Abstract
OBJECTIVE
Vancomycin is commonly used in the neonatal population to treat Gram-positive bacterial infections. Despite frequent use, consensus on the ideal dosing regimen in low birth weight (LBW) neonates is lacking. The objective of this research is to determine how frequently vancomycin troughs within goal range (10–20 mg/L) are achieved with empiric dosing in critically ill neonates and infants weighing less than 2500 g.
METHODS
This retrospective review evaluated LBW infants who were admitted to a level IV NICU from January 2015 to December 2016. Patients were included if they had a vancomycin trough sample collected at steady state (after at least 3 doses). Three trough cohorts (subtherapeutic: <10 mg/L, therapeutic: 10–20 mg/L, and supratherapeutic: >20 mg/L) were compared with 1-way ANOVA for continuous data and a chi-square analysis for categorical data.
RESULTS
A total of 74 patients were included, with a mean birth weight (BW) of 819.7 ± 355.4 g and a mean gestational age (GA) of 26.4 ± 3.7 weeks. Only 27 patients (36.5%) had therapeutic vancomycin trough concentrations. Subtherapeutic troughs were recorded in 40 patients (54.1%), while supratherapeutic troughs were recorded in 7 patients (9.5%). Although there was no difference between the initial dose, initial frequency was significantly different between cohorts (p = 0.04).
CONCLUSION
Empiric dosing regimens do not produce vancomycin troughs within the goal range in most LBW patients.
Keywords: birth weight; gestational age; infant, newborn; intensive care units, neonatal; research; vancomycin
Introduction
Vancomycin is a glycopeptide antibiotic that is frequently used in neonates with suspected or confirmed Gram-positive bacterial infections. Despite published description of vancomycin pharmacokinetics in the neonatal population, there is a lack of consensus on the most appropriate dosage regimen. Pharmacokinetics of vancomycin is altered in neonates owing to increased volume of distribution and decreased clearance relative to pediatric patients.1 Factors such as fluctuating renal function in the neonatal period and concomitant use of nephrotoxic medications can further complicate vancomycin dosing.2,3 Dosing recommendations for low birth weight (LBW) patients, such as those outlined in Lexicomp (Wolters Kluwer Clinical Drug Information Inc, Hudson, OH), NeoFax (IBM Corporation, Greenwood Village, CO), and in primary literature are highly variable, ranging from 10 to 20 mg/kg per dose given every 8 to 48 hours, depending on the patient's renal function,1,4,5 weight,6 and indication for vancomycin.
Therapeutic drug monitoring (TDM) is a useful tool in evaluating appropriate dosing regimens for neonates and infants. The ratio of the 24-hour area under the concentration-time curve (AUC24) to the MIC has been suggested as a parameter of vancomycin efficacy in invasive methicillin-resistant Staphylococcus aureus (MRSA) infections.7 Although this method is being used more frequently, owing to the relative difficulty of calculating AUC24 for individual patients, the most commonly used marker for vancomycin efficacy and toxicity is trough concentration.8,9 However, suggested vancomycin trough concentration goals have changed over time.
Initially, 5 to 10 mg/L was suggested as an adequate trough concentration goal,10 but this benchmark likely falls short of achieving necessary vancomycin concentrations in many types of infections. In 2009, a consensus guideline of vancomycin dosing in adults determined that minimum concentrations should be maintained above 10 mg/L to avoid development of resistance.11 Adult data also indicate that for infections with a MIC of 1 mg/L, the vancomycin trough of at least 15 mg/L should be maintained to achieve the target AUC24/MIC ratio of 400 mg*hr/L.12–14 In particular, higher trough concentrations of 15 to 20 mg/L are recommended for complicated infections such as bacteremia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia caused by S aureus.11 However, neonatal studies have found that a trough of 10 to 11 mg/L correlates with an AUC24 of >400 mg*hr/L, meaning a goal concentration around 10 mg/L would adequately treat an infection with MIC ≤1 mg/L.8,9 Recently, a revised consensus guideline of therapeutic monitoring for serious vancomycin for MRSA infections was published.15 In it the authors15 warn that using a surrogate goal of trough concentrations of 15 to 20 mg/L instead of the AUC24/MIC target of 400 mg*hr/L may lead to increased nephrotoxicity in adult and pediatric patients.
A trend of low vancomycin trough concentrations has been observed at our NICU in LBW infants. The purpose of this study was to retrospectively assess how often vancomycin troughs within goal range (10–20 mg/L) were achieved from the initial vancomycin dosing regimen in critically ill infants weighing less than 2500 g. To our knowledge, it is the first study focused on vancomycin dosing in LBW infants, without regard to gestational age (GA) at birth.
Methods
Study Design and Patient Population. A retrospective observational study was conducted of patients admitted to the 48-bed, level IV Children's of Alabama (COA) NICU in Birmingham, Alabama, from January 2015 to December 2016. COA is a private, not-for-profit, teaching hospital staffed by University of Alabama at Birmingham neonatologists. It is the only free-standing pediatric hospital in Alabama. As such, patients are not in-born but are transferred from referring hospitals based on severity of illness and need for care by specialists.
Vancomycin doses are infused over 60 minutes, and empiric vancomycin dosing at COA is age based. Patients 28 days of age or younger receive 15 mg/kg/dose of vancomycin with the interval chosen by the prescriber, based on clinical factors including renal function and disease state. Patients 28 days of age and older receive 20 mg/kg/dose, and again, the interval is chosen by the prescriber. This introduces considerable variability in the amount of vancomycin LBW infants receive.
Therapeutic drug monitoring is performed in NICU patients under the discretion of the neonatology and surgery teams with guidance provided by 2 NICU clinical pharmacists as part of routine care. Unless an increase in serum creatinine or a decrease in urine output causes concern for renal dysfunction, trough concentrations are collected at steady state (drawn after a minimum of 3 vancomycin doses, within 1 hour of the next dose). In patients receiving antibiotics for a sepsis “rule out,” trough concentration collection is often deferred until after the initial 48-hour treatment course, as antimicrobials are ideally discontinued if blood cultures are negative at that time. Goal troughs in our NICU are indication based and are either 10 to 15 mg/L or 15 to 20 mg/L, depending on factors such as MIC of the bacterial isolate, location of infection, and ability to remove sources of infection such as catheters from the patient. As such, the goal vancomycin trough range for this study was 10 to 20 mg/L. Trough concentrations <10 mg/L were considered subtherapeutic, and concentrations >20 mg/L were considered supratherapeutic.
The patients included in this review were ≤44 weeks' postmenstrual age, weighing ≤2500 g, and received ≥3 doses of vancomycin with at least 1 trough concentration during the study period. Exclusion criteria included clinically significant cardiovascular diagnoses or support with dialysis or extracorporeal membrane oxygenation. Demographic data were collected including GA at birth, postmenstrual age at time of vancomycin initiation, BW, weight at time of vancomycin initiation, sex, NICU survival, and primary diagnosis (reason for transfer to a level IV NICU). Data related to the vancomycin course were also collected including serum creatinine at baseline, maximum serum creatinine during the vancomycin course, number of vancomycin doses given prior to the trough concentration, vancomycin dose (milligrams per kilogram per dose) and frequency, and first trough concentration. All data collection was performed via electronic medical health record review. The study received institutional review board approval from the University of Alabama at Birmingham.
Study Variables and Measured Outcomes. The primary outcome was the percentage of patients achieving a trough concentration between 10 and 20 mg/L with initial vancomycin dosing. Blood samples for trough concentrations were drawn by the nursing staff in accordance with standards of care, and the trough values were determined by the hospital laboratory, using Abbott Architect i1000SR (Abbott Park, IL). Secondary outcomes included mortality during the index NICU admission and statistically significant serum creatinine increase during vancomycin therapy.
Statistical Analyses. Descriptive statistics were performed for each of the vancomycin trough cohorts. Continuous data were expressed as mean, standard deviation (mean ± SD). Means were compared with 1-way ANOVA. Categorical data were compared with a chi-square analysis, except when expected counts were <5, for which Fischer exact test was used. Missing data were removed from analysis. A p value <0.05 was considered statistically significant. Data analysis was performed by using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Results
Study Population. There were 143 patients weighing ≤2500 g and ≤44 weeks' postmenstrual age who received vancomycin in the designated time frame. One patient was excluded for receiving dialysis support, and another 17 patients were excluded for having clinically significant cardiac diagnoses. Each vancomycin order was analyzed for inclusion as a discrete record. There were 654 records for the remaining 125 patients. Of these, 244 records were excluded for having fewer than 3 doses of vancomycin given, leaving 410 records. Lastly, records without a trough recorded were removed, leaving 184 records. Of the remaining 184 records, the first record for each patient (n = 74) was included in analysis (Figure). Patients were compared with one another based on 3 trough groupings: <10 mg/L, 10 to 20 mg/L, and >20 mg/L.
Figure.
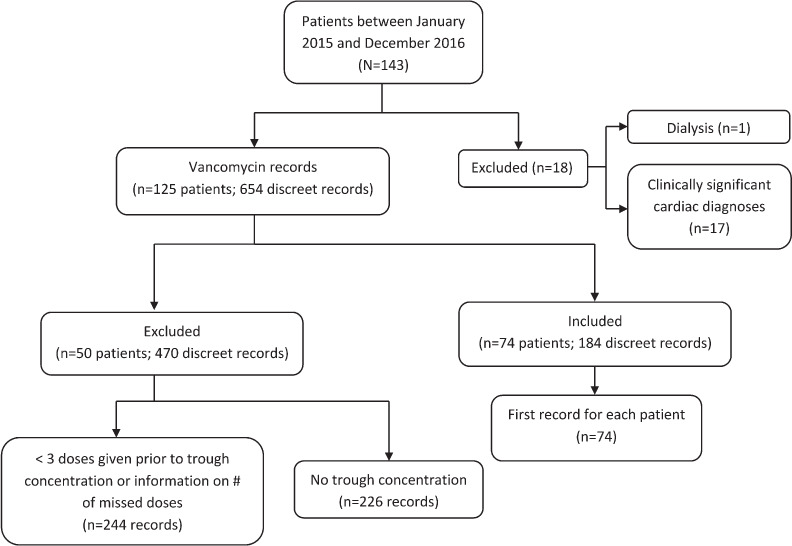
Flow diagram of study participant inclusion.
Measured Outcomes. The first trough concentration for each included patient's vancomycin course was analyzed. Among the 74 patients included, 40 patients (54.1%) had subtherapeutic trough concentrations, 27 patients (36.5%) achieved goal trough values, and 7 patients (9.5%) had supratherapeutic trough concentrations (Table 1). There was no significant difference in the mean individual dose given between groups, but there was a significant difference (p = 0.04) in frequency, with patients in the <10 mg/L trough group more likely to have a frequency of every 12 hours or greater (Table 1). Baseline serum creatinine was similar between groups, but the maximum serum creatinine recorded during vancomycin treatment was significantly different: 0.45 ± 0.23 mg/dL for trough <10 mg/L; 0.62 ± 0.34 mg/dL for trough of 10 to 20 mg/L; and 0.77 ± 0.3 mg/dL for trough >20 mg/L (p = 0.004).
Table 1.
Vancomycin Data
| Characteristics | Vancomycin Trough Serum Concentration, mg/L | p value | ||
|---|---|---|---|---|
| Trough <10 (n = 40) | Trough 10–20 (n = 27) | Trough >20 (n = 7) | ||
| Dose, mean ± SD, mg/kg | 14.9 ± 1.8 | 14.6 ± 2.6 | 16.4 ± 2.4 | 0.14 |
| Frequency, n (%), hr | ||||
| 6 | 0 | 1 (3.7) | 0 | |
| 8 | 1 (2.5) | 6 (22.2) | 3 (42.9) | |
| 12 | 25 (62.5) | 15 (55.6) | 3 (42.9) | 0.04 |
| 18 | 7 (17.5) | 2 (7.4) | 1 (14.3) | |
| 24 | 7 (17.5) | 3 (11.1) | 0 | |
| Trough concentration, mean ± SD (range), mg/L | 5.9 ± 2 (3–9.4) | 14 ± 3.4 (10.4–20.8) | 28.2 ± 5.3 (23.1–35.7) | <0.001 |
| Doses given prior to trough, mean ± SD (range), n | 4.1 ± 1.6 (3–10) | 3.6 ± 0.9 (3–6) | 3 ± 0 (3–3) | 0.08 |
Patient Demographics and Clinical Characteristics. There was no difference in GA at birth, corrected GA, BW, weight at time of vancomycin initiation, sex, or primary diagnosis (Table 2). The mortality rate for all patients was 21.6%, as 16 of 74 patients died during the index NICU admission.
Table 2.
Comparison of Patient Demographics by Trough Concentration
| Characteristics | Vancomycin Trough Serum Concentration, mg/L | p value | ||
|---|---|---|---|---|
| Trough <10 (n = 40) | Trough 10–20 (n = 27) | Trough >20 (n = 7) | ||
| Gestational age at birth, mean ± SD (range), wk | 26.7 ± 4 (22.4–35.6) | 26.2 ± 3.7 (22–38.6) | 25.5 ± 1.6 (22.4–27.2) | 0.71 |
| Corrected gestation age, mean ± SD (range), wk | 31.1 ± 4.4 (24.2–40.4) | 30.7 ± 3.8 (25.1–42.3) | 32.4± 2 (29.1–35.3) | 0.61 |
| Birth weight, mean ± SD (range), g | 859.4 ± 384 (470–1895) | 799.4 ± 348.2 (450–1775) | 677.1 ± 143.8 (480–880) | 0.43 |
| Weight at time of vancomycin initiation, mean ± SD (range), kg | 1.2 ± 0.5 (0.5–2.2) | 1.1 ± 0.4 (0.6–1.9) | 1.1 ± 0.3 (0.6–1.5) | 0.52 |
| Sex, n (%) | ||||
| Male | 24 (60) | 14 (51.9) | 3 (42.9) | 0.66 |
| Female | 16 (40) | 13 (48.2) | 4 (57.1) | |
| Diagnosis, n (%) | ||||
| Gastrointestinal | 27 (67.5) | 18 (66.7) | 5 (71.4) | |
| Neurosurgical | 8 (20) | 4 (14.8) | 1 (14.3) | |
| Infectious | 1 (2.5) | 2 (7.4) | 1 (14.3) | 0.92 |
| Miscellaneous* | 2 (5) | 2 (7.4) | 0 (0) | |
| Respiratory | 2 (5) | 1 (3.7) | 0 (0) | |
* Miscellaneous diagnoses: choanal atresia, congenital anomalies, incarcerated hernia, and neonatal hemochromatosis.
Discussion
Preterm and LBW infants are at risk for sepsis16 and acute kidney injury, both of which independently increase risk of mortality.17 There is a paucity of data on vancomycin for these infants, despite the fact that vancomycin is the treatment of choice for neonatal infection caused by coagulase-negative Staphylococcus species and MRSA.18,19
Vancomycin is the most frequently used antibiotic at this institution. In 2017, institution-wide vancomycin usage ranged between 80 and 100 days of therapy per 1000 patient days, with the second highest used antibiotic ranging from 28 to 52 days of therapy per 1000 patient days. The hospital's antimicrobial susceptibility report from January 2017 to December 2017 indicates 100% vancomycin susceptibility for Gram-positive organisms isolated from sterile sites (Enterococcus faecalis, S aureus, and coagulase-negative Staphylococcus) for both inpatient and community (outpatient) isolates.
In the present study, we determined that only 36.5% of LBW, critically ill neonates achieved vancomycin troughs within goal range with empiric therapy, whereas 54.1% had subtherapeutic troughs and 9.5% had supratherapeutic troughs. Of note, the 2011 Infectious Disease Society of America guidelines for the treatment of MRSA recommend an even higher trough concentration of 15 to 20 mg/L for serious infections in patients of all ages, although the authors recommend additional studies be performed in the pediatric population to provide efficacy and safety data of this higher trough range.7
Previous studies conducted in similar populations found that goal vancomycin troughs are often not achieved with empiric therapy. In 2014, Sinkeler et al18 analyzed initial trough concentrations in a retrospective cohort of preterm and term neonates admitted to a tertiary NICU from 2009–2012. The authors determined that of these 112 patients, 53 (47%) had a subtherapeutic concentration, 37 (33%) had a concentration at goal, and 22 (20%) had a supratherapeutic concentration. Of note, a therapeutic vancomycin trough was defined as 10 to 15 mg/L, with anything less than 10 mg/L deemed subtherapeutic, and greater than 15 mg/L, supratherapeutic. Mortality rate was similar to that seen in the present study at 18% (21/112 patients).
Ringeberg et al19 studied initial trough concentrations in a multi-institutional retrospective chart review of neonatal patients treated with vancomycin, using dosing recommendations from a common neonatal reference. There were 4 level III NICUs included in the study. The average GA was 28.2 ± 4.1 weeks, average postnatal age at start of vancomycin therapy was 34.1 ± 34.6 days, and average weight of the patients at start of vancomycin was 1602 ± 1014.5 g. They found that only 25.1% of n = 171 vancomycin serum trough concentrations represented an initial vancomycin trough within goal range of 10 to 20 mg/L with empiric dosing.
Madigan et al20 performed retrospective analysis of 57 preterm, very LBW neonates receiving vancomycin in the NICU before and after implementation of a new vancomycin dosing protocol. Both vancomycin daily dose and frequency of administration were increased in the new protocol. The postimplementation group experienced lower rates of undetectable trough concentrations (24% vs 50%, p = 0.04), higher median trough concentrations (10.8 vs 5.9 mg/L), a higher proportion of trough concentrations in goal range of 10 to 20 mg/L (35% vs 4%, p = 0.005), and a significantly higher vancomycin AUC24 than historical controls. Despite improvements seen in the intervention group, most patients did not achieve goal trough concentrations.20 This further demonstrates the difficulty of safely and effectively dosing vancomycin in small neonates.
Nephrotoxic medications are a modifiable risk factor for acute kidney injury, while other risk factors such as premature birth and congenital heart disease are not, so a multidisciplinary effort to reduce nephrotoxin-induced acute kidney injury AKI is imperative.17 Not surprisingly, the patients with higher than desired trough results also had a significantly higher serum creatinine maximum (defined as the highest serum creatinine concentration measured during each patient's vancomycin course). The same correlation was noted in a 2017 retrospective review of premature neonates exposed to vancomycin.21
The patients included in the present study had a low mean GA (26.4 ± 3.7 weeks) and BW (819.7 ± 355.4 g). They are representative of populations served in level IV NICUs throughout the United States, with exclusion of the fewest number of patients feasible. Patients with significant cardiac diagnoses were excluded owing to the fluctuating circulation these patients experience and the reduction in vancomycin clearance that has been noted with concomitant use of indomethacin and ibuprofen (40%–50% and 28% reduction, respectively).2 Infants weighing ≤2500 g were included regardless of GA at birth, which allowed for a diverse patient population. Gestational age at birth ranged from 22 weeks to 38 weeks, 6 days. This differs from the studies of Sinkeler18 and Ringeberg19 that included term and preterm infants, regardless of weight, and the study by Madigan20 that focused on very LBW preterm neonates.
The present study has some limitations, including its retrospective nature. It was completed in a single level IV NICU with a small sample size. During the study period, no standard dosing protocol was in place for vancomycin, therefore the initial dosing regimen was at the discretion of the prescriber with assistance from 2 clinical pharmacists. Renal dysfunction was not identified according to the neonatal acute kidney injury KDIGO classification22 or the RIFLE criteria23 and urine output was not assessed. Instead, an increase in serum creatinine from baseline to the maximum recorded serum creatinine while on vancomycin therapy was noted in terms of statistical significance. Severity of illness and risk of mortality data were not available, and information about ethnicity, concomitant nephrotoxic medications, vancomycin AUC, and presence of bacterial infections was not recorded. Although most concentrations (81.08%) were drawn within 1 hour of the intended frequency (0.31 ± 2.34 hours), concentrations that were drawn outside of the ideal 1-hour time frame were included in final analysis.
In conclusion, this study was designed to determine how frequently vancomycin troughs within goal range were collected in patients admitted to a level IV NICU. Of the 74 patients meeting study criteria for whom vancomycin troughs were analyzed, only 27 (36.5%) achieved an initial vancomycin trough in goal range. Patients with subtherapeutic troughs more frequently received vancomycin at a frequency of every 12 hours or greater. Although more studies are warranted to determine the optimal vancomycin regimen in the infant population, the results of this study show that dosing vancomycin every 8 hours in our NICU population more often produces troughs within goal range. Prospective studies are needed, and TDM continues to be a very important aspect of vancomycin therapy.
Acknowledgments
Neonatal Intensive Care Unit and Infectious Disease physicians, pharmacists, nurse practitioners, and nurses at Children's of Alabama. Portions of this work were presented at the American Society of Health-Systems Pharmacists, 52nd Midyear Clinical Meeting in Orlando, Florida, on December 3–7, 2017.
ABBREVIATIONS
- AUC24
24-hour area under the concentration-time curve
- BW
birth weight
- GA
gestational age
- LBW
low birth weight
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- NICU
neonatal intensive care unit
- TDM
therapeutic drug monitoring
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation. The study was approved by appropriate institutional committees. Given the nature of the study, the committee did not require informed consent or patient assent.
References
- 1.Capparelli EV, Lane JR, Romanowski GL et al. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41(9):927–934. doi: 10.1177/00912700122010898. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici GM, Allegaert K. Clinical pharmacokinetics of vancomycin in the neonate: a review. Clinics (Sao Paulo) 2012;67(7):831–837. doi: 10.6061/clinics/2012(07)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43(7):417–440. doi: 10.2165/00003088-200443070-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JS, Nelson JD, Barnett ED, Cantey JB, editors. Nelson's Pediatric Antimicrobial Therapy. 24th ed. Vol. 53 American Academy of Pediatrics; 2018. Antimicrobial therapy for newborns. [Google Scholar]
- 5.Kimberlin DW, Brady MT, Jackson MA, Long SA, editors. Red Book 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018. Antimicrobial agents and related therapy; pp. 918–919. [Google Scholar]
- 6.Pickering LK, Baker CJ, Kimberlin DW, et al., editors. Red Book 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. Antimicrobial agents and related therapy; p. 746. [Google Scholar]
- 7.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Disease Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 8.Stockmann C, Hersh AL, Roberts JK et al. Predictive performance of a vancomycin population pharmacokinetic model in neonates. Infect Dis Ther. 2015;4(2):187–198. doi: 10.1007/s40121-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frymoyer A, Hersh AL, El-Komy MH et al. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother. 2014;58(11):6454–6461. doi: 10.1128/AAC.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geraci JE. Vancomycin. Mayo Clin Proc. 1977;52(10):631–634. [PubMed] [Google Scholar]
- 11.Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 12.Soriano A, Marco F, Martinez JA et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(suppl 2):193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 13.Hsu DI, Hidayat LK, Quist R et al. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008;32(suppl 5):378–385. doi: 10.1016/j.ijantimicag.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Le J, Bradley JS, Murray W et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(suppl 4):e155–e163. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybak M, Le J, Lodise TP et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 16.Schelonka RL, Infante AJ. Neonatal immunology. Semin Perinatol. 1998;22(1):2–14. doi: 10.1016/s0146-0005(98)80003-7. [DOI] [PubMed] [Google Scholar]
- 17.Hanna MH, Askenazi DJ, Selewski DT. Drug-induced acute kidney injury in neonates. Curr Opin Pediatr. 2016;28(2):180–187. doi: 10.1097/MOP.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinkeler FS, de Haan TR, Hodiamont CJ et al. Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations. BMC Pediatr. 2014;14:193. doi: 10.1186/1471-2431-14-193. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringenberg T, Robinson C, Meyers R et al. Achievement of therapeutic vancomycin trough serum concentrations with empiric dosing in neonatal intensive care unit patients. Pediatr Infect Dis J. 2015;34(7):742–747. doi: 10.1097/INF.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 20.Madigan T, Teng CB, Koshaish J et al. Optimization of vancomycin dosing in very low-birth-weight preterm neonates. Am J Perinatol. 2015;32(1):83–86. doi: 10.1055/s-0034-1376183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhargava V, Malloy M, Fonseca R. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr. 2017;17(1):50. doi: 10.1186/s12887-017-0777-0. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24:191–196. doi: 10.1097/MOP.0b013e32834f62d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellomo R, Ronco C, Kellum JA, et al. the ADQI work-group Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]


