Abstract
BACKGROUND
The mechanisms underlying the effect of preconditioning on remote microvasculature remains undisclosed. The primary objective was to document the remote effect of ischemic preconditioning on microvascular function in humans. The secondary objective was to test if exercise also induces remote microvascular effects.
METHODS AND RESULTS
A total of 12 healthy young men and women participated in 2 experimental days in a random counterbalanced order. On one day the participants underwent 4×5 minutes of forearm ischemic preconditioning, and on the other day they completed 4×5 minutes of hand‐grip exercise. On both days, catheters were placed in the brachial and femoral artery and vein for infusion of acetylcholine, sodium nitroprusside, and epoprostenol. Vascular conductance was calculated from blood flow measurements with ultrasound Doppler and arterial and venous blood pressures. Ischemic preconditioning enhanced (P<0.05) the remote vasodilator response to intra‐arterial acetylcholine in the leg at 5 and 90 minutes after application. The enhanced response was associated with a 6‐fold increase (P<0.05) in femoral venous plasma prostacyclin levels and with a transient increase (P<0.05) in arterial plasma levels of brain‐derived neurotrophic factor and vascular endothelial growth factor. In contrast, hand‐grip exercise did not influence remote microvascular function.
CONCLUSIONS
These findings demonstrate that ischemic preconditioning of the forearm improves remote microvascular endothelial function and suggest that one of the underlying mechanisms is a humoral‐mediated potentiation of prostacyclin formation.
Keywords: ischemic preconditioning, microvascular endothelial function, platelets, prostacyclin, vasodilation
Subject Categories: Basic Science Research, Vascular Biology, Endothelium/Vascular Type/Nitric Oxide
Nonstandard Abbreviations and Acronyms
- BDNF
brain‐derived neutrophic factor
- 6‐ketoPGF1α
6‐keto prostaglandin F1α
- VEGF
vascular endothelial growth factor
CLINICAL PERSPECTIVE
What Is New?
This is the first study to explore the acute effect of ischemic preconditioning on remote microcirculation in humans.
We demonstrate that the vasodilator response to intra‐arterial acetylcholine is enhanced in the leg after ischemic preconditioning applied at the arm.
The enhanced vasodilatory responsiveness to acetylcholine in remote vascular tissue is associated with increased prostacyclin production.
What Are the Clinical Implications?
The findings suggest that ischemic preconditioning may be an effective treatment strategy to improve remote microvascular endothelial function and endogenous prostacyclin production, which are essential steps in the prevention of cardiovascular morbidity and mortality.
Repeated bouts of ischemia followed by reperfusion, known as ischemic preconditioning, is a procedure that originally was shown to reduce myocardial infarct size in animals 1 and subsequently also to protect against vascular injury. 2 The protective effect was demonstrated to be remote, that is, effective also in organs not directly exposed to ischemia. 3 , 4 In addition, ischemic preconditioning has been reported to induce improved vascular endothelial function of remote conduit arteries in human studies. 5 , 6 These findings suggest a systemic vascular effect of ischemic preconditioning, which could be related to either neural or humoral signaling. 7 Interestingly, a single session of exercise has recently also been shown to induce a remote cardioprotective effect, 8 , 9 similar to that of ischemic preconditioning, but whether exercise can lead to improved remote microvascular function remains unclear.
Although the abovementioned effects of remote ischemic preconditioning are likely to be of important clinical relevance, 10 , 11 there are several critical aspects that remain to be resolved. First, it is unknown whether ischemic preconditioning improves not only macrovascular but also microvascular function, and thereby the control of vascular resistance. Second, the mechanism by which ischemic preconditioning induces its beneficial effects on the vasculature remains to be identified. Further knowledge of mechanisms would bring us closer to the development of a pharmacological intervention that can simulate or potentiate the effect. Third, it is unknown whether an acute exercise session can induce a beneficial effect on the remote microvasculature. This knowledge would provide further insight into the mechanisms of preconditioning. In this study, we address these 3 aspects.
The observation that vascular protection can be transferred through blood from a preconditioned animal to a naïve animal emphasizes that remote ischemic preconditioning is mediated through humoral signaling via plasma or cells such as platelets. 12 Platelets are likely candidates for conveying signaling molecules to remote parts of the vasculature as they can take up and release compounds where needed. 13 , 14 In animal studies, platelets have been found to carry and release serotonin, BDNF (brain‐derived neurotrophic factor) , and VEGF (vascular endothelial growth factor), which are all compounds that can modulate vascular tone by promoting the formation of vasodilators, including prostacyclin, in vascular endothelium. 15 , 16 , 17 , 18 , 19 Combined with our findings that prostacyclin is an important regulator of vascular tone in humans 20 , 21 and with findings from animal models showing that acute ischemic preconditioning improves endogenous formation of prostaglandins, 22 , 23 these data suggest that preconditioning could in part be mediated through this pathway.
The primary objective of the present study was to document the remote effect of ischemic preconditioning on microvascular endothelial function in humans as assessed by vasodilatory responsiveness to intra‐arterial acetylcholine. Moreover, we aimed to elucidate if endogenous formation of prostacyclin was involved in the microvascular response to ischemic preconditioning. A secondary objective was to assess whether a remote microvascular effect could be achieved by exercise preconditioning. We hypothesized that forearm ischemic preconditioning would improve remote microvascular function as determined in the leg and that this effect would be mediated via enhanced endogenous prostacyclin formation. In addition, it was hypothesized that a session of hand‐grip exercise would improve remote microvascular function.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Participants
A total of 12 healthy young participants (7 men, 5 women) were included in the study (Table S1). All participants were nonsmokers, showed no signs of cardiovascular diseases, and were not taking any chronic medications. The study was approved by the Ethics Committee of the Capital Region of Copenhagen (H‐18000977), and all procedures were carried out in accordance with the latest guidelines of the Declaration of Helsinki. Written and oral informed consent was obtained from all participants before final enrollment.
Experimental Design
Study Overview
The experimental design included 2 separate visits to the laboratory in randomized order, where the subjects underwent either (1) ischemic preconditioning or (2) hand‐grip exercise (Figure 1). Measurements of microvascular function were conducted, and blood samples were drawn, simultaneously in 1 arm and 1 leg.
Figure 1. Experimental protocol.
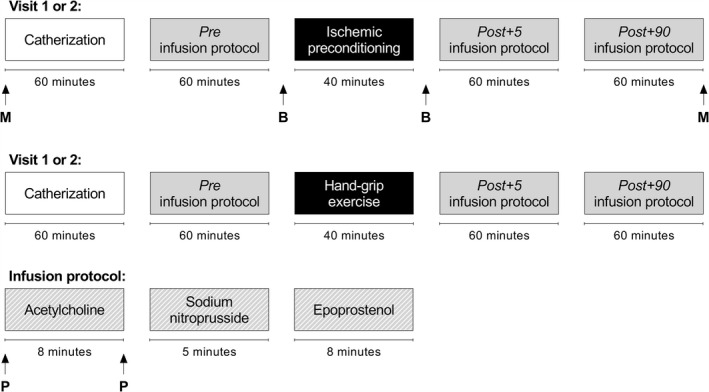
Vertical arrows refer to sampling of muscle biopsies for gene expression analysis (M), arterial blood for isolation of platelets and platelet‐free plasma (B), and venous plasma for analysis of vasoactive compounds (P).
To address the primary aim (to elucidate whether ischemic preconditioning induces a remote effect on microvascular function), the vasodilator response to acetylcholine was determined in the leg at baseline and after ischemic preconditioning of the arm. Measurements in the arm were conducted simultaneously to determine the local effect.
To explore the potential mechanisms underlying the effect of the intervention, plasma prostacyclin levels were determined in blood samples obtained before and after acetylcholine infusion given that acetylcholine stimulates prostacyclin formation.
To further evaluate mechanisms underlying changes in microvascular function, serotonin, BDNF, and VEGF were determined in platelets and platelet‐free plasma obtained before and after ischemic conditioning. In addition, skeletal muscle biopsies were obtained from the leg to assess a potential influence of ischemic preconditioning on the expression of vascular proteins and to assess whether changes related to microvascular function occurred at the transcriptional level.
To address the secondary aim (to determine the effect of hand‐grip exercise on remote microvascular function for comparison with that of the ischemic intervention), the same experimental protocol as for ischemic preconditioning was conducted with hand‐grip exercise.
Intervention
Participants were instructed to refrain from physical activity as well as caffeine and alcohol intake for 24 hours before the experiments and to arrive in the laboratory at 8 am after a light breakfast (similar between visits).
The ischemic preconditioning protocol consisted of 4 repetitive bouts of forearm occlusion, which were accomplished by rapidly inflating a pneumatic cuff (E20, D.E. Hokanson Inc., Bellevue, WA) to 220 mm Hg for 5 minutes, followed by rapid deflation and 5 minutes of rest. The hand‐grip exercise protocol was conducted as 4 intermittent bouts, where participants performed 5 minutes of dynamic hand‐grip exercise (SH5001, Saehan Corp., Masan, South Korea) with 30 contractions per minute, followed by 5 minutes of rest. The workload was based on the participants' maximal voluntary isometric contraction and set to ensure that the participants were barely able to complete the 150 contractions in 1 bout. All sessions of ischemic preconditioning and hand‐grip exercise were supervised by the same person, ensuring consistency between experiments.
Experimental Protocol
Under aseptic conditions and local anesthesia (lidocaine, 20 mg mL−1; Astra Zeneca, Cambridge, UK), catheters (20G, Arrow International, Reading, PA) were inserted in the femoral artery and vein 2 to 4 cm below the inguinal ligament of the experimental leg. Under guidance of ultrasound, the tip of the catheter was placed in same position in the common femoral artery. A catheter was inserted in the brachial artery (20G, arterial cannula; Becton, Dickinson and Company, Sandy, UT) and in the basilic vein (18G, peripheral catheter; Becton, Dickinson and Company) of the experimental arm. A 3‐way cock was placed on the arterial line so that infusions and blood pressure measurements could be performed simultaneously. In the supine position with the experimental arm extended laterally, participants received simultaneous intra‐arterial (brachial and femoral) infusion of acetylcholine (10 and 25 µg min−1 L tissue volume−1; miochol‐E, Bausch & Lomb Inc., Bridgewater, NJ), sodium nitroprusside (3 µg min−1 L tissue volume−1; Hospira Inc., Lake Forest, IL), and epoprostenol (25 and 50 µg min−1 L tissue volume−1; Flolan, GSK Pharma, Brentford, UK).
On the first visit in the laboratory, leg and forearm tissue volumes were estimated by anthropometric measures. The infusion doses were determined based on these measures. Infusions were separated by at least 15 minutes, and each dose was infused for ≈3 minutes. Measurements (blood flow and blood pressure) were performed in the last minute of respective infusion.
The entire infusion protocol was performed before (pre), 5 minutes after (post+5), and 90 minutes after (post+90) ischemic preconditioning or hand‐grip exercise (Figure 1).
Venous blood samples were collected at rest and during the highest dose of acetylcholine at pre and post+5. On the experimental day involving ischemic preconditioning, arterial blood for the isolation of platelets and platelet‐free plasma was collected before (pre), in the arm immediately after (post arm) and in the leg 10 minutes after (post leg) the last cuff deflation.
In addition, skeletal muscle biopsies were obtained from the m. vastus lateralis before (pre) and 2.5 hours after (post) ischemic preconditioning (Figure 1).
Blood and Tissue Sampling
Venous Plasma
Venous blood was drawn in EDTA anticoagulated tubes and immediately centrifuged (5 minutes at 4000g and 4°C), and aliquots of plasma were stored at −80°C until later analysis.
Platelets and Platelet‐Free Plasma
Arterial blood was drawn in citrate‐anticoagulated tubes and kept at room temperature until centrifuged (10 minutes at 200g and 20°C) to obtain platelet‐rich plasma. The platelet‐rich plasma was layered on top of a gradient density medium (iodixanol solution, OptiPrep, Sigma‐Aldrich, St. Louis, MO) and centrifuged (15 minutes at 450g and 20°C), after which a fraction of isolated platelets and platelet‐poor plasma was collected. To obtain plasma completely free of platelets, the platelet‐poor plasma was centrifuged (15 minutes at 450g and 20°C) once more on the density gradient medium, and a fraction of the plasma was collected as platelet‐free plasma. In all collected samples, platelets were counted using a hematology analyzer (XP‐300; Sysmex, Kobe, Japan). Isolated platelets were lysed in a fresh batch buffer (Meso Scale Diagnostics Tris lysis buffer, Meso Scale Diagnostics, Rockville, MD) on ice before being centrifuged (10 minutes at 10 000g and 4°C), and the supernatant was kept as platelet lysate. All samples were stored at −80°C until later analysis.
Skeletal Muscle Biopsies
Under local anesthesia (lidocaine, 20 mg mL−1), skeletal muscle biopsies were obtained using the percutaneous needle biopsy technique with suction. 24 The biopsies were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Measurements
Hemodynamics
Arterial blood flow (leg, femoral arterial blood flow; forearm, brachial arterial blood flow) was measured with ultrasound Doppler (Vivid E9, GE Healthcare, Chicago, IL) equipped with a linear probe (L9) operating at an imaging frequency of 4/8 MHz and a Doppler frequency of 4.2 MHz. In the femoral artery, the site of measurement was distal to the inguinal ligament but above the bifurcation into the superficial and profound femoral branch to avoid turbulence from the bifurcation. For measurements of brachial arterial flow, the probe was positioned approximately half way up the upper arm. All recordings were obtained at the lowest possible insonation angle but always below 60°. Sample volume was maximized by choosing the widest section of the artery, and recordings were made without interference of the vascular wall. A low‐velocity filter (<1.8 m s−1) rejected noise caused by turbulence at the vascular wall. Doppler traces and B‐mode images were recorded continuously and averaged over ≈30 seconds. Arterial diameter was assessed during systole from B‐mode images for each Doppler recording. Intra‐arterial and venous blood pressure and heart rate were monitored with transducers (Pressure Monitoring Set, Edwards Lifesciences, Irvine, CA) positioned at the level of the heart. Vascular conductance was calculated by use of the following formula:
Vasoactive Compounds in Venous Plasma
Plasma levels of the stable metabolite of prostacyclin, 6‐keto prostaglandin F1α (PGF1α), and norepinephrine were measured with enzyme immunoassay kits (6‐keto PGF1α, Cayman Chemical Co., Ann Arbor, MI; norepinephrine; LDN, Nordhorn, Germany) in accordance with the manufacturer's instructions.
Serotonin, VEGF, and BDNF in Platelets and Platelet‐Free Plasma
The content of serotonin in platelets and platelet‐free plasma was measured with an enzyme immunoassay kit (IBL International, Hamburg, Germany) in accordance with the manufacturer's instructions, and VEGF and BDNF content were measured with an electrochemiluminescent assay kit (U‐PLEX platform, Meso Scale Diagnostics).
Gene Expressions in Skeletal Muscle Biopsies
Total RNA was isolated from the muscle biopsies using a TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instructions. First‐strand cDNA was synthesized from 1 μg of total RNA by SuperScript II reverse transcriptase (Invitrogen) as described previously. 25 The mRNA content of cyclooxygenase 1 (Hs00377726_m1), prostacyclin synthase (Hs00153133_m1), and endothelial NO synthase (Hs00167166_m1) was determined by real‐time reverse transcription polymerase chain reaction (ABI PRISM 7900 Sequence Detection System, Applied Biosystems, Foster City, CA). The cDNAs were amplified using the TaqMan gene expression assays from Applied Biosystems. For each sample, the amount of target gene mRNA was normalized to GAPDH mRNA content. The effect of the interventions on the level of GAPDH mRNA was determined statistically, and no significant effect was found with ischemic preconditioning.
Statistical Analysis
A priori sample size determination was performed based on previous data for the primary outcome, the difference in vasodilatory responsiveness to intra‐arterial acetylcholine with ischemic preconditioning. 26 Significance level was set to P<0.05 at a power level of 0.8. To detect a significant difference of 5.0±5.0 mL min−1 mm Hg−1 in vascular conductance with acetylcholine infusion, 12 participants were needed. Statistical analyses were performed with R (version 3.4.1, R Foundation for Statistical Computing, Vienna, Austria) using the interface RStudio (version 1.1.463, RStudio Team, Boston, MA).
To estimate limb and intervention‐specific differences in the vasodilator response to intra‐arterial infusions, a linear mixed model approach 27 was used with “time” (pre, post+5, post+90) and “infusion dose” (dose1, dose2) as fixed factors. Likewise, a linear mixed model was used for differences in baseline hemodynamics, venous plasma norepinephrine, and 6‐keto PGF1α levels and platelet and platelet‐free plasma levels of serotonin, VEGF, and BDNF with “time” (pre, post+5, post+90) as the fixed factor. For all analysis, participants were specified as a repeated factor and identifier of random variation. Model checking as well as homogeneity of variance and normal distribution were confirmed through residual and Q‐Q plots. To control for type I error regarding multiple statistical testing on the response to intra‐arterial infusions and venous plasma prostacyclin levels, a Bonferroni correction was applied within the family of statistical tests by multiplying the P value with the number of statistical tests performed (e.g., the vasodilator response to intra‐arterial acetylcholine, which was analyzed in 4 separate models). If significant main effects or interactions were observed, the Tukey honestly significant difference post hoc procedure was used to detect all pairwise differences performed with multiple comparisons, 28 and adjusted P values are reported. A paired Student t test was used to estimate differences in mRNA levels with ischemic preconditioning.
Two participants (1 man and 1 woman) failed to attend their second visit but completed the initial visit. Consequently, the data presented for all measurements before and after the hand‐grip exercise include only 10 of the 12 participants. Furthermore, because of technical difficulties with sample preparation, data presented for changes in mRNA levels with ischemic preconditioning and data presented for platelet and platelet‐free plasma levels include 11 of the 12 participants (clarified in figure labels). The influence of sex and timing of the visits were initially evaluated statistically with a linear mixed model approach; however, no effect of either variable on “time” was found (absence of significant interaction). Data are presented as mean±SEM.
RESULTS
Effect of Ischemic Preconditioning on Local and Remote Microvascular Function
Baseline
Mean arterial blood pressure, blood flow, and vascular conductance in the femoral and brachial arteries were unaltered by ischemic preconditioning (Table S2).
Leg and Forearm Microvascular Endothelial Cell Responsiveness to Acetylcholine Infusion
In the leg, the vasodilator response to intra‐arterial acetylcholine was increased (P<0.05) at post+5 and post+90 compared with pre following ischemic preconditioning (Figure 2). In the forearm, no differences were detected in vasodilatory responsiveness to intra‐arterial acetylcholine after ischemic preconditioning. All changes in blood flow, vascular conductance, mean arterial blood pressure, and venous blood pressure for both extremities are presented in Table S3.
Figure 2. Leg and forearm vasodilator response to intra‐arterial acetylcholine and epoprostenol before and after ischemic preconditioning of the forearm.
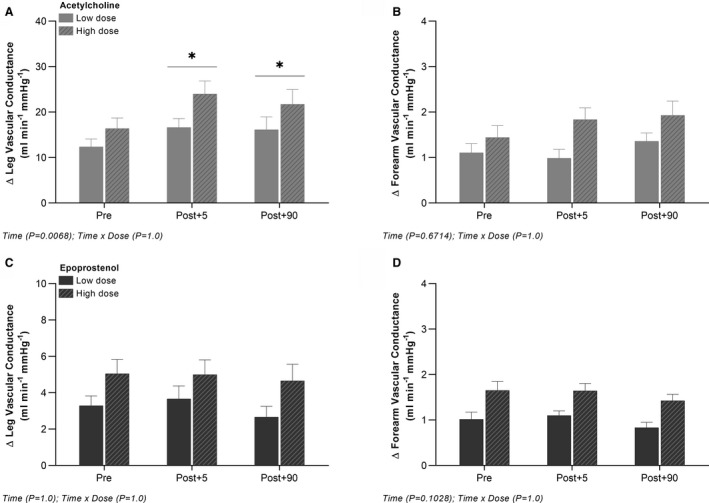
Absolute changes from baseline in leg and forearm vascular conductance with arterial infusion of acetylcholine (A and B) and epoprostenol (C and D). Data are presented as mean±SEM (n=12). *Significantly different from pre.
Leg and Forearm Microvascular Smooth Muscle Cell Responsiveness to Epoprostenol and Sodium Nitroprusside Infusions
In the leg and forearm, no differences were detected in vasodilatory responsiveness to intra‐arterial epoprostenol or sodium nitroprusside following ischemic preconditioning (Figure 2; Table S4). Baseline values and all changes in blood flow, vascular conductance, mean arterial blood pressure, and venous blood pressure for both extremities are presented in Tables S4 and S5.
Venous Plasma 6‐keto PGF1α and Norepinephrine Levels Before and After Ischemic Preconditioning
Baseline
In the leg, no differences were detected in venous plasma 6‐keto PGF1α or norepinephrine levels with ischemic preconditioning. In the forearm, venous plasma 6‐keto PGF1α levels were increased (P<0.05) at post+5 compared with pre, whereas venous plasma norepinephrine levels were increased (P<0.05) at post+5 and post+90 compared with pre following ischemic preconditioning (Table S2).
Changes in Venous Plasma 6‐keto PGF1α Levels With Acetylcholine Infusion
In the leg, the change in venous plasma 6‐keto PGF1α levels from baseline to the highest dose of acetylcholine was increased (P<0.05) at post+5 compared with pre following ischemic preconditioning, whereas no change was observed in the forearm venous plasma 6‐keto PGF1α level (Figure 3).
Figure 3. Changes in leg and forearm venous plasma 6‐keto PGF1α levels with intra‐arterial acetylcholine.
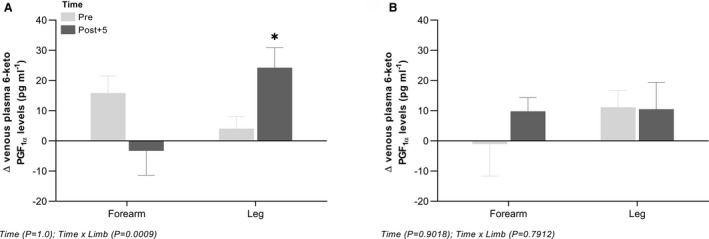
Absolute changes from baseline to the highest dose of acetylcholine in venous plasma levels of 6‐keto PGF1α before and after ischemic preconditioning of the forearm (n=12) (A) and hand‐grip exercise (n=10) (B). Data are presented as mean±SEM. *Significantly different from pre. 6‐keto PGF1α, 6‐keto prostaglandin F1α.
Effect of Ischemic Preconditioning on Serotonin, BDNF, and VEGF in Platelets and Platelet‐Free Plasma
In platelets, no differences were detected in either serotonin, BDNF, or VEGF with ischemic preconditioning. Platelet‐free plasma levels of BDNF and VEGF were increased (P<0.05) at post arm compared with pre and post leg, whereas serotonin levels were unaltered with ischemic preconditioning (Figure 4).
Figure 4. Platelet content and platelet‐free plasma levels of serotonin, VEGF, and BDNF before and after ischemic preconditioning of the forearm.
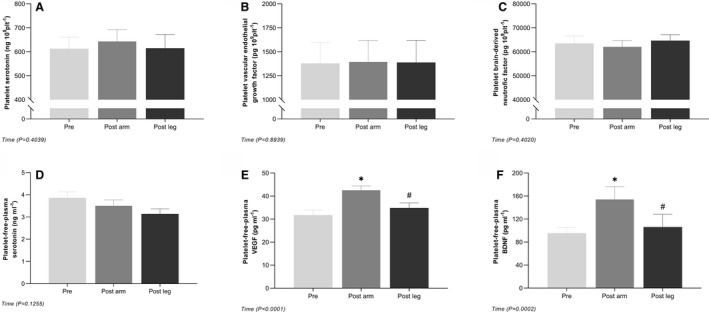
Platelet content (A through C) and platelet‐free plasma levels (D through F) of serotonin, VEGF, and BDNF. Data are presented as mean±SEM (n=11). *Significantly different from pre; #significantly different from post+5. BDNF, brain‐derived neurotrophic factor; and VEGF, vascular endothelial growth factor.
Skeletal Muscle mRNA Levels Before and After Ischemic Preconditioning
Endothelial NO synthase, prostacyclin synthase, and cyclooxygenase 1 mRNA levels in skeletal muscle were similar before and after ischemic preconditioning (Figure 5).
Figure 5. Skeletal muscle mRNA levels of eNOS, PGIS, and COX1 before and after ischemic preconditioning of the forearm.
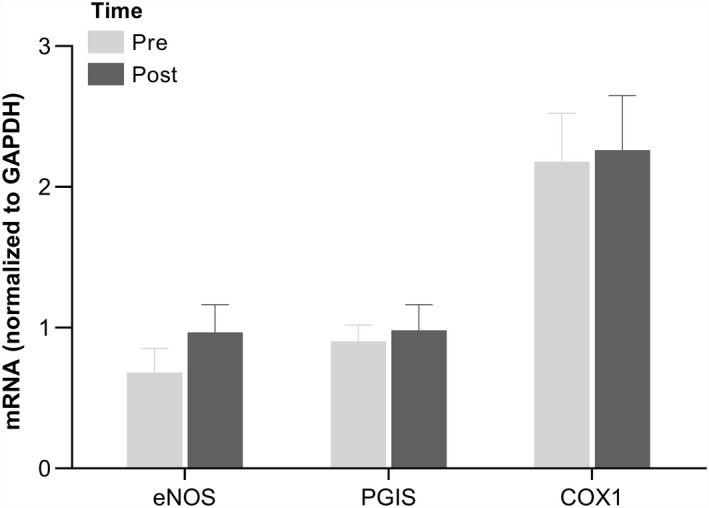
mRNA levels of eNOS, PGIS, and COX1 in skeletal muscle homogenates from m. vastus lateralis. Data are presented as mean±SEM (n=11). COX1, cyclooxygenase 1; eNOS, endothelial nitric oxide synthase; and PGIS, prostacyclin synthase.
Effect of Hand‐Grip Exercise on Local and Remote Microvascular Function
Baseline
Mean arterial blood pressure in the femoral and brachial arteries remained unaltered by hand‐grip exercise. In the leg, blood flow and vascular conductance were similar after hand‐grip exercise, whereas in the forearm blood flow and vascular conductance were increased (P<0.05) at post+5 compared with pre and post+90 (Table S2).
Leg and Forearm Microvascular Endothelial Cell Responsiveness to Acetylcholine Infusion
In the leg, no differences were detected in vasodilatory responsiveness to intra‐arterial acetylcholine with hand‐grip exercise. In the forearm, the vasodilator response to intra‐arterial acetylcholine was increased (P<0.05) at post+5 and post+90 compared with pre following hand‐grip exercise (Figure 6). All changes in blood flow, vascular conductance, mean arterial blood pressure, and venous blood pressure for both extremities are presented in Table S3.
Figure 6. Leg and forearm vasodilator response to intra‐arterial acetylcholine and epoprostenol before and after hand‐grip exercise.
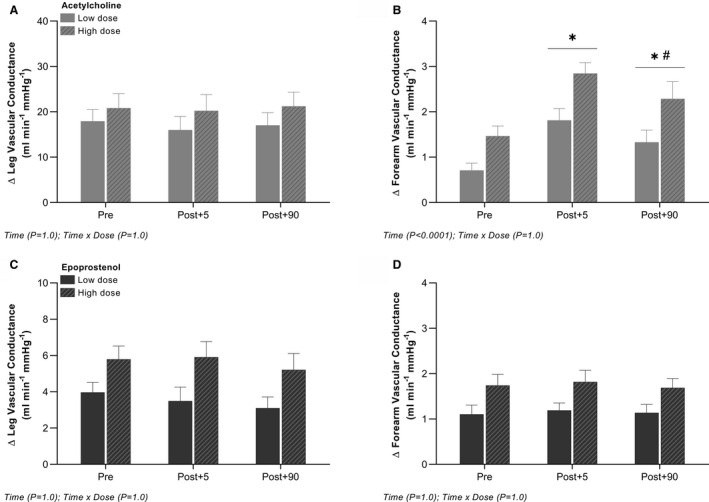
Absolute changes from baseline in leg and forearm vascular conductance with arterial infusion of acetylcholine (A and B) and epoprostenol (C and D). Data are presented as mean±SEM (n=10). *Significantly different from pre; #significantly different from post+5.
Leg and Forearm Microvascular Smooth Muscle Cell Responsiveness to Epoprostenol and Sodium Nitroprusside Infusions
In the leg and forearm, no differences were detected in vasodilatory responsiveness to intra‐arterial epoprostenol or sodium nitroprusside with hand‐grip exercise (Figure 6; Table S4). Baseline values and all changes in blood flow, vascular conductance, mean arterial blood pressure, and venous blood pressure for both extremities are presented in Tables S4 and S5.
Venous Plasma 6‐keto PGF1α and Norepinephrine Levels Before and After Hand‐Grip Exercise
Baseline
In the leg and forearm, no differences were detected in venous plasma 6‐keto PGF1α or norepinephrine levels with hand‐grip exercise (Table S2).
Changes in Venous Plasma 6‐keto PGF1α Levels With Acetylcholine Infusion
In the leg and forearm, no differences were detected in changes in venous plasma 6‐keto PGF1α levels from baseline to the highest dose of acetylcholine with hand‐grip exercise (Figure 3).
DISCUSSION
The principal finding of this study was that an acute bout of ischemic preconditioning performed on the arm induced a sustained improvement in microvascular endothelial function in the leg, that is, a remote effect, as indicated by an enhanced vasodilator response to arterial acetylcholine infusion. The enhanced acetylcholine induced response in leg vascular conductance was paralleled by a greater increase in venous plasma prostacyclin levels, suggesting that the increased sensitivity to acetylcholine was related to enhanced formation of prostacyclin. The enhancement in prostacyclin levels was in turn associated with transiently increased platelet‐free plasma levels of VEGF and BDNF, compounds known to stimulate endothelial prostacyclin formation. Our findings demonstrate, for the first time in humans, that ischemic preconditioning leads to improved remote microvascular endothelial function, an effect that may be related to increased prostacyclin production. Hand‐grip exercise did not significantly alter remote microvascular endothelial function. This finding indicates a different mechanistic effect of exercise versus ischemic preconditioning and underlines the unique effect of ischemic preconditioning for promoting remote vascular protection.
Effect of Ischemic Preconditioning on Remote Microvascular Function
Remote ischemic preconditioning was originally shown to prevent vascular damage associated with prolonged tissue ischemia, 4 indicating that the procedure may induce a systemic stimulus leading to functional adaptations in vascular endothelium. We demonstrate that acute exposure to ischemic preconditioning of the arm enhances the acetylcholine‐induced change in leg vascular conductance. The effect was present within 5 minutes after the procedure and persisted for at least 90 minutes. The enhanced vasodilator response was not paralleled by changes in smooth muscle cell sensitivity, as indicated by an unaltered responsiveness to the NO donor, sodium nitroprusside, and the prostacyclin analog, epoprostenol, emphasizing that the improvement was localized to the endothelium. In support of an improvement at the endothelial level, the acetylcholine‐induced increase in plasma prostacyclin levels was higher in the leg after ischemic preconditioning, suggesting that the procedure potentiated prostacyclin production. This finding is in line with the proposition that prostacyclin is important for vascular protection induced by remote ischemic preconditioning 22 , 23 and that targeting prostacyclin formation may play a central role in treating patients with endothelial dysfunction. 29
Increased prostacyclin production by ischemic preconditioning could be explained by an enhanced receptor sensitivity to acetylcholine, a higher substrate availability or, alternatively, increased enzyme activity in the prostacyclin system. In the current study, we examined local and systemic alterations in 3 compounds known to enhance endothelial prostaglandin formation: serotonin, BDNF, and VEGF. 16 , 30 , 31 , 32 , 33 These compounds can be transported both in platelets and in plasma, and therefore these fractions were analyzed separately. The purpose was to assess whether the remote effect of the ischemic preconditioning intervention was associated with an increase in these compounds either by platelets or in platelet‐free plasma. We observed increased levels of BDNF and VEGF in platelet‐free plasma after ischemic preconditioning, indicating that these compounds could have been transported in plasma to the remote vasculature and influenced the prostacyclin production, although further evidence for this possibility is clearly needed. Transport of BDNF and VEGF by platelets could not be verified as the level of these compounds in the platelet fraction remained unaltered after the ischemic preconditioning. Combined, our findings suggest that ischemic preconditioning leads to an improvement in remote microvascular endothelial function through humoral signaling, leading to increased prostacyclin production.
Sustained Effect of Remote Ischemic Preconditioning
Previous studies have documented a sustained effect of ischemic preconditioning: a second window, lasting for up to 48 hours after the procedure. 34 Such a sustained effect could be attributed to alterations at the transcriptional level. We therefore determined the mRNA levels of prostacyclin synthase, cyclooxygenase 1, and endothelial NO synthase in skeletal muscle biopsies obtained before and 2.5 hours after ischemic preconditioning. A potential increase in mRNA levels of proteins related to the formation of vasoactive compounds is also of interest in relation to the observed beneficial effect of a period of daily treatment with ischemic preconditioning on vascular function. 35 , 36 , 37 No significant alterations in mRNA levels were, however, detected for either of the vasodilator enzymes, and the present study was not able to confirm the hypothesis that the sustained effect of ischemic preconditioning would be attributed to the upregulation of these vasodilator enzymes. It is, however, worth noting that endothelial NO synthase mRNA was numerically higher after ischemic preconditioning in 9 of the 11 participants, which may indicate that ischemic preconditioning can lead to upregulation of endothelial NO synthase.
It should also be clarified that our finding does not exclude that an increased mRNA expression could occur at a later time than measured here or that other vasoactive proteins could be influenced.
Effect of Ischemic Preconditioning on Local Microvascular Function
Ischemic preconditioning was not found to influence the vasodilator response to acetylcholine locally in the forearm, a finding that may have been related to an opposing negative effect of the procedure attributed to local ischemia. Ischemia reperfusion can lead to an increased formation of reactive oxygen species in skeletal muscle, 38 which readily remove NO and interfere with prostacyclin formation. 39 , 40 Thus, the availability of these vasoactive compounds may have been reduced locally in the arm, counteracting the enhanced vasodilatory capacity observed in the leg.
Effect of Exercise Preconditioning on Local and Remote Microvascular Function
We examined the functional effect of exercise preconditioning to assess whether exercise could also induce a remote effect. Previous studies have reported that exercise can induce a preconditioning effect similar to that of ischemic preconditioning at the macrovascular level, 8 however, it is unclear whether exercise improves remote microvascular vasodilatory responsiveness. The remote microvascular response to acetylcholine infusion was not detectably altered by hand‐grip exercise, whereas an improved response was found locally in the arm. Smooth muscle sensitivity to NO and prostacyclin was not significantly altered locally in the arm, suggesting that primarily endothelial function was improved locally by the exercise. The improved vasodilator response to acetylcholine in the arm was not paralleled by a greater change in plasma prostacyclin levels during arterial acetylcholine infusion. This finding suggests that the mechanism mediating vascular changes with exercise may be different from that of ischemic preconditioning.
The lack of effect of hand‐grip exercise on remote microvascular responsiveness and prostacyclin formation indicates that the previously observed effect of exercise preconditioning on remote endothelial injury 8 is not likely caused by changes in microvascular endothelial function. The explanation for the difference between exercise and ischemia can only be speculated on, but it appears plausible that the observed remote signaling specifically is dependent on an ischemic insult that cannot be induced by dynamic hand‐grip exercise. Overall, our current observations suggest that the mechanism by which ischemic preconditioning influences vascular function is different from that of exercise. The precise mechanism underlying the local effect of exercise remains to be elucidated.
Study Limitations
To assess the acute effect of remote ischemic preconditioning on microvascular function, infusions of the vasoactive compounds were repeated 3 times during the experimental day. The sequence of drugs was kept the same in the protocols to keep the time after the interventions constant. This procedure raises the possibility that the observed changes may have been influenced by the prior infusion. However, it is our experience from many years of conducting infusion with these same drugs that the short‐term infusions used do not impact the vasodilatory response of other subsequent drugs. Moreover, the lack of influence of previously infused drugs was verified by the clear difference in remote and local vasodilator responses on the 2 experimental days involving ischemic preconditioning and hand‐grip exercise, respectively.
The improved sensitivity to acetylcholine after ischemic preconditioning could, in addition to increased prostacyclin production, also have been due to an enhanced formation of NO. As NO is short lived, venous plasma levels can only be indirectly assessed as the stable metabolites nitrite and nitrate. However, because nitrite and nitrate are rather insensitive markers, limited changes in formation would not have been possible to detect in plasma. As a result, we cannot exclude the possibility that a potentiation of NO formation contributed to the improved vasodilator response to acetylcholine after ischemic preconditioning. It should also be mentioned that signaling proteins may be carried by microvesicles or cells in the blood 41 ; however, this was not studied in the current investigation and thus their role cannot be ruled out.
Conclusions
The present study demonstrates for the first time in humans that ischemic preconditioning of the forearm leads to an improved vasodilator response to arterial acetylcholine infusion in the leg and to an associated increase in formation of prostacyclin. This finding indicates that the remote effect of ischemic preconditioning is related to a potentiation of the prostacyclin system. Furthermore, in contrast to our hypothesis, the study did not detect an improved remote microvascular function in response to preconditioning by hand‐grip exercise. Collectively, these findings suggest that ischemic preconditioning may be an effective new treatment strategy in conditions associated with microvascular endothelial dysfunction.
Sources of Funding
The study was funded by The Independent Research Fund Denmark and by The Danish Ministry of Culture Fund for Sports Research. Howard Carter was supported by a grant from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Disclosures
None.
Supporting information
Tables S1–S5
Acknowledgments
The technical assistance of Gemma Kroos is gratefully acknowledged.
(J Am Heart Assoc. 2020;9:e016017 DOI: 10.1161/JAHA.120.016017.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016017
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 2. Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia‐reperfusion in humans in vivo. Circulation. 2001;103:1624–1630. [DOI] [PubMed] [Google Scholar]
- 3. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 4. Kharbanda RK, Morten UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. [DOI] [PubMed] [Google Scholar]
- 5. Enko K, Nakamura K, Yunoki K, Miyoshi T, Akagi S, Yoshida M, Toh N, Sangawa M, Nishii N, Nagase S, et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J Physiol Sci. 2011;61:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moro L, Pedone C, Mondì A, Nunziata E, Antonelli Incalzi R, Mondí A, Nunziata E, Antonelli IR. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis. 2011;219:750–752. [DOI] [PubMed] [Google Scholar]
- 7. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seeger JPH, Lenting CJ, Schreuder THA, Landman TRJ, Cable NT, Hopman MTE, Thijssen DHJ. Interval exercise, but not endurance exercise, prevents endothelial ischemia‐reperfusion injury in healthy subjects. Am J Physiol Heart Circ Physiol. 2015;308:H351–H357. [DOI] [PubMed] [Google Scholar]
- 9. Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of exercise preconditioning with immediate cardioprotection: a review. JAMA Cardiol. 2018;3:169–176. [DOI] [PubMed] [Google Scholar]
- 10. Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. [DOI] [PubMed] [Google Scholar]
- 11. Ekeloef S, Homilius M, Stilling M, Ekeloef P, Koyuncu S, Münster A‐MB, Meyhoff CS, Gundel O, Holst‐Knudsen J, Mathiesen O, et al. The effect of remote ischaemic preconditioning on myocardial injury in emergency hip fracture surgery (PIXIE trial): phase II randomised clinical trial. BMJ. 2019;367:l6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis. 1999;8:123–129. [DOI] [PubMed] [Google Scholar]
- 13. Battinelli EM, Markens BA, Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcus AJ, Weksler BB, Jaffe EA, Broekman MJ. Synthesis of prostacyclin from platelet‐derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980;66:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meuchel LW, Thompson MA, Cassivi SD, Pabelick CM, Prakash YS. Neurotrophins induce nitric oxide generation in human pulmonary artery endothelial cells. Cardiovasc Res. 2011;91:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Totoson P, Pedard M, Marie C, Demougeot C. Activation of endothelial TrkB receptors induces relaxation of resistance arteries. Vascul Pharmacol. 2018;106:46–53. [DOI] [PubMed] [Google Scholar]
- 17. Knapp AE, Goldberg D, Delavar H, Trisko BM, Tang K, Hogan MC, Wagner PD, Breen EC. Skeletal myofiber VEGF regulates contraction‐induced perfusion and exercise capacity but not muscle capillarity in adult mice. Am J Physiol Regul Integr Comp Physiol. 2016;311:R192–R199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroll J, Waltenberger J. A novel function of VEGF receptor‐2 (KDR): rapid release of nitric oxide in response to VEGF‐A stimulation in endothelial cells. Biochem Biophys Res Commun. 1999;265:636–639. [DOI] [PubMed] [Google Scholar]
- 19. Oberkofler CE, Limani P, Jang J‐H, Rickenbacher A, Lehmann K, Raptis DA, Ungethuem U, Tian Y, Grabliauskaite K, Humar R, et al. Systemic protection through remote ischemic preconditioning is spread by platelet‐dependent signaling in mice. Hepatology. 2014;60:1409–1417. [DOI] [PubMed] [Google Scholar]
- 20. Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, Hellsten Y. Early postmenopausal phase is associated with reduced prostacyclin‐induced vasodilation that is reversed by exercise training. Hypertension. 2016;68:1011–1020. [DOI] [PubMed] [Google Scholar]
- 21. Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen S. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J Hypertens. 2012;30:2007–2014. [DOI] [PubMed] [Google Scholar]
- 22. Bouchard JF, Chouinard J, Lamontagne D. Participation of prostaglandin E2 in the endothelial protective effect of ischaemic preconditioning in isolated rat heart. Cardiovasc Res. 2000;45:418–427. [DOI] [PubMed] [Google Scholar]
- 23. Gres P, Schulz R, Jansen J, Umschlag C, Heusch G. Involvement of endogenous prostaglandins in ischemic preconditioning in pigs. Cardiovasc Res. 2002;55:626–632. [DOI] [PubMed] [Google Scholar]
- 24. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 25. Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. [DOI] [PubMed] [Google Scholar]
- 26. Altman D. How large a sample? BMJ. 1980;281:1336–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bates D, Mächler M, Bolker B, Fitting WS. Linear mixed‐effects models using lme4. J Stat Softw. 2015;67 DOI: 10.18637/jss.v067.i01. [Google Scholar]
- 28. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. [DOI] [PubMed] [Google Scholar]
- 29. Meyer ASP, Johansson PI, Kjaergaard J, Frydland M, Meyer MAS, Henriksen HH, Thomsen JH, Wiberg SC, Hassager C, Ostrowski SR. "Endothelial Dysfunction in Resuscitated Cardiac Arrest (ENDO‐RCA): safety and efficacy of low‐dose Iloprost, a prostacyclin analogue, in addition to standard therapy, as compared to standard therapy alone, in post‐cardiac‐arrest‐syndrome patients." Am Heart J. 2020;219:9–20. [DOI] [PubMed] [Google Scholar]
- 30. Gliki G, Abu‐Ghazaleh R, Jezequel S, Wheeler‐Jones C, Zachary I. Vascular endothelial growth factor‐induced prostacyclin production is mediated by a protein kinase C (PKC)‐dependent activation of extracellular signal‐regulated protein kinases 1 and 2 involving PKC‐delta and by mobilization of intracellular Ca2+ . Biochem J. 2001;353:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wheeler‐Jones C, Abu‐Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A 2 in endothelial cells via p42/p44 mitogen‐activated protein kinase. FEBS Lett. 1997;420:28–32. [DOI] [PubMed] [Google Scholar]
- 32. Santhanam AVR, Smith LA, Katusic ZS. Brain‐derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke. 2010;41:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackshear JL, Orlandi C, Hollenberg NK. Serotonin and the renal blood supply: role of prostaglandins and the 5HT‐2 receptor. Kidney Int. 1986;30:304–310. [DOI] [PubMed] [Google Scholar]
- 34. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia‐reperfusion injury in humans. J Am Coll Cardiol. 2005;46:450–456. [DOI] [PubMed] [Google Scholar]
- 35. Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DHJ. Seven‐day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27:918–925. [DOI] [PubMed] [Google Scholar]
- 36. Jones H, Nyakayiru J, Bailey TG, Green DJ, Cable NT, Sprung VS, Hopkins ND, Thijssen DHJ. Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. Eur J Prev Cardiol. 2015;22:1083–1087. [DOI] [PubMed] [Google Scholar]
- 37. Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther. 2014;8:1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998;179:169–187. [DOI] [PubMed] [Google Scholar]
- 39. Gamez‐Mendez AM, Vargas‐Robles H, Ríos A, Escalante B. Oxidative stress‐dependent coronary endothelial dysfunction in obese mice. PLoS One. 2015;10:e0138609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi Y, Vanhoutte PM. Macro‐ and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9:434–449. [DOI] [PubMed] [Google Scholar]
- 41. Davidson SM, Andreadou I, Barile L, Birnbaum Y, Cabrera‐Fuentes HA, Cohen MV, Downey JM, Girao H, Pagliaro P, Penna C, et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc Res. 2019;115:1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


