Abstract
Background
Killian polyp (KP) is a benign lesion that arises from the maxillary sinus. The etiology of KP is unknown. The aim of this study was to investigate the potential involvement of human papilloma- (HPV) and polyoma-viruses (HPyV) infections in the onset of KP.
Methods
DNA from antral (n = 14) and nasal (n = 14) KP fractions were analyzed for HPV and HPyV sequences, genotypes, viral DNA load and physical status along with expression of viral proteins and p16 cellular protein.
Results
The oncogenic HPV16 was detected in 3/14 (21.4%) antral KPs, whilst nasal KPs tested HPV-negative (0/14). The mean HPV16 DNA load was 4.65 ± 2.64 copy/104 cell. The whole HPV16 episomal genome was detected in one KP sample, whereas HPV16 DNA integration in two KPs. P16 mRNA level was lower in the KP sample carrying HPV16 episome than in KPs carrying integrated HPV16 and HPV- negative KPs (p< 0.001). None of the antral and nasal KP samples tested positive for HPyV DNA (0/28).
Conclusions
A fraction of KP tested positive for the oncogenic HPV16. HPV16 detection in the KP antral portion may be consistent with HPV16 infection derived from the maxillary sinus. HPV16 DNA integration represents a novel finding. Altogether, these data improve our knowledge on the association between KP and HPV infection, whereas it indicates that the KP onset is heterogeneous.
Keywords: Killian polyp, Human papillomavirus, Polyomavirus, Infection, Nasal polyps
Introduction
Killian polyp (KP), or antrochoanal polyp, is a benign lesion of the upper respiratory tract arising from the maxillary anthrum, which may extend through the nasal cavity to the choana. KP represents about 5 and 33% of nasal polyps in adults and children, respectively [1, 2]. KP usually presents as unilateral pedunculated mass composed by an antral portion, which is usually cystic, and a nasal/choanal fraction, emerging through an enlarged maxillary accessory ostium [3].
The etiopathogenesis of KP is not known. Several studies have suggested that autoreactivity, allergies and/or chronic inflammation could be risk factors for KP onset [4–6]. KPs are indeed inflammatory polyps [7]. Schryver et al. questioned if autoreactivity contributed to the KP onset or it resulted from a chronic inflammation, and proposed to investigate other inflammation causes, such as viral infections [8]. In fact, KP recurrence after its incomplete surgical removal suggests that viral infections may play a role [3, 9, 10].
Different viruses are able to infect the oropharyngeal region, and play a role in various head and neck diseases [11, 12]. Specifically, human papillomaviruses (HPV) and polyomaviruses (HPyV) such as BKPyV, JCPyV and Merkel cell polyomavirus (MCPyV), are DNA viruses infecting the tonsillar tissues [13–16], and have been associated to the development of respiratory diseases as well as to head and neck cancer [17–20]. HPV and HPyV display similar biological behavior in infected target tissues. After infection of epithelial cells, HPV and HPyV may multiply and spread in different anatomical sites, or enter lifelong latent phase, whereby viral DNA is maintained at low copy number [21, 22]. In some instance, long term latency of the oncogenic HPV and HPyV types may result in viral DNA integration into the host cell genome, leading to cell transformation upon viral oncoprotein overexpression [21–25].
The association between HPV infection and KP has been poorly investigated, whereas studies on HPyV in KP are missing. HPV sequences have been found at different prevalence, ranging 0–54% [26–28]. Moreover, oncogenic HPV genotypes such as HPV16, have been found to be prevalent in KPs, raising the question if HPV may play role in cell transformation. One recent study focusing on tumor marker expression, such as p16 and viral oncoproteins, did not find any correlation between HPV DNA positivity and KP development, concluding that HPV latently infects KP [27]. However, HPV DNA load and physical status, which are two main hallmarks of latent or active infection, have not been assessed yet in KP [29].
Even though maxillary sinus viral infections are considered risk factors for KP, there is no evidence proving the KP etiopathogenesis from this infection. So far, studies focusing on the identification of viral infections have analyzed bulk KP tissues without diversifying between the antral and nasal components. This distinction would be particularly important to understand if viral infections may play a role in the KP onset. In fact, any viral sequences detected in the antral region might account for maxillary sinus infections, and therefore potentially involved in the onset of KP, while those in the nasal region might be due to nasopharyngeal infections after the KP formation, thus not relevant for KP onset.
The aim of this study was to investigate the potential involvement of HPV and HPyV infections in the onset of KP. To this purpose, tissue samples from KP were divided into antral and nasal parts, and analyzed separately for HPVs and HPyVs sequences, genotypes, DNA load and physical status (episomal vs integrated), and expression, along with expression levels of p16, which is a cell protein strictly associated to active HPV infection.
Materials and methods
Samples
Killian polyp (KP) tissue specimens were collected from 14 patients (Mean age ± SD; 44 ± 18 years) who underwent surgical removal at the Ear, Nose and Throat Unit, University Hospital of Ferrara (Italy). Inclusion criteria were unilateral polyp with histopathological diagnosis of KP and age between 18 and 80 yrs. Exclusion criteria were bilateral polyps not coincident with KP. Written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the County Ethical Committee (ID:160986).
Nucleic acids extraction
KP tissue samples (n=14) were divided into two portions: the antral (n=14) and the nasal portion (n=14). Samples (n=28) were incubated overnight with proteinase K at 56 °C to allow tissue digestion. Then, nucleic acids were simultaneously extracted from samples using the All Prep DNA/RNA extraction kit (Qiagen, Milan, Italy). DNA from KPs was isolated/purified together with a salmon sperm DNA (ssDNA) sample and a mock sample lacking DNA [30]. After purification, DNAs/RNAs were quantified spectrophotometrically (NanoDrop 2000, Thermo Scientific) [31]. DNA amplification suitability was evaluated by β-globin gene PCR [32]. DNA/RNAs were stored at − 80 °C until time of analysis.
Detection of HPV and HPyV DNAs
KP tissue samples were tested for HPV and BKPyV, JCPyV and MCPyV DNA sequences, by quantitative PCR (qPCR). Fifty ng of human genomic DNA were used in 10 μl qPCR reactions. For HPV DNA detection the universal primers GP5+/GP6+ (Table 1) were used, as previously reported [33, 34]. These primers allow simultaneous amplification of several HPV types [35, 36], including those frequently detected in KP, such as HPV6/11/16/18 [27, 28]. QPCR reactions included 2x of the SsoAdvanced Universal SYBR Green Supermix, Bio-Rad (Hercules, CA, USA) and a final concentration of 0.5 μM for each GP5+/GP6+ primer. For HPyV DNA detection, specific primers for BKPyV, JCPyV and MCPyV were employed [25, 38, 39]. QPCR reactions included 2x of the TaqMan Universal Master Mix II, no UNG, Thermo Fisher Scientific (Waltham, MA, USA), and 1X of primers and probe assays (Table 1). Recombinant plasmids containing HPV16 genome and BKPyV, JCPyV, and MCPyV genomes were used as positive controls [25, 38, 39], whereas ssDNA and mock samples lacking of DNA, as negative controls of DNA extraction and PCR amplification. Each assay was run in triplicate.
Table 1.
Primers used in qPCR to detect and quantify HPV, PyV DNA, viral, cellular genes
| Target | Primers names | Primers sequence (5′→ 3′) | Amplicon size (bp) | Annealing temp. (°C) | References | |
|---|---|---|---|---|---|---|
| DNA | ||||||
| Viral | HPV L1 | GP5+ | TTTGTTACTGTGGTAGATACTAC | 139–145 | 48 | Malagutti et al. 2020 [33]; Tognon et al. 2020 [34]; Rotondo et al. 2020a [35] |
| GP6+ | GAAAAATAAACTGTAAATCATATTC | |||||
| HPV16 E2 | E- HPV16 E2 F | AACGAAGTATCCTCTCCTGAAATTATTAG | 82 | 60 | Peitsaro, Johansson, e Syrjänen 2002 [37] | |
| E- HPV16 E2 R | CCAAGGCGACGGCTTTG | |||||
| E Probe 16E2PRO | [ROX] CACCCCGCCGCGACCCATA [BHQ2] | |||||
| HPV16 E6 | I+E- HPV16 E6 F | GAGAACTGCAATGTTTCAGGACC | 81 | 60 | ||
| I+E- HPV16 E6 R | TGTATAGTTGTTTGCAGCTCTGTGC | |||||
| I+E Probe 16E6PRO | [6FAM] CAGGAGCGACCCAGAAAGTTACCACAGTT [BHQ1] | |||||
| MCPyV | RQ MCPyV_LT.1F | CCACAGCCAGAGCTCTTCCT | Tagliapietra et al. 2020 [38] | |||
| RQ MCPyV_LT.1R | TGGTGGTCTCCTCTCTGCTACTG | |||||
| RQ MCPyV_LT Probe | [6FAM] TCCTTCTCAGCGTCCCAGGCTTCA [MGB] | |||||
| JCPyV | Assay_JCyV | AI1RWNE | Tagliapietra et al. 2019 [39] | |||
| BKPyV | Assay_BKyV | AI20UTM | ||||
| Host | β-Globin | β-Globin F | TGGGTTTCTGATAGGCACTGACT | 152 | 56 | Contini et al. 2018 [32] |
| β-Globin R | AACAGCATCAGGAGTGGACAGAT | |||||
| RNA | ||||||
| Viral | HPV16 E2 | HPV16 E2 F | AACGAAGTATCCTCTCCTGAAATTATTAG | 82 | 60 | Peitsaro, Johansson, e Syrjänen 2002 [37] |
| HPV16 E2 R | CCAAGGCGACGGCTTTG | |||||
| HPV16 E6 | HPV16 E6 F | GAGAACTGCAATGTTTCAGGACC | 81 | 60 | ||
| HPV16 E6 R | TGTATAGTTGTTTGCAGCTCTGTGC | |||||
| HPV16 E5 | 16-E5 FWD | CGTCCGCTGCTTTTGTCTGTGTCTACATAC | 89 | 60 | Weyn et al. 2011 [40] | |
| 16-E5 REV | CACCTAAACGCAGAGGCTGCTGTTATCCAC | |||||
| HPV16 E7 | E7 FWD | AGGAGGATGAAATAGATGGTCCAG | 112 | 60 | Pett et al. 2006 [41] | |
| E7 REV | CTTTGTACGCACAACCGAAGC | |||||
| Host | P16INK4A | p16 ink4a FWD | CCAACGCACCGAATAGTTACG | 58 | 60 | Marcoux et al. 2013 [42] |
| p16 ink4a REV | GCGCTGCCCATCATCATG | |||||
| GAPDH | GAPDH F | GAAGGTGAAGGTCGGAGTC | 226 | 60 | Xiao et al. 2011 [43] | |
| GAPDH R | GAAGATGGTGATGGGATTTC | |||||
HPV DNA load, genotype and physical status analyses
HPV DNA load was quantified by qPCR assay using the GP5+/GP6+ primers and a 10-fold dilutions standard curve, from 108 to 102 copies, of recombinant plasmids. HPV DNA load values were reported as viral copies per human cell equivalents (viral copy/cell). Samples were normalized vs. HPV16-positive SiHa cell line, which contains one HPV16 copy/cell. Human β-globin gene was used to determine the human cell equivalents of each sample [32]. HPV genotype was determined by differential melting temperature (Tm), adding a high resolution melting (HRM) step, from 65 °C to 95 °C (ramping 0.1 °C every 0.03 s), to the qPCR analysis, as done before for detection of the HPV16 and HPV18 genotypes [44]. HPV6/11/16/18 plasmids were used as positive controls. HPV DNA physical status was investigated using the E2/E6 ratio by qPCR, as previously described (Table 1) [37]. Briefly, 50 ng of template DNA were analyzed in 10 μl multiplex PCR reactions, 2x TaqMan Universal Master Mix II, no UNG, Thermo Fisher Scientific (Waltham, MA, USA); 0.3 μM of each HPV16 E2 primer; 0.5 μM of each HPV16 E6 primer; and 0.1 μM of each E2 and E6 probe. E2/E6 ratio equal to 1 indicated episomal form, less or more than 1 mixed forms, i.e. episomal and integrated, whereas no E2 DNA detection indicated full integration. Each assay was run in triplicate.
Rolling circle amplification (RCA) assay
The episomal viral DNAs were detected by rolling circle amplification (RCA) assay using the TempliPhi™ 100 Amplification Kit (GE Healthcare, Chicago, USA) [45], and in accordance with manufacturer’s instructions. Briefly, reactions were prepared with 25 ng of genomic DNA and 175 μM of dNTP mix (Thermo Scientific, Massachusetts, USA). The specificity of the RCA products was assessed by DNA restriction enzyme digestion in a final volume of 10 μL (Thermo Scientific, Massachusetts, USA). RCA and digested RCA products were visualized onto a 0.8% agarose gel. Positive and negative controls were used in the RCA assay.
Gene expression analysis
Total RNA was retrotranscribed using the Improm II (Promega, Wisconsin, USA) reverse transcription system [46]. cDNAs were analyzed for the expression of HPV16 E2, E6, E7 and E5 genes and p16 cellular gene (Table 1) [37, 40–42]. Briefly, 50 ng of cDNA were used in 10 μl reaction, 2x of the SsoAdvanced Universal SYBR Green Supermix, Bio-Rad (Hercules, CA, USA) and a final concentration of 0.5 μM for each primer [47]. GAPDH gene was employed as control for the gene expression analysis [43]. SiHa cell line was used as positive control for HPV gene expression and mock sample as negative control. Each assay was run in triplicate.
Statistical analyses
Statistical analyses were performed using the GraphPad Prism for Windows (version 6.0, GraphPad, California, USA) [48, 49]. For mRNA, fold change was calculated by the 2-ΔΔCt method and represented in Log2 scale, using HPV-negative samples as controls [31]. One-way analysis of variance was used to compare fold-change among samples [50]. P values less than 0.05 were considered statistically significant (p< 0.05) [51].
Results
Prevalence of HPV and HPyV sequences
DNAs isolated from KP tissue samples (n=28) represented by antral (n=14) and nasal (n=14) portions were tested for viral DNA sequences of HPV and HPyVs. The qPCR analyses showed that 3/14 (21.4%) of the antral KP tissues were positive for HPV DNA (Table 2). None of the nasal KP samples (n=14) tested positive for HPV DNA (0/14; 0%) (Table 2). KP tissue samples analyzed for BKPyV, JCPyV and MCPyV DNA sequences gave negative results in both antral (n=14) and nasal (n=14) portions (0/14; 0%) (Table 2).
Table 2.
Prevalence of HPV and HPyV in antral and nasal KP tissues
| Tissue sample | Number of positive samples/samples analyzed (%) | |||
|---|---|---|---|---|
| HPV | MCPyV | JCPyV | BKPyV | |
| Antral KP | 3/14 (21.4) | 0/14 (0) | 0/14 (0) | 0/14 (0) |
| Nasal KP | 0/14 (0) | 0/14 (0) | 0/14 (0) | 0/14 (0) |
HPV DNA load, genotyping, and physical status analyses
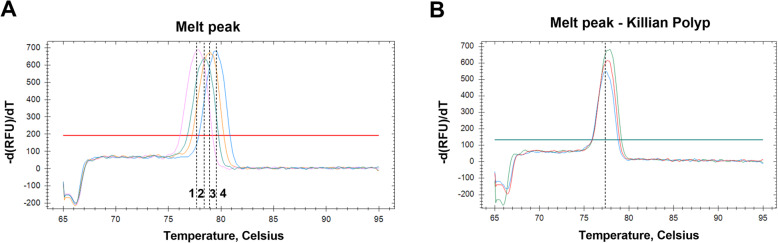
HPV DNA load was determined by comparison to the HPV plasmid standard curve in qPCR assay. The mean viral DNA load in HPV-positive antral KPs (n=3) was 4.65±2.64 copy/104 cell. In detail, in the three HPV-positive antral KP samples, the viral DNA load was 8.32 copy/104 cell, 3.43 copy/104 cell, and 2.21 copy/104 cell. HPV genotype analyses were carried out by HRM qPCR assay. Firstly, the optimal Tm range for discriminating HPV6/11/16/18 types from GP5+/GP6+ amplicons was identified, which was between 75.4–79.5±0.2 °C (Fig. 1a). HPV genotype analyses were carried out by comparing qPCR Tm with the positive controls. Results indicated that the three HPV-positive antral KP samples carried the HPV type 16 (3/3; 100%) (Fig. 1b).
Fig. 1.
HPV differential melt peaks. a Melting temperature (Tm) for; 1) pUC19_HPV16; 2) pUC19_HPV11; 3) pUC19_HPV6 and 4) pUC19_HPV18. b Tm for KP samples, corresponding to that of pUC19_HPV16
HPV16 DNA physical status was assessed by E2/E6 ratio in the three HPV16-positive antral KP samples. The E2/E6 ratio was 1.01 in one sample (1/3; 33.3%) indicating presence of HPV16 in the episomal form. In the two other samples only the E6 sequence was found (2/3; 66.6%), indicating that HPV16 was integrated into the host cell genome.
HPV physical status validation by RCA
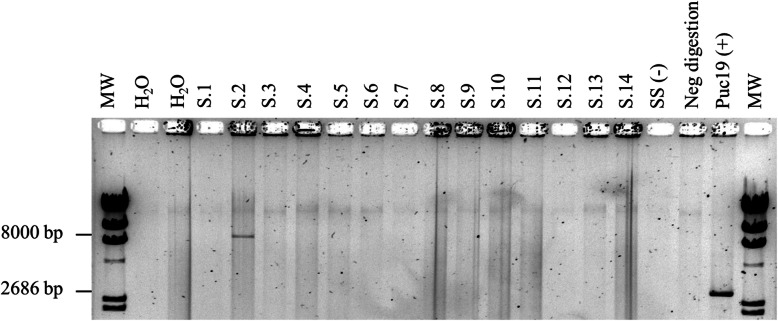
Antral KP DNAs (n=14) were investigated by RCA for validating the HPV DNA episomal physical status. Successfully amplification was obtained only in the KP sample detected with E2/E6 ratio of 1.01, that was predictable of the episomal form. The molecular weight for the positive band corresponding to approximately 8000 bp was consistent of the HPV genome (Fig. 2, lane S2). Digestion with Bam HI enzyme, which cuts once into HPV16 genome, further confirmed the positivity for HPV16.
Fig. 2.
Rolling circle amplification assay performed on antral KP DNAs. MW: Molecular Weight. Negative controls: H2O, Salmon Sperm DNA (SS), Neg digestion (H2O). KP DNAs 1–14
Gene expression analysis
Viral gene expression was studied for the HPV-positive antral KP samples (n=3). No expression for HPV16 E2, E5, E6, E7 genes was detectable in any of the samples analyzed, indicating either that HPV16 is not transcriptionally active in KP or that viral mRNA levels were too low to be detected under qPCR conditions. To gain insight into this topic, p16, which is considered a surrogate marker of active HPV infection, was analyzed for mRNA expression in the three antral HPV-positive KP samples, containing the episomal HPV16 (n=1) and the integrated HPV16 (n=2). Results indicated that p16 mRNA level was 8.01-fold lower in HPV16-episome KP sample than in HPV16-integrated KP samples and 7.05-fold lower in HPV16-episome KP sample than in HPV-negative samples (p< 0.0001, Fig. 3). Although not statistically significant, p16 expression was slightly higher in HPV16-integrated KP samples compared to HPV-negative samples (p> 0.05, Fig. 3).
Fig. 3.
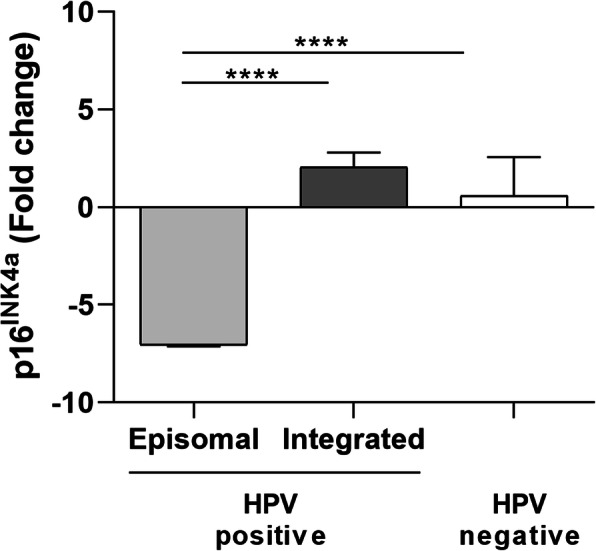
p16 mRNA expression. KPs (n=14) were stratified according to HPV positivity/negativity. HPV-positive were further divided in episomal (n=1) and integrated (n=2). ****p< 0.0001
Discussion
The etiopathogenesis of KP is still not completely understood. Viral infections have been suggested to be involved in KP onset [52, 53]. Herein, with the aim to verify the putative role of the viral infections, HPV and HPyV were investigated in KP samples. Independent analyses of the antral and nasal region were useful in understanding whether the KP infections depended on the maxillary sinus or the nasal cavity.
HPV sequences were detected in 21% of the antral KP samples, while none of the nasal samples tested positive for HPV. This result indicates that the KP antral region is target of HPV infection and suggests a possible link between maxillary sinus infections and KP development. In term of prevalence, our data are in agreement with previous studies reporting HPV rates ranging from 0 to 54% in KP samples, although the new methodological approach used herein does not allow our and previous data to be compared adequately [26, 27, 54–60].
HPV genotypes have been investigated in two previous studies reporting HPV16 to be frequently detected at higher rate, 61.9 and 85.72%, respectively, than HPV11, 14.3 and 14.28%, respectively [27, 28]. In this study HPV16 was the only viral genotype detected in KP. These results are of interest as HPV16 is the high risk oncogenic type involved in development of different tumors [24, 61–63], including head and neck cancer [64, 65].
HPV viral load and physical status are indicative of active or latent infection in the infected tissues [29, 66]. For the first time, DNA load and physical status was investigated in HPV-positive KP samples. The viral DNA load was lower than 1 copy/cell, which is consistent with latent or persistent infection occurring in normal tissues [67, 68]. When HPV physical status was analyzed a heterogeneous trend was found among the HPV-positive KP samples. One sample carried HPV16 in episomal form, which was confirmed amplifying the whole HPV genome by RCA assay. Instead, two KP samples showed the HPV16 DNA in integrated form. This is an interesting finding because high risk HPV integration into the host cell genome is a common event preceding cell transformation [69]. On the other hand, HPV integration occurs up to 42.8% of normal tissues, as previously reported in HPV-positive normal cervical samples [70]. Regard KP, evidences proving its neoplastic transformation do not exist, although some cases mimicking malignant transformation have been reported [71]. Nevertheless, HPV carcinogenesis in KP, if any, could be difficult to be assessed, since KPs are removed early after presentation, whereas HPV transformation process occurs in long lasting time, needing many years to be detected. Altogether, our data indicate that HPV16 is present at low DNA load in both episomal and integrated form, consistent with latent/persistent infection in the antral KP. Nevertheless, the detection of the oncogenic HPV16 combined with its DNA integration in the KP is intriguing. Further studies are needed to assess the HPV DNA integration in KP over the time.
HPV mRNA expressions occur during active viral infection. Accordingly, in this study, viral expression of E2, E5, E6 and E7 sequences was not detected in the HPV-positive KP samples. Although E6/E7 expression in HPV-positive KP samples carrying viral DNA integration would be expected, HPV latency in normal and pathological tissues presenting viral DNA integration is also common [72]. Some other explanations may account for lack of viral expression. For instance, KPs are covered by ciliated cylindrical epithelium, which may be not permissive for HPV E6/E7 gene expression [7, 67]. Also, it is possible that viral mRNA levels were too low to be detected under our qPCR conditions. Further studies with more sensitive assays may clarify this matter [46].
Since no HPV transcriptional activity was found, the surrogate marker of active HPV infection, the p16, was studied in correlation to infection. During HPV infection the viral E7 protein inactivates pRb tumor suppressor protein leading to p16 overexpression [73]. In this study, no difference between HPV16-positive KP samples carrying integrated viral DNA and HPV-negative KPs was observed (p> 0.05), although a slightly higher p16 mRNA level was found in HPV16-positive KPs. Likely, the small samples size used in the study did not allow statistical significance to be reached. In contrast, the KP sample carrying episomal HPV DNA showed stronger p16 down-expression compared to HPV-positive and HPV-negative KP samples (p< 0.001). Mutations at the p16 coding gene may explain its down-expression [74, 75]. Alternatively, methylation at p16 promoter may silence the gene leading to decrease expression, as previously shown in HPV-positive samples carrying HPV in episomal form [76].
Finally, HPyV DNA sequences were analyzed in KP. HPyVs have been found associated to different diseases, including cancer and polyposis [77]. Specifically, JCPyV has been studied in correlation to colon polyposis [78], whereas BKPyV has been investigated in the prostate and colon cancer onset [79]. MCPyV is the main cause of the Merkel cell carcinoma, a rare but very aggressive non-melanoma skin cancer [25]. Moreover, MCPyV is considered to be a part of the skin microbiota, and viral DNA sequences have been found in nasal swabs, blood, chorionic villi, eyebrows and adrenal glands [38, 77, 80]. In this study, HPyV sequences were not found neither in antral nor nasal KPs, thus excluding their role in KP formation.
Conclusions
The present study investigated HPV and HPyV as potential pathogenic risk factors in KP. While no implication was found for HPyV, a fraction of KPs showed positivity for HPV16. New information on HPV DNA load and physical status in KPs were also provided. Specifically, HPV16-positive KPs presented viral DNA at low load and in episomal or integrated form. The reduced sample size employed in this pilot study could be considered a limitation, and further studies in a larger samples size are needed, especially for clarifying the oncogenic HPV16 integration into the KPs. Of note, KP samples were divided in antral and nasal portions, whereas HPV sequences were found only in the antral region, providing a possible explanation for polyp formation from sinus maxillary infections. We suggest that a HPV latent infection of the maxillary sinus might be responsible for its recurrence, after KP surgical removal, highlighting the importance of complete surgical removal of the HPV-positive pathological tissue to prevent further recurrences.
Abbreviations
- KP
Killian polyp
- HPV
Human papillomavirus
- HPyV
Polyomaviruses
- RCA
Rolling circle amplifications
- MCPyV
Merkel cell polyomavirus
- ssDNA
Salmon sperm DNA
- qPCR
quantitative PCR
Authors’ contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, F.M. and S.P.; methodology, L.O.G. and J.C.R..; software, M.D.M.; validation, M.T., S.P., F.M.; formal analysis, L.O.G., J.C.R.; investigation, L.O.G., J.C.R., C.L., C.M., I.B. M.D.M.,; resources, L.C., N.M., A.C., C.B., S.P.; data curation L.O.G., J.C.R., C.L., C.M., I.B..; writing-original draft preparation, L.O.G., J.C.R..; writing, review and editing, C.L., M.T., S.P., F.M..; visualization, L.O.G..; supervision, M.T., S.P., F.M..; project administration, M.T., S.P., F.M..; funding acquisition, J.C.R., M.T., F.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Ferrara, FAR grants (2017/2018 to MT and FM) and FIR grants 2016, 2017, 2018 to FM; Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Contract grant number: IG 21617 to M.T. and 21956 to J.C.R.. J.C.R was a post-doctoral fellow of the Fondazione Umberto Veronesi, Milan, Italy (2019–2020).
Availability of data and materials
Data and material will be available upon request to the corresponding author.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lucia Oton-Gonzalez and John Charles Rotondo contributed equally to this work.
Contributor Information
Mauro Tognon, Email: tgm@unife.it.
Fernanda Martini, Email: mrf@unife.it.
References
- 1.Chaiyasate S, Roongrotwattanasiri K, Patumanond J, Fooanant S. Antrochoanal polyps: how long should follow-up be after surgery? Int J Otolaryngol. 2015;2015:297417. doi: 10.1155/2015/297417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksakal C. Bilateral antrochoanal polyp in a child. J Craniofac Surg. 2018;29(8):2368–2369. doi: 10.1097/SCS.0000000000004947. [DOI] [PubMed] [Google Scholar]
- 3.Frosini P, Picarella G, De Campora E. Antrochoanal polyp: analysis of 200 cases. Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-facc. 2009;29(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- 4.Piquet JJ, Chevalier D, Leger GP, Rouquette I, Leconte-Houcke M. Endonasal microsurgery of antro-choanal polyps. Acta Otorhinolaryngol Belg. 1992;46(3):267–271. [PubMed] [Google Scholar]
- 5.Cook PR, Davis WE, McDonald R, McKinsey JP. Antrochoanal polyposis: a review of 33 cases. Ear Nose Throat J. 1993;72(6):401–402. doi: 10.1177/014556139307200607. [DOI] [PubMed] [Google Scholar]
- 6.Lee T-J, Huang S-F. Endoscopic sinus surgery for antrochoanal polyps in children. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2006;135(5):688–692. doi: 10.1016/j.otohns.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Hirshoren N, Neuman T, Gross M, Eliashar R. Angiogenesis in chronic rhinosinusitis with nasal polyps and in antrochoanal polyps. Inflamm Res. 2011;60(4):321–327. doi: 10.1007/s00011-010-0271-8. [DOI] [PubMed] [Google Scholar]
- 8.Schryver ED, Calus L, Bonte H, Natalie DR, Gould H, Donovan E, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016;138(3):893–895.e5. doi: 10.1016/j.jaci.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stierna PL. Nasal polyps: relationship to infection and inflammation. Allergy Asthma Proc. 1996;17(5):251–257. doi: 10.2500/108854196778662282. [DOI] [PubMed] [Google Scholar]
- 10.Yaman H, Yilmaz S, Karali E, Guclu E, Ozturk O. Evaluation and management of antrochoanal polyps. Clin Exp Otorhinolaryngol. 2010;3(2):110. doi: 10.3342/ceo.2010.3.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A review of HPV-related head and neck cancer. J Clin Med. 2018;7(9). [DOI] [PMC free article] [PubMed]
- 12.Poluschkin L, Rautava J, Turunen A, Wang Y, Hedman K, Syrjänen K, et al. Polyomaviruses detectable in head and neck carcinomas. Oncotarget. 2018;9(32):22642–22652. doi: 10.18632/oncotarget.25202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- 14.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72(12):9918–9923. doi: 10.1128/JVI.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantola K, Sadeghi M, Lahtinen A, Koskenvuo M, Aaltonen L-M, Möttönen M, et al. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2009;45(4):292–295. doi: 10.1016/j.jcv.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieth KKS, Gill SR, Lott-Limbach AA, Merkley MA, Botero N, Allen PD, et al. Prevalence of high-risk human papillomavirus in tonsil tissue in healthy adults and colocalization in biofilm of tonsillar crypts. JAMA Otolaryngol-- Head Neck Surg. 2018;144(3):231–237. doi: 10.1001/jamaoto.2017.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialasiewicz S, Lambert SB, Whiley DM, Nissen MD, Sloots TP. Merkel cell polyomavirus DNA in respiratory specimens from children and adults. Emerg Infect Dis. 2009;15(3):492–494. doi: 10.3201/eid1503.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyomavirus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83(12):2220–2224. doi: 10.1002/jmv.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillison ML, Alemany L, Snijders PJF, Chaturvedi A, Steinberg BM, Schwartz S, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–F54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 20.Shikova E, Emin D, Alexandrova D, Shindov M, Kumanova А, Lekov A, et al. Detection of merkel cell polyomavirus in respiratory tract specimens. Intervirology. 2017;60(1–2):28–32. doi: 10.1159/000479372. [DOI] [PubMed] [Google Scholar]
- 21.Rotondo JC, Mazzoni E, Bononi I, Tognon M, Martini F. Association between human tumours and simian virus 40. Fontiers Oncol. 2019;9 In press. [DOI] [PMC free article] [PubMed]
- 22.Krump NA, Liu W, You J. Mechanisms of persistence by small DNA tumor viruses. Curr Opin Virol. 2018;32:71–79. doi: 10.1016/j.coviro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa N, Egawa K, Griffin H, Doorbar J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7(7):3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preti M, Rotondo JC, Holzinger D, Micheletti L, Gallio N, McKay-Chopin S, et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer. 2020;15:20. doi: 10.1186/s13027-020-00286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotondo JC, Bononi I, Puozzo A, Govoni M, Foschi V, Lanza G, et al. Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs, including anti-TNF. Clin Cancer Res. 2017;23(14):3929–3934. doi: 10.1158/1078-0432.CCR-16-2899. [DOI] [PubMed] [Google Scholar]
- 26.Pei F, Chen X-P, Zhang Y, Wang Y, Chen Q, Tan X-J, et al. Human papillomavirus infection in nasal polyps in a Chinese population. J Gen Virol. 2011;92(Pt 8):1795–1799. doi: 10.1099/vir.0.031955-0. [DOI] [PubMed] [Google Scholar]
- 27.Knör M, Tziridis K, Agaimy A, Zenk J, Wendler O. Human papillomavirus (HPV) prevalence in nasal and antrochoanal polyps and association with clinical data. PLoS One. 2015;10(10):e0141722. doi: 10.1371/journal.pone.0141722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yılmaz E, Alatas N, Ucar F, Cora T, Buruk K, Unlu Y. Investigation of human papillomavirus (HPV) and epstein-barr virus (EBV) in antrochoanal polyps. Am J Otolaryngol. 2019;40(3):389–392. doi: 10.1016/j.amjoto.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 29.McBride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017;13(4):e1006211. doi: 10.1371/journal.ppat.1006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotondo JC, Candian T, Selvatici R, Mazzoni E, Bonaccorsi G, Greco P, et al. Tracing males from different continents by genotyping JC polyomavirus in DNA from semen samples. J Cell Physiol. 2017;232(5):982–985. doi: 10.1002/jcp.25686. [DOI] [PubMed] [Google Scholar]
- 31.Rotondo JC, Giari L, Guerranti C, Tognon M, Castaldelli G, Fano EA, et al. Environmental doses of perfluorooctanoic acid change the expression of genes in target tissues of common carp. Environ Toxicol Chem. 2018;37(3):942–948. doi: 10.1002/etc.4029. [DOI] [PubMed] [Google Scholar]
- 32.Contini C, Rotondo JC, Magagnoli F, Maritati M, Seraceni S, Graziano A, et al. Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J Cell Physiol. 2018;234(1):100–107. doi: 10.1002/jcp.26952. [DOI] [PubMed] [Google Scholar]
- 33.Malagutti N, Rotondo JC, Cerritelli L, Melchiorri C, De Mattei M, Selvatici R, et al. High human papillomavirus DNA loads in inflammatory middle ear diseases. Pathog Basel Switz. 2020;9(3). [DOI] [PMC free article] [PubMed]
- 34.Tognon M, Tagliapietra A, Magagnoli F, Mazziotta C, Oton-Gonzalez L, Lanzillotti C, et al. Investigation on spontaneous abortion and human papillomavirus infection. Vaccines. 2020;8(3):473. doi: 10.3390/vaccines8030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotondo JC, Oton-Gonzalez L, Mazziotta C, Lanzillotti C, Iaquinta MR, Tognon M, et al. Simultaneous detection and viral DNA load quantification of different human papillomavirus types in clinical specimens by the high analytical droplet digital PCR method. Front Microbiol. 2020; In press. Available at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.591452/abstract. [DOI] [PMC free article] [PubMed]
- 36.Evans MF, Adamson CSC, Simmons-Arnold L, Cooper K. Touchdown General Primer (GP5+/GP6+) PCR and optimized sample DNA concentration support the sensitive detection of human papillomavirus. BMC Clin Pathol. 2005;5:10. doi: 10.1186/1472-6890-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peitsaro P, Johansson B, Syrjanen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol. 2002;40(3):886–891. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Oton Gonzalez L, et al. Droplet-digital PCR assay to detect Merkel cell polyomavirus sequences in chorionic villi from spontaneous abortion affected females. J Cell Physiol. 2020. [DOI] [PubMed]
- 39.Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M, et al. Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion. Hum Reprod Oxf Engl. 2019. [DOI] [PubMed]
- 40.Weyn C, Vanderwinden J-M, Rasschaert J, Englert Y, Fontaine V. Regulation of human papillomavirus type 16 early gene expression in trophoblastic and cervical cells. Virology. 2011;412(1):146–155. doi: 10.1016/j.virol.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 41.Pett MR, Herdman MT, Palmer RD, Yeo GSH, Shivji MK, Stanley MA, et al. Selection of cervical keratinocytes containing integrated HPV16 associates with episome loss and an endogenous antiviral response. Proc Natl Acad Sci. 2006;103(10):3822–3827. doi: 10.1073/pnas.0600078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcoux S, Le ONL, Langlois-Pelletier C, Laverdière C, Hatami A, Robaey P, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol Lond Engl. 2013;8:252. doi: 10.1186/1748-717X-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Z, Liu Q, Mao F, Wu J, Lei T. TNF-α-induced VEGF and MMP-9 expression promotes hemorrhagic transformation in pituitary adenomas. Int J Mol Sci. 2011;12(6):4165–4179. doi: 10.3390/ijms12064165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Araujo MR, De Marco L, Santos CF, Rubira-Bullen IRF, Ronco G, Pennini I, et al. GP5+/6+ SYBR green methodology for simultaneous screening and quantification of human papillomavirus. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2009;45(2):90–95. doi: 10.1016/j.jcv.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 45.da Silva FRC, Cibulski SP, Daudt C, Weber MN, Guimarães LLB, Streck AF, et al. Novel bovine papillomavirus type discovered by rolling-circle amplification coupled with next-generation sequencing. Aguayo FR, curatore. PLoS ONE. 2016;11(9):e0162345. doi: 10.1371/journal.pone.0162345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torreggiani E, Rossini M, Bononi I, Pietrobon S, Mazzoni E, Iaquinta MR, et al. Protocol for the long-term culture of human primary keratinocytes from the normal colorectal mucosa. J Cell Physiol. 2019;234(7):9895–9905. doi: 10.1002/jcp.28300. [DOI] [PubMed] [Google Scholar]
- 47.Rotondo JC, Borghi A, Selvatici R, Mazzoni E, Bononi I, Corazza M, et al. Association of retinoic acid receptor β gene with onset and progression of lichen sclerosus–associated vulvar squamous cell carcinoma. JAMA Dermatol. 2018;154(7):819. doi: 10.1001/jamadermatol.2018.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzoni E, Martini F, Corallini A, Taronna A, Barbanti-Brodano G, Querzoli P, et al. Serologic investigation of undifferentiated nasopharyngeal carcinoma and simian virus 40 infection. Head Neck. 2016;38(2):232–236. doi: 10.1002/hed.23879. [DOI] [PubMed] [Google Scholar]
- 49.Rotondo JC, Oton-Gonzalez L, Selvatici R, Rizzo P, Pavasini R, Campo GC, et al. SERPINA1 gene promoter is differentially methylated in peripheral blood mononuclear cells of pregnant women. Front Cell Dev Biol. 2020;8 Available at: https://www.frontiersin.org/article/10.3389/fcell.2020.550543/full. [citato 3 settembre 2020]. [DOI] [PMC free article] [PubMed]
- 50.Mazzoni E, Pietrobon S, Masini I, Rotondo JC, Gentile M, Fainardi E, et al. Significant low prevalence of antibodies reacting with simian virus 40 mimotopes in serum samples from patients affected by inflammatory neurologic diseases, including multiple sclerosis. PLoS One. 2014;9(11):e110923. doi: 10.1371/journal.pone.0110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzoni E, Di Stefano M, Fiore JR, Destro F, Manfrini M, Rotondo JC, et al. Serum IgG antibodies from pregnant women reacting to mimotopes of simian virus 40 large T antigen, the viral oncoprotein. Front Immunol. 2017;8:411. doi: 10.3389/fimmu.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galluzzi F, Pignataro L, Maddalone M, Garavello W. Recurrences of surgery for antrochoanal polyps in children: A systematic review. Int J Pediatr Otorhinolaryngol. 2018;106:26–30. doi: 10.1016/j.ijporl.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Hong SK, Min YG, Kim CN, Byun SW. Endoscopic removal of the antral portion of antrochoanal polyp by powered instrumentation. Laryngoscope. 2001;111(10):1774–1778. doi: 10.1097/00005537-200110000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Becker M, Forslund O, Hansson BG, Malm L. Search for the human papillomavirus in nasal polyps, using a polymerase chain reaction-method. J Otolaryngol. 1994;23(5):344–346. [PubMed] [Google Scholar]
- 55.Hoffmann M, Kahn T, Goeroegh T, Lohrey C, Gottschlich S, Meyer J, et al. Tracing human papillomavirus DNA in nasal polyps by polymerase chain reaction. Acta Otolaryngol (Stockh) 2000;120(7):872–875. doi: 10.1080/000164800750061750. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann M, Klose N, Gottschlich S, Görögh T, Fazel A, Lohrey C, et al. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;239(1):64–70. doi: 10.1016/j.canlet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Zaravinos A, Bizakis J, Spandidos DA. Prevalence of human papilloma virus and human herpes virus types 1-7 in human nasal polyposis. J Med Virol. 2009;81(9):1613–1619. doi: 10.1002/jmv.21534. [DOI] [PubMed] [Google Scholar]
- 58.Sham CL, Tol KF, Chan PKS, Lee DLY, Tong MCF, van Hasselt CA. Prevalence of human papillomavirus, Epstein-Barr virus, p21, and p53 expression in sinonasal inverted papilloma, nasal polyp, and hypertrophied turbinate in Hong Kong patients. Head Neck. 2012;34(4):520–533. doi: 10.1002/hed.21772. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo R, Malagutti N, Bortolotti D, Gentili V, Rotola A, Fainardi E, et al. Infection and HLA-G molecules in nasal polyposis. J Immunol Res. 2014;2014:407430. doi: 10.1155/2014/407430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pagella F, Emanuelli E, Pusateri A, Borsetto D, Cazzador D, Marangoni R, et al. Clinical features and management of antrochoanal polyps in children: cues from a clinical series of 58 patients. Int J Pediatr Otorhinolaryngol. 2018;114:87–91. doi: 10.1016/j.ijporl.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 61.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramakrishnan S, Partricia S, Mathan G. Overview of high-risk HPV’s 16 and 18 infected cervical cancer: pathogenesis to prevention. Biomed Pharmacother Biomedecine Pharmacother. 2015;70:103–110. doi: 10.1016/j.biopha.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 63.Nicolás-Párraga S, Gandini C, Pimenoff VN, Alemany L, de Sanjosé S, Xavier Bosch F, et al. HPV16 variants distribution in invasive cancers of the cervix, vulva, vagina, penis, and anus. Cancer Med. 2016;5(10):2909–2919. doi: 10.1002/cam4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geißler C, Tahtali A, Diensthuber M, Gassner D, Stöver T, Wagenblast J. The role of p16 expression as a predictive marker in HPV-positive oral SCCHN--a retrospective single-center study. Anticancer Res. 2013;33(3):913–916. [PubMed] [Google Scholar]
- 65.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 66.Shukla S, Mahata S, Shishodia G, Pande S, Verma G, Hedau S, et al. Physical state & copy number of high risk human papillomavirus type 16 DNA in progression of cervical cancer. Indian J Med Res. 2014;139(4):531–543. [PMC free article] [PubMed] [Google Scholar]
- 67.Abramson AL, Nouri M, Mullooly V, Fisch G, Steinberg BM. Latent human papillomavirus infection is comparable in the larynx and trachea. J Med Virol. 2004;72(3):473–477. doi: 10.1002/jmv.20013. [DOI] [PubMed] [Google Scholar]
- 68.Kalantari M, Garcia-Carranca A, Morales-Vazquez CD, Zuna R, Montiel DP, Calleja-Macias IE, et al. Laser capture microdissection of cervical human papillomavirus infections: Copy number of the virus in cancerous and normal tissue and heterogeneous DNA methylation. Virology. 2009;390(2):261–267. doi: 10.1016/j.virol.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Qian Z, Gong Y, Wang Y, Guan Y, Han Y, et al. Comprehensive genomic variation profiling of cervical intraepithelial neoplasia and cervical cancer identifies potential targets for cervical cancer early warning. J Med Genet. 2019;56(3):186–194. doi: 10.1136/jmedgenet-2018-105745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thakur JS, Chaitanya A, Minhas RS, Azad RK, Sharma DR, Mohindroo NK. Killian’s polyp mimicking malignant tumor. Ann Maxillofac Surg. 2015;5(2):281–283. doi: 10.4103/2231-0746.175775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leonard SM, Pereira M, Roberts S, Cuschieri K, Nuovo G, Athavale R, et al. Evidence of disrupted high-risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847. doi: 10.1038/srep20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romagosa C, Simonetti S, López-Vicente L, Mazo A, Lleonart ME, Castellvi J, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30(18):2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 74.Hibi K, Koike M, Nakayama H, Fujitake S, Kasai Y, Ito K, et al. A cancer-prone case with a background of methylation of p16 tumor suppressor gene. Clin Cancer Res Off J Am Assoc Cancer Res. 2003;9(3):1053–1056. [PubMed] [Google Scholar]
- 75.Wong DJ, Paulson TG, Prevo LJ, Galipeau PC, Longton G, Blount PL, et al. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61(22):8284–8289. [PubMed] [Google Scholar]
- 76.Carestiato FN, Amaro-Filho SM, Moreira MAM, Cavalcanti SMB. Methylation of p16 ink4a promoter is independent of human papillomavirus DNA physical state: a comparison between cervical pre-neoplastic and neoplastic samples. Mem Inst Oswaldo Cruz. 2018;114:e180456. doi: 10.1590/0074-02760180456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prado JCM, Monezi TA, Amorim AT, Lino V, Paladino A, Boccardo E. Human polyomaviruses and cancer: an overview. Clin Sao Paulo Braz. 2018;73(suppl 1):e558s. doi: 10.6061/clinics/2018/e558s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coelho TR, Gaspar R, Figueiredo P, Mendonça C, Lazo PA, Almeida L. Human JC polyomavirus in normal colorectal mucosa, hyperplastic polyps, sporadic adenomas, and adenocarcinomas in Portugal: JCV presence in normal or abnormal colorectal mucosa. J Med Virol. 2013;85(12):2119–2127. doi: 10.1002/jmv.23705. [DOI] [PubMed] [Google Scholar]
- 79.Tognon M, Corallini A, Martini F, Negrini M, Barbanti-Brodano G. Oncogenic transformation by BK virus and association with human tumors. Oncogene. 2003;22(33):5192–5200. doi: 10.1038/sj.onc.1206550. [DOI] [PubMed] [Google Scholar]
- 80.Mazzoni E, Rotondo JC, Marracino L, Selvatici R, Bononi I, Torreggiani E, et al. Detection of merkel cell polyomavirus DNA in serum samples of healthy blood donors. Front Oncol. 2017;7:294. doi: 10.3389/fonc.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material will be available upon request to the corresponding author.