Abstract
Background
The aim of this study was to compare the predictive accuracy of acute kidney injury (AKI) after cardiac surgery using cardiopulmonary bypass for the largest area under the curve (AUC) below the oxygen delivery (DO2) threshold and the cumulative AUC below the DO2 threshold.
Methods and Results
From March 2017 to October 2019, 202 patients who had undergone cardiac surgery with cardiopulmonary bypass were enrolled. The perfusion parameters were recorded every 20 seconds, and the DO2 (10×pump flow index [L/min per m2]×[hemoglobin (g/dL)×1.36×arterial oxygen saturation (%)+partial pressure of arterial oxygen (mm Hg)×0.003]) threshold of 300 mL/min per m2 was considered to define sufficient DO2. The nadir DO2, the cumulative AUC below the , and the largest AUC below the were used to predict the incidence of AKI. Postoperative AKI was observed in 12.4% of patients (25/202). By multivariable analysis, the largest AUC below the ≥880 (odds ratio [OR], 4.9; 95% CI, 1.2–21.5 [P=0.022]), preoperative hemoglobin concentration ≤11.6 g/dL (OR, 7.6; 95% CI, 2.0–32.3 [P=0.004]), and red blood cell transfusions during cardiopulmonary bypass ≥2 U (OR, 3.3; 95% CI, 1.0–11.1 [P=0.041]) were detected as independent risk factors for AKI. Receiver operating curve analysis revealed that the largest AUC below the was more accurate to predict postoperative AKI compared with the nadir DO2 and the cumulative AUC below the (differences between areas, 0.0691 [P=0.006] and 0.0395 [P=0.001]).
Conclusions
These data suggest that a high AUC below the is an important independent risk factor for AKI after cardiopulmonary bypass, which could be considered for risk prediction models of AKI.
Keywords: acute kidney injury, area under the curve, cardiac surgery, cardiopulmonary bypass, oxygen delivery
Subject Categories: Complications, Cardiovascular Surgery, Imaging
Nonstandard Abbreviations and Acronyms
- AKI
acute kidney injury
- AUC
area under the curve
- CaO2
oxygen content of arterial blood
- CPB
cardiopulmonary bypass
- DO2
oxygen delivery
oxygen delivery 300 mL/min per m2
- eGFR
estimated glomerular filtration rate
- KDIGO
Kidney Disease: Improving Global Outcome
- RBC
red blood cell
Clinical Perspective
What Is New?
The predictive accuracy of acute kidney injury after cardiac surgery using cardiopulmonary bypass was compared for the largest area under the curve (AUC) below the oxygen delivery (DO2) threshold and cumulative AUC below the DO2 threshold.
The largest AUC below the DO2 300 mL/min per m2 threshold was detected as a more accurate predictor of postoperative acute kidney injury compared with nadir DO2 and cumulative AUC below the DO2 300 mL/min per m2 threshold.
What Are the Clinical Implications?
Potentially, these data suggest that avoiding a continuous or severe decrease of DO2 (largest AUC below the DO2 300 mL/min per m2 threshold <880) could reduce the risk of acute kidney injury after cardiac surgery, and minimization of hemodilution and transfusion and adjustment of pump flow are important for preservation of DO2 above 300 mL/min per m2 in clinical practice.
Acute kidney injury (AKI) is a common and serious complication with an occurrence rate ranging from 20% to 40% after cardiac surgery with cardiopulmonary bypass (CPB). 1 , 2 , 3 Several studies have shown that even minimal changes in postoperative creatinine values can predict early and long‐term mortality after cardiac surgery. 4 , 5 , 6 Age, sex, obesity, preoperative renal function, preoperative anemia, diabetes mellitus, chronic lung disease, and postoperative hypotension were reported as risk factors of AKI. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Regarding CPB management, prolonged CPB duration, severe hemodilution, and low oxygen delivery (DO2) were identified as predictors of AKI. 16 , 17 , 18 , 19 , 20 DO2 is one of the few important modifiable parameters related to AKI. It is the amount of oxygen delivered to tissues throughout the whole body per minute and is expressed as the product of cardiac output and oxygen content of arterial blood. DO2 during CPB is dependent on hemoglobin concentration, oxygen saturation, pump flow, and partial pressure of arterial oxygen. When either of these factors drop and DO2 falls below critical levels, oxygen consumption cannot be maintained using aerobic energy production, activating the anaerobic mechanism to supply energy to the cells and increasing lactate levels. Previous retrospective studies have shown the association between nadir DO2 during CPB and postoperative AKI, and the DO2 level to prevent AKI must be maintained at >262 to 272 mL/min per m2 under moderate hypothermia (>32°C). 21 , 22 , 23 However, in these studies, the nadir DO2 level was defined by using an intermittent measurement value at 10‐ to 20‐minute intervals. Since DO2 level is constantly changing during CPB, nadir DO2 level may not be accurately reflected depending on the timing of measurement. Moreover, DO2 level should be >262 to 272 mL/min per m2 under mild hypothermia (>34°C).
In recent years, Mukaida et al 24 evaluated the cumulative area under the curve (AUC) below the DO2 threshold in patients receiving mild hypothermic (35°C) CPB. The AUC represents the area integrated with the time and depth from below the DO2 threshold to return to above the DO2 threshold, the cumulative AUC below the DO2 threshold represents the accumulation of all AUCs (Figure 1). They concluded that a cumulative AUC below the DO2 300 mL/min per m2 () threshold was a good indicator to predict postoperative AKI compared with nadir DO2. 24 However, for cumulative AUC below the DO2 threshold, it was not possible to accurately assess whether the time and depth below the DO2 threshold persisted for a long time. We hypothesized that evaluating the magnitude of the time and depth of continuously falling below the DO2 threshold would be important to predict AKI development after cardiac surgery compared with cumulative AUC below the DO2 threshold. In this study, the largest AUC below the DO2 threshold was evaluated as an indicator of the continuous time and depth below the DO2 threshold during CPB. The largest AUC below the DO2 threshold represents the one with the largest area among all AUCs (Figure 1). DO2, hemoglobin, pump flow rate, arterial oxygen saturation, and partial pressure of arterial oxygen were measured at 20‐second intervals during CPB. Based on this background, the aim of this study was to compare the predictive accuracy of AKI after cardiac surgery using CPB for the largest AUC below the DO2 threshold and cumulative AUC below the DO2 threshold.
Figure 1. Typical case of oxygen delivery (DO2) change during cardiopulmonary bypass based on DO2 300 mL/min per m2 threshold.
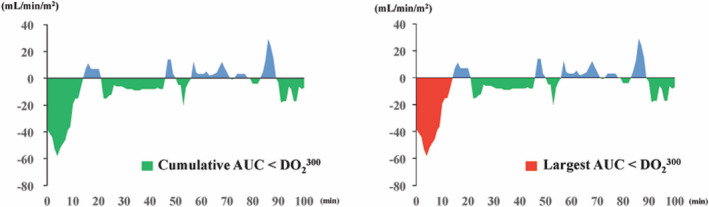
The green area shows the cumulative area below the DO2 threshold=300 mL/min per m2 and the blue area shows the cumulative area above the DO2 threshold=300 mL/min per m2. The red area shows the largest area below the DO2 threshold=300 mL/min per m2. AUC indicates area under the curve.
Methods
Patient Population
This retrospective, case‐controlled study was approved by our institutional ethics committee. 015566‐T3 for using patients' data was obtained from all patients. The data that support the findings of this study are available from the corresponding author upon reasonable request. Between March 2017 and October 2019, 244 patients (≥20 years) underwent cardiac surgery under CPB support at the Sakakibara Heart Institute of Okayama. Of these, 202 patients were enrolled, excluding patients with preoperative moderate renal insufficiency (preoperative estimated glomerular filtration rate [eGFR] <45 mL/min per 1.73m2) and emergent operation. The procedures included 22 isolated coronary artery bypass grafting, 125 isolated valve surgery, 46 coronary artery bypass grafting+valve surgery, 2 ascending aorta replacement+valve surgery, 5 adult congenital surgery, and 2 myxoma resections. Postoperative AKI occurrence was evaluated, and we compared perioperative data between patients with and without AKI.
Definition of AKI
AKI was defined according to the creatinine changes specified in the Kidney Disease: Improving Global Outcome (KDIGO) classification. 25 AKI stage 1 is defined as an absolute increase of 0.3 mg/dL within 48 hours or an increase in serum creatinine level of 150% to 200% of baseline within the past 7 days, and AKI stage 2 is defined as an increase in serum creatinine level of >200% of baseline within the past 7 days.
The eGFR was calculated using the following equations, where sCr is serum creatinine:
Anesthetic Protocol
Perioperative care was performed according to a standard protocol, to which all anesthesiologists adhered. Anesthesia induction was performed with dexmedetomidine hydrochloride, fentanyl, and propofol followed by rocuronium for skeletal muscle relaxation. Sevoflurane, propofol, and supplemental doses of remifentanil were used for maintenance of anesthesia, based on clinical criteria.
CPB Management
The pump circuit was primed with 800 mL of bicarbonate Ringer solution, 300 mL of 20% mannitol, and 4000 U of heparin. The hemoconcentrator was used for all patients. Anticoagulation was given at an initial dose of 300 U/kg to achieve a goal activated clotting time of at least 480 seconds and an additional dose of 4000 U was given per hour. Roller pumps were used. The institutional standard pump flow target was 2.5 L/min per m2. Phenylephrine was administrated to maintain a mean blood pressure >50 mm Hg. Red blood cell (RBC) transfusion was considered when hemoglobin concentration could not be maintained at 8 g/dL during CPB. A CDI Blood Parameter Monitoring System 500 (Terumo) was recalibrated every 30 minutes, and an arterial blood gas sample was also checked every 30 minutes. Body temperature was maintained at 34°C. Cold crystalloid cardioplegia was given to all patients requiring cardiac arrest, and terminal warm blood cardioplegia was administered before declamping in the case of prolonged cardiac arrest (>3 hours) or low ejection fraction (<30%). After CPB, all remaining perfusate was used by an intraoperative blood salvage system (Xtra, LivaNova).
Monitoring of DO2 During CPB
The DO2 was calculated according to the equation: DO2 (mL/min per m2)=10×pump flow index (L/min per m2)×(hemoglobin [g/dL]×1.36×arterial oxygen saturation [%]+partial pressure of arterial oxygen [mm Hg]×0.003). 26 Hemoglobin, arterial oxygen saturation, partial pressure of arterial oxygen, and venous oxygen saturation were measured using the CDI Blood Parameter Monitoring System 500 every 20 seconds. The threshold was considered to define sufficient DO2. 24 , 27 Nadir DO2, cumulative AUC below the , and the largest AUC below the were identified using the LivaNova Connect data management system (LivaNova), which recorded the data every 20 seconds. Figure 1 shows the respective parameters in a typical case.
Statistical Analysis
Continuous data are presented as mean±SD and were compared with a Mann–Whitney test. Categorical variables are given as count and percentage of patients and were compared using chi‐square test. When any expected frequency was <1, or 20% of expected frequencies were ≤5, a Fisher exact test was used. All factors associated (P<0.1) with AKI were entered in a multivariable analysis. Univariate analysis was performed on all variables to detect potential risk factors for postoperative AKI. All continuous parameters were dichotomized at the best cutoff value obtained by receiver operating characteristic analysis as the threshold for logistic regression analysis. The best cutoff values obtained by receiver operating characteristic analysis were calculated by Youden index. The univariate predictors with a P<0.1 were selected by the stepwise method and entered into the multivariate analysis. To avoid multicollinearity, variables affected by mathematical coupling were separately entered into different models. P<0.05 was considered to be statistically significant. All data were analyzed using Statistical Analysis Systems software JMP 10.0 (SAS Institute Inc.).
Results
Comparison of Preoperative Characteristics
Postoperative AKI was observed in 12.4% of patients (25/202). The comparison of preoperative data between patients with and without AKI is shown in Table 1. There were no significant differences in age (72.5±8.7 years versus 70.0±12.0 years [P=0.49]) and percentage of women (40.0% [10/25] versus 37.3% [66/177], P=0.79) between patients with and without AKI, respectively. Preoperative hemoglobin concentration was significantly lower in patients with AKI (13.0±1.5 g/dL versus 13.7±1.3 g/dL, P=0.019) and percentage of diabetes mellitus was insignificantly higher in patients with AKI (44.0% [11/25] versus 26.6% [47/177], P=0.07). On the other hand, the baseline serum creatinine and the eGFR were equivalent between the AKI+ and AKI− groups (0.82±0.20 mg/dL versus 0.79±0.17 mg/dL [P=0.49] and 66.0±12.7 mL/min per 1.73 m2 versus 70.2±14.7 mL/min per 1.73 m2 [P=0.21]).
Table 1.
Preoperative Characteristics of Patients With and Without AKI
| Variables |
All Patients (N=202) |
AKI+ (n=25) |
AKI− (n=177) |
P Value |
|---|---|---|---|---|
| Age, y | 70.3±11.7 | 72.5±8.7 | 70.0±12.0 | 0.49 |
| Women, No. (%) | 76 (37.6) | 10 (40.0) | 66 (37.3) | 0.79 |
| Body surface area, m2 | 1.60±0.20 | 1.55±0.27 | 1.61±0.18 | 0.28 |
| Body mass index, kg/m2 | 23.2±3.4 | 24.2±4.2 | 23.1±3.3 | 0.27 |
| Hypertension, No. (%) | 103 (51.0) | 13 (52.0) | 90 (50.9) | 0.91 |
| Diabetes mellitus, No. (%) | 59 (29.2) | 11 (44.0) | 47 (26.6) | 0.07 |
| Chronic lung disease, No. (%) | 21 (10.4) | 1 (4.0) | 20 (11.3) | 0.26 |
| Preoperative atrial fibrillation, No. (%) | 53 (26.2) | 5 (20.0) | 48 (27.1) | 0.45 |
| Redo operation, No. (%) | 15 (7.4) | 1 (4.0) | 14 (7.9) | 0.49 |
| Serum creatinine, mg/dL | 0.79±0.17 | 0.82±0.20 | 0.79±0.17 | 0.49 |
| eGFR, mL/min per 1.73m2 | 69.6±14.5 | 66.0±12.7 | 70.2±14.7 | 0.21 |
| Hemoglobin, g/dL | 13.6±1.4 | 13.0±1.5 | 13.7±1.3 | 0.019 |
| Brain natriuretic peptide, pg/mL | 74 (33–215) | 111 (47–213) | 69 (33–214) | 0.19 |
| LVEF, % | 60.3±12.7 | 57.1±14.1 | 60.8±12.5 | 0.23 |
| EuroSCORE II | 2.5±1.7 | 2.5±1.9 | 2.5±1.7 | 0.98 |
| Cleveland Clinical Foundation Score | 2.7±0.9 | 2.7±0.8 | 2.7±0.9 | 0.66 |
AKI indicates acute kidney injury; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation; and LVEF, left ventricular ejection fraction.
Comparison of Intraoperative Data Including CPB Management
Comparison of the intraoperative data is shown in Table 2. There were no significant differences in CPB duration and cross‐clamp time between patients with and without AKI (170±50 minutes versus 165±50 minutes [P=0.51] and 123±39 minutes versus 117±41 minutes [P=0.27]). RBC transfusions during CPB were significantly greater (2 U [2–4] versus 0 U [0–2]; P<0.001) and nadir hemoglobin level during CPB was significantly lower (7.6±1.2 g/dL versus 8.0±0.9 g/dL, P=0.027) in the AKI+ group. Additionally, significantly lower nadir DO2 during CPB (259±34 mL/min per m2 versus 274±31 mL/min per m2, P=0.015), greater cumulative AUC below the (2490 [261–5466] versus 441 [6–2085], P=0.002), and largest AUC below the (1914 [621–4632] versus 237 [0–825], P<0.001) were observed in the AKI+ group. There were no significant differences in the maximum lactate levels, maximum oxygen extraction ratio, and nadir venous oxygen saturation between the groups.
Table 2.
Perioperative Data of Patients With and Without AKI
| Variables |
All Patients (N=202) |
AKI+ (n=25) |
AKI− (n=177) |
P Value |
|---|---|---|---|---|
| CPB duration, min | 165±50 | 170±50 | 165±50 | 0.51 |
| Aortic cross‐clamp time, min | 117±41 | 123±39 | 117±41 | 0.27 |
| Nadir bladder temperature, °C | 34 (34–34) | 34 (34–34) | 34 (34–34) | 0.88 |
| Maximum lactate level during CPB, mg/dL | 15.9±4.2 | 16.8±4.9 | 15.7±4.1 | 0.42 |
| RBC transfusions during CPB, U | 0 (0–2) | 2 (2–4) | 0 (0–2) | <0.001 |
| Phenylephrine administered during CPB, mg | 0.8 (0.5–1.4) | 0.7 (0.4–1.3) | 0.9 (0.5–1.4) | 0.25 |
| Hemolysis during CPB, No. (%) | 44 (21.8) | 8 (32.0) | 36 (20.3) | 0.19 |
| Mean arterial pressure during CPB, mm Hg | 55.7±8.6 | 55.9±9.7 | 55.7±8.5 | 0.92 |
| Urine output during CPB, mL/kg per h | 1.9 (1.2–3.4) | 2.3 (0.9–4.3) | 1.9 (1.2–3.5) | 0.89 |
| Nadir hemoglobin during CPB, g/dL | 8.0±1.0 | 7.6±1.2 | 8.0±0.9 | 0.027 |
| Nadir DO2 during CPB, mL/min per m2 | 272±32 | 259±34 | 274±31 | 0.015 |
| Maximum O2ER, % | 27.1±5.9 | 27.5±4.7 | 27.0±6.0 | 0.66 |
| Nadir SvO2, % | 70.2±5.4 | 70.2±4.9 | 70.2±5.5 | 0.84 |
| Cumulative AUC below the | 549 (24–2472) | 2490 (261–5466) | 441 (18–2082) | 0.002 |
| Largest AUC below the | 303 (21–1173) | 1293 (222–4080) | 273 (15–849) | <0.001 |
AKI indicates acute kidney injury; AUC, area under the curve; CPB, cardiopulmonary bypass; DO2, oxygen delivery; O2ER, oxygen extraction ratio; RBC, red blood cell; and SvO2, venous oxygen saturation.
Comparison of Postoperative Data
The results of the postoperative outcomes are shown in Table 3. AKI was diagnosed by using the maximum increase during the first 7 postoperative days based on the definition of the KDIGO classification. AKI occurred in 25 patients (12.4%) postoperatively. Of these patients, 23 developed KDIGO stage 1 (92.0%) and 2 patients developed stage 2 (8.0%). There was a case requiring continuous renal replacement therapy in the AKI+ group. The change in creatinine was significantly greater in the AKI+ group (0.43 [0.38–0.56] mg/dL versus 0.10 [0.03–0.19] mg/dL, P<0.001). Intubation time (17 [15–21] hours versus 7 [5–16] hours, P<0.001), intensive care unit stay (4.0 [2.6–5.8] days versus 2.8 [1.8–3.8] days, P=0.010), and postoperative hospital stay (28 [20–39] days versus 21 [16–27] days, P=0.024) were significantly prolonged in patients with AKI. There was no in‐hospital death in either group.
Table 3.
Postoperative Outcomes of Patients With and Without AKI
| Variables |
AKI+ (n=25) |
AKI− (n=177) |
P Value |
|---|---|---|---|
| KDIGO stage, No. (%) | |||
| Stage 1 | 23 (92.0) | ||
| Stage 2 | 2 (8.0) | ||
| Continuous renal replacement therapy, No. (%) | 1 (4.0) | ||
| Delta creatinine from baseline, mg/dL | 0.43 (0.38–0.56) | 0.10 (0.03–0.19) | <0.001 |
| Creatinine increase from baseline, No. (%) | 60 (49–66) | 12 (4–24) | <0.001 |
| Intubation time, h | 17 (15–21) | 7 (5–16) | <0.001 |
| ICU stay, d | 4.0 (2.6–5.8) | 2.8 (1.8–3.8) | 0.010 |
| Postoperative hospital stay, d | 28 (20–39) | 21 (16–27) | 0.024 |
| Hospital mortality, No. (%) | 0 (0) | 0 (0) | 1.00 |
AKI indicates acute kidney injury; ICU, intensive care unit; and KDIGO, Kidney Disease: Improving Global Outcomes.
Respective Parameters Regarding DO2 as a Predictor of Postoperative AKI
Receiver operating characteristic analysis was performed to predict AKI in nadir DO2, cumulative AUC below the , and largest AUC below the . The calculated AUC was 0.663 (95% CI, 0.534–0.772), 0.693 (95% CI, 0.568–0.795), and 0.733 (95% CI, 0.608–0.829), respectively, and the largest AUC below the was more accurate to predict postoperative AKI compared with nadir DO2 and cumulative AUC below the (differences between areas, 0.0691 [P=0.0061] and 0.0395 [P=0.0013]). There was no significant difference in the area between nadir DO2 and cumulative AUC below the (differences between areas, 0.0297; P=0.25). According to the Youden index, the best cutoff values were the nadir DO2 ≤265 mL/min per m2 (sensitivity, 68.0%; specificity, 58.8%), the cumulative AUC below the ≥1555 (sensitivity, 60.0%; specificity, 67.8%), and the largest AUC below the ≥880 (sensitivity, 72.0%; specificity, 72.3%) (Figure 2).
Figure 2. Comparison of receiver operating characteristic curve to predict acute kidney injury between the nadir oxygen delivery (DO2), cumulative area under the curve (AUC) below the , and largest AUC below the .
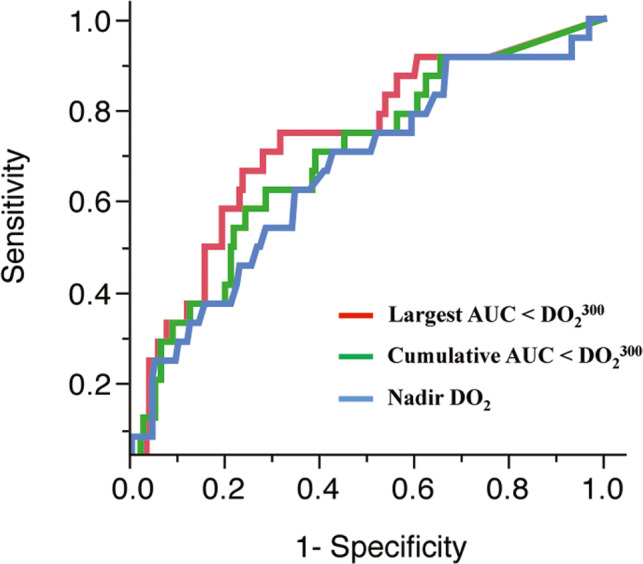
AUCs and P values are 0.663 (95% CI, 0.534–0.772) for the nadir DO2, 0.693 (95% CI, 0.568–0.795) for the cumulative AUC below the , and 0.733 (95% CI, 0.608–0.829) for the largest AUC below the , respectively. Differences between areas are 0.0691 (P=0.0061) for the nadir DO2 and the largest AUC below the , 0.0395 (P=0.0013) for the cumulative AUC below the and the largest AUC below the , and 0.0297 (P=0.25) for the nadir DO2 and the cumulative AUC below the , respectively.
Multivariable Analysis for Risk of Postoperative AKI
The multivariable analysis was performed with AKI as dependent variables. For each model, the largest AUC below the and cumulative AUC below the were separately analyzed because of mathematical coupling between the 2 variables. By univariable analysis, diabetes mellitus, preoperative hemoglobin concentration, nadir hemoglobin during CPB, RBC transfusions during CPB, nadir DO2, cumulative AUC below the , and the largest AUC below the were obtained as risk factors for AKI. In the multivariable analysis for AKI, we tested the following variables: diabetes mellitus, preoperative hemoglobin concentration ≤11.6 g/dL, RBC transfusions during CPB ≥2 U, nadir hemoglobin during CPB ≤7.4 g/dL, nadir DO2 ≤265 mL/min per m2 and largest AUC below the ≥880 (model 1), diabetes mellitus, preoperative hemoglobin concentration ≤11.6 g/dL, RBC transfusions during CPB ≥2 U, nadir hemoglobin during CPB ≤7.4 g/dL, nadir DO2 ≤265 mL/min per m2, and cumulative AUC below the ≥1555 (model 2). After multivariable analysis, in the multivariable model including the largest AUC below the ≥880, preoperative hemoglobin concentration ≤11.6 g/dL (odds ratio [OR], 7.6; 95% CI, 2.0–32.3 [P=0.004]), RBC transfusions during CPB ≥2 U (OR, 3.3; 95% CI, 1.0–11.1 [P=0.041]), and the largest AUC below the ≥880 (OR, 4.9; 95% CI, 1.2–21.5 [P=0.022]) were identified as independent risk factors for postoperative AKI. In the multivariable model including the cumulative AUC below the ≥1555, preoperative hemoglobin concentration ≤11.6 g/dL (OR, 8.2; 95% CI, 2.1–34.3 [P=0.002]) and RBC transfusions during CPB ≥2 U (OR, 3.2; 95% CI, 1.0–10.8 [P=0.048]) were identified as independent risk factors for postoperative AKI, but cumulative AUC below the ≥1555 was not identified as an independent risk factor for postoperative AKI (Table 4).
Table 4.
Univariate and Multivariate Analysis for Predictor of Postoperative AKI
| Variables | Univariate Analysis | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Multivariate Analysis | Multivariate Analysis | |||||
| OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Diabetes mellitus | 2.5 (1.1–5.9) | 0.030 | 2.4 (0.9–6.6) | 0.08 | 2.5 (0.9–6.7) | 0.06 |
| Preoperative hemoglobin ≤11.6 g/dL | 13.4 (3.9–46) | <0.001 | 7.6 (2.0–32.3) | 0.004 | 8.2 (2.1–34.3) | 0.002 |
| RBC transfusions during CPB ≥2 U | 5.2 (1.9–13.7) | <0.001 | 3.3 (1.0–11.1) | 0.041 | 3.2 (1.0–10.8) | 0.048 |
| Nadir hemoglobin ≤7.4 g/dL | 3.0 (1.3–7.0) | 0.009 | 1.4 (0.4–5.3) | 0.58 | 1.0 (0.3–3.9) | 0.99 |
| Nadir DO2 ≤265 mL/min per m2 | 3.0 (1.2–7.4) | 0.012 | 1.7 (0.4–7.7) | 0.48 | 1.4 (0.3–5.3) | 0.65 |
| Largest AUC below the ≥880 | 5.4 (2.1–13.7) | <0.001 | 4.9 (1.2–21.5) | 0.022 | ||
| Cumulative AUC below the ≥1555 | 3.3 (1.4–7.8) | 0.006 | 1.0 (0.3–4.3) | 0.95 | ||
AKI indicates acute kidney injury; AUC, area under the curve; DO2, oxygen delivery; OR, odds ratio; and RBC, red blood cell.
Discussion
The rationale of the study was to investigate whether the prolonged and excessive decrease of DO2 below the threshold of 300 mL/min per m2 is superior in predicting AKI after cardiac surgery compared with the established parameters nadir DO2 or cumulative AUC below the threshold. The major findings of this study are as follows: (1) postoperative AKI was observed in 12.4% of patients after cardiac surgeries with CPB support; (2) diabetes mellitus, preoperative hemoglobin concentration, nadir hemoglobin, RBC transfusions during CPB, nadir DO2, cumulative AUC below the , and largest AUC below the were identified as risk factors for AKI; (3) the largest AUC below the ≥880, RBC transfusion ≥2 U, and preoperative hemoglobin concentration ≤11.6 g/dL were identified as risk factors of AKI by multivariate analysis; and (4) regarding management of DO2 during CPB, the largest AUC below the was the most accurate assessment index compared with other valuation methods.
Even a slight increase in postoperative creatinine was reported to worsen short‐ and long‐term prognosis. 4 , 5 , 6 Although there were only 2 cases of severe renal dysfunction (KDIGO stage 2), the intubation time, intensive care unit stay, and postoperative hospital stay were significantly prolonged in the AKI group. Many factors are implicated in the complex mechanisms leading to postoperative kidney function impairment. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 However, many of these risk factors were intrinsic to the individual patient. In the present study, preoperative anemia was detected as an independent risk factor by multivariate analysis. Administration of erythropoietin has been suggested as a way to promote erythropoiesis in anemic patients before cardiac surgery. 28 Although recombinant erythropoietin significantly increased preoperative hemoglobin levels and hematocrit, it has not been demonstrated to reduce the need for RBC transfusion in cardiac surgery. 29 In anemic patients, prophylactic RBC transfusion was reported by Karkouti et al 30 to be an effective option to reduce perioperative anemia and RBC transfusions and may reduce plasma iron levels. They demonstrated that perioperative RBC transfusions were directly related to postoperative transferrin saturation, and high transferrin saturation (>80%) was associated with AKI, implicating transfusion‐related iron overload as a cause of AKI.
On the other hand, appropriate CPB management has been focused on to predict postoperative AKI as a modifiable approach. Nadir DO2 during CPB was recognized as an important index and must be maintained higher than 262 to 272 mL/min per m2 at moderate hypothermia. 21 , 22 , 23 However, in these studies, the nadir DO2 level was defined using an intermittent measurement value at 10‐ to 20‐minute intervals. Since DO2 level is dynamically changing during CPB, the intermittently calculated nadir DO2 level may be overestimated. Additionally, intermittent measurement of DO2 levels cannot reflect the exposure time of low DO2. In our study, the nadir DO2 calculated every 20 seconds was significantly lower in the AKI group and the best cutoff value obtained by receiver operating characteristic analysis was 265 mL/min per m2. This result confirms some important values identified in previous studies, 21 , 22 , 23 but had less accuracy with predicting postoperative AKI than largest AUC below the .
In a recent study, Lannemyr and associates 31 demonstrated that renal DO2 during CPB is reduced by about 20% as a result of renal vasoconstriction and hemodilution, leading to renal oxygen supply/demand mismatch. Especially in the outer medulla, oxygen tissue partial pressure is as low as 10 to 20 mm Hg even under normal conditions compared with 50 mm Hg in the cortex. 32 Thus, as the outer medulla is on the border of hypoxia already under normal conditions, low renal DO2 episodes are likely to cause ischemic conditions. Sustained ischemia causes subsequent ischemia‐reperfusion kidney injury, resulting in impaired renal oxygenation and proximal tubular dysfunction. 33 , 34 Therefore, risk factors for postoperative AKI may not be assessed accurately without considering the continuous duration of low DO2.
Recently, Mukaida et al 24 evaluated time and depth below the DO2 threshold and reported that cumulative AUC below the and cumulative time below the were more sensitive indicators for predicting AKI after cardiac surgery with CPB compared with nadir DO2. They concluded that the incidence of AKI significantly increased when the cumulative time below the exceeded 15 minutes. However, this parameter included both temporary and continuous decreases in DO2, and the rates of temporary and continuous decreases in DO2 were not accurately assessed. Studies on kidney ischemia have shown that repeated temporary renal ischemia (4 cycles of 4 minutes of ischemia with a cumulative ischemia time of 16 minutes) does not damage the kidneys. On the other hand, continuous ischemia for 20 minutes caused mild renal tubular injury as a result of ischemia reperfusion injury. 35 Therefore, it may be necessary to accurately assess the extent of continuous oxygen debt during CPB in predicting postoperative AKI. In this study, the largest AUC below the was evaluated as an indicator of the degree of continuous oxygen debt during CPB. In multivariate analysis, the largest AUC below the was identified as an independent risk factor for postoperative AKI, but cumulative AUC below the was not identified as an independent risk factor.
Based on the findings of this study, the largest AUC below the is a modified index to reflect both the depth and duration of continuous decrease in DO2. The largest AUC below the can be a good indicator to take intraoperative measures to avoid a continuous or severe decrease in DO2. The largest AUC below the had a significantly larger area in the AKI group, and the highest accuracy of predicting postoperative AKI was obtained compared with nadir DO2 and cumulative AUC below the . The cutoff value of the largest AUC below the was 880, and this value can be an improved index to avoid postoperative AKI. DO2 during CPB mainly depends on hemoglobin concentration and pump flow (ignoring partial pressure of oxygen as it is only a minor contribution). Lannemyr et al 36 demonstrated that the renal oxygen supply/demand mismatch caused by CPB hemodilution and vasoconstriction was improved by increasing CPB flow rate above 2.7 to 3.0 L/min per m2. On the other hand, it is known that RBCs undergo irreversible morphological and biochemical changes during storage. As a result, RBC transfusions can cause AKI as a result of impaired tissue DO2 and exacerbate inflammatory responses and tissue oxidative stress. 37 , 38 Therefore, in clinical practice, we try to minimize hemodilution and RBC transfusion by reducing the priming volume and using a hemoconcentrator for preservation of DO2 above 300 mL/min per m2. Additionally, pump flow is adjusted according to the level of hemodilution.
Study Limitations
This study has several limitations. First, this study was a retrospective observational study in a single center. Therefore, our models need to be validated at multiple centers for broad applicability. Second, patients with preoperative chronic renal insufficiency were excluded, so the development rate of AKI and a number of cases presenting with severe renal injury was small. Thus, the clinical impacts of this index are still undetermined. Finally, although this study defined AKI only by the change in creatinine within 7 days after surgery, creatinine production is affected by muscle mass and can vary greatly depending on factors such as age, sex, and nutritional status. The effectiveness of DO2 management should be evaluated using urinary tissue inhibitor of metalloproteinases‐2/insulin‐like growth factor–binding protein 7 as biomarkers for AKI. 39
Conclusions
The present data suggest that the largest AUC below the during CPB is an important independent risk factor for AKI. Potentially, these data suggest that avoiding a continuous or severe decrease of DO2 (largest AUC below the <880) could reduce the risk of AKI after cardiac surgery.
Sources of Funding
None.
Disclosures
None.
J Am Heart Assoc. 2020;9:e015566 DOI: 10.1161/JAHA.119.015566.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Provenchere S, Plantefeve G, Hufnagel G, Vicaut E, De Vaumas C, Lecharny JB, Depoix JP, Vrtovsnik F, Desmonts JM, Philip I. Renal dysfunction after cardiac surgery with normothermic cardiopulmonary bypass: incidence, risk factors, and effect on clinical outcome. Anesth Analg. 2003;96:1258–1264. [DOI] [PubMed] [Google Scholar]
- 2. Brown JR, Cochran RP, MacKenzie TA, Furnary AP, Kunzelman KS, Ross CS, Langner CW, Charlesworth DC, Leavitt BJ, Dacey LJ, et al. Long‐term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg. 2008;86:4–11. [DOI] [PubMed] [Google Scholar]
- 3. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long‐term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. [DOI] [PubMed] [Google Scholar]
- 4. Liotta M, Olsson D, Sartipy U, Holzmann MJ. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol. 2014;113:70–75. [DOI] [PubMed] [Google Scholar]
- 5. Tolpin DA, Collard CD, Lee VV, Virani SS, Allison PM, Elayda MA, Pan W. Subclinical changes in serum creatinine and mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143:682–688. [DOI] [PubMed] [Google Scholar]
- 6. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. [DOI] [PubMed] [Google Scholar]
- 7. Sirvinskas E, Andrejaitiene J, Raliene L, Nasvytis L, Karbonskiene A, Pilvinis V, Sakalauskas J. Cardiopulmonary bypass management and acute renal failure: risk factors and prognosis. Perfusion. 2008;23:323–327. [DOI] [PubMed] [Google Scholar]
- 8. O’Sullivan KE, Byrne JS, Hudson A, Murphy AM, Sadlier DM, Hurley JP. The effect of obesity on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:1622–1628. [DOI] [PubMed] [Google Scholar]
- 9. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. [DOI] [PubMed] [Google Scholar]
- 10. Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, Cheung D, Das SR, He GW. Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology. 1998;49:789–800. [DOI] [PubMed] [Google Scholar]
- 11. Mangos GJ, Brown MA, Chan WY, Horton D, Trew P, Whitworth JA. Acute renal failure following cardiac surgery: incidence, outcomes and risk factors. Aust N Z J Med. 1995;25:284–289. [DOI] [PubMed] [Google Scholar]
- 12. Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–631. [DOI] [PubMed] [Google Scholar]
- 13. Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis. 2003;41:76–83. [DOI] [PubMed] [Google Scholar]
- 14. Padmanabhan H, Siau K, Curtis J, Ng A, Menon S, Luckraz H, Brookes MJ. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta‐analysis. Ann Thorac surg. 2019;108:1840–1848. [DOI] [PubMed] [Google Scholar]
- 15. De Santo L, Romano G, Della Corte A, de Simone V, Grimaldi F, Cotrufo M, de Feo M. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg. 2009;138:965–970. [DOI] [PubMed] [Google Scholar]
- 16. Magruder JT, Dungan SP, Grimm JC, Harness HL, Wierschke C, Castillejo S, Barodka V, Katz N, Shah AS, Whitman GJ. Nadir oxygen delivery on bypass and hypotension increase acute kidney injury risk after cardiac operations. Ann Thorac Surg. 2015;100:1697–1703. [DOI] [PubMed] [Google Scholar]
- 17. Fischer UM, Weissenberger WK, Warters RD, Geissler HJ, Allen SJ, Mehlhorn U. Impact of cardiopulmonary bypass management on post cardiac surgery renal function. Perfusion. 2002;17:401–406. [DOI] [PubMed] [Google Scholar]
- 18. Ranucci M, Menicanti L, Frigiola A. Acute renal injury and lowest hematocrit during cardiopulmonary bypass: not only a matter of cellular hypoxemia. Ann Thorac Surg. 2004;78:1880–1881. [DOI] [PubMed] [Google Scholar]
- 19. Fang WC, Helm RE, Krieger KH, Rosengart TK, DuBois WJ, Sason C, Lesser ML, Isom OW, Gold JP. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96(suppl II):194–199. [PubMed] [Google Scholar]
- 20. Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–1450. [DOI] [PubMed] [Google Scholar]
- 21. Ranucci M, Romitti M, Isgrò G, Cotza M, Brozzi S, Boncilli A, Ditta A. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–2220. [DOI] [PubMed] [Google Scholar]
- 22. de Somer F, Mulholland JW, Bryan MR, Aloisio T, Van Nooten GJ, Ranucci M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: time for a goal‐directed perfusion management? Crit Care. 2011;15:R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newland RF, Baker RA, Woodman RJ, Barnes MB, Willcox TW; Australian and New Zealand Collaborative Perfusion Registry . Predictive capacity of oxygen delivery during cardiopulmonary bypass on acute kidney injury. Ann Thorac Surg. 2019;108:1807–1814. [DOI] [PubMed] [Google Scholar]
- 24. Mukaida H, Matsushita S, Kuwaki K, Inotani T, Minami Y, Saigusa A, Amano A. Time–dose response of oxygen delivery during cardiopulmonary bypass predicts acute kidney injury. J Thorac Cardiovasc Surg. 2019;158:492–499. [DOI] [PubMed] [Google Scholar]
- 25. Uhlig K, Berns J, Carville S, Chan W, Cheung M, Guyatt G, Hart A, Lewis A, Tonelli M, Webster A, et al. Recommendations for kidney disease guideline updating: a report by the KDIGO Methods Committee. Kidney Int. 2016;89:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baker RA. Variation in measurement and reporting of goal directed perfusion parameters. J Extra Corpor Technol. 2017;49:2–7. [PMC free article] [PubMed] [Google Scholar]
- 27. Magruder JT, Crawford TC, Harness HL, Grimm JC, Suarez‐Pierre A, Wierschke C, Biewer J, Hogue C, Whitman GR, Shah AS, et al. A pilot goal‐directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore‐Lesserson LJ, Goodnough LT, et al. 2011 update to the Society of Thoracic Surgeons and the Society of cardiovascular Anesthesiologists blood conservation clinical practice guidelined. Ann Thorac Surg. 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 29. D’Ambra MN, Gray RJ, Hillman R, Jones JW, Kim HC, Rawitscher R, Schnaper H, Szymanski I, Vlahakes GJ, Kaplan D, et al. Effect of recombinant human erythropoietin on transfusion risk in coronary bypass patients. Ann Thorac Surg. 1997;64:1686–1693. [DOI] [PubMed] [Google Scholar]
- 30. Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, Crowther MA, Hozhabri S, Beattie WS. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology. 2012;116:613–621. [DOI] [PubMed] [Google Scholar]
- 31. Lannemyr L, Bragadottir G, Krumbholz V, Redfors B, Sellgren J, Ricksten SE. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology. 2017;126:205–213. [DOI] [PubMed] [Google Scholar]
- 32. Brezis M, Rosen S. Hypoxia of the renal medulla‐its implications for disease. N Engl J Med. 1995;332:647–655. [DOI] [PubMed] [Google Scholar]
- 33. Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M. Mass spectrometry‐based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case‐control study. Am J Kidney Dis. 2009;53:584–595. [DOI] [PubMed] [Google Scholar]
- 34. Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE. Acute renal failure is NOT an ‘acute renal success’‐a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit Care Med. 2010;38:1695–1701. [DOI] [PubMed] [Google Scholar]
- 35. Jefayri MK, Grace PA, Mathie RT. Attenuation of reperfusion injury by renal ischaemic preconditioning: the role of nitric oxide. BJU Int. 2000;85:1007–1013. [DOI] [PubMed] [Google Scholar]
- 36. Lannemyr L, Bragadottir G, Hjärpe A, Redfors B, Ricksten SE. Impact of cardiopulmonary bypass flow on renal oxygenation in patients undergoing cardiac operations. Ann Thorac Surg. 2019;107:505–511. [DOI] [PubMed] [Google Scholar]
- 37. Almac E, Ince C. The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol. 2007;21:195–208. [DOI] [PubMed] [Google Scholar]
- 38. Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. [DOI] [PubMed] [Google Scholar]
- 39. Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, et al. Clinical use of the urine[ TIMP‐2]×[IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


