Abstract
Background
Coronary artery aneurysms (CAAs) may occur after Kawasaki disease (KD) and lead to important morbidity and mortality. As CAA in patients with KD are rare and heterogeneous lesions, prognostication and risk stratification are difficult. We sought to derive the cumulative risk and associated factors for cardiovascular complications in patients with CAAs after KD.
Methods and Results
A 34‐institution international registry of 1651 patients with KD who had CAAs (maximum CAA Z score ≥2.5) was used. Time‐to‐event analyses were performed using the Kaplan–Meier method and Cox proportional hazard models for risk factor analysis. In patients with CAA Z scores ≥10, the cumulative incidence of luminal narrowing (>50% of lumen diameter), coronary artery thrombosis, and composite major adverse cardiovascular complications at 10 years was 20±3%, 18±2%, and 14±2%, respectively. No complications were observed in patients with a CAA Z score <10. Higher CAA Z score and a greater number of coronary artery branches affected were associated with increased risk of all types of complications. At 10 years, normalization of luminal diameter was noted in 99±4% of patients with small (2.5≤Z<5.0), 92±1% with medium (5.0≤Z<10), and 57±3% with large CAAs (Z≥10). CAAs in the left anterior descending and circumflex coronary artery branches were more likely to normalize. Risk factor analysis of coronary artery branch level outcomes was performed with a total of 893 affected branches with Z score ≥10 in 440 patients. In multivariable regression models, hazards of luminal narrowing and thrombosis were higher for patients with CAAs of the right coronary artery and left anterior descending branches, those with CAAs that had complex architecture (other than isolated aneurysms), and those with CAAs with Z scores ≥20.
Conclusions
For patients with CAA after KD, medium‐term risk of complications is confined to those with maximum CAA Z scores ≥10. Further risk stratification and close follow‐up, including advanced imaging, in patients with large CAAs is warranted.
Keywords: cardiovascular outcomes, coronary artery, Kawasaki disease, risk factors
Subject Categories: Clinical Studies, Coronary Circulation
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CAA
coronary artery aneurysm
- CI
cumulative incidence
- IKDR
International Kawasaki Disease Registry
- IQR
interquartile range
- IVIG
intravenous immunoglobulin
- KD
Kawasaki disease
- LAD
left anterior descending coronary artery
- LCX
left circumflex artery
- LMCA
left main coronary artery
- MI
myocardial infarction
- RCA
right coronary artery
- REDCap
Research Electronic Data Capture
Clinical Perspective
What Is New?
Medium‐term cardiac complications of coronary artery aneurysms secondary to Kawasaki disease are relatively frequent but generally limited to patients with maximum coronary artery Z scores >10.
While patients with coronary artery Z scores >10 were previously thought to represent a common risk category, increased risk profile was observed for patients with coronary artery Z scores >20.
Physical properties of coronary artery aneurysms are associated with the risk of complications potentially by affecting blood rheology.
What Are the Clinical Implications?
While still requiring ongoing follow‐up, patients with small or medium coronary artery aneurysms can likely be managed with low‐intensity, antiplatelet‐based thromboprophylaxis and are unlikely to need anticoagulation.
Given the substantial risk of complications, combined thromboprophylaxis with both anticoagulation and antiplatelet agents should be considered in patients with giant coronary artery aneurysms, particularly those with coronary artery Z scores >20.
Kawasaki disease (KD) is a pediatric systemic vasculitis with a predilection for the coronary arteries. Coronary artery aneurysms (CAAs) are recognized as the most important complication of KD, leading to significant morbidity and mortality. As a result, KD is now thought to be the leading cause of acquired heart disease in children of developed countries. 1 , 2 Despite optimal acute management, CAAs still develop in ≈4% of cases. The prevalence of CAAs increases to 25% if the diagnosis of KD is missed or treatment is delayed. 1 Thrombosis in large CAAs can lead to total occlusion and myocardial infarction (MI) and sudden death. 1 In the long‐term, CAAs accompanied by chronic inflammation and luminal myofibroblastic proliferation have the potential to reduce the size of CAA, but the process may eventually cause luminal narrowing. Additionally, nonocclusive organized thrombus and recanalized occlusive thrombus can contribute to luminal narrowing. 1 An angiographic study published in 1986 found that there was low prevalence of occlusion and stenosis early on after the acute KD period, and that complications often occurred at least 1 year from disease onset. 3 These findings were later supported by a large historical Japanese cohort reported by Kato et al. 4 This highlights the importance of uninterrupted long‐term follow‐up with serial imaging to determine the risks of cardiac complications in patients with KD and inform tailored management strategies.
Given the heterogeneity of patients with CAAs and their rarity at any single institution, there are a paucity of data available to inform more precise risk stratification for outcomes in this population. In addition, a limited number of risk factors have been identified for adverse outcomes, which has impeded the development and evaluation of long‐term surveillance and management strategies calibrated to individual risk. Therefore, we sought to determine the cumulative risk and associated factors for adverse cardiovascular outcomes for patients with KD and CAAs using data from the IKDR (International Kawasaki Disease Registry), a large, curated, multi‐institutional registry including medium‐ to long‐term clinical follow‐up.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the research ethics board of The Hospital for Sick Children at researchethics.board2@sickkids.ca and to the corresponding author of this study to initiate the process. Authorization from all institutions who participated in this study will be required to access the data set.
Study Population
Data for this study was collected by the IKDR, which included 34 pediatric referral centers in Canada, the United States, and Taiwan. To be eligible for the registry, patients younger than 18 years must have been diagnosed with complete or incomplete KD between 1999 and 2017 and have at least 1 CAA with a maximum coronary artery Z score ≥2.5 at any time after diagnosis based on the American Heart Association (AHA) recommendations for risk stratification. 1 Patients seen at participating centers only for a single consultation (or in the acute phase only) and patients seen for the first time at the reporting center >3 months after the acute phase were not eligible for the registry. For the purpose of this study, data harvest was performed on March 1, 2018, and included all follow‐up information up to December 31, 2017. Participating centers were required to submit all cases that met all inclusion criteria and none of the exclusion criteria, thus preventing potential selection bias.
Data Acquisition
Data were retrospectively collected by participating centers and entered using centralized Research Electronic Data Capture (REDCap) tools, which is a secure, web‐based application designed to support data capture for research studies, hosted at SickKids Hospital (Toronto, Canada). 5 Personnel from the data coordinating center (also hosted at SickKids Hospital) reviewed patient data for eligibility and performed data validation and verification. Participation in the IKDR requires institutional review board or research ethics board approval for the study at the US and Canadian/Taiwanese sites, respectively, with the institutional review board/research ethics board approving a waiver of consent for the collection of retrospective data. SickKids Hospital was responsible for managing all bilateral data sharing agreements.
Data Collection
The IKDR collects deidentified information regarding patient demographics, details of hospitalization for the acute KD episode, short‐ and long‐term management, imaging data, and complications. As per AHA guidelines, patients with prolonged fever but ≤3 of the classic clinical features of KD were classified as having incomplete KD. Copies of all imaging reports (echocardiograms, angiograms, computed tomography scans, and magnetic resonance imaging) were submitted to the data coordinating center for centralized data extraction, and the coronary arteries of all patients were characterized and classified. Serial absolute internal luminal diameters were retrieved from echocardiography reports, and the measurements were adjusted for body surface area (recalculated using the Haycock formula for all patients to ensure consistency) by calculation of Z scores using the Boston coronary artery Z score equations (the left anterior descending artery [LAD] equation was used for the left circumflex artery [LCX]). 2 Based on maximal Z score, CAAs were classified as small (2.5≤Z<5), medium (5≤Z<10), and large (Z≥10), as per 2017 AHA guidelines. 1 Data extracted from written reports of imaging study included the number of coronary artery branches with CAAs and the maximum CAA Z score ever reached. The coronary artery architecture was classified as dilatation only, isolated CAA in an otherwise normal vessel, isolated CAA in a dilated vessel, or complex CAAs. Complex CAA was defined as any coronary artery branch with >1 discrete lesion or with CAAs extending into the bifurcation of the left main coronary artery. The CAA location within the branch, the CAA shape and length of the CAA when reported, the CAA diameter to length ratio, and the estimated CAA ellipsoid area (diameter multiplied by length×0.80) were also assessed. Normalization of CAA diameter was defined as the reduction of the internal diameter of a CAA during follow‐up to achieve a Z score <2.5.
Patient Outcomes
Complications of CAAs were defined as: (1) luminal narrowing clinically reported as significant coronary artery stenosis/obstruction confirmed on coronary artery angiography (>50% narrowing of lumen diameter); (2) coronary artery revascularization either by catheterization or surgery; (3) coronary artery thrombosis (occlusive or nonocclusive) diagnosed on any imaging modality; (4) MI reported clinically; and (5) major adverse cardiac complications, including a composite of surgical or catheter‐based revascularization, MI, cardiac ischemia, heart transplantation, or death from cardiac causes.
Statistical Analysis
Data are presented as means with SDs, medians with interquartile ranges (IQRs), and frequencies as appropriate. Time‐related freedom from outcomes was calculated using the Kaplan–Meier method (presented as cumulative incidence rate rather than freedom from event). Patient follow‐up intervals were censored at last follow‐up or death. Multivariable risk factor analysis was performed using Cox proportional hazard analysis, with variable selection informed using bootstrap resampling (500 random samples, P<0.05 to enter and P<0.10 to remain). 6 Variables with high reliability (ie, selected in >50% of the random samples) were then included in a multivariable Cox proportional hazard model. Backward selection of variables was then used to obtain a final model. Proportional hazard assumption was tested by assessing the statistical significance of the time‐dependent covariate (interaction between the time component and each parameters) in the regression models; none of which demonstrated a violation of the proportional hazard assumption. A complete list of variables considered in the risk factor analysis can be found in Table 1. Note that acetylsalicylic acid use was not included in risk factor analysis given that >99% of patients were taking this medication, and long‐term treatments (eg, antithrombotics, β‐blockers, and statins) were not included given their time‐varying nature. Pertinent negatives are reported. For the analysis by affected coronary artery branch, only branches with CAA maximal Z score ≥10 were included. While each branch is unique, patients could have had >1 branch with CAA Z score ≥10. For this analysis, adjustment for repeated measures (multiple branches included from the same patient) was performed by including the model‐based estimate for the covariance matrix as a regression term in the Cox proportional hazard model. Kaplan–Meier analyses were performed for up to 10 years postdiagnosis, after which the number of patients remaining at risk was deemed too low to generate valid estimates. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Table 1.
Patients and CAA Characteristics Considered in the Risk Factor Analysis for Cardiac Complications and Outcomes
| Risk Factors |
|---|
| Age at KD |
| Sex |
| Type of KD (complete/incomplete) |
| Arthritis at diagnosis |
| Laboratory values during acute phase |
| Hemoglobin, g/L |
| Hematocrit, % |
| ESR, mm/h |
| White blood cell count, ×109/L |
| Platelets, ×109/L |
| Albumin, g/L |
| C‐reactive protein, mg/L |
| Red blood cell count, ×1012/L |
| Aspartate transaminase, U/L |
| Alanine aminotransferase, U/L |
| Duration of fever before IVIGs |
| Total duration of fever |
| IVIG treatment |
| No. of IVIG doses |
| Intravenous steroids in the acute phase |
| Oral steroids in the acute phase |
| Infliximab in the acute phase |
| Any use of nonsteroidal anti‐inflammatory drugs in the acute phase |
| Coronary artery branch |
| Maximum coronary artery Z score |
| Involvement of the LAD/LMCA/LCX bifurcation |
| Aneurysm architecture |
| Aneurysm location within coronary branch |
| Aneurysm shape |
| Aneurysm width to height ratio |
| Aneurysm estimated area |
CAA indicates coronary artery aneurysm; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; LAD, left anterior descending artery; LCX, left circumflex artery; and LMCA, left main coronary artery.
Results
Patient and CAA Characteristics
A total of 1651 patients were included in this analysis, of whom 1184 (72%) were men, as expected given the sex distribution of KD. The median age at diagnosis was 2 years (IQR, 0.67–4.67 years; range 1 month–17.9 years), with 744 (45%) aged between 1 and 5 years. Patients had a median fever duration of 9 days (IQR, 7–13 days), and 973 of 1633 (60%) had complete KD. Maximum Z scores of any of the 4 coronary artery branches (right coronary artery [RCA], left main coronary artery [LMCA], LAD, or LCX) were used to categorize patients as having small 854 (52%), medium 357 (22%), and large 440 (27%) CAAs. Notably, a greater degree of coronary artery complications was associated with extremes of the age spectrum (younger and older), having incomplete rather than complete KD, longer duration of fever before treatment, nonresponse to first treatment with intravenous immunoglobulins (IVIGs) as indicated by need for immunosuppressive therapy beyond the initial IVIG cycle, and longer total duration of fever. Complete patient characteristics stratified by degree of coronary artery involvement are presented in Table 2.
Table 2.
Patient Characteristics Stratified by Maximum Degree of Coronary Artery Involvement
| No. | All Patients | No. | Small CAA | No. | Medium CAA | No. | Large CAA | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Acute phase presentation | |||||||||
| Men | 1651 | 1184 (72%) | 854 | 623 (73%) | 357 | 245 (69%) | 440 | 316 (72%) | 0.32 |
| Age at diagnosis, y | 1651 | 2.0 (0.7–4.7) | 854 | 2.4 (1.1–5.0) | 357 | 1.5 (0.5–3.8) | 440 | 1.4 (0.4–4.7) | <0.001 |
| Age at diagnosis category, y | 1651 | 854 | 357 | 440 | <0.001 | ||||
| <0.5 | 282 (17%) | 78 (9%) | 76 (21%) | 128 (29%) | |||||
| 0.5 to <1 | 240 (15%) | 113 (13%) | 61 (17%) | 66 (15%) | |||||
| 1 to <5 | 744 (45%) | 447 (52%) | 155 (43%) | 142 (32%) | |||||
| 5 to <10 | 306 (19%) | 171 (20%) | 56 (16%) | 79 (18%) | |||||
| ≥10 | 79 (5%) | 45 (5%) | 9 (3%) | 25 (6%) | |||||
| Any acute readmission | 1508 | 223 (15%) | 757 | 79 (10%) | 331 | 44 (13%) | 420 | 100 (23%) | <0.001 |
| Fever pre‐IVIGs, d | 1578 | 7 (5–10) | 824 | 6 (5–8) | 348 | 7 (5–10) | 406 | 9 (6–14) | <0.001 |
| Fever post‐IVIGs, d | 1527 | 1 (0–3) | 783 | 1 (0–2) | 344 | 1 (0–3) | 400 | 1 (0–5) | <0.001 |
| Total fever duration, d | 1553 | 9 (7–13) | 798 | 8 (6–10) | 348 | 9 (7–12) | 407 | 13 (9–17) | <0.001 |
| Length of hospital stay, d | 1576 | 5 (3–8) | 802 | 4 (3–6) | 352 | 5 (3–9) | 422 | 8 (4–15) | <0.001 |
| Conjunctivitis | 1616 | 1462 (91%) | 835 | 756 (91%) | 354 | 317 (90%) | 427 | 389 (91%) | 0.75 |
| Cervical lymphadenopathy | 1616 | 653 (40%) | 835 | 367 (44%) | 354 | 130 (37%) | 427 | 156 (37%) | 0.01 |
| Rash | 1618 | 1386 (86%) | 837 | 717 (86%) | 354 | 315 (89%) | 427 | 354 (83%) | 0.05 |
| Erythema/edema of extremities | 1593 | 978 (61%) | 823 | 527 (64%) | 348 | 206 (59%) | 422 | 245 (58%) | 0.08 |
| Mucosal changes | 1616 | 1237 (77%) | 835 | 677 (81%) | 354 | 256 (72%) | 427 | 304 (71%) | <0.001 |
| No. of KD symptoms | 1597 | 828 | 351 | 418 | 0.006 | ||||
| 1 | 56 (3%) | 26 (3%) | 12 (3%) | 18 (4%) | |||||
| 2 | 175 (11%) | 71 (8%) | 48 (13%) | 56 (13%) | |||||
| 3 | 436 (26%) | 208 (24%) | 101 (28%) | 127 (29%) | |||||
| 4 | 648 (39%) | 363 (43%) | 137 (38%) | 148 (34%) | |||||
| 5 | 282 (17%) | 160 (19%) | 53 (15%) | 69 (16%) | |||||
| Type of KD reported | 1633 | 845 | 355 | 433 | <0.001 | ||||
| Complete | 973 (60%) | 549 (65%) | 199 (56%) | 225 (52%) | |||||
| Incomplete | 660 (40%) | 296 (35%) | 156 (44%) | 208 (48%) | |||||
| Arthritis | 1384 | 170 (12%) | 718 | 87 (12%) | 310 | 24 (8%) | 356 | 59 (17%) | 0.002 |
| Weight before first IVIG dose, kg | 1401 | 13 (9–19) | 700 | 13 (10–19) | 309 | 12 (8–17) | 392 | 11 (8–20) | <0.001 |
| Duration of follow‐up, y | 1651 | 2.1 (0.9–6.4) | 854 | 1.2 (0.4–3.4) | 357 | 2.8 (1.0–6.3) | 440 | 5.2 (1.9–8.9) | <0.001 |
| Laboratory values at admission | |||||||||
| Hemoglobin, g/L | 1350 | 105.7±14.2 | 732 | 108.6±12.8 | 295 | 103.6±14.2 | 323 | 101.2±15.7 | <0.001 |
| Hematocrit, % | 1300 | 0.31±0.04 | 716 | 0.32±0.04 | 282 | 0.31±0.04 | 302 | 0.30±0.04 | <0.001 |
| Red blood cell count, ×1012/L | 1042 | 4.0 (3.6–4.3) | 526 | 4.1 (3.8–4.4) | 246 | 3.9 (3.6–4.3) | 270 | 3.8 (3.4–4.2) | <0.001 |
| White blood cell count, ×109/L | 1358 | 16.1±6.8 | 729 | 14.9±5.7 | 303 | 16.6±6.7 | 326 | 18.2±8.4 | <0.001 |
| Platelets, ×109/L | 1339 | 442±220 | 719 | 395±170 | 295 | 467±242 | 325 | 523±265 | <0.001 |
| ESR, mm/h | 1166 | 62 (46–88) | 647 | 60 (45–85) | 257 | 61 (46–85) | 262 | 70 (50–100) | <0.001 |
| C‐reactive protein, mg/L | 1140 | 20 (9–84) | 629 | 18 (8– 69) | 246 | 20 (8–96) | 265 | 25 (12–106) | 0.004 |
| Albumin, g/L | 1003 | 31.6±6.4 | 553 | 33.1±6.2 | 222 | 30.8±6.1 | 228 | 28.8±6.2 | <0.001 |
| Aspartate transaminase, U/L | 946 | 36 (25–58) | 494 | 37 (27–61) | 220 | 34 (24–55) | 232 | 33 (24–53) | 0.02 |
| Alanine aminotransferase, U/L | 1202 | 35 (19–74) | 684 | 38 (20–90) | 259 | 33 (18–64) | 259 | 32 (19–57) | 0.04 |
| Acute treatment | |||||||||
| At least 1 IVIG course | 1646 | 1592 (97%) | 852 | 828 (97%) | 357 | 350 (98%) | 437 | 414 (95%) | 0.03 |
| No. of IVIG doses | 1643 | 852 | 357 | 434 | <0.001 | ||||
| 0 | 54 (3%) | 24 (3%) | 7 (2%) | 23 (5%) | |||||
| 1 | 1009 (61%) | 600 (70%) | 207 (58%) | 202 (46%) | |||||
| 2 | 506 (31%) | 214 (25%) | 125 (35%) | 167 (38%) | |||||
| 3 | 66 (4%) | 14 (2%) | 16 (5%) | 36 (8%) | |||||
| ≥4 | 8 (1%) | 0 (0%) | 2 (1%) | 6 (1%) | |||||
| Infliximab | 1642 | 214 (13%) | 851 | 86 (10%) | 355 | 51 (14%) | 436 | 77 (18%) | <0.001 |
| Pentoxifylline | 1591 | 7 (0.4%) | 816 | 0 (0%) | 346 | 2 (1%) | 429 | 5 (1%) | 0.01 |
| Immunotherapy | 1591 | 46 (3%) | 816 | 10 (1%) | 346 | 11 (3%) | 429 | 25 (6%) | <0.001 |
| Any steroids | 1594 | 342 (21%) | 817 | 104 (13%) | 348 | 73 (21%) | 429 | 165 (38%) | <0.001 |
| Oral steroids | 1592 | 216 (14%) | 817 | 58 (7%) | 346 | 44 (13%) | 429 | 114 (27%) | <0.001 |
| Intravenous steroids | 1525 | 288 (19%) | 761 | 85 (11%) | 336 | 62 (19%) | 428 | 141 (33%) | <0.001 |
Small CAA: 2.5 ≤ Z < 5; Medium CAA: 5 ≤ Z < 10; Large CAA: Z ≥ 10.
CAA indicates coronary artery aneurysm; ESR, erythrocyte sedimentation rate; IVIG, intravenous immunoglobulin; and KD, Kawasaki disease.
Complications of CAAs
Median duration of follow‐up was 2.1 years (IQR, 0.9–6.4; 5th–95th percentile: 0.1–12.5) (total of 6366 patient‐years of follow‐up) for all patients. Follow‐up was substantially longer for patients with large CAAs, with a median of 5.2 years (IQR, 1.9–8.9; 5th–95th percentile: 0.3–14.3) (P<0.001). No complications were reported for patients with small CAAs. Only 1 patient with a medium CAA (maximum Z score of 8.5) experienced a cardiac complication (chronic cardiac ischemia resulting in heart failure and death); this patient happened to have CAAs in all 4 coronary artery branches. Figure 1 presents the cumulative incidence (CI) of 6 major complications in patients with large CAAs, including luminal narrowing (10‐year CI, 20±3%), coronary artery interventions (10‐year CI, 10±2%), coronary artery thrombosis (10‐year CI, 18±2%), MI (10‐year CI, 5±2%), composite major adverse cardiac complications (10‐year CI, 14±2%), and death from cardiac causes or heart transplantation (10‐year CI, 2±1%).
Figure 1. Cumulative incidence calculated using Kaplan–Meier survival analysis of complications for patients with coronary artery aneurysms after Kawasaki disease in patients with at least 1 lesion with a coronary artery Z score ≥10.
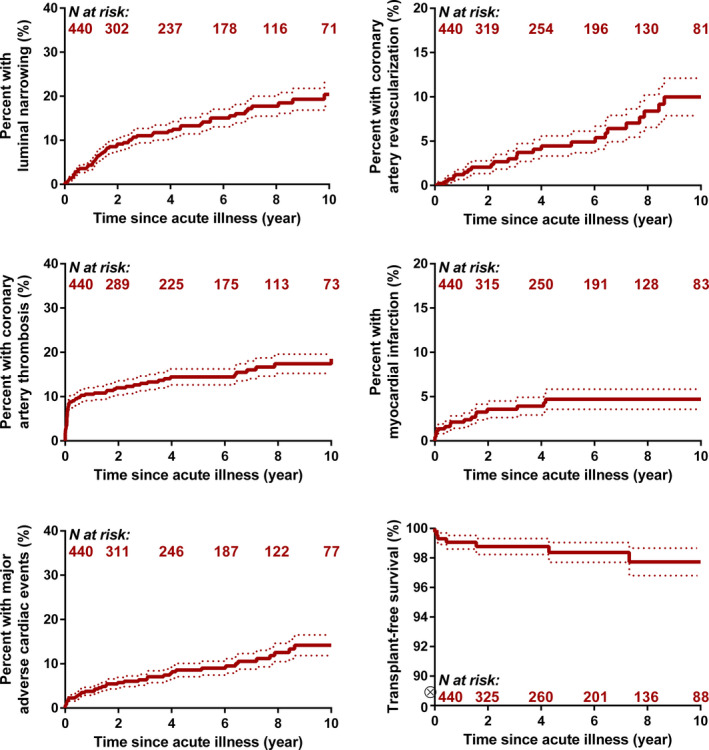
Dotted lines represent the estimated standard error around the cumulative incidence.
There were 8 deaths overall: 1 with medium and 7 with large CAAs. The 1 patient with medium CAAs in all 4 branches (mentioned above) died of chronic ischemia 6 months after the acute phase. Four deaths were secondary to MI (3 within 6 weeks of the acute phase and 1 that occurred 18 months after the acute phase). The other 3 patients died of noncardiac causes (1 mitochondrial disorder and 2 trauma). In addition, 2 patients with large CAAs underwent heart transplantation at 5 months and 4 years after the acute phase. A higher maximum CAA Z score and higher number of coronary artery branches affected were strongly associated with the composite outcome of major cardiac complications (Table 3).
Table 3.
Univariable Factors Associated With Composite Risk Major Adverse Cardiac Complications in Cox Proportional Hazard Analysis for All Patients With KD and Maximum Coronary Artery Z Score ≥2.5
| Univariable Risk Factors | HR | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|
| Maximum CAA Z score | ||||
| ≥2.5 to <10 | 0.19 | 0.09 | 0.42 | <0.001 |
| ≥10 to <20 | Reference | |||
| ≥20 to <30 | 2.33 | 1.01 | 5.40 | 0.05 |
| ≥30 | 7.63 | 3.12 | 18.7 | <0.001 |
| No. of CAAs with Z score ≥10 | ||||
| 1 | 0.05 | 0.02 | 0.16 | <0.001 |
| 2 | Reference | |||
| 3 | 1.98 | 0.91 | 4.33 | 0.09 |
| 4 | 3.13 | 1.38 | 7.11 | 0.006 |
CAA indicates coronary artery aneurysm; HR, hazard ratio; KD, Kawasaki disease.
Branch Level Outcomes
Given that the risk of complications was associated with specific CAA characteristics (as opposed to being determined solely at the patient level), risk factor analyses were performed at the coronary artery branch level for branches with at least 1 CAA with a Z score ≥10. CAAs with Z scores <10 were not included in this analysis because of our finding that there was no substantial risk of complications in those patients. A total of 893 CAAs in 440 patients were included in this analysis, with 370 (42%) in the LAD, 297 (33%) in the RCA, 135 (15%) in the LCX, and 91 (10%) in the LMCA. The majority (603, 68%) of branches included had maximum CAA Z scores 10 to 19.9, 195 (22%) had Z scores 20 to 29.9, 48 (5%) had Z scores 30 to 39.9 (5%), and 47 (5%) had Z scores ≥40. Complete details of the architecture of CAAs by branch are presented in Table 4. This analysis focused on 3 branch‐level outcomes: narrowing of luminal diameter >50% of normal, coronary artery thrombosis, and normalization of luminal diameter. Given that vascular interventions and MIs may be a consequence of the first 2 categories, risk factors are presumed likely to be the same. Vascular interventions and MIs were not analyzed as separate outcomes.
Table 4.
Characteristics of Coronary Artery Branches With Aneurysms With Maximum Coronary Artery Z Score ≥10
| Branch | |
| RCA | 297 (33%) |
| LMCA | 91 (10%) |
| LAD | 370 (42%) |
| LCX | 135 (15%) |
| Maximum coronary artery Z score | Median (IQR) 15.7 (12.3–22.3) |
| 10.0–19.9 | 603 (68%) |
| 20.0–29.9 | 195 (22%) |
| 30.0–39.9 | 48 (5%) |
| 40.0+ | 47 (5%) |
| Involvement of the LAD/LMCA/LCX bifurcation | |
| Yes | 338 (38%) |
| No | 555 (62%) |
| Aneurysm architecture | |
| Isolated aneurysm in normal vessel | 161 (18%) |
| Isolated aneurysm in a dilated vessel | 332 (37%) |
| Complex aneurysms* | 251 (28%) |
| Undefined/unreported | 149 (17%) |
| Aneurysm location within branch | |
| Proximal segment | 343 (38%) |
| Distal segment | 79 (9%) |
| Extending throughout segment | 221 (25%) |
| Undefined/unreported | 250 (28%) |
| Aneurysm shape | |
| Saccular | 171 (20%) |
| Fusiform/irregular | 306 (34%) |
| Undefined/unreported | 416 (47%) |
| Aneurysm diameter to height ratio (n=312) | Median (IQR) 0.73 (0.53–1.06) |
| Aneurysm estimated area (n=312) | Median (IQR) 0.72 (0.44–1.34) |
IQR indicates interquartile range; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; and RCA, right coronary artery.
Architecture other than a single isolated aneurysm in a coronary artery branch.
At 10 years, luminal narrowing affected 12±1% of all branches with large CAAs, with substantially higher rates of narrowing for CAAs in the RCA and the LAD (versus LMCA and LCX) and in branches with CAAs with Z scores ≥20 (Figure 2). The complete list of factors associated with luminal narrowing in multivariable Cox regression models is provided in Table 5. Other than CAA branch and higher CAA size, complex CAA architecture was also associated with greater hazard of luminal narrowing. Some characteristics of the acute phase were also associated with an increased hazard of luminal narrowing, namely lower number of IVIG doses (ie, cycles) during the acute phase, lower serum albumin levels, and higher total number of days of fever before treatment with IVIGs.
Figure 2. Cumulative incidence of luminal narrowing, coronary artery thrombosis, and normalization of luminal diameter, calculated using Kaplan–Meier survival analysis, for coronary artery aneurysms after Kawasaki disease in coronary artery branches with coronary artery Z score ≥10 stratified by coronary artery branches.
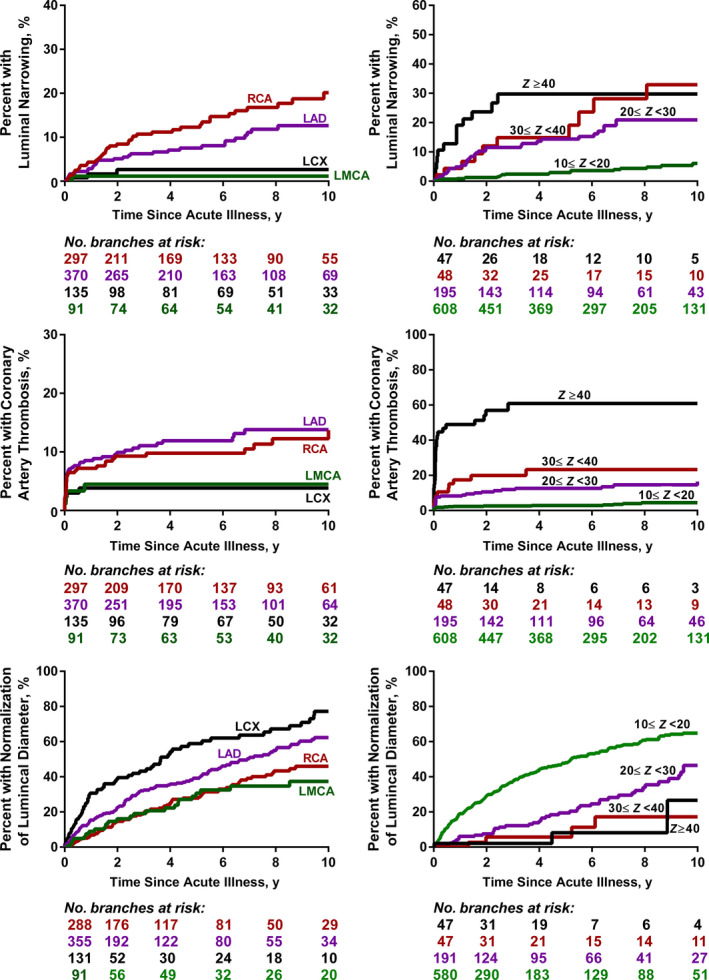
LAD indicates left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery; and maximum coronary artery Z scores.
Table 5.
Multivariable Factors Associated With Risk Luminal Narrowing Using Cox Proportional Hazard Analysis in Branches With CAAs With Z Score ≥10
| Multivariable Risk Factors | Reliability, % | HR | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|---|
| Maximum CAA Z score | 100 | ||||
| ≥10 to <20 | Reference | ||||
| ≥20 to <30 | 3.65 | 1.91 | 6.98 | <0.001 | |
| ≥30 to <40 | 4.24 | 2.00 | 9.01 | <.0001 | |
| ≥40 | 7.56 | 3.19 | 17.9 | <0.001 | |
| No. of d of fever before IVIGs | 98 | 1.04 | 1.01 | 1.07 | 0.01 |
| Complex aneurysms architecture | 97 | 1.93 | 1.24 | 3.01 | 0.004 |
| Albumin level, g/L | 94 | 0.92 | 0.86 | 0.98 | 0.008 |
| No. of IVIG cycles | 93 | ||||
| 0 | Reference | ||||
| 1 | 0.13 | 0.03 | 0.54 | 0.005 | |
| 2 | 0.34 | 0.12 | 0.96 | 0.04 | |
| 3+ | 0.48 | 0.18 | 1.29 | 0.15 | |
| Branch | 78 | ||||
| RCA | Reference | ||||
| LMCA | 0.11 | 0.01 | 0.82 | 0.03 | |
| LAD | 0.58 | 0.38 | 0.89 | 0.01 | |
| LCX | 0.22 | 0.07 | 0.72 | 0.01 |
CAA indicates coronary artery aneurysm; HR, hazard ratio; IVIG, intravenous immunoglobulin; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; and RCA, right coronary artery.
Thrombosis affected 11±1% of all branches with large CAAs by 10 years, with a predilection for the RCA and the LAD, and for branches with CAAs with Z score ≥20 (Figure 2). The complete list of factors associated with coronary artery thrombosis in multivariable Cox regression models is provided in Table 6. Factors associated with an increased risk of thrombosis included greater maximum CAA Z score, no treatment with IVIGs in the acute phase, complex CAA architecture, and lower serum C‐reactive protein in the acute phase.
Table 6.
Multivariable Factors Associated With Risk Coronary Artery Thrombosis, Using Cox Proportional Hazard Analysis, in Branches With CAAs With Z Score ≥10
| Multivariable Risk Factors | Reliability, % | HR | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|---|
| Maximum CAA Z score | 100 | ||||
| ≥10 to <20 | Reference | ||||
| ≥20 to <30 | 3.51 | 1.84 | 6.69 | <0.001 | |
| ≥30 to <40 | 5.74 | 2.50 | 13.16 | <0.001 | |
| ≥40 | 29.5 | 14.4 | 60.3 | <0.001 | |
| Complex aneurysms architecture | 88 | 2.08 | 1.32 | 3.26 | 0.002 |
| Treatment with IVIG | 87 | 0.38 | 0.19 | 0.76 | 0.006 |
| C‐reactive protein, 10 mg/L | 76 | 0.95 | 0.90 | 0.99 | 0.02 |
CAA indicates coronary artery aneurysm; HR, hazard ratio; and IVIG, intravenous immunoglobulin.
Normalization of luminal diameter at 10 years occurred in 57±3% of branches with large CAAs, which was substantially lower than the rate of normalization for branches with medium (92±1%) or small CAAs (99±4%) (Figure 3). Large CAAs in the LAD and LCX were more likely to normalize in dimensions compared with those in the RCA or LMCA, and the hazard of normalization was inversely proportional to the maximum Z score of the CAA (Figure 2). The complete list of factors associated with normalization of luminal diameter in the multivariable regression model are shown in Table 7. Other than coronary artery branch and the maximum size of the CAA, factors associated with increased risk of normalization were lower total CAA ellipsoid area, treatment with IVIGs in the acute phase, female sex, younger age at acute KD, incomplete KD, and lower initial erythrocyte sedimentation rate.
Figure 3. Cumulative incidence of normalization of luminal diameter, calculated using Kaplan–Meier survival analysis, for coronary artery aneurysms (CAAs) after Kawasaki disease stratified by level of coronary artery involvement.
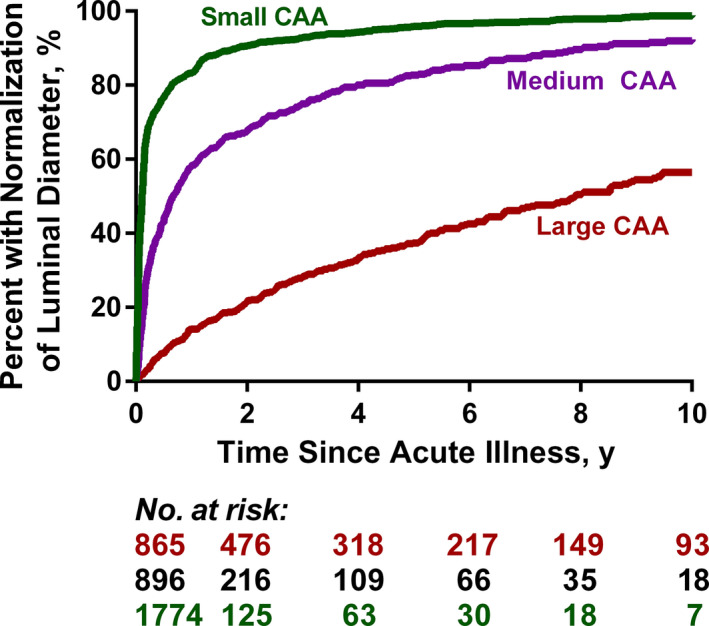
Table 7.
Multivariable Factors Associated With Risk of Normalization of Luminal Diameter, Using Cox Proportional Hazard Analysis, in Branches With CAAs With Z Score ≥10
| Multivariable Risk Factors | Reliability, % | HR | Lower 95% CI | Upper 95% CI | P Value |
|---|---|---|---|---|---|
| Maximum CAA Z score | 100 | ||||
| ≥10 to <20 | Reference | ||||
| ≥20 to <30 | 0.50 | 0.38 | 0.66 | 0.002 | |
| ≥30 to <40 | 0.24 | 0.12 | 0.51 | <0.001 | |
| ≥40 | 0.25 | 0.10 | 0.61 | <0.001 | |
| Aneurysm area, cm2 | 100 | ||||
| >1.35 | 7.01 | 2.38 | 20.6 | <.001 | |
| 0.71 to 1.35 | 4.50 | 1.61 | 12.6 | 0.004 | |
| 0.46 to 0.70 | 1.59 | 0.46 | 5.58 | 0.47 | |
| ≤0.45 | Reference | ||||
| Branch | 99 | ||||
| RCA | Reference | ||||
| LMCA | 0.60 | 0.39 | 0.91 | 0.02 | |
| LAD | 1.94 | 1.51 | 2.48 | <.001 | |
| LCX | 2.16 | 1.55 | 3.00 | <.001 | |
| Treatment with IVIGs | 94 | 3.22 | 1.28 | 8.13 | 0.01 |
| Age at acute KD, y | 94 | 0.93 | 0.89 | 0.97 | 0.006 |
| Incomplete KD | 85 | 1.31 | 0.99 | 1.73 | 0.056 |
| ESR, 10 mm/h | 78 | 0.95 | 0.91 | 0.99 | 0.03 |
| Women | 67 | 1.33 | 1.03 | 1.72 | 0.03 |
CAA indicates coronary artery aneurysm; ESR, erythrocyte sedimentation rate; HR, hazard ratio; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; and RCA, right coronary artery.
CAA shape (saccular or fusiform/irregular), location in the vessel (distal, extending, or proximal), and width to height ratio were not associated with any cardiac complications in multivariable models (although some associations were statistically significant at the univariable level) (data not shown).
Discussion
In this study, we report that the occurrence of luminal narrowing, coronary artery thrombosis, and major adverse cardiac complications were nearly completely confined to patients with CAAs with Z scores ≥10. Importantly, in those patients, the risk continued to increase over time, highlighting the importance of long‐term and uninterrupted clinical follow‐up. The highest risks were associated with CAAs in the LAD and RCA with maximum Z scores ≥20 and complex CAA architecture. Luminal normalization of coronary artery dimension was more likely to occur for CAAs in the LAD and LCX branches, with the likelihood of normalization inversely related to the initial maximum Z score of CAA. Additional factors such as IVIG treatment during the acute phase, lower erythrocyte sedimentation rate, and lower total CAA area were also associated with an increased likelihood of luminal normalization. This study has the advantages of being a large, multi‐institutional, international study, which more precisely defines the prevalence of CAA‐related cardiovascular complications, uses longitudinal Z scores, and defines associations with coronary artery branch, with over a 10‐year follow‐up.
Risk Stratification
Manlhiot et al 7 first demonstrated that, regardless of absolute dimensions, a CAA Z score ≥10 represented the effective lower bound of risk for cardiac complications in the medium term after KD. However, we found that within groups with large CAAs (Z scores ≥10), the risk profile of large aneurysms with Z scores <20 differs significantly from aneurysms with Z scores ≥20, with the latter being at substantially higher risk of complications. This is a novel finding that may have important implications in the surveillance and management of patients with CAA Z scores ≥20. We, therefore, propose that patients with CAAs that have Z scores ≥20 be further distinguished and be regarded as a separate and higher risk category than those with CAAs that have Z scores between ≥10 and <20. The change in risk stratification has important implications for the intensity and type of thromboprophylaxis that should be used in those patients. In small (2.5≤Z<5) to medium (5≤Z≤10) CAAs, where platelet activation is a key factor in initiating thrombus formation, antiplatelet therapy is likely to be effective and is currently the mainstay of therapy. However, more aggressive thromboprophylactic therapy with the addition of an anticoagulant is necessary in patients with large (Z score ≥10, and even more so in those with Z score ≥20) or progressive CAAs, where the low shear stress environment and flow stasis leads to activation of the clotting cascade. 8
The 2017 AHA guidelines considered omitting echocardiographic evaluation in children with small aneurysms who had normalization of the dimension of their coronary artery lumen (risk level 3.2). No patient with small CAAs had cardiac complications during the follow‐up period, and 99% had normalization of their CAA luminal diameter. Given our data and a previous study by Dionne et al, 9 which showed preserved endothelium continuity in patients with small CAAs but no actual anatomical aneurysms (ie, dilatation only), it would be reasonable to consider discharging these patients from cardiology follow‐up. This recommendation is currently only true for children with lower risk levels (risk level 1 or 2). 1 As cardiologists and whenever evidence supports, we ought to empower our patients to achieve their maximal potential and not leave a cloud of being different for those with no evidence of complications. Such a label might impact “self‐image and lifestyle.” 10
Examination of Additional Risk Factors
While Manlhiot et al proposed a risk classification system based solely on coronary artery Z scores, we found evidence that branch‐level factors play an important role in determining the risk of luminal narrowing and thrombosis, as well as the likelihood of luminal diameter normalization. 7 This supports findings from Tsuda et al 11 that branch CAAs had a higher risk of stenosis compared with bifurcation aneurysms. Rheological studies have reported a strong negative correlation between shear stress and risk of thrombus formation. 8 , 12 In angiographic studies with Doppler flow, the average peak velocity in the CAA was found to decrease as the size of the CAA increased, suggesting that flow stagnation was also a risk factor for thrombosis. 12 , 13 , 14 , 15 , 16
In our analysis of CAA architecture, we found that both a higher number of CAAs within a coronary artery branch, and CAAs of the LAD/LCX involving the left bifurcation, increased the risk of complications in patients with large CAAs. Interestingly, research on atherosclerotic heart disease has found that the localization of atherosclerotic lesions to certain areas within the arterial system, particularly the branching sites, could be associated with rheological characteristics. 14 , 15
In terms of CAA location, the risk of thrombosis and luminal narrowing was higher in CAAs affecting the RCA and LAD coronary artery branches. We speculate that this could be a result of several reasons. First, reliability of LMCA measurements has been of concern, because of its anatomical variation. 17 Second, being short and high‐pressured, the walls of the LMCA may be less likely to weaken and facilitate sluggish flow. In terms of the LCX branch, its distal anatomical placement hinders visualization by echocardiography, which creates the possibility that stenosis and thrombus development in the area were missed.
Taken together, our findings support that these additional coronary artery branch–level risk factors should be taken into account when decisions are made regarding risk classification. While we conclude that CAA architecture was an important feature to include for risk stratification, we did not find any association between CAA shape (saccular versus fusiform) and any of the cardiac complications considered in this study, despite this finding being reported in previous studies. This could be associated with the fact that almost half of all imaging reports did not indicate the shape of the CAAs and, as such, the number of missing data were substantial to identify a pattern. The development and utilization of better methods to characterize CAA lesions would be an important next step to further refine risk stratification models.
Outcome Comparisons With Previous Studies
In a 10‐ to 21‐year follow‐up study by Kato et al 4 of 146 patients with KD and CAAs, 90% of patients showed normalization of luminal dimensions within the first 2 years from onset; however, none of the large or giant CAAs, defined as having an absolute diameter of at least 8 mm, showed normalization of luminal dimension. A study from the United States by Friedman et al 18 found an overall CAA proportion of normalization of luminal dimensions of 75%, which they believed was higher than those reported in previous cohorts attributable to more aggressive treatment. Similar to Kato et al, they also found that the probability of normalization of luminal dimensions was inversely proportional to CAA Z score at diagnosis. 18 This was in line with our findings, with >80% of small to medium CAAs and ≈50% of large CAAs achieving luminal diameter normalization. Friedman et al also reported that major adverse cardiovascular events occurred exclusively in large CAAs, which was similar to our findings, where all complications occurred in patients with large CAAs, with the exception of 1 patient with medium CAAs who had CAAs in all 4 branches. In our study, the rate of CAA normalization was similar to Friedman et al and larger than Kato et al (55%–60%), which speaks to potentially more aggressive management in the current era. However, the rate of luminal narrowing for large CAAs with Z score ≥10 at ≈20% was similar to the data from Kato et al 4 (despite the different definitions of large CAAs), indicating a need for more aggressive management and/or novel approaches in this patient population.
Limitations
This study used echocardiography reports rather than an imaging core laboratory; thus, differences in methods of measurement by participating cardiologists may have contributed to data variability. A secondary analysis of the Pediatric Heart Network’s randomized Kawasaki disease trial has previously shown moderately good agreement (interclass correlation between 0.60 and 0.75 for all segments other than the circumflex) between coronary artery segment dimensions measured at local centers versus core laboratory assessment in this context. 19 Regardless of the method of interpretation, echocardiography is inferior to advanced imaging modalities (eg, computed tomography, magnetic resonance imaging, or invasive angiography) in estimating coronary artery diameter, particularly the distal coronary artery tree, although it is still the standard of care for most patients. Not all patients had such advanced imaging studies (1% of patients with small CAAs, 5% to 8% of patients with medium CAAs, and ≈50% of patients with large CAAs divided roughly equally between angiography and magnetic resonance imaging). In terms of coronary artery branch anatomy, assessment based on reports was required to obtain features, which is subject to interpretation error. Another source of variation is that outcomes were often detected at the time of imaging rather than when they actually occurred, particularly when asymptomatic. Last, our study design was retrospective, and there was no auditing of data at centers, so we cannot exclude the possibility of selection bias in cases submitted, although this would be unlikely.
Conclusions
In this large, multi‐institutional international study conducted in patients with KD who had CAAs, we report that the risks of luminal narrowing, thrombosis, and major adverse cardiovascular complications were nearly completely confined to those with maximum CAA Z scores ≥10. We can also propose that CAAs with Z scores ≥20 should now be considered a separate risk strata because of their distinctly greater risk profile. The presence of complex CAAs, as well as the involvement of LAD and RCA branches, increases an individual's risk for CAA‐related complications, and these factors should be taken into account on an individual basis when considering surveillance and management strategies. Future studies could focus on rheology to investigate patient‐specific hemodynamic factors, which could increase the risk of complications and assess the effectiveness of more aggressive thromboprophylactic regimens, including triple therapy, in patients with CAAs with Z scores ≥20.
Sources of Funding
Funding for the data coordinating center was partially provided by the CIBC World Market Chair in Child Health Research (Brian McCrindle) and the Labatt Family Heart Centre at SickKids Hospital. Additional local funding for participation in the IKDR was provided by les Fonds BoBeau Coeur of the Ste‐Justine Hospital Foundation (Nagib Dahdah), the McCance Family Foundation (Jane Newburger), the Vella Fund (Jane Newburger), and the Children’s Health Foundation of London, Ontario (Kambiz Norozi).
Disclosures
None.
Acknowledgments
The IKDR is grateful for the hard work of the multiple research coordinators, research nurses, and students who collected the data for this registry across all participating centers. The IKDR specifically wishes to thank Annette L. Baker (Boston Children's Hospital), Tanveer Collins (The Hospital for Sick Children, Toronto), Amy Cooper (Nationwide Children's Hospital), Catherine Dimes (Nationwide Children's Hospital), Anne Fournier (CHU Ste‐Justine, Montreal), David R. Fulton (Boston Children's Hospital), Sunita O'Shea (The Hospital for Sick Children, Toronto), Mary Beth Son (Boston Children's Hospital), and Devin D. Thinker (Cincinnati Children's Hospital Medical Center).
The members of the International Kawasaki Disease Registry include: Carolyn A. Altman MD—Texas Children's Hospital, Baylor College of Medicine, Houston, TX; Brett R. Anderson MD—Columbia University, College of Physicians and Surgeons, New York, NY; Emilie Beaulieu MD—Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Quebec, Canada; Carolyn E. Boychuk BSc—Stollery Children's Hospital, Edmonton, Alberta, Canada; Elizabeth Braunlin MD, PhD—University of Minnesota, Minneapolis, MN; Jane C. Burns MD—Department of Pediatrics, University of California San Diego, Rady Children's Hospital‐San Diego, CA; Michael R. Carr MD—Ann and Robert H. Lurie Children's Hospital of Chicago, IL; Andrew Crean MRCP, MPH—Toronto General Hospital, Toronto, Ontario, Canada; Jessica H. Colyer MD—Pediatrics Cardiology, Children's National Health System/George Washington University, Washington, DC; Adam Dempsey PhD—Department of Paediatrics, Western University, London, Ontario, Canada; Laurent Desjardins MD—Division of Pediatric Cardiology, Centre Hospitalier Universitaire Ste‐Justine, University of Montreal, Quebec, Canada; Rejane Dillenburg MD—McMaster Children's Hospital, Hamilton, Ontario, Canada; Audrey Dionne MD—Division of Pediatric Cardiology, Centre Hospitalier Universitaire Ste‐Justine, University of Montreal, Quebec, Canada; Anna Ferris MBBS—Columbia University ‐ College of Physicians and Surgeons, New York, NY; Michael Gewitz MD—Maria Fareri Children’s Hospital at Westchester Medical Center (WMC) Health, New York Medical College, Valhalla, New York; Michelle M. Grcic RN, MSN, CNP—The Heart Center at Nationwide Children's Hospital, Columbus, OH; Steven C. Greenway MD—Alberta Children's Hospital, Calgary, Alberta, Canada; Kevin C. Harris MD, MHSc—Children's Heart Centre, University of British Columbia, Vancouver, British Columbia; Christina Hayden‐Rush BSN—The Children's Hospital of Philadelphia, Philadelphia, PA; Kevin D. Hill MD—Duke University Medical Center, Durham, NC; Supriya Jain MD—Maria Fareri Children’s Hospital at Westchester Medical Center (WMC) Health, New York Medical College, Valhalla, New York; Thomas R. Kimball MD—Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Sean M. Lang MD—Heart Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Ming‐Tai Lin MD, PhD—National Taiwan University, Taipei, Taiwan; William T. Mahle MD—Children's Healthcare of Atlanta, GA; Tapas Mondal MD—McMaster Children's Hospital, Hamilton, Ontario, Canada; Michael A. Portman MD—Seattle Children's Research Institute, Seattle, WA; Claudia Renaud MD—Montreal Children's Hospital, McGill University, Montreal, Quebec, Canada; S. Kristen Sexson Tejitel MD, PhD, MPH—Texas Children's Hospital, Baylor College of Medicine, Houston, TX; Jacqueline R. Szmuszkovicz MD—Children's Hospital of Los Angeles, CA; Karen M. Texter MD—The Heart Center at Nationwide Children's Hospital, Columbus, OH; Deepika Thacker MD—Nemours Cardiac Center, Nemours/Alfred I. duPont Hospital for Children, New Castle County, DE; Elif Seda Selamet Tierney MD—Division of Pediatric Cardiology, Department of Pediatrics, Stanford University, School of Medicine, Palo Alto, CA; Thomas Thomas MD—Pediatric Cardiology, Children's Hospital Colorado, University of Colorado School of Medicine, Aurora, CO; Adriana H. Tremoulet MS, MAS—Department of Pediatrics, University of California San Diego, Rady Children's Hospital‐San Diego, CA; Sharon Wagner‐Lees RN‐BC, BSN, MBA—Children's Hospital of Los Angeles, CA; Andrew Warren MD—IWK Health Centre, Halifax, Nova Scotia, Canada.
J Am Heart Assoc. 2020;9:e016440 DOI: 10.1161/JAHA.119.016440.
This work was presented at the 11th International Kawasaki Disease Symposium, February 3 to 6, 2015, in Honolulu, HI, and at the 12th International Kawasaki Disease Symposium, June 12 to 15, 2018, in Yokohama, Japan.
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Brian W. McCrindle, Email: brian.mccrindle@sickkids.ca.
for the International Kawasaki Disease Registry:
Carolyn A. Altman, Brett R. Anderson, Emilie Beaulieu, Carolyn E. Boychuk, Elizabeth Braunlin, Jane C. Burns, Michael R. Carr, Andrew Crean, Jessica H. Colyer, Adam Dempsey, Laurent Desjardins, Rejane Dillenburg, Audrey Dionne, Anna Ferris, Michael Gewitz, Michelle M. Grcic, Steven C. Greenway, Kevin C. Harris, Christina Hayden‐Rush, Kevin D. Hill, Supriya Jain, Thomas R. Kimball, Sean M. Lang, Ming‐Tai Lin, William T. Mahle, Tapas Mondal, Michael A. Portman, Claudia Renaud, S. Kristen Sexson Tejitel, Jacqueline R. Szmuszkovicz, Karen M. Texter, Deepika Thacker, Elif Seda Selamet Tierney, Thomas Thomas, Adriana H. Tremoulet, Sharon Wagner‐Lees, and Andrew Warren
References
- 1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, et al. Diagnosis, treatment, an d long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. [DOI] [PubMed] [Google Scholar]
- 2. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW, et al.; Pediatric Heart Network Investigators . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, Kimura K, Takamiya M. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7:3–9. [DOI] [PubMed] [Google Scholar]
- 4. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long‐term consequences of Kawasaki disease. A 10‐ to 21‐year follow‐up study of 594 patients. Circulation. 1996;94:1379–1385. [DOI] [PubMed] [Google Scholar]
- 5. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Austin PC. Bootstrap model selection had similar performance for selecting authentic and noise variables compared to backward variable elimination: a simulation study. J Clin Epidemiol. 2008;61:1009–1017.e1. [DOI] [PubMed] [Google Scholar]
- 7. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z‐scores after Kawasaki disease. Pediatr Cardiol. 2010;31:242–249. [DOI] [PubMed] [Google Scholar]
- 8. Ohkubo T, Fukazawa R, Ikegami E, Ogawa S. Reduced shear stress and disturbed flow may lead to coronary aneurysm and thrombus formations. Pediatr Int. 2007;49:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Dionne A, Ibrahim R, Gebhard C, Benovoy M, Leye M, Dery J, Lapierre C, Girard P, Fournier A, Dahdah N. Difference between persistent aneurysm, regressed aneurysm, and coronary dilation in Kawasaki disease: an optical coherence tomography study. Can J Cardiol. 2018;34:1120–1128. [DOI] [PubMed] [Google Scholar]
- 10. Gersony WM. The adult after Kawasaki disease the risks for late coronary events. J Am Coll Cardiol. 2009;54:1921–1923. [DOI] [PubMed] [Google Scholar]
- 11. Tsuda E, Kamiya T, Ono Y, Kimura K, Kurosaki K, Echigo S. Incidence of stenotic lesions predicted by acute phase changes in coronary arterial diameter during Kawasaki disease. Pediatr Cardiol. 2005;26:73–79. [DOI] [PubMed] [Google Scholar]
- 12. Kuramochi Y, Ohkubo T, Takechi N, Fukumi D, Uchikoba Y, Ogawa S. Hemodynamic factors of thrombus formation in coronary aneurysms associated with Kawasaki disease. Pediatr Int. 2000;42:470–475. [DOI] [PubMed] [Google Scholar]
- 13. Hamaoka K, Onouchi Z. Effects of coronary artery aneurysms on intracoronary flow velocity dynamics in Kawasaki disease. Am J Cardiol. 1996;77:873–875. [DOI] [PubMed] [Google Scholar]
- 14. Hamaoka K, Onouchi Z, Kamiya Y, Sakata K. Evaluation of coronary flow velocity dynamics and flow reserve in patients with Kawasaki disease by means of a Doppler guide wire. J Am Coll Cardiol. 1998;31:833–840. [DOI] [PubMed] [Google Scholar]
- 15. Montenegro MR, Eggen DA. Topography of atherosclerosis in the coronary arteries. Lab Invest. 1968;18:586–593. [PubMed] [Google Scholar]
- 16. Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. [DOI] [PubMed] [Google Scholar]
- 17. Ronai C, Hamaoka‐Okamoto A, Baker AL, de Ferranti SD, Colan SD, Newburger JW, Friedman KG. Coronary artery aneurysm measurement and Z score variability in Kawasaki disease. J Am Soc Echocardiogr. 2016;29:150–157. [DOI] [PubMed] [Google Scholar]
- 18. Friedman KG, Gauvreau K, Hamaoka‐Okamoto A, Tang A, Berry E, Tremoulet AH, Mahavadi VS, Baker A, deFerranti SD, Fulton DR, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5:e003289 DOI: 10.1161/JAHA.116.003289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Margossian R, Lu M, Minich LL, Bradley TJ, Cohen MS, Li JS, Printz BF, Shirali GS, Sleeper LA, Newburger JW, et al.; Pediatric Heart Network Investigators . Predictors of coronary artery visualization in Kawasaki disease. J Am Soc Echocardiogr. 2011;24:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


