems. Am J Health Syst Pharm. 2018;75(15):1151–1165. doi: 10.2146/ajhp170827. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt-Mehta V, Buck ML, Chung AM et al. Recommendations for meeting the pediatric patient's need for a clinical pharmacist: a joint opinion of the Pediatrics Practice and Research Network of the American College of Clinical Pharmacy and the Pediatric Pharmacy Advocacy Group. Pharmacotherapy. 2013;33(2):243–251. doi: 10.1002/phar.1246. [DOI] [PubMed] [Google Scholar]
- 39.Niznik JD, He H, Kane-Gill SL. Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: a systematic review. Res Social Adm Pharm. 2018;14(8):707–717. doi: 10.1016/j.sapharm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Singh LG, Accursi M, Korch Black K. Implementation and outcomes of a pharmacist-managed clinical video telehealth anticoagulation clinic. Am J Health Syst Pharm. 2015;72(1):70–73. doi: 10.2146/ajhp130750. [DOI] [PubMed] [Google Scholar]
- 41.Cohen LB, Taveira TH, Wu WC, Pirraglia PA. Pharmacist-led telehealth disease management for patients with diabetes and depression. J Telemed Telecare. 2020 Jun;26(5):294–302. doi: 10.1177/1357633X18822575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


