Abstract
Background
Stroke is a serious complication of hypertensive disorders of pregnancy (HDP), with potentially severe and long‐term sequelae. However, the temporal trends, predictors, and outcomes of stroke in women with HDP at delivery remain unknown.
Methods and Results
All HDP delivery hospitalizations with or without stroke event (ischemic, hemorrhagic, or unspecified) between 2004 and 2014 in the United States National Inpatient Sample were analyzed to examine incidence, predictors, and prognostic impact of stroke. Of 4 240 284 HDP delivery hospitalizations, 3391 (0.08%) women had stroke. While the prevalence of HDP increased over time, incident stroke rates decreased from 10 to 6 per 10 000 HDP delivery hospitalizations between 2004 and 2014. Women with stroke were increasingly multimorbid, with some risk factors being more strongly associated with ischemic strokes, including congenital heart disease, peripheral vascular disease, dyslipidemia, and sickle cell disease. Delivery complications were also associated with stroke, including cesarean section (odds ratio [OR], 1.58; 95% CI, 1.33–1.86), postpartum hemorrhage (OR, 1.91; 95% CI, 1.33–1.86), and maternal mortality (OR, 99.78; 95% CI, 59.15–168.31), independently of potential confounders. Women with stroke had longer hospital stays (median, 6 versus 3 days), higher hospital charges (median, $14 655 versus $4762), and a higher proportion of nonroutine discharge locations (38% versus 4%).
Conclusions
The incidence of stroke in women with HDP has declined over time. While a relatively rare event, identification of women at highest risk of ischemic or hemorrhagic stroke on admission for delivery is important to reduce long‐term sequelae.
Keywords: preeclampsia/pregnancy, pregnancy, stroke in young adults
Subject Categories: Epidemiology
Nonstandard Abbreviations and Acronyms
- CVD
cardiovascular disease
- HDP
hypertensive disorders of pregnancy
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- NIS
National Inpatient Sample
- OR
odds ratio
Clinical Perspective
What Is New?
The incidence of peripartum stroke in women with hypertensive disorders of pregnancy has declined over time.
In this population, women with ischemic versus hemorrhagic strokes had moderately different clinical profiles whereby some stroke predictors were more strongly associated with ischemic than hemorrhagic strokes, including congenital heart disease, peripheral vascular disease, and dyslipidemia.
Peripartum stroke is associated with increased odds of cesarean section and excessive in‐hospital mortality risk in women with hypertensive disorders of pregnancy at 1.5‐ and 100‐fold, respectively.
What Are the Clinical Implications?
Clinicians should be encouraged to actively investigate and treat pregnant women with features suggestive of stroke, especially in the high‐risk population of women with hypertensive disorders of pregnancy.
The assessment of these women for their risk of ischemic or hemorrhagic stroke on admission for delivery is needed so that measures, such as closer blood pressure monitoring, may be instigated to improve their intrapartum care.
Hypertensive disorders of pregnancy (HDP) are a leading cause of maternal morbidity and mortality worldwide, 1 , 2 , 3 , 4 affecting almost 10% of all pregnancies. 5 Chronic hypertension is defined as hypertension diagnosed before pregnancy or before 20 weeks of gestation; gestational hypertension is hypertension diagnosed during pregnancy at or after 20 weeks of gestation, delivery, or postpartum; while preeclampsia or eclampsia is hypertension diagnosed during pregnancy at or after 20 weeks of gestation, delivery, or postpartum with proteinuria or multisystem organ failure. 6 Women with HDP are at increased risk of stroke long‐term, 7 while during the pregnancy almost 50% of pregnancy‐associated strokes are associated with preeclampsia or eclampsia. 8
Pregnancy‐associated stroke is the most common cause of serious long‐term disability following pregnancy 9 and accounts for 7.7% of maternal deaths in the United States. Furthermore, maternal deaths from stroke in women with HDP may be underestimated, as they may be categorized as deaths attributable to HDP. 10 In the United States, 6.9% of maternal mortality is attributable to HDP. 10 Although there are known risk factors for peripartum strokes in preeclampsia, including older age, black race, infections, and prothrombotic or inflammatory disorders, 11 pregnancy‐associated strokes continue to be difficult to predict and prevent.
Approximately 40% of pregnancy‐associated strokes occur during hospital admissions for delivery, 1 , 12 , 13 with the highest risk occurring the day before or 2 days after delivery. 14 Most of the literature has not assessed the risk of stroke in women with HDP during this high‐risk period, 15 when it may be possible to implement preventative strategies for these devastating events. The few larger studies in the context of HDP delivery outcomes are limited by the fact that they reported outcomes from selected preeclampsia cohorts, 11 , 16 lacked specific delivery admissions data, 1 included only selected risk factors and comorbidities, 1 , 16 were derived from limited geographic areas, 11 , 16 and lacked stroke subtype comparisons. 11 , 16 A nationally representative database, such as the National Inpatient Sample (NIS) containing discharge data from US hospitals, offers the opportunity to study rare events such as pregnancy‐associated strokes during hospital delivery and fill current knowledge gaps to accelerate the progress in peripartum stroke prevention.
The current study used a national cohort of over 4 million delivery hospitalization episodes with HDP that occurred between 2004 and 2014. We aimed to assess the temporal trends in the incidence of stroke, patient characteristics, and comorbidities, as well as the associations of stroke with delivery complications, stratified by type of stroke.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. We conducted a cross‐sectional study using the nationally representative NIS database, the largest all‐payer inpatient healthcare database within the United States sponsored by the Agency for Healthcare Research and Quality as a part of the Healthcare Cost and Utilization Project. 17 It contains information on 7 million to 8 million hospital discharges per year.
We identified all women with a delivery hospitalization over 11 calendar years between January 2004 and December 2014 using a validated protocol that has been previously published (Data S1). 18 Following this, we established delivery hospitalizations that also had diagnosis codes for HDP using codes from previous publications on the NIS (Table S1). 19 , 20 , 21 , 22 Within these hospitalizations, we extracted records of a stroke event during the admission episode. This was stratified into ischemic (acute ischemic stroke, cerebral venous thrombosis, and transient ischemic attack), hemorrhagic (acute hemorrhagic stroke) and unspecified (stroke in puerperium or iatrogenic stroke, unspecific in nature) stroke (Table S1). In parallel, we also stratified the hospitalizations into HDP subgroups (chronic hypertension, gestational hypertension, preeclampsia, and superimposed preeclampsia on chronic hypertension).
Relevant treatments (angiography, thrombolysis, and thrombectomy), delivery complications (maternal mortality, preterm birth, stillbirth, cesarean section, postpartum hemorrhage), and cost outcomes (length of stay and total hospital charge) were determined from the data set using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes from previous studies (Table S1). 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 28 We grouped the years (2004–2007, 2008–2011, and 2012–2014) in the temporal trend analyses.
Covariates on patient demographics, obstetric factors, and all Agency for Healthcare Research and Quality Elixhauser comorbidity measures were extracted, except for weight loss, metastatic cancer, solid tumor without metastasis, lymphoma, and blood loss, which are deemed either too uncommon in pregnancy or too common in our delivery cohort (Data S1). Neurological disorders included multiple sclerosis and epilepsy. Cardiovascular disease (CVD) was defined as a composite of arrhythmia, valvular disease, ischemic heart disease, peripheral vascular disease, heart failure, or peripartum cardiomyopathy. The ICD‐9‐CM codes used were based on previous publications and presented in Table S1. 26 , 27 , 29 , 30 This study involved the analysis of deidentified data and therefore did not require institutional review board review in accordance with the Code of Federal Regulations, 45 CFR 46.
Stata/MP version 14.0 statistical package was used to perform all analyses. Continuous variables are presented as medians and interquartile ranges (IQRs), and categorical data are presented as numbers and percentages. As recommended by Agency for Healthcare Research and Quality, to account for the survey design of the NIS database, the survey estimation commands were used (svy prefix in Stata) for all analyses.
We conducted binary logistic regression analyses to determine the association of potential risk factors with pregnancy‐associated stroke, as well as the association between stroke and delivery complications of interest. The following potential risk factors were adjusted for in all fully adjusted analyses: year of admission, age, weekday/weekend admission, race and ethnicity, median zip code income quartile, hospital region, smoking, congenital heart disease, dyslipidemia, ischemic heart disease, peripartum cardiomyopathy, arrhythmias, previous stroke, sickle cell disease, obstetric factors associated with gestational hypertension or coagulopathy (gestational diabetes mellitus, fetal growth restriction, placenta previa, and multiple pregnancy), and selected Agency for Healthcare Research and Quality Elixhauser comorbidity measures (obesity, heart failure, diabetes mellitus, valvular disease, pulmonary circulation disorders, peripheral vascular disease, neurological disorders, chronic pulmonary disease, hypothyroidism, renal failure, liver disease, HIV and AIDS, rheumatoid arthritis/collagen vascular diseases, fluid and electrolyte disorders, deficiency anemias, alcohol abuse, drug abuse, depression, psychosis, coagulopathy, paralysis, and peptic ulcer). All odds ratios (ORs) were presented with the corresponding 95% CIs. We ensured that our study adhered to the recommended methodology standards 31 and an extension of the Strengthening the Reporting of Observational Studies in Epidemiology checklist, the Reporting of Studies Conducted Using Observational Routinely Collected Data checklist, 32 is shown in Table S2.
Results
A total of 4 240 284 delivery hospitalization episodes with HDP, including 3391 (0.08%) women with stroke, between 2004 and 2014 were included (Figure 1). There was an increase in the proportion of HDP delivery hospitalizations episodes from 8.4% to 10.9% of a total of 44 801 002 hospitalizations between 2004 and 2014 (Figure 2A). However, the proportion of HDP delivery hospitalizations with a recorded stroke diagnosis decreased from 10 per 10 000 HDP delivery hospitalizations in 2004 to 6 per 10 000 HDP delivery hospitalizations in 2008, then remained stable until 2014 (Figure 2B). Next, we examined the temporal trends of demographic factors that may affect women with HDP with stroke, such as age, race and ethnicity, median income, and prevalent CVD. In both the HDP population and its stroke subpopulation, the median age and the composition of race groups remained relatively constant over time (Table S3). Although median income (Figure S1A) and prevalent CVD (Figure 2C) within the HDP population have remained unchanged over the decade, there was a proportional increase of women in the wealthiest income quartile (6%–17%; Figure S1B) and with CVD (6%–18%; Figure 2D) in women with HDP with stroke during this study period.
Figure 1.

Flow diagram of included/excluded records. HDP indicates hypertensive disorders of pregnancy; and NIS, National Inpatient Sample.
Figure 2.
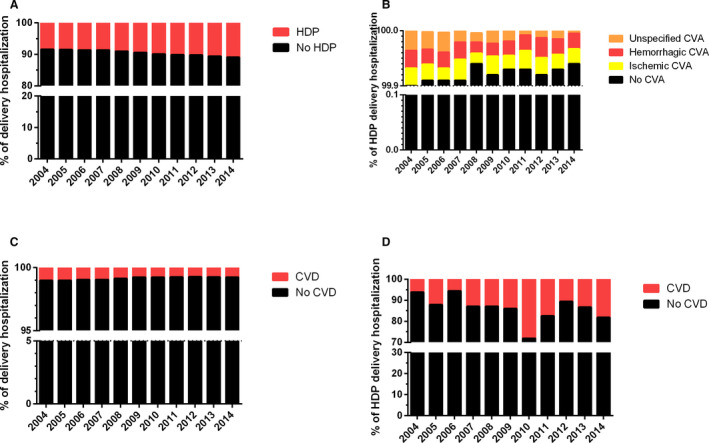
Comparison of hypertensive disorders of pregnancy (HDP) population in the delivery hospitalizations and stroke subpopulation in the HDP delivery hospitalizations over 1 decade. A, Percentage of HDP diagnosis in delivery hospitalizations. B, Percentage of stroke (cerebrovascular accident [CVA]) diagnosis within the HDP delivery hospitalization population. A comparison of cardiovascular disease (CVD) diagnosis was also made between (C) HDP and (D) stroke populations.
Table S4 shows the characteristics of our study population. Women with stroke comprised 0.08% of the HDP delivery population. They were older (median age, 30 versus 28) and had a higher proportion of black ethnicity (24% versus 17%). These women had more comorbidities, such as congenital heart disease, ischemic heart disease, peripheral vascular disease, heart failure, peripartum cardiomyopathy, coagulopathy, dyslipidemia, and previous stroke. Longer hospital stays (median, 6 versus 3 days) and higher hospital charges (median, $14 655 versus $4762), compared with women without stroke, were evident in women within the stroke group.
More women with stroke had ischemic or hemorrhagic strokes (36% or 35% of all stroke population), while the remaining 28% had unspecified strokes. The ischemic stroke group had a higher proportion of Hispanic women, and women with ischemic heart disease, peripheral vascular disease, heart failure, peripartum cardiomyopathy, previous stroke, dyslipidemia, sickle cell disease, and obesity. Conversely, the prevalence of valvular disease and coagulopathy was higher in the hemorrhagic compared with the ischemic group.
The temporal changes in the prevalence of recorded stroke risk factors and comorbidities in the HDP delivery hospitalization episodes within the stroke and no‐stroke groups showed increased recorded prevalence of all risk factors and comorbidities over time in women with stroke (Table S5), except for valvular disease, ischemic heart disease, renal failure, and rheumatoid arthritis or collagen vascular diseases. No obvious differences in stroke risk factors or comorbidities were found between the stroke subgroups.
Angiography was most commonly conducted in hemorrhagic strokes, while for ischemic strokes, thrombolysis was performed more frequently than thrombectomy (Table S6). The prevalence of all delivery complications was higher in women with strokes compared with women without strokes (Table S6). Maternal mortality, stillbirth, and postpartum hemorrhage occurred most frequently in hemorrhagic strokes, whereas preterm birth occurred most frequently in ischemic strokes. No obvious temporal patterns were detected for treatments and delivery complications both overall and in the stroke subgroups (Table S5).
Multivariable analyses were conducted to examine the independent association of risk factors and stroke subgroups (ischemic, hemorrhagic, and unspecified) (Table 1). Preexisting neurological disorders (adjusted OR, 17.35; 95% CI, 13.42–22.43), peripheral vascular disease (OR, 10.03; 95% CI, 3.98–25.25), congenital heart disease (OR, 7.38; 95% CI, 3.85–14.16), fluid and electrolyte disorder (OR, 5.90; 95% CI, 4.65–7.49), and previous stroke (OR, 4.77; 95% CI, 2.09–10.87) had the highest ORs in association with all stroke. We performed a separate analysis to study the independent predictors of ischemic and hemorrhagic strokes. Generally, the risk factors associated with stroke were the same for ischemic and hemorrhagic strokes, although congenital heart disease, peripheral vascular disease, dyslipidemia, and sickle cell disease were more strongly associated with ischemic stroke.
Table 1.
Association Between Stroke Risk Factors and Comorbidities With Subgroups of Stroke
| Stroke | No Stroke (n=4 236 893) | ||||
|---|---|---|---|---|---|
| All Stroke (n=3391) | Ischemic (n=1229) | Hemorrhagic (n=1187) | Unspecified (n=975) | ||
| OR (95% CI); n | |||||
| Neurological disorders |
17.35 (13.42–22.43) n=730 |
15.99 (10.82, 23.62) n=255 |
18.94 (12.87–27.86) n=276 |
17.23 (10.63–27.92) n=199 |
1.00 (reference) n=34 319 |
| Peripheral vascular disease |
10.03 (3.98–25.25) n=54 |
24.16 (8.47–68.90) n=40 |
6.26 (1.18–33.18) n=10 |
1.40 (0.09–21.21) n=4 |
1.00 (reference) n=1695 |
| Congenital heart disease |
7.38 (3.85–14.16) n=81 |
7.70 (2.90–20.43) n=29 |
3.49 (0.93–13.05) n=14 |
12.31 (4.46–33.96) n=38 |
1.00 (reference) n=6779 |
| Fluid and electrolyte disorders |
5.90 (4.65–7.49) n=661 |
6.66 (4.39–10.09) n=269 |
8.50 (6.03–11.96) n=291 |
2.46 (1.41–4.30) n=101 |
1.00 (reference) n=72 875 |
| Previous stroke |
4.77 (2.09–10.87) n=55 |
3.67 (0.96–14.03) n=20 |
n=0 |
14.09 (5.20–38.18) n=35 |
1.00 (reference) n=36 |
| Coagulopathy |
4.71 (3.80–5.84) n=639 |
3.92 (2.74–5.61) n=210 |
6.71 (4.83–9.31) n=300 |
3.45 (2.18–5.46) n=129 |
1.00 (reference) n=129 225 |
| Arrhythmia |
2.86 (1.94–4.21) n=203 |
2.58 (1.41–4.72) n=77 |
2.77 (1.50–5.14) n=67 |
3.39 (1.57–7.29) n=59 |
1.00 (reference) n=30 082 |
| Ischaemic heart disease |
2.84 (1.25–6.46) n=53 |
3.21 (0.84–12.26) n=29 |
2.61 (0.65–10.48) n=9 |
2.54 (0.58–11.09) n=15 |
1.00 (reference) n=3813 |
| Drug abuse |
1.99 (1.35–2.94) n=173 |
1.87 (0.92–3.79) n=63 |
1.94 (1.07–3.53) n=57 |
2.23 (1.09–4.54) n=53 |
1.00 (reference) n=72 451 |
| Sickle cell disease |
1.94 (0.68–5.50) n=23 |
6.81 (2.21–20.99) n=23 |
n=0 |
n=0 |
1.00 (reference) n=4237 |
| Peripartum cardiomyopathy |
1.89 (0.70–5.06) n=82 |
2.54 (0.64, 10.14) n=44 |
0.55 (0.03–9.95) n=9 |
2.30 (0.43, 12.23) n=29 |
1.00 (reference) n=11 016 |
| Heart failure |
1.66 (0.74–3.69) n=91 |
2.51 (0.79–7.94) n=49 |
0.48 (0.01–15.22) n=9 |
1.80 (0.51–6.40) n=33 |
1.00 (reference) n=12 287 |
| Renal failure |
1.36 (0.68–2.73) n=65 |
0.94 (0.26–3.44) n=20 |
0.89 (0.25–3.15) n=15 |
2.86 (0.99–8.25) n=30 |
1.00 (reference) n=13 558 |
| Rheumatoid arthritis/collagen vascular diseases |
1.23 (0.64–2.36) n=55 |
0.30 (0.04–2.27) n=5 |
0.96 (0.29–3.21) n=14 |
2.85 (1.25–6.51) n=36 |
1.00 (reference) n=20 761 |
| Depression |
1.02 (0.68–1.52) n=147 |
0.63 (0.29–1.38) n=35 |
1.12 (0.56–2.25) n=54 |
1.44 (0.77–2.69) n=58 |
1.00 (reference) n=117 362 |
| Obesity |
0.87 (0.67–1.13) n=342 |
1.01 (0.67–1.54) n=153 |
0.75 (0.47–1.20) n=98 |
0.82 (0.48–1.40) n=91 |
1.00 (reference) n=469 024 |
| Smoking |
0.87 (0.62–1.21) n=259 |
1.01 (0.63–1.64) n=111 |
0.78 (0.43–1.40) n=75 |
0.78 (0.41–1.46) n=73 |
1.00 (reference) n=286 838 |
| Valvular disease |
0.87 (0.39–1.94) n=69 |
0.33 (0.06–1.68) n=15 |
1.81 (0.62–5.28) n=26 |
1.03 (0.31–3.42) n=28 |
1.00 (reference) n=26 269 |
| Diabetes mellitus |
0.57 (0.38–0.86) n=152 |
0.54 (0.26–1.10) n=59 |
0.38 (0.18–0.82) n=34 |
0.85 (0.42–1.73) n=59 |
1.00 (reference) n=165 239 |
| Gestational diabetes mellitus |
0.49 (0.36–0.68) n=192 |
0.53 (0.32–0.88) n=73 |
0.38 (0.20–0.70) n=52 |
0.60 (0.34–1.06) n=67 |
1.00 (reference) n=446 145 |
| Alcohol abuse |
0.16 (0.02–1.51) n=5 |
n=0 |
n=0 |
0.63 (0.08–5.07) n=5 |
1.00 (reference) n=6355 |
Data expressed as odds ratios (OR) and 95% CIs. n indicates weighted number of cases.
An odds ratio could not be calculated because of lack of cases in subgroup.
We also examined the association of stroke with delivery complications (Table 2). This showed that all stroke was associated with a 100‐fold increase in risk of maternal mortality (OR, 99.78; 95% CI, 59.15–168.31), which was even greater in the case of hemorrhagic stroke (OR, 260.80; 95% CI, 138.10–492.51). There was an almost double risk of postpartum hemorrhage (OR, 1.91; 95% CI, 1.54–2.37) and 1.5‐fold risk for cesarean section (OR, 1.58; 95% CI, 1.33–1.86) for women with strokes. Over the 11‐year study period, there was no change in the association between stroke and delivery complications (Figure S2).
Table 2.
Association Between Subgroups of Stroke and Delivery Complications
| All stroke (n=3391) | Stroke | No Stroke (n=4 236 893) | |||
|---|---|---|---|---|---|
| Ischemic (n=1229) | Hemorrhagic (n=1187) | Unspecified (n=975) | |||
| OR (95% CI); n | |||||
| Maternal mortality |
99.78 (59.15–168.31) n=297 |
30.34 (12.32–74.73) n=59 |
260.80 (138.10–492.51) n=193 |
40.34 (14.16–114.87) n=45 |
1.00 (reference) n=847 |
| Postpartum hemorrhage |
1.91 (1.54–2.37) n=531 |
1.98 (1.38–2.83) n=194 |
2.03 (1.46–2.82) n=223 |
1.65 (1.01–2.68) n=114 |
1.00 (reference) n=205 913 |
| Stillbirth |
1.68 (1.00–2.82) n=96 |
0.93 (0.34–2.69) n=21 |
1.67 (0.70–4.00) n=35 |
2.84 (1.33–6.07) n=40 |
1.00 (reference) n=37 285 |
| Cesarean section |
1.58 (1.33–1.86) n=2084 |
1.62 (1.22–2.16) n=761 |
1.44 (1.08–1.91) n=708 |
1.71 (1.26–2.30) n=615 |
1.00 (reference) n=1 961 258 |
| Preterm birth |
1.22 (0.99–1.49) n=797 |
1.34 (0.98–1.82) n=303 |
1.25 (0.91–1.73) n=283 |
1.02 (0.68–1.54) n=211 |
1.00 (reference) n=637 229 |
Data expressed as odds ratios (OR) and 95% CIs. n indicates weighted number of cases.
Women with stroke had longer lengths of hospital stays (6 days; IQR, 3–10) compared with women without stroke (3 days; IQR, 2–4), with hemorrhagic stroke being associated with the longest duration (7 days; IQR, 3–12) out of all stroke subgroups (ischemic, hemorrhagic, unspecified) (Table S4). Similarly, the total charge was higher for women with stroke ($14 655; IQR, $8494–$27 895) compared with women without stroke ($4762; IQR, $3278–$7036), with the highest charge seen in hemorrhagic stroke ($20 532; IQR, $10 256–$41 042) (Table S4). No temporal changes were detected in the length of stay and total charge outcomes (Table S5). Additional sensitivity analyses on delivery complications and cost outcomes were conducted to examine for the effects of excluding records with missing data (Table S7). This showed no important changes in the ORs.
As a surrogate of disability following stroke, we examined the discharge locations following delivery hospitalizations (Table S4, Figure 3). Women with stroke (Figure 3B) had a higher proportion of discharges to facilities other than routine at own home compared with women without stroke (Figure 3A). The hemorrhagic stroke population (Figure 3D) had a greater proportion of discharges to short‐term hospitals and other care facilities, compared with ischemic (Figure 3C) and unspecified (Figure 3E) stroke populations. In contrast, there was a greater proportion of discharges to home care in women with ischemic (Figure 3C) and unspecified (Figure 3E) compared with hemorrhagic strokes (Figure 3D).
Figure 3.
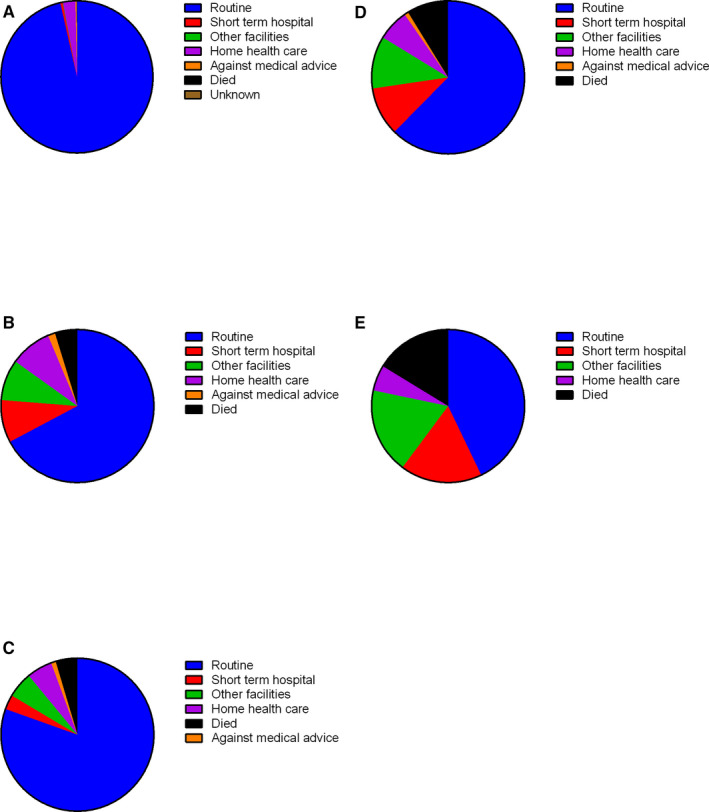
Discharge locations of women with hypertensive disorders of pregnancy and (A) no stroke, (B) any stroke, (C) ischemic stroke, (D) hemorrhagic stroke, or (E) unspecified stroke.
We also stratified the data according to subgroups of HDP (chronic hypertension, gestational hypertension, preeclampsia, and superimposed preeclampsia). Over half of the strokes (52.5%) occurred in women with preeclampsia (Table S8). We then reassessed the prognostic association of stroke risk factors with stroke and the delivery complications associated with strokes for each HDP subgroup (Table S9). The gestational hypertension subgroup had the highest increase in risk of maternal mortality and preterm birth following stroke, while for the preeclampsia group, the highest increase in risk occurred in postpartum hemorrhage and cesarean section.
Discussion
Our study is the first to consider the temporal trends of the clinical profile and delivery complications of women with HDP with stroke during hospital admissions for delivery, a time of increased stroke risk. Our analysis of over 4.2 million HDP delivery hospitalizations show that while the prevalence of HDP has increased over time, incident stroke rates have declined but remain important predictors of delivery complications, including mortality and cesarean section. Women with stroke are increasingly multimorbid with distinctive risk profiles for ischemic and hemorrhagic complications. Congenital heart disease, peripheral vascular disease, dyslipidemia, and sickle cell disease were more strongly associated with ischemic stroke compared with hemorrhagic stroke.
Previous studies have suggested the incidence of peripartum stroke is increasing over time but without considering the effects of concurrent increase in the incidence of HDP. We are the first to compare the temporal trends in the characteristics of the HDP population with its stroke subpopulation. Our analysis shows that the proportion of incident stroke within the HDP delivery population is actually decreasing. This finding is consistent with previous research showing a decline in overall stroke hospitalizations in the general population over time, which was more pronounced in women. 33 , 34 This may be attributable to improvements in CVD prevention efforts. However, this downward trend in stroke incidence is at risk of being lost because of other emerging patterns such as increasing sedentary lifestyle, substance abuse, and social isolation, as well as the obesity/metabolic syndrome epidemic.
We comprehensively assessed comorbidities using Elixhauser comorbidity measures and showed that an increasing proportion of women with multimorbidity with HDP suffer strokes, despite the reduction in overall incident stroke in HDP over time. However, this may simply reflect the general trend of more women with multimorbidity conceiving successfully. 22 There was an increase in HDP pregnancy admissions in women who reside in wealthier zip codes over the years, this may be related to increasing access to fertility treatments, as HDP is associated with fertility treatments. 35 , 36
There is limited literature on peripartum outcomes of women with HDP with stroke, as the majority have studied stroke in the wider pregnant population. 15 , 37 , 38 A previous study using the 1994–2011 NIS database examined hospitalizations with HDP and stroke during the whole pregnancy, rather than the delivery period that we have studied, and showed an event rate of 0.02% for stroke. 1 In contrast to our study, they found that stroke in HDP pregnancy hospitalizations increased over their study period. 1 This discrepancy may be attributable to the different study population. Furthermore, different clinical outcomes were examined, with ours focusing on delivery complications, while the other study assessed stroke‐related outcomes, such as mechanical ventilation, pneumonia, and seizure. 1 Other national studies on HDP delivery hospitalizations evaluated only women with preeclampsia without other HDP subgroups. 11 , 16 , 39 , 40 These focused on stroke risk factors and did not consider adverse outcomes following stroke. A regional study on HDP delivery hospitalizations in New York showed an incidence rate of 0.13% for stroke. 41 However, most of the analyses in this study included stroke only in a composite cardiovascular morbidity outcome.
Traditional stroke risk factors have been shown to increase the risk for any stroke in women with HDP during pregnancy. 1 We demonstrated that there are some risk factors that are more strongly associated with ischemic than hemorrhagic strokes. A previous study on the whole pregnancy period also identified sickle cell disease as a risk factor for ischemic stroke. 1 Consistent with our findings, diabetes mellitus was not found to increase the risk for pregnancy‐associated stroke in this study on HDP pregnancy hospitalizations. 1 Unique to our study is the fact that we are the first to stratify stroke predictors by specific HDP subgroups.
There are some possible mechanisms for the stroke predictors we identified. The most common mechanism of stroke associated with congenital heart disease is paradoxical embolism, where right‐to‐left shunts allow thromboemboli to reach into the arterial circulation without traversing the lungs. 42 Moreover, while pregnancy increases the risk of thromboembolism 6‐fold, this risk is further increased in women with HDP. 43 , 44 Both peripheral vascular disease and dyslipidemia increase the risk of atherosclerosis, which in turn causes ischemic stroke. In addition to the physiological hyperlipidemia of pregnancy, 45 women with dyslipidemia have increased risk of preeclampsia. 46 Therefore, women with HDP are a particularly high‐risk group for ischemic stroke. Women with sickle cell disease are at high risk for HDP and stroke. Moreover, the stress of delivery can precipitate vaso‐occlusive crisis, a cause of stroke in sickle cell disease. 47
We are the first to show that peripartum stroke is independently associated with increased odds of cesarean section and excessive in‐hospital mortality risk in the HDP population at 1.5‐ and 100‐fold, respectively. It is worth noting that pregnant women suffering stroke usually undergo cesarean sections. Comparable to our findings, prior research that examined stroke in pregnancy admissions showed a 1.8‐fold risk of postpartum hemorrhage. 13 It is possible that postpartum hemorrhage is caused by stroke treatment; however, as there are no data on chronicity in our data set, we can only speculate. In a longer‐term setting, the adjusted incident rate ratio for death from stroke for preeclampsia/eclampsia was 3.59 (95% CI, 1.04–12.4). 48
Stroke is often misdiagnosed in pregnant women as representing more benign conditions, such as migraines or seizures. 49 Compounded with clinicians’ general reluctance to give medication and/or perform nonobstetric surgery in pregnant women, these patients may miss the chance to receive timely effective treatment. Prior research has shown that even for minor symptoms of nausea and vomiting, general practitioners were reluctant to start antiemetic treatment in pregnancy unless the symptoms have progressed to a severe stage. 50 Therefore, clinicians should be encouraged to actively investigate and treat pregnant women with features suggestive of stroke, especially in the high‐risk HDP population.
The strengths of this study include the large number of HDP hospital admission episodes, the comprehensive capture of delivery hospitalizations, and the diversity of the HDP population in terms of geography and race or ethnicity. This allows us to have statistical power to examine disease patterns of rare events such as stroke. With 3391 stroke events in our population, we were also able to examine the temporal trends in the prevalence, comorbidities, and associated delivery complications.
A limitation of our study is that our results are national estimates based on sampling weights. As the unweighted events for stroke subtype are low, some of the calculated ORs in the subgroup analyses have wide CIs. Another limitation is the lack of information relevant to patient prognosis after stroke, for example, time to diagnosis, imaging modality, and pharmacotherapy. However, other available data could provide further information. For example, the arrhythmia comorbidity may be a surrogate for warfarin use. We captured data only on women admitted to the hospital without considering stroke in the community. Similarly, we have not captured births in the community. However, US national statistics show that >98% of births occur in hospitals. 51 Because of the design of the NIS, we were unable to track patients over the years and could consider only in‐hospital outcomes. Therefore, one woman could have had multiple deliveries during the study period. As there was no information on timing of events, we could not conduct analyses on time to events, such as delivery complications, or effects of chronicity on comorbidities. In addition to mortality, we examined other delivery complications, such as cesarean section and postpartum hemorrhage, which could have contributed to the cause of mortality. However, we did not consider mortality as a competing risk for other delivery complications. Since we did not adjust for multiple testing, some of the statistically significant results may be attributable to chance. Errors may arise from inaccurate physician and administrative reporting of ICD codes. Furthermore, chronic conditions are usually undercoded in administrative data sets, with low to moderate sensitivity for the majority of conditions. Finally, for the temporal analyses, the accuracy may have improved over time because of improved diagnosis or better coding from changes in guidelines or incentives.
In conclusion, our analysis showed that over a decade, the incidence of stroke reduced in an increasingly complex HDP delivery population. Women with HDP with ischemic versus hemorrhagic strokes had moderately different clinical profiles whereby some stroke predictors were more strongly associated with ischemic than hemorrhagic strokes and therefore represent underlying differences in the populations at risk. The assessment of women with HDP for their risk of ischemic or hemorrhagic stroke on admission for delivery is needed so that measures such as closer blood pressure monitoring may be instigated to improve their intrapartum care.
Sources of Funding
Dr Wu is funded by a National Institute for Health Research (NIHR) Transitional Research Fellowship (TRF‐2017‐10‐005). Dr Chew‐Graham is part‐funded by West Midlands ARC. Dr Chappell is funded by a NIHR Professorship (RP‐2014‐05‐019). This paper presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The funders had no involvement in the conduct of this research.
Disclosures
None.
Supporting information
Data S1
Tables S1–S9
Figures S1–S2
(J Am Heart Assoc. 2020;9:e016182 DOI: 10.1161/JAHA.120.016182.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy‐related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grear KE, Bushnell CD. Stroke and pregnancy: clinical presentation, evaluation, treatment, and epidemiology. Clin Obstet Gynecol. 2013;56:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McClure JH, Cooper GM, Clutton‐Brock TH. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–8: a review. Br J Anaesth. 2011;107:127–132. [DOI] [PubMed] [Google Scholar]
- 4. Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 5. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192. [DOI] [PubMed] [Google Scholar]
- 6. ACOG . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 7. Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199:133.e1–133.e8. [DOI] [PubMed] [Google Scholar]
- 8. Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke. 1995;26:930–936. [DOI] [PubMed] [Google Scholar]
- 9. Treadwell SD, Thanvi B, Robinson TG. Stroke in pregnancy and the puerperium. Postgrad Med J. 2008;84:238–245. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Pregnancy mortality surveillance system: causes of pregnancy‐related death in the United States: 2011–2016. Available at: https://www.cdc.gov/reproductivehealth/maternal‐mortality/pregnancy‐mortality‐surveillance‐system.htm. Accessed May 5, 2020.
- 11. Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Marshall RS, Elkind MSV, Willey JZ. Risk factors for pregnancy‐associated stroke in women with preeclampsia. Stroke. 2017;48:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu S, Chan WS, Ray JG, Kramer MS, Joseph KS. Stroke and cerebrovascular disease in pregnancy. Stroke. 2019;50:13–20. [Google Scholar]
- 13. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516. [DOI] [PubMed] [Google Scholar]
- 14. Salonen Ros H, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology. 2001;12:456–460. [DOI] [PubMed] [Google Scholar]
- 15. Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy‐related stroke: a systematic review and meta‐analysis. Int J Stroke. 2017;12:687–697. [DOI] [PubMed] [Google Scholar]
- 16. Miller EC, Gallo M, Kulick ER, Friedman AM, Elkind MSV, Boehme AK. Infections and risk of peripartum stroke during delivery admissions. Stroke. 2018;49:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Healthcare Cost and Utilization Project . National Inpatient Sample. Available at: https://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed June 17, 2020.
- 18. Kuklina EV, Whiteman MK, Hillis SD, Jamieson JD, Meikle SF, Posner SF, Marchbanks PA. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–477. [DOI] [PubMed] [Google Scholar]
- 19. Lima FV, Parikh PB, Zhu J, Yang J, Stergiopoulos K. Association of cardiomyopathy with adverse cardiac events in pregnant women at the time of delivery. JACC Heart Fail. 2015;3:257–266. [DOI] [PubMed] [Google Scholar]
- 20. Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, Macones GA, Sibai BM, Jena AB. Short‐term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217:237–248.e16. [DOI] [PubMed] [Google Scholar]
- 21. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–134.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fingar K, Mabry‐Hernandez I, Ngo‐Metzger Q, Wolff T, Steiner C, Elixhauser A. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. HCUP Statistical Brief #222. 2017. [PubMed]
- 23. Kuklina EV, Callaghan WM. Cardiomyopathy and other myocardial disorders among hospitalizations for pregnancy in the United States: 2004–2006. Obstet Gynecol. 2010;115:93–100. [DOI] [PubMed] [Google Scholar]
- 24. Collins RT II, Chang D, Sandlin A, Goudie A, Robbins JM. National in‐hospital outcomes of pregnancy in women with single ventricle congenital heart disease. Am J Cardiol. 2017;119:1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidya VR, Arora S, Patel N, Badheka AO, Patel N, Agnihotri K, Billimoria Z, Turakhia MP, Friedman PA, Madhavan M, et al. Burden of arrhythmia in pregnancy. Circulation. 2017;135:619–621. [DOI] [PubMed] [Google Scholar]
- 26. Zhong QY, Gelaye B, Smoller JW, Avillach P, Cai T, Williams MA. Adverse obstetric outcomes during delivery hospitalizations complicated by suicidal behavior among US pregnant women. PLoS One. 2018;13:e0192943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL, Briller JE. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail. 2018;11:e004005. [DOI] [PubMed] [Google Scholar]
- 28. Rougerie M, Czuzoj‐Shulman N, Abenhaim HA. Diabetic ketoacidosis among pregnant and non‐pregnant women: a comparison of morbidity and mortality. J Matern Fetal Neonatal Med. 2018;40:1–4. [DOI] [PubMed] [Google Scholar]
- 29. Agarwal MA, Garg L, Lavie CJ, Reed GL, Khouzam RN. Impact of family history of coronary artery disease on in‐hospital clinical outcomes in ST‐segment myocardial infarction. Ann Transl Med. 2018;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smilowitz NR, Gupta N, Guo Y, Beckman JA, Bangalore S, Berger JS. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non‐cardiac surgery. Heart. 2018;104:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318:2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) Statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramirez L, Kim‐Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ, Towfighi A. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc. 2016;5:e003233 DOI: 10.1161/JAHA.116.003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tong X, George MG, Gillespie C, Merritt R. Trends in hospitalizations and cost associated with stroke by age, United States 2003–2012. Int J Stroke. 2016;11:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, Archontakis S, Argyri O, Tsioufis C, Makris TK, et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta‐analysis. J Clin Hypertens (Greenwich). 2017;19:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta‐analysis. Hum Reprod Update. 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- 37. Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42:2564–2570. [DOI] [PubMed] [Google Scholar]
- 38. Miller EC, Leffert L. Stroke in pregnancy: a focused update. Anesth Analg. 2020;130:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, Chu PH. Preeclampsia‐eclampsia and the risk of stroke among peripartum in Taiwan. Stroke. 2009;40:1162–1168. [DOI] [PubMed] [Google Scholar]
- 40. Park Y, Cho GJ, Kim LY, Lee TS, Oh MJ, Kim YH. Preeclampsia increases the incidence of postpartum cerebrovascular disease in Korean population. J Korean Med Sci. 2018;33:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ackerman CM, Platner MH, Spatz ES, Illuzzi JL, Xu X, Campbell KH, Smith GN, Paidas MJ, Lipkind HS. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. Am J Obstet Gynecol. 2019;220:582.e1–582.e11. [DOI] [PubMed] [Google Scholar]
- 42. Attar H, Sachdeva A, Sundararajan S. Cardioembolic stroke in adults with a history of congenital heart disease. Stroke. 2016;47:e79–e81. [DOI] [PubMed] [Google Scholar]
- 43. Kane EV, Calderwood C, Dobbie R, Morris C, Roman E, Greer IA. A population‐based study of venous thrombosis in pregnancy in Scotland 1980–2005. Eur J Obstet Gynecol Reprod Biol. 2013;169:223–229. [DOI] [PubMed] [Google Scholar]
- 44. Won HS, Kim DY, Yang MS, Lee SJ, Shin HH, Park JB. Pregnancy‐induced hypertension, but not gestational diabetes mellitus, is a risk factor for venous thromboembolism in pregnancy. Korean Circ J. 2011;41:23–27. Available at: https://www.endotext.org/chapter/effect‐of‐pregnancy‐on‐lipid‐metabolism‐and‐lipoprotein‐levels. Accessed June 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grimes SB and Wild R. Effect of pregnancy on lipid metabolism and lipoprotein levels In: Feingold KR, et al., eds. Endotext. South Darthmouth: MDText.com Inc; 2018. Available at: https://www.endotext.org/chapter/effect‐of‐pregnancy‐on‐lipid‐metabolism‐and‐lipoprotein‐levels. Accessed June 17, 2020. [Google Scholar]
- 46. Baumfeld Y, Novack L, Wiznitzer A, Sheiner E, Henkin Y, Sherf M, Novack V. Pre‐conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 2015;10:e0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yale SH, Nagib N, Guthrie T. Approach to the vaso‐occlusive crisis in adults with sickle cell disease. Am Fam Physician. 2000;61:1349–1356, 1363–1364. [PubMed] [Google Scholar]
- 48. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuruvilla A, Bhattacharya P, Rajamani K, Chaturvedi S. Factors associated with misdiagnosis of acute stroke in young adults. J Stroke Cerebrovasc Dis. 2011;20:523–527. [DOI] [PubMed] [Google Scholar]
- 50. Heitmann K, Svendsen HC, Sporsheim IH, Holst L. Nausea in pregnancy: attitudes among pregnant women and general practitioners on treatment and pregnancy care. Scand J Prim Health. 2016;34:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacDorman MF, Matthews TJ, Declercq E. Trends in Out‐of‐Hospital Births in the United States, 1990–2012. NCHS Data Brief, No 144. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S9
Figures S1–S2


