Abstract
Background
A 2018 American Heart Association science advisory indicated that, pending further research, artificially sweetened beverages (ASBs) may be an appropriate initial replacement for sugar‐sweetened beverages (SSBs) during transition to unsweetened beverages (USBs).
Methods and Results
We randomly assigned 203 adults (121 males, 82 females; 91.6% retention), who habitually consumed SSBs, to 3 groups and delivered free SSBs, ASBs, or USBs to their homes for 12 months. Outcomes included serum triglyceride to high‐density lipoprotein cholesterol ratio (primary), body weight, and sweet taste preference (experimental assessment, 0%–18% sucrose solutions). Change in serum triglyceride to high‐density lipoprotein cholesterol ratio was not different between groups. Although overall change in weight also was not different between groups, we found effect modification (P=0.006) by central adiposity. Among participants in the highest tertile of baseline trunk fat but not other tertiles, weight gain was greater (P=0.002) for the SSB (4.4±1.0 kg, estimate±SE) compared with ASB (0.5±0.9 kg) or USB (−0.2±0.9 kg) group. Both sweetness threshold (–1.0±0.2% m/v; P=0.005) and favorite concentration (–2.3±0.4% m/v; P<0.0001) decreased in the USB group; neither changed in the SSB group. In the ASB group, sweetness threshold did not change, and favorite concentration decreased (–1.1±0.5% m/v; P=0.02). Pairwise comparison between the ASB and USB groups indicated a difference in sweetness threshold (P=0.015).
Conclusions
Replacing SSBs with noncaloric beverages for 12 months did not affect serum triglyceride to high‐density lipoprotein cholesterol ratio. Among individuals with central adiposity, replacing SSBs with either ASBs or USBs lowered body weight. However, USBs may have the most favorable effect on sweet taste preference.
Registration
URL: https://www.clinicaltrials.gov; unique identifier: NCT01295671.
Keywords: beverages, body weight, diet, dyslipidemia, sweet taste preference
Subject Categories: Diet and Nutrition, Lifestyle, Obesity, Primary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- ASB
artificially sweetened beverage
- HDL‐C
high‐density lipoprotein cholesterol
- RCT
randomized controlled trial
- SSB
sugar‐sweetened beverage
- TG
triglyceride
- USB
unsweetened beverage
Clinical Perspective
What Is New?
In this 12‐month intervention study, replacing sugar‐sweetened beverages (SSBs) with artificially sweetened beverages (ASBs) or unsweetened beverages (USBs) had no effect on cardiometabolic risk factors.
In a subgroup analysis, individuals with fat around the midsection lost more weight with replacement of SSBs by either ASBs or USBs, and sweet taste preference decreased more when SSBs were replaced with USBs compared with ASBs.
The null findings for cardiometabolic risk factors may relate to compensatory changes in other dietary sources of refined carbohydrate; in comparison with randomized controlled trials, the positive associations in observational studies typically include statistical control for carbohydrate source and other measures of dietary quality.
What Are the Clinical Implications?
Overall dietary quality should be considered in interventions involving SSBs to reduce risk for cardiometabolic disease.
Consistent with American Heart Association recommendations, substitution of SSBs with ASBs or USBs may help to control body weight in susceptible individuals (eg, those with central adiposity).
As replacements for SSBs, USBs decrease sweet taste preference more compared with ASBs, an effect that may have implications for promoting adherence to low‐sugar diets.
With increasing awareness of the adverse health outcomes of consuming added sugars, 1 , 2 particularly sugar‐sweetened beverages (SSBs), 3 much attention has been directed toward the effects of replacing SSBs with noncaloric options. The Dietary Guidelines for Americans 2015–2020 emphasizes the importance of drinking unsweetened beverages (USBs), most notably plain water. 4 Pending further research, a science advisory from the American Heart Association indicated that artificially sweetened beverages (ASBs) may be an appropriate initial replacement for SSBs in adults who are habitual consumers, have a strong sweet taste preference, and consider USBs an undesirable alternative. 5
Data are limited regarding the effects of consuming ASBs compared with USBs as replacements for SSBs. Randomized controlled trials (RCTs) of beverage interventions have compared consumption of SSBs versus a combination of ASBs and USBs, 6 , 7 only ASBs, 8 , 9 , 10 and either ASBs or USBs in just 1 study, 11 , 12 with results showing a beneficial effect of various noncaloric beverages on body weight. Prospective observational studies of ASB consumption have yielded mixed results, with some indicating negative associations with weight gain, 13 , 14 , 15 consistent with the cited RCTs, 6 , 7 , 8 , 9 , 10 , 12 and others indicating positive associations with weight gain, 16 , 17 dyslipidemia, 18 , 19 metabolic syndrome, 18 , 20 , 21 type 2 diabetes mellitus, 22 , 23 , 24 , 25 and cardiovascular disease. 26 , 27 , 28 , 29 These positive associations, if causal, may relate to the metabolic effects of the synthetic sweeteners in ASBs (eg, acesulfame potassium, aspartame, and sucralose) that potently activate chemoreceptors for sweet taste at very low concentrations. 30 Of special concern is the possibility that the high‐intensity sweetness of ASBs may increase the preference for sweet foods, in a manner similar to SSBs, with implications for long‐term food choices and dietary quality. 31 , 32 , 33
The aim of this RCT was to compare effects of consuming SSBs, ASBs, and USBs in adults who habitually consumed SSBs. We hypothesized that, as replacements for SSBs, USBs versus ASBs would produce greater reductions in cardiometabolic risk factors, body weight and adiposity, and sweet taste preference. We also explored whether replacing SSBs with ASBs or USBs would be especially beneficial for individuals with high trunk fat mass, in view of the relationship between central adiposity and insulin secretion. 34
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We randomly assigned participants to 3 groups (SSB, ASB, USB) for 12 months. The randomization was stratified by sex, ethnicity/race (non‐Hispanic white versus other), and baseline body mass index (normal weight, 18.5–24.9; overweight, 25–29.9; obese, ≥30). Following randomization, participants received a 12‐month intervention to promote consumption of beverages consistent with group assignment. Study outcomes were assessed at baseline and 12 months. The institutional review board at Boston Children’s Hospital approved the study protocol. Participants provided written informed consent. The study was conducted at Boston Children’s Hospital between February 2011 and November 2015. The protocol history, documented in institutional review board amendments, with no change in the primary outcome, is presented in Table S1.
Participants
We screened adults aged 18 to 40 years who reported consuming at least 1 serving (12 fl oz) per day of SSBs and had a body mass index ranging from 18.5 to 40.0 kg/m2. Exclusion criteria included fasting blood glucose ≥110 mg/dL, physician diagnosis of a major medical or psychiatric illness, chronic use of any medication that could affect ≥1 study outcomes, and heavy smoking (>10 cigarettes/d). Additional exclusion criteria for females included: pregnancy (preceding 12 months) or plans to become pregnant during the study period, lactation (preceding 3 months), and change in hormonal contraceptives (preceding 3 months). Before baseline assessments, we obtained medical clearance from a primary care provider. Demographic information based on participant self‐report included: sex, date of birth, ethnicity (Hispanic or non‐Hispanic), race (white, black, Asian, multiple, or other), and total annual household income. We provided monetary incentive upon completion of assessments at baseline ($25) and 12 months ($275).
Interventions
The interventions comprised home deliveries of free beverages, specific messages pertaining to beverage consumption, and check‐in telephone calls. We asked each participant to select a desired beverage combination, consistent with group assignment, from a menu of options and ordered the desired combination from an online delivery service affiliated with a supermarket chain (Peapod, Stop & Shop, Quincy, MA). Unsweetened beverages included spring water, purified water, and sparkling water (with or without flavoring). Beverages were ordered at a frequency to ensure continuous availability, such that delivered beverages could be replacements for the SSBs consumed at baseline, as determined using a beverage frequency questionnaire adapted from a validated instrument. 35 The intervention messages presented in Table 1 were conveyed to study participants in writing and during biweekly check‐in telephone calls. Core messages regarding consumption of delivered beverages were consistent across groups. Group‐specific messages provided instructions on which beverages to avoid. During the check‐in telephone calls, we reviewed beverage consumption, reiterated intervention messages, and encouraged compliance with intervention protocols. Discussions focused exclusively on beverage consumption, with no attention to other dietary behaviors. The target duration of each call was 10 minutes.
Table 1.
Intervention Messages
| Core Messages (For All Groups) | Group‐Specific Messages |
|---|---|
|
SSB Group
|
|
ASB Group
| |
|
USB Group
|
ASB indicates artificially sweetened beverage; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
To establish an individualized point of reference for the target number of delivered beverages to be consumed daily, we assessed baseline intake of SSBs using a beverage frequency questionnaire adapted from a validated instrument. 35
Intervention Fidelity
Procedures to maintain intervention fidelity included group‐specific scripts for reiterating intervention messages, obtaining beverage orders, and negotiating delivery times; systems for cross‐checking beverage orders with supermarket receipts; guides to provide structure for check‐in calls, with adequate flexibility for addressing situations unique to each participant; protocols for documenting each check‐in call; and regular study team meetings to discuss strategies for promoting adherence without compromising differentiation among groups. We also digitally recorded check‐in calls for periodic review.
Process Evaluation
Implementation of the beverage interventions was evaluated based on numbers of completed beverage deliveries and check‐in calls. Three unannounced telephone interviews (2 weekdays, 1 weekend day) were conducted at baseline and again at 12 months to assess dietary intake and physical activity for the day preceding each call. The interviewer was masked to group assignment. Dietary data were collected using the Nutrition Data System for Research software versions 2010–2014, and final data were generated with version 2015 (Nutrition Coordinating Center, University of Minnesota, Minneapolis). Healthy Eating Index 2015 scores (total and subcomponent) were calculated according to a standardized protocol 36 using Nutrition Data System for Research output and code from the Nutrition Coordinating Center. Physical activity data were collected, and daily metabolic equivalent was calculated as previously described. 34
Outcomes
Study outcomes were measured during 2 visits at each time point (baseline, 12 months), following a 12‐hour overnight fast. Personnel who assessed study outcomes were masked to group assignment. A secure web‐based application (Research Electronic Data Capture), 37 hosted at Boston Children’s Hospital, was used for data management.
The prespecified primary outcome was ratio of serum triglyceride (TG) to high‐density lipoprotein cholesterol (HDL‐C) concentration (TG:HDL‐C). Reducing refined sources of dietary carbohydrate, including sugar, has been shown to decrease TG and increase HDL‐C, 34 , 38 , 39 possibly mediated by attenuated postprandial insulinemia. 40 A lipid profile characterized by hypertriglyceridemia and low HDL‐C correlates with insulin resistance 41 and is a component of the metabolic syndrome. 42 Previous studies indicate that the TG:HDL‐C is an independent predictor of coronary atherosclerosis, 43 risk of myocardial infarction, 44 and cardiovascular events and all‐cause mortality. 45 Other prespecified outcomes included blood levels of low‐density lipoprotein cholesterol (direct determination by enzymatic spectrophotometric assay), high‐sensitivity C‐reactive protein, fibrinogen, uric acid, alanine aminotransferase, glucose, and insulin. Insulin sensitivity and β‐cell function, expressed as percentages of values for a normal reference population, were evaluated by homeostasis model assessment using glucose and insulin data. 46 All biochemical analyses were done in facilities certified by the Clinical Laboratory Improvement Amendments. Resting blood pressure was measured by auscultation. 47
Body weight and height were measured using an electronic scale and a wall‐mounted stadiometer. Whole‐body fat mass (henceforth referred to as fat mass) and trunk fat mass (henceforth referred to as trunk fat) were assessed by dual‐energy X‐ray absorptiometry (Discovery A, Hologic, Inc., Bedford, MA). According to our original proposal, insulin secretion was specified as an effect modifier; however, because of scheduling challenges and the need to streamline study visits, we did not measure insulin secretion. In a previous study, 34 we measured insulin concentration 30 minutes after a 75‐g dose of oral glucose (insulin‐30), as a proxy measure of insulin secretion, and noted a significant association between trunk fat and log insulin‐30 (C.B. Ebbeling, H.A. Feldman, and D.S. Ludwig, unpublished data, 2007: N=97; Pearson r=0.46; P<0.0001). 48 Based on this relationship, we conducted exploratory analyses to evaluate effect modification by trunk fat when comparing effects of consuming SSBs, ASBs, or USBs on body weight and fat mass. These analyses are consistent with the American Heart Association science advisory, which notes that replacing SSBs with noncaloric options may be particularly beneficial for weight control among individuals with central adiposity due to high levels of visceral fat. 5
We evaluated sweet taste preference using 10 samples of solutions ranging in sucrose concentration (%m/v) from 0% to 18%. The samples were made using distilled water, flavored with lemon juice, and served at 4°C. On the morning of the taste testing protocol, personnel in the clinical research center at Boston Children’s Hospital prepared and served a standard breakfast (Table S2). Ninety minutes after breakfast, we instructed participants to sip, taste, swallow, and rate the sweetness of each sample in order of increasing sucrose concentration. Sweetness ratings were obtained using a 10‐cm visual analog scale, with verbal anchors at 0 (not at all sweet) and 10 (extremely sweet). Participants consumed a small cracker and sip of water after rating each solution. At the end of the protocol, participants tasted all of the samples again and selected 1 as an overall favorite concentration.
Adverse Events
An adverse event was defined as any symptom or safety concern requiring medical attention, as reported by a participant.
Statistical Analysis
Baseline characteristics and process data were compared across the three study groups by 1‐way ANOVA for continuous measures and Fisher’s exact test for discrete variables. Longitudinal analysis of anthropometric, dietary, and biochemical measures was conducted by repeated‐measures ANOVA. The independent variables were study group and visit (baseline or 12 months), with an interaction term to test for differential change across groups. All models were adjusted for sex, ethnicity, race, and baseline age. A compound‐symmetric covariance structure was employed for the repeated‐measures model, chosen over alternatives that either failed to account for within‐participant correlation (independence), were equivalent to compound symmetry in this 2‐point design (autoregressive, Toeplitz, spatial power), or required additional parameters unjustified by evidence of nonuniform variance (heterogeneous, unstructured). From parameters of the fitted model, we calculated covariate‐adjusted mean changes for each group, with standard error. The mean changes were compared within group to 0 by t test (mean÷standard error), and across groups by the F‐test for interaction.
Variables displaying a sharply skewed distribution were log‐transformed for analysis. For descriptive purposes, in place of the unadjusted mean and standard deviation, we retransformed the mean and quartile boundaries of the log‐transformed distribution and reported them as median and interquartile range. The covariate‐adjusted mean changes and standard errors from analysis of log‐transformed variables (Δ±SE) were retransformed for reporting as percentages (100%×exp(Δ)±100%×exp(Δ)×(exp(SE)−1)).
To investigate effect modification, we employed analysis of variance with 12‐month change as the dependent variable. The independent variables were study group and the value of the effect modifier at baseline, expressed as tertiles (irrespective of study group), with an interaction term to test whether the group differences varied by tertile. The model was adjusted for sex, ethnicity, race, and baseline age.
To characterize sweet taste preference at each time point, we used nonlinear regression analysis to fit a sigmoid curve to each participant's ratings of the 10 sucrose solutions, and calculated sweetness threshold as the concentration corresponding to 5 cm on the 10‐cm visual analog scale (Figure S1).We calculated each participant’s 12‐month change for this parameter and the designated favorite concentration, and then compared the mean changes across study groups. To minimize the influence of extreme values, we applied robust (outlier‐resistant) regression, 39 an iterative procedure by which data points with greater deviation from the fitted mean are given lower weight on subsequent iterations. Among the available variants of this procedure, we employed M‐estimation, most suitable for a discrete independent variable; the quasi‐triangular bisquare weighting function; and a modified Wald test for comparing study groups. We repeated the analysis with adjustment for sex, ethnicity, race, and baseline age, and corroborated the results with the nonparametric Kruskal–Wallis test.
We used SAS software (version 9.4, SAS Institute, Cary, NC) for all computations and specified P<0.05 as indicating statistical significance. In cases where the hypothesis of 3 equal groups was rejected, the principle of closed testing allowed us to conduct pairwise comparisons between groups using P<0.05 as critical value while preserving the family‐wise type I error rate of 5% per outcome variable. 49 We applied the 5% family‐wise type I error rate to each study outcome independently, without adjustment for multiple comparisons, following the rationale of Glantz and Slinker. 50 When designing the RCT, we specified a sample size of 270 participants to detect, with 80% power, a mean difference for change in TG:HDL‐C between groups as small as 24%, based on associations observed in the Framingham Heart Study (and a magnitude of effect deemed to have clear clinical implications). 18
Drs Ebbeling, Feldman, and Ludwig had full access to all the data and take responsibility for its integrity and data analysis.
RESULTS
Participants
We randomly assigned 203 participants (121 males and 82 females) to an intervention group (Figure 1). The overall retention rate at 12 months was 91.6%. The 17 dropouts were uniformly distributed among study groups (7/67 SSB group, 7/67 ASB group, 3/69 USB group; P=0.32). Dropouts did not differ significantly from the 186 completers with respect to the baseline characteristics shown in Table 2, with minor exceptions. Compared with completers, dropouts more often declined to report household income (11/17 versus 39/186; P<0.001) or education (9/17 versus 7/186; P<0.001), but these discrepancies did not vary significantly across study groups. Dropouts were on average slightly younger than completers (24.2±4.7 versus 27.1±5.7 years, mean±SD; P=0.04), but this difference likewise did not vary significantly across study groups. All parametric analyses were adjusted for age, further obviating any selection bias from this source.
Figure 1.
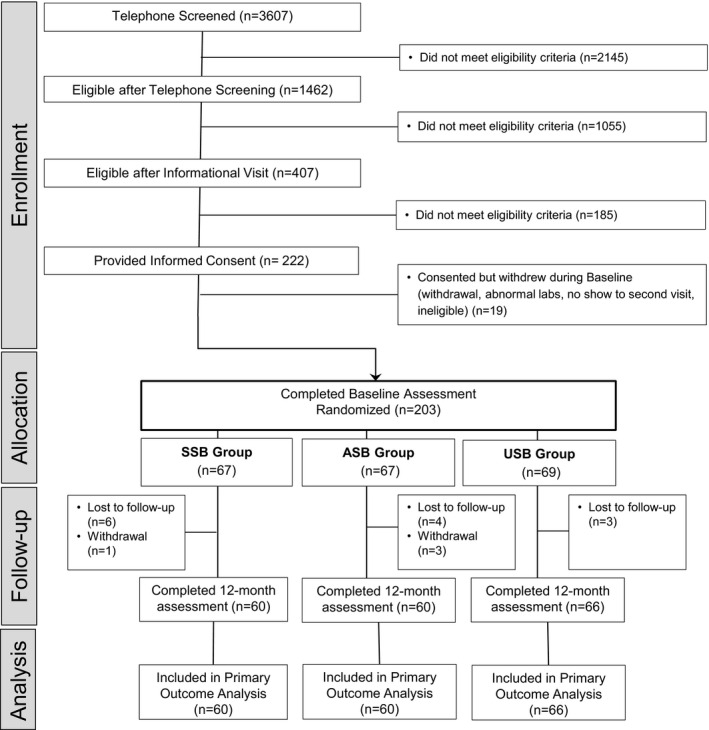
Participant flow. ASB indicates artificially sweetened beverage; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
Table 2.
Baseline Characteristics of Study Participants by Beverage Group
| Beverage Group | |||
|---|---|---|---|
| SSB | ASB | USB | |
| N | 67 | 67 | 69 |
| Categorical variables* | N (%) | ||
| Sex | |||
| Male | 40 (59.7) | 40 (59.7) | 41 (59.4) |
| Female | 27 (40.3) | 27 (40.3) | 28 (40.6) |
| Ethnicity † | |||
| Hispanic | 10 (14.9) | 7 (10.4) | 8 (11.6) |
| Race † | |||
| White | 38 (56.7) | 33 (49.3) | 32 (46.4) |
| Black | 14 (20.9) | 15 (22.4) | 11 (15.9) |
| Asian | 6 (9.0) | 6 (9.0) | 14 (20.3) |
| Multiple/unknown/other | 9 (13.4) | 13 (19.4) | 12 (17.4) |
| Annual household income | |||
| <$30 000 | 13 (19.4) | 19 (28.4) | 20 (29.0) |
| $30 000–$59 999 | 18 (26.9) | 16 (23.9) | 12 (17.4) |
| $60 000–$89 999 | 8 (11.9) | 9 (13.4) | 7 (10.1) |
| ≥$90 000 | 10 (14.9) | 5 (7.5) | 16 (23.2) |
| Not reported/unknown | 18 (26.9) | 18 (26.9) | 14 (20.3) |
| Education | |||
| Some high school | 1 (1.5) | 0 (0.0) | 3 (4.4) |
| High school or GED | 5 (7.5) | 5 (7.5) | 4 (5.8) |
| Some college or vocational school | 17 (25.4) | 20 (29.9) | 14 (20.3) |
| Associate’s degree | 4 (6.0) | 4 (6.0) | 3 (4.4) |
| Bachelor’s degree | 19 (28.4) | 19 (28.4) | 20 (29.0) |
| Some graduate school or degree | 16 (23.9) | 14 (20.9) | 19 (27.5) |
| Not reported/unknown | 5 (7.5) | 5 (7.5) | 6 (8.7) |
| Continuous variables | Mean±SD | ||
| Age, y | 25.9±5.1 | 26.7±5.7 | 27.9±6.0 |
| Weight, kg | 75.5±15.6 | 76.8±16.7 | 77.5±16.1 |
| Height, cm | 170.8±9.2 | 171.3±9.6 | 170.3±10.5 |
| Body mass index, kg/m2 | 25.8±4.7 | 26.1±5.2 | 26.6±4.6 |
ASB indicates artificially sweetened beverage; GED, General Educational Development; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
Percentages may not sum to 100 because of rounding.
Ethnicity and race reported by participants.
Process Data
Intervention intensity did not differ among groups (Table S3). Participants retained in the study (N=186) received 7.4±4.1 beverage deliveries and completed 19.2±4.5 of 24 planned check‐in calls (mean±SD).
Changes in dietary intake are presented in Table 3. Daily consumption of SSBs increased by 1 serving in the SSB group (P<0.001) and declined to almost 0 in the ASB and USB groups (P<0.001). Reflecting these changes, consumption of added sugars and energy from SSBs increased in the SSB group (P=0.002 for added sugars; P<0.001 for energy from SSB) and declined in the ASB and USB groups (P<0.001). Consumption of ASBs increased by 1.5 servings (12 fl oz/serving) in the ASB group (P<0.001) and did not change in the SSB and USB groups. At 12 months, participants in the ASB group consumed (mean±SD) 185.2±202.1 mg/day of aspartame, 268.35±559.89 mg/day of sucralose, and negligible amounts of other artificial sweeteners (including acesulfame potassium and saccharine). Consumption of USBs increased for the USB group (P<0.001) and remained constant for the SSB and ASB groups.
Table 3.
Self‐Reported Dietary Intake and Physical Activity
| Variable | Study Group | Unadjusted Data | Adjusted Data* | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | Mean Change±SE | P Within Group | P Between Groups | ||||
| N | Mean±SD | N † | Mean±SD | |||||
| Beverages (12 fl oz/serving) | ||||||||
| SSBs, servings/d | SSB | 67 | 1.4±1.1 | 60 | 2.5±1.8 | 1.0±0.2 | <0.001 | <0.001 |
| ASB | 67 | 1.5±1.4 | 60 | 0.1±0.3 | −1.4±0.2 | <0.001 | ||
| USB | 69 | 1.8±1.3 | 65 | 0.2±0.5 | −1.6±0.2 | <0.001 | ||
| ASBs, servings/d | SSB | 67 | 0.2±0.4 | 60 | 0.1±0.2 | −0.2±0.1 | 0.17 | <0.001 |
| ASB | 67 | 0.1±0.3 | 60 | 1.6±1.4 | 1.5±0.1 | <0.001 | ||
| USB | 69 | 0.2±0.7 | 65 | 0.0±0.3 | −0.2±0.1 | 0.14 | ||
| USBs, servings/d | SSB | 67 | 1.6±1.5 | 60 | 1.6±1.5 | −0.0±0.3 | 0.92 | <0.001 |
| ASB | 67 | 1.8±1.6 | 60 | 1.9±1.9 | 0.1±0.3 | 0.70 | ||
| USB | 69 | 2.1±1.6 | 65 | 4.3±2.1 | 2.2±0.3 | <0.001 | ||
| Sugar | ||||||||
| Total, g/d | SSB | 67 | 115.8±50.7 | 60 | 139.0±64.2 | 23.7±7.4 | 0.002 | <0.001 |
| ASB | 67 | 122.4±57.9 | 60 | 63.6±37.0 | −57.6±7.4 | <0.001 | ||
| USB | 69 | 123.2±57.0 | 65 | 62.8±34.6 | −60.3±7.1 | <0.001 | ||
| Added, g/d | SSB | 67 | 75.7±44.0 | 60 | 98.5±61.7 | 23.2±7.4 | 0.002 | <0.001 |
| ASB | 67 | 80.9±51.5 | 60 | 34.1±28.4 | −46.6±7.4 | <0.001 | ||
| USB | 69 | 86.2±47.3 | 65 | 30.0±23.1 | −56.1±7.2 | <0.001 | ||
| Energy | ||||||||
| Total, kcal/d | SSB | 67 | 2053±638 | 60 | 2207±577 | 130±79 | 0.10 | <0.001 |
| ASB | 67 | 2225±676 | 60 | 1894±576 | −312±79 | <0.001 | ||
| USB | 69 | 2113±689 | 65 | 1874±591 | −247±76 | 0.002 | ||
| From SSBs, kcal/d | SSB | 67 | 202±167 | 60 | 338±256 | 138±25 | <0.001 | <0.001 |
| ASB | 67 | 207±190 | 60 | 21±43 | −186±25 | <0.001 | ||
| USB | 69 | 242±164 | 65 | 9±31 | −232±24 | <0.001 | ||
| Physical activity | ||||||||
| Total physical activity (MET) | SSB | 67 | 1.58±0.19 | 60 | 1.59±0.21 | 0.01±0.03 | 0.68 | 0.90 |
| ASB | 67 | 1.64±0.21 | 60 | 1.65±0.26 | 0.01±0.03 | 0.80 | ||
| USB | 69 | 1.56±0.19 | 65 | 1.58±0.24 | 0.03±0.03 | 0.37 | ||
ASB indicates artificially sweetened beverage; MET, metabolic equivalent; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
Data analyzed using repeated measures analysis of variance, adjusted for covariates (sex, ethnicity, race, age).
Dietary recall data missing for one participant in USB group, who could not be reached for telephone interviews at 12 months.
Changes in Healthy Eating Index total score and subcomponent scores are presented in Table S4. Change in Healthy Eating Index total score did not differ between groups (P=0.14) but improved in the USB group (P=0.03) on the basis of exploratory within‐group analyses. Changes in the added sugars score differed between groups (P<0.001), consistent with data presented in Table 3. The between‐group effect for the refined grains score was of borderline significance (P=0.06), attributable to decreased consumption in the SSB group (P=0.05) and no change in the ASB and USB groups.
Study Outcomes
Blood lipids, homeostasis model assessment variables, and body weight and fat mass are presented in Table 4. Change in TG:HDL‐C from baseline to 12 months, the prespecified primary outcome, was not significantly different between groups (P=0.65). Likewise, changes in homeostasis model assessment variables (insulin sensitivity, P=0.38; β‐cell function, P=0.49), body weight (P=0.66), fat mass (P=0.27), and other outcomes (Table S5) did not differ significantly between groups. We observed mean (±SE) differences for change in TG:HDL‐C of –5.4%±7.5 for the ASB versus SSB group and –6.2%±7.2 for the USB versus SSB group. We had only 8% power to detect a group effect of this magnitude with the attained sample size, which was smaller than proposed because of recruitment challenges. Nevertheless, enrollment of additional participants to attain the proposed sample size would not have substantially enhanced power to detect such small differences.
Table 4.
Blood Lipids, Homeostasis Model Assessment Variables, and Body Weight and Fat Mass
| Variable* | Study Group | Unadjusted Data | Adjusted Data § | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | Mean Change±SE | P Within Group | P Between Groups | ||||
| N † | Mean±SD Median (IQR) ‡ | N † | Mean±SD or Median (IQR) ‡ | |||||
| Blood lipids | ||||||||
| TG:HDL‐C (primary outcome)* | SSB | 67 | 1.37 (0.87–2.16) | 60 | 1.46 (0.98–2.19) | 3.2±5.7 | 0.56 | 0.65 |
| ASB | 67 | 1.30 (0.78–1.98) | 60 | 1.19 (0.86–1.87) | −2.4±5.4 | 0.65 | ||
| USB | 69 | 1.51 (1.08–2.51) | 66 | 1.45 (0.99–2.31) | −3.2±5.1 | 0.52 | ||
| LDL‐C, mg/dL | SSB | 67 | 99.6±26.8 | 60 | 102.5±30.5 | 1.2±2.6 | 0.65 | 0.67 |
| ASB | 67 | 101.5±28.0 | 60 | 104.0±30.2 | 1.7±2.6 | 0.51 | ||
| USB | 69 | 109.9±33.3 | 66 | 109.1±29.4 | −1.3±2.5 | 0.61 | ||
| Homeostasis Model Assessment | ||||||||
| Insulin sensitivity, %* | SSB | 67 | 89.1 (69.9–161.5) | 60 | 84.3 (59.3–143.0) | −7.5±6.1 | 0.22 | 0.38 |
| ASB | 66 | 81.8 (58.7–125.0) | 60 | 87.5 (60.4–148.8) | 4.9±6.9 | 0.46 | ||
| USB | 69 | 85.2 (55.8–122.9) | 65 | 87.4 (57.5–126.5) | −0.6±6.3 | 0.93 | ||
| β‐cell function, %* | SSB | 67 | 120.6 (89.7–141.9) | 60 | 127.6 (88.4–153.8) | 0.7±4.2 | 0.86 | 0.49 |
| ASB | 66 | 126.3 (98.6–155.8) | 60 | 122.1 (90.9–147.5) | −5.7±4.0 | 0.15 | ||
| USB | 69 | 122.3 (99.2–163.9) | 65 | 117.7 (96.4–154.1) | −4.3±3.9 | 0.27 | ||
| Body weight and fat mass | ||||||||
| Weight, kg | SSB | 67 | 75.5±15.6 | 60 | 78.0±17.4 | 1.2±0.6 | 0.03 | 0.66 |
| ASB | 67 | 76.8±16.7 | 60 | 77.0±17.1 | 0.6±0.6 | 0.32 | ||
| USB | 69 | 77.5±16.1 | 66 | 78.3±17.1 | 0.7±0.5 | 0.22 | ||
| Whole‐body fat mass, kg | SSB | 64 | 22.0±9.6 | 44 | 24.2±11.1 | 1.0±0.5 | 0.03 | 0.27 |
| ASB | 65 | 22.5±9.8 | 44 | 22.8±10.2 | 0.1±0.5 | 0.81 | ||
| USB | 66 | 24.0±8.8 | 52 | 23.5±8.2 | 0.1±0.4 | 0.81 | ||
ASB indicates artificially sweetened beverage; LDL‐C, low‐density lipoprotein cholesterol; SSB, sugar‐sweetened beverage; TG:HDL‐C, triglyceride to high‐density lipoprotein cholesterol ratio; and USB, unsweetened beverage.
TG:HDL‐C and homeostasis model assessment log‐transformed for analysis, results retransformed for reporting as described below.
Missing data: Homeostasis model assessment missing for 1 participant in ASB group at baseline (missing glucose) and 1 participant in USB group at 12 months (missing insulin); dual‐energy x‐ray absorptiometry whole body fat mass missing for 8 participants at baseline (including 1 dropout in SSB group) and 44 participants at 12 months (scanned on a replacement scanner that was not adequately calibrated with the original scanner); 2 additional participants did not complete the dual‐energy x‐ray absorptiometry scan at 12 months (1 in SSB group, 1 in USB group).
Unadjusted mean±standard deviation; in the case of variables log‐transformed for analysis, median and interquartile range (25th, 75th percentiles).
Mean change±standard error and P‐values, from repeated‐measures analysis of variance, adjusted for prespecified covariates (sex, ethnicity, race, age). For variables log‐transformed for analysis, adjusted change in mean log value and its standard error (Δ±SE) expressed as percentages: 100%×exp(Δ)±100%×exp(Δ)×(exp(SE)−1).
To explore individual differences in response according central adiposity, we divided the cohort into tertiles of baseline trunk fat. As shown in Figure 2 (and Table S6), trunk fat was a significant effect modifier (group×time×trunk fat tertile) for change in body weight (P=0.006) and fat mass (P=0.004). Among participants with the most trunk fat (tertile 3), body weight and fat mass increased significantly more in the SSB group compared with the other 2 groups (P=0.002). There were no discernible group effects for these outcomes among participants in the lower tertiles of trunk fat.
Figure 2.
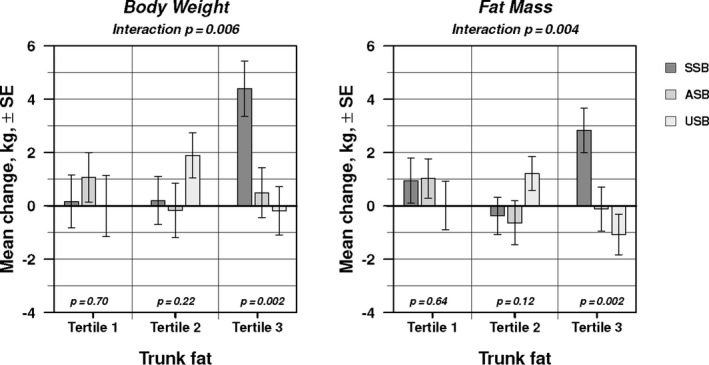
Effect modification by baseline trunk fat for changes in body weight and fat mass. Each bar indicates 12‐month mean change±standard error, from repeated‐measures analysis of variance, adjusted for prespecified covariates (sex, ethnicity, race, age). Within each tertile of trunk fat, P (bottom) tests for difference in mean change across beverage groups. Interaction P (top) tests for difference in beverage effect across tertiles. ASB indicates artificially sweetened beverage; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
Changes in sweet taste preference are shown in Figure 3. Comparison across the 3 beverage groups by robust regression analysis showed a significant difference in the mean change for both sweetness threshold (P=0.008) and favorite concentration (P=0.006). Sweetness threshold did not change discernibly in the SSB or ASB group but decreased significantly in the USB group (P=0.001), with pairwise comparison indicating a significant difference between the ASB and USB groups (P=0.015). Similarly, favorite concentration did not change discernibly in the SSB group but decreased significantly in the USB group (P<0.0001) and ASB group (P=0.02), with the difference between the ASB and USB groups of borderline statistical significance (P=0.06). Adjustment for covariates left the between‐group difference significant for both measures (P=0.01 and P=0.003, respectively). The nonparametric Kruskal–Wallis test confirmed the group differences in distribution (P=0.03 for sweetness threshold; P=0.009 for favorite concentration; Table S7).
Figure 3.
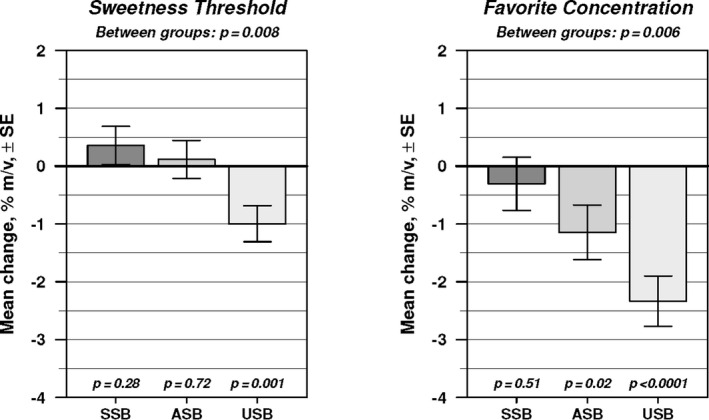
Changes in sweet taste preference. Robust (outlier‐resistant) regression analysis provided 12‐month mean change±standard error and P values testing for difference in mean change between groups (top) and within groups (bottom). ASB indicates artificially sweetened beverage; SSB, sugar‐sweetened beverage; and USB, unsweetened beverage.
Adverse Events
Three adverse events were documented during the study (hospitalization for body aches; hospitalization for asthma; headache and mood swings attributable to a toothache). Each of these events was deemed unrelated to participation in the RCT.
DISCUSSION
Our study aimed to address a major controversy of relevance to dietary guidelines for the public: Are artificially sweetened beverages equivalent to unsweetened beverages as replacements for sugar‐sweetened beverages? To address this controversy, we conducted an RCT of well‐differentiated interventions, controlling for intervention intensity. We found no overall group differences for changes in TG:HDL‐C and other prespecified cardiometabolic risk factors. Although body weight and fat mass also did not differ among groups, baseline trunk fat was a significant effect modifier for these outcomes. As such, among individuals with central adiposity, replacing SSBs with either ASBs or USBs had a favorable effect on body weight and fat mass. Overall, USBs were a better replacement than ASBs for decreasing sweet taste preference. Length of the intervention period, level of beverage exposure, and individual susceptibility warrant careful consideration when comparing results to data from prospective observational studies 25 , 29 , 51 , 52 and previous RCTs. 6 , 7 , 11 , 12 , 34 , 53 , 54 , 55
The benefits of replacing consumption of SSBs with ASBs or USBs on cardiometabolic risk factors may require longer periods of study for the general population. In prospective observational studies, significant associations between SSB or ASB consumption and mortality have been observed in several cohorts over long‐term follow‐up periods. 25 , 29 , 51 , 52 For example, SSB consumption was positively associated with all‐cause and cardiovascular disease mortality in the Health Professionals’ Follow‐up Study (28 years of follow‐up) and Nurses’ Health Study (34 years of follow‐up), with mortality increasing by 10% with each additional daily serving of SSB. 51 In addition, ASB consumption was associated with all‐cause and cardiovascular disease mortality in the Nurses’ Health study among women who were consuming at least 4 servings per day. 51 Statistical models of beverage substitutions in the Women’s Health Initiative (mean follow‐up of 8.4 years) 25 and European Prospective Investigation into Cancer and Nutrition (mean follow‐up of 10.8 years) 52 indicate reductions in risk for type 2 diabetes mellitus when replacing SSBs with USBs but no benefit when replacing SSBs with ASBs.
The intervention messages in the present RCT focused on replacing SSBs consumed at baseline with provided beverages. While relevant from a public health perspective, these messages may have resulted in more variability in consumption and thus less consistent exposure compared with interventions specifying an absolute daily intake. In a Danish RCT of healthy adults, participants were instructed to consume provided beverages at a rate of 1 L (≈36 fl oz) per day. 11 , 12 , 55 At this level of daily consumption for just 6 months, regular cola compared with aspartame‐sweetened cola or water caused greater increases in triglycerides, total cholesterol, and uric acid, but not HDL‐cholesterol and insulin sensitivity. On average, participants in the present RCT did not consume the daily volumes specified in the Danish RCT, even with the unintended increase in consumption of SSBs (in the SSB group) leading to a total volume of ≈30 fl oz per day on average (≈2.5 servings, rather than ≈1.5 servings reflecting baseline consumption).
Certain individuals may be particularly susceptible to the adverse effects of dietary carbohydrate on deposition of fat tissue and weight gain and thus more likely to benefit from replacing consumption of SSBs with noncaloric options. 6 , 7 , 34 , 53 , 54 The present RCT indicates that consumption of SSBs had an adverse effect on body weight and fat mass among individuals with high baseline trunk fat, likely attributable in part to increased consumption in the SSB group. Insulin secretion may be one key component of complex mechanisms underlying susceptibility in that individuals with higher central adiposity are more likely to have high initial insulin secretion in response to sugar consumption. 48 As summarized previously, 56 consumption of high‐glycemic‐load sources of carbohydrate, such as SSBs, may promote weight gain by raising the postprandial ratio of serum insulin to glucagon, resulting in increased hunger and decreased energy expenditure. Dietary changes to reduce glycemic load may have the most pronounced effect among individuals with high trunk fat, in whom the postprandial insulin response to oral glucose may be greatest. 34 , 56 In the present RCT, similar changes in body weight among participants with high trunk fat who consumed ASBs or USBs are consistent with studies indicating that mixed meals containing sucralose or aspartame do not raise postprandial blood glucose or insulin levels to the same extent as those containing sucrose. 57 , 58
The observed decrease in sweetness threshold among participants who consumed USBs is consistent with the findings of Wise et al. 59 In their RCT, a dietary intervention to reduce consumption of sugar for 3 months altered perceived sweet taste intensity, such that puddings and beverages containing specified sucrose concentrations were perceived as more sweet in the intervention compared with control group. In the present RCT, favorite concentration also decreased with USBs, and to a lesser extent ASBs. Change in sweet taste preference, achieved by replacing SSBs with USBs, may provide a mechanism for promoting adherence to prescribed low‐sugar diets in the context of comprehensive behavioral intervention programs. 60 Improved Healthy Eating Index total score for the USB group is consistent with the change in overall dietary quality observed by Hedrick et al 61 with an intervention aimed solely at reducing consumption of SSBs.
Group effects must be interpreted in the context of the unintended mean increase in consumption of SSBs. With delivery of free SSBs to their homes, participants in the SSB group exhibited propensity to increase, rather than maintain, baseline levels of consumption. Neither sweetness threshold nor favorite concentration changed for the SSB group, suggesting that increased exposure to SSBs did not have an effect on sweet taste preference. While contributing to differentiation in beverage intake among groups (and thus confidence in the null outcomes), increased intake in the SSB group arguably would not threaten external validity for the positive outcomes, as consumption remained within the prevailing range for 60% of adults aged 20 to 39 years who consume SSBs in the United States. 62
In light of the effects of dietary carbohydrate on TG and HDL‐C observed in previous studies, 34 , 39 the spontaneous decrease in consumption of refined grains for the SSB group may have contributed to the null finding. In a recent epidemiologic study, 19 consumption of SSBs was directly associated with adverse changes in TG and HDL‐C in analyses adjusted for dietary quality. To detect the potential effects of beverage consumption on these variables in RCTs, more attention may be needed toward behavioral strategies for controlling intake of other foods (most notably, sources of refined carbohydrate) that could attenuate the independent effects of beverage consumption in intervention studies.
Strengths of this RCT include an intervention targeting a single dietary behavior (beverage consumption), home delivery of beverages to promote differentiation in consumption across study groups, examination of several biomarkers of cardiometabolic risk, evaluation of effect modification by baseline central adiposity, a novel protocol for assessing sweet taste preference, a diverse sample (≈50% nonwhite), and high retention rates across groups. Limitations include limited power to observe small effects because of study design (single site rather than multisite study) and recruitment challenges, inability to mask participants to study group assignments, lack of biomarkers of compliance, and reliance on self‐report to assess dietary intake and physical activity (with high likelihood of inaccurate reporting and possibly differential misreporting among intervention groups related to factors such as social desirability bias). To speculate, participants with a strong desire to be viewed favorably by others (high social desirability bias) may have demonstrated more underreporting of actual energy intake in response to interventions focusing on consumption of noncaloric beverages (ASBs and USBs) compared with SSBs. 63 Also, the study was not designed to compare the effects of different artificial sweeteners on study outcomes. Emerging data indicate that, while some metabolic effects are consistent, others vary depending on choice of artificial sweetener. 30
In conclusion, replacing consumption of SSBs with either ASBs or USBs for 12 months had no effect on cardiometabolic risk factors. Among individuals with central adiposity, replacing SSBs with either ASBs or USBs had a favorable effect on body weight and adiposity, consistent with prior findings. 6 , 7 , 8 , 9 , 10 As stated in the American Heart Association science advisory, replacing SSBs with ASBs may be an appropriate initial recommendation for susceptible adults who habitually consume SSBs and consider USBs an undesirable alternative because of a strong sweet taste preference. 5 However, USBs were a better replacement than ASBs for decreasing sweet taste preference, particularly sweetness threshold, a finding with plausible implications for promoting adherence to prescribed low‐sugar diets. In light of epidemiologic data, 25 , 51 , 52 the benefits of eliminating consumption of SSBs and the differential effects of ASBs and USBs on cardiometabolic risk factors may require longer periods of study for the general population.
Sources of Funding
The RCT was funded by grants from the National Heart, Lung, and Blood Institute (R01HL104215) and National Institute of Diabetes and Digestive and Kidney Diseases (K24DK082730 awarded to Dr Ludwig); the National Center for Research Resources (M01RR02172); the Harvard Catalyst Clinical and Translational Science Center (UL1RR025758), and the New Balance Foundation. The views expressed in this article are those of the authors and do not necessarily represent the official views of the sponsors.
Disclosures
Dr Ludwig received royalties for books on obesity and nutrition that recommend a low‐glycemic‐load diet. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figure S1
Acknowledgments
We thank the study participants for their time and commitment to advancing science; Catherine Matero (Clinical Research Specialist) for her effort directed toward enrolling participants, assessing outcomes, managing data, and implementing intervention protocols; and research assistants, nurses, and technologists at Boston Children’s Hospital, who provided technical support.
(J Am Heart Assoc. 2020;9:e015668 DOI: 10.1161/JAHA.119.015668.)
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie‐Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. [DOI] [PubMed] [Google Scholar]
- 2. Vos MB, Kaar JL, Welsh JA, Van Horn LV, Feig DI, Anderson CAM, Patel MJ, Cruz Munos J, Krebs NF, Xanthakos SA, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2017;135:e1017–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malik VS, Pan A, Willett WC, Hu FB. Sugar‐sweetened beverages and weight gain in children and adults: a systematic review and meta‐analysis. Am J Clin Nutr. 2013;98:1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. U.S. Department of Health and Human Services and U.S. Department of Agriculture . Dietary Guidelines for Americans 2015 ‐ 2020. 8th ed December 2015. Available at: http://health.gov/dietaryguidelines/2015/guidelines/. Accessed October 31, 2019. [Google Scholar]
- 5. Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Despres JP, Hu FB, Kris‐Etherton PM, Otten JJ, Towfighi A, Wylie‐Rosett J. Low‐calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138:e126–e140. [DOI] [PubMed] [Google Scholar]
- 6. Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar‐sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680. [DOI] [PubMed] [Google Scholar]
- 7. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar‐sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high‐fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51:963–969. [DOI] [PubMed] [Google Scholar]
- 9. Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. [DOI] [PubMed] [Google Scholar]
- 10. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar‐free or sugar‐sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. [DOI] [PubMed] [Google Scholar]
- 11. Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose‐sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6‐month randomised controlled trial. Eur J Clin Nutr. 2015;69:949–953. [DOI] [PubMed] [Google Scholar]
- 12. Maersk M, Belza A, Stodkilde‐Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose‐sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6‐mo randomized intervention study. Am J Clin Nutr. 2012;95:283–289. [DOI] [PubMed] [Google Scholar]
- 13. Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar‐sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. [DOI] [PubMed] [Google Scholar]
- 14. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long‐term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar‐sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle‐aged women. JAMA. 2004;292:927–934. [DOI] [PubMed] [Google Scholar]
- 16. Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long‐term weight gain. Obesity (Silver Spring). 2008;16:1894–1900. [DOI] [PubMed] [Google Scholar]
- 17. Sylvetsky AC, Rother KI. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity (Silver Spring). 2018;26:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle‐aged adults in the community. Circulation. 2007;116:480–488. [DOI] [PubMed] [Google Scholar]
- 19. Haslam DE, Peloso GM, Herman MA, Dupuis J, Lichtenstein AH, Smith CE, Beverage MNM. Consumption and longitudinal changes in lipoprotein concentrations and incident dyslipidemia in US adults. The Framingham Heart Study. J Am Heart Assoc. 2020;9:e014083 DOI: 10.1161/JAHA.119.014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. [DOI] [PubMed] [Google Scholar]
- 21. Ferreira‐Pego C, Babio N, Bes‐Rastrollo M, Corella D, Estruch R, Ros E, Fito M, Serra‐Majem L, Aros F, Fiol M, et al. Frequent consumption of sugar‐ and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a Mediterranean population at high cardiovascular disease risk. J Nutr. 2016;146:1528–1536. [DOI] [PubMed] [Google Scholar]
- 22. de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar‐sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel‐Chapelon F. Consumption of artificially and sugar‐sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l'Education Nationale‐European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2013;97:517–523. [DOI] [PubMed] [Google Scholar]
- 24. Hirahatake KM, Jacobs DR, Shikany JM, Jiang L, Wong ND, Steffen LM, Odegaard AO. Cumulative intake of artificially sweetened and sugar‐sweetened beverages and risk of incident type 2 diabetes in young adults: the Coronary Artery Risk Development In Young Adults (CARDIA) Study. Am J Clin Nutr. 2019;110:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang M, Quddus A, Stinson L, Shikany JM, Howard BV, Kutob RM, Lu B, Manson JE, Eaton CB. Artificially sweetened beverages, sugar‐sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: the prospective Women's Health Initiative observational study. Am J Clin Nutr. 2017;106:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med. 2012;27:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pase MP, Himali JJ, Beiser AS, Aparicio HJ, Satizabal CL, Vasan RS, Seshadri S, Jacques PF. Sweetened beverages and the risks of incident stroke and dementia: a prospective cohort study. Stroke. 2017;48:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mossavar‐Rahmani Y, Kamensky V, Manson JE, Silver B, Rapp SR, Haring B, Beresford SAA, Snetselaar L, Wassertheil‐Smoller S. Artificially sweetened beverages and stroke, coronary heart disease, and all‐cause mortality in the Women’s Health Initiative. Stroke. 2019;50:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rother KI, Conway EM, Sylvetsky AC. How non‐nutritive sweeteners influence hormones and health. Trends Endocrinol Metab. 2018;29:455–467. [DOI] [PubMed] [Google Scholar]
- 31. Bartolotto C. Does consuming sugar and artificial sweeteners change taste preferences? Perm J. 2015;19:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low‐calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebbeling CB, Willett WC, Ludwig DS. The special case of sugar‐sweetened beverages In: KD B, MS G, eds. Food and Addiction: A Comprehensive Handbook. New York: Oxford University Press; 2012:147–153. [Google Scholar]
- 34. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low‐glycemic load vs low‐fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. [DOI] [PubMed] [Google Scholar]
- 35. Hu FB, Rimm E, Smith‐Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food‐frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. [DOI] [PubMed] [Google Scholar]
- 36. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC. Effects of low‐carbohydrate vs low‐fat diets on weight loss and cardiovascular risk factors: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. [DOI] [PubMed] [Google Scholar]
- 39. Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. [DOI] [PubMed] [Google Scholar]
- 41. McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin‐resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. [DOI] [PubMed] [Google Scholar]
- 42. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 43. Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL‐cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49:1873–1880. [DOI] [PubMed] [Google Scholar]
- 44. Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high‐density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. [DOI] [PubMed] [Google Scholar]
- 45. Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey‐Merz CN, Sopko G. The triglyceride/high‐density lipoprotein cholesterol ratio predicts all‐cause mortality in women with suspected myocardial ischemia: a report from the Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2009;157:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. [DOI] [PubMed] [Google Scholar]
- 47. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97:1517–1525. [DOI] [PubMed] [Google Scholar]
- 49. Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 50. Glantz SA, Slinker BK. Primer of Applied Regression and Analysis of Variance. New York: McGraw‐Hill; 1990. [Google Scholar]
- 51. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long‐term consumption of sugar‐sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia. 2015;58:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katan MB, de Ruyter JC, Kuijper LD, Chow CC, Hall KD, Olthof MR. Impact of masked replacement of sugar‐sweetened with sugar‐free beverages on body weight increases with initial BMI: secondary analysis of data from an 18 month double‐blind trial in children. PLoS One. 2016;11:e0159771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, Greenberg AS, Roberts SB. A low‐glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28:2939–2941. [DOI] [PubMed] [Google Scholar]
- 55. Engel S, Tholstrup T, Bruun JM, Astrup A, Richelsen B, Raben A. Effect of high milk and sugar‐sweetened and non‐caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr. 2018;72:358–366. [DOI] [PubMed] [Google Scholar]
- 56. Ludwig DS, Ebbeling CB. The carbohydrate‐insulin model of obesity: beyond “calories in, calories out.” JAMA Intern Med. 2018;178:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi‐Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19:1004–1005. [DOI] [PubMed] [Google Scholar]
- 59. Wise PM, Nattress L, Flammer LJ, Beauchamp GK. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am J Clin Nutr. 2016;103:50–60. [DOI] [PubMed] [Google Scholar]
- 60. Demos KE, McCaffery JM, Thomas JG, Mailloux KA, Hare TA, Wing RR. Identifying the mechanisms through which behavioral weight‐loss treatment improves food decision‐making in obesity. Appetite. 2017;114:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hedrick VE, Davy BM, You W, Porter KJ, Estabrooks PA, Zoellner JM. Dietary quality changes in response to a sugar‐sweetened beverage‐reduction intervention: results from the Talking Health randomized controlled clinical trial. Am J Clin Nutr. 2017;105:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in beverage consumption among children and adults, 2003–2014. Obesity (Silver Spring). 2018;26:432–441. [DOI] [PubMed] [Google Scholar]
- 63. Kirkpatrick SI, Collins CE, Keogh RH, Krebs‐Smith SM, Neuhouser ML, Wallace A. Assessing dietary outcomes in intervention studies: pitfalls, strategies, and research needs. Nutrients. 2018;10:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figure S1


