Abstract
Background
Bempedoic acid (BA) is a novel lipid‐lowering drug. We performed a systematic review and meta‐analysis on efficacy and safety of BA compared with standard treatment in patients with hypercholesterolemia.
Methods and Results
Studies were systematically searched in the PubMed, Web of Science, Scopus, and EMBASE databases. Efficacy outcome was represented by percentage changes (mean difference [MD] with pertinent 95% CIs) in total cholesterol, low‐density lipoprotein cholesterol, triglycerides, high‐density lipoprotein cholesterol, apolipoprotein B, non–high‐density lipoprotein cholesterol, and hs‐CRP (high‐sensitivity C‐reactive protein) in BA patients and controls. Seven studies were included (2767 BA‐treated patients and 1469 controls), showing a more significant reduction in low‐density lipoprotein cholesterol (MD, −17.5%; 95% CI, −22.9% to −12.0%), total cholesterol (MD, −10.9%; 95% CI, −13.3% to −8.5%), non–high‐density lipoprotein cholesterol (MD, −12.3%; 95% CI, −15.3% to −9.20%), apolipoprotein B (MD, −10.6%; 95% CI, −13.2% to −8.02%), and hs‐CRP (MD, −13.2%; 95% CI, −16.7% to −9.79%) in BA‐treated patients compared with controls. Results were confirmed when separately analyzing studies on patients with high cardiovascular risk, studies on statin‐intolerant patients, and studies on patients with hypercholesterolemia on maximally tolerated lipid‐lowering therapy. BA‐treated subjects reported a higher rate of treatment discontinuation caused by adverse effects, of gout flare, and of increase in uric acid compared with controls. On the other hand, BA‐treated patients showed a lower incidence of new‐onset diabetes mellitus than controls.
Conclusions
BA is associated with a significant reduction in low‐density lipoprotein cholesterol, total cholesterol, non–high‐density lipoprotein cholesterol, apolipoprotein B, and hs‐CRP compared with standard treatment. Documented efficacy is accompanied by an acceptable safety profile.
Keywords: bempedoic acid, hypercholesterolemia, low‐density lipoprotein cholesterol
Subject Categories: Epidemiology, Primary Prevention, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- Apo
Bapolipoprotein B
- BA
bempedoic acid
- HDL‐C
high‐density lipoprotein cholesterol
- hs‐CRP
high‐sensitivity C‐reactive protein
- LDL‐C
low‐density lipoprotein cholesterol
- MD
mean difference
- OR
odds ratio
- RCT
randomized controlled trial
- TC
total cholesterol
Clinical Perspective.
What Is New?
Bempedoic acid is a safe and effective lipid‐lowering agent for the treatment of hypercholesterolemia, associated with a significant reduction in total cholesterol, low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, apolipoprotein B, and hs‐CRP (high‐sensitivity C‐reactive protein).
Bempedoic acid is a valuable treatment option (1) for patients with statin intolerance, not able to receive an adequate lipid‐lowering treatment; and (2) for patients with high cardiovascular risk, not reaching desired target of low‐density lipoprotein cholesterol despite a maximally tolerated lipid‐lowering treatment, including both statin and ezetimibe.
What Are the Clinical Implications?
Although data are currently lacking, a treatment with bempedoic acid on top of maximally tolerated lipid‐lowering treatment might reduce the need of treatment with proprotein convertase subtilisin/kexin type 9 inhibitors.
Several studies emphasize the role of high levels of low‐density lipoprotein cholesterol (LDL‐C) as the main causative factor in atherosclerosis development.1, 2 Among patients with hypercholesterolemia, those with high levels of LDL‐C exhibit increased prevalence of subclinical atherosclerosis and a more rapid atherosclerosis progression, thus leading to a significantly higher cardiovascular risk1 and related disability.3, 4 Although statin treatment represented for years the gold standard as lipid‐lowering therapy and helped reduce cardiovascular risk in patients with hypercholesterolemia, the target LDL‐C is not always achieved.5 More recently, proprotein convertase subtilisin/kexin type 9 inhibitors demonstrated efficacy in LDL‐C reduction, in the prevention from cardiovascular events, and in atherosclerotic burden regression.6 Nonetheless, despite the development of these innovative therapeutic options, many patients fail to achieve adequate lowering of LDL‐C.7, 8, 9, 10 As a result, patients remain at elevated cardiovascular risk because of persistently increased LDL‐C levels, particularly long‐term patients with familial hypercholesterolemia or multiple vascular risk factors.1, 11 The limitations of available therapies in terms of effectiveness as well as tolerability, adherence, and access highlight the unmet need for additional therapeutic options for lipid lowering.
Bempedoic acid (BA) is a once‐daily, oral, first‐in‐class ATP–citrate lyase inhibitor. ATP–citrate lyase is a cytosolic enzyme integral to the cholesterol synthesis pathway that acts upstream of statin reductase.12 This mechanism of action is distinct from other lipid‐lowering therapies, including statins (which target statin reductase) and ezetimibe (an inhibitor of intestinal cholesterol absorption). By inhibiting ATP–citrate lyase, BA suppresses cholesterol synthesis,12 thereby triggering upregulation of low‐density lipoprotein receptor expression in the liver, resulting in increased clearance of low‐density lipoprotein particles and lowering of LDL‐C.1 Both phase 2 and phase 3 clinical trials showed that BA as monotherapy or when added to background lipid‐lowering therapy significantly lowered LDL‐C as well as other relevant lipids and biomarkers.13
The only available meta‐analysis on this topic14 only included phase 2 studies, with BA dosages other than the 180 mg, which was the standard dose in phase 3 pivotal trials. Thus, in the present study, we performed a systematic review with meta‐analysis of randomized controlled trials (RCTs) to assess safety and efficacy of 180‐mg BA in patients with hypercholesterolemia.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. A protocol for this review was prospectively developed, detailing the specific objectives, the criteria for study selection, the approach to assess study quality, the outcomes, and the statistical methods (registered in PROSPERO (International prospective register of systematic reviews), CRD42020162733)
Search Strategy
To identify all available studies, a detailed search pertaining safety and efficacy of BA in patients with hypercholesterolemia was conducted according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.15 A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, and EMBASE), using the following search terms in all possible combinations: bempedoic acid, ETC.‐1002, cholesterol, hypercholesterolemia, hypercholesterolemic, lipoprotein, low‐density lipoprotein, LDL, high‐density lipoprotein, HDL‐C, triglycerides, apolipoprotein B, C‐reactive protein. The last search was performed on November 14, 2019. The search strategy was developed without any language or publication year restriction.
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e‐mail to try to retrieve original data. Two independent authors (A.D.M., R.L.) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (M.N.D.D.M.). Discrepancies were resolved by consensus. Selection results showed a high interreader agreement (κ=0.99) and have been reported according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart (Figure S1).
Data Extraction and Quality Assessment
According to the prespecified protocol, all phase 2–phase 3 RCTs evaluating safety or efficacy of BA in patients with hypercholesterolemia were included. Only studies including data on BA, 180 mg, were included, considering that other dosages were not included in registrative trials and will not be licensed for the use in clinical practice. Nonrandomized controlled trials, case reports, case series without a control group, reviews, and animal studies were excluded. We included in the analysis all studies providing values (means with SD or SE) of total cholesterol (TC), LDL‐C, high‐density lipoprotein cholesterol (HDL‐C), triglycerides, apolipoprotein B (ApoB), non–HDL‐C, hs‐CRP (high‐sensitivity C‐reactive protein, or rate of adverse effects (any adverse events, serious adverse events, muscle‐related adverse events, discontinuation of treatment because of adverse effect, new‐onset diabetes mellitus, gout flare, and changes in uric acid) in patients receiving BA or control treatment. In each study, data on sample size, major clinical and demographic variables, values of changes in TC, LDL‐C, HDL‐C, non–HDL‐C, triglycerides, ApoB, hs‐CRP, and adverse effects were extracted.
As primary efficacy outcome, we evaluated mean changes in LDL‐C cholesterol at 12 weeks in subjects receiving BA and in control treatment group. As secondary efficacy outcomes, we evaluated changes in TC, HDL‐C, triglycerides, ApoB, non–HDL‐C, and hs‐CRP at 12 weeks in subjects receiving BA and in control group. In addition, outcomes included in primary and secondary efficacy analyses were also evaluated after 24 and 52 weeks of treatment.
As safety outcomes, we evaluated the incidence of any adverse event, severe adverse events, muscle‐related adverse effects, discontinuation because of adverse effect, new‐onset diabetes mellitus, gout flare, and changes in uric acid in subjects receiving BA and in control treatment group. Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Cochrane risk of bias assessment tool,16 and results are reported in Table S1.
Statistical Analysis and Risk of Bias Assessment
Statistical analysis was performed using Comprehensive Meta‐Analysis (Version 2; Biostat, Englewood, NJ [2005]). Differences among cases and controls were expressed as mean difference (MD) with pertinent 95% CIs for continuous variables, and as odds ratio (OR) with pertinent 95% CI for dichotomous variables. Changes in TC, LDL‐C, triglycerides, HDL‐C, ApoB, non–HDL‐C, and hs‐CRP have been expressed as percentage change from baseline values in BA‐treated patients compared with control treatment group.
The overall effect was tested using Z scores, and significance was set at P<0.05. Statistical heterogeneity between studies was assessed with χ2 Cochran's Q test and with I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates that is caused by heterogeneity rather than sampling error. In detail, I2 values of 0% indicates no heterogeneity; 25%, low heterogeneity; 25% to 50%, moderate heterogeneity; and 50%, high heterogeneity.17
Publication bias was assessed by the Egger's test and represented graphically by funnel plots of the standard difference in means versus the SE. Visual inspection of funnel plot asymmetry was performed to address for possible small‐study effect, as well as Egger's test to address publication bias, over and above any subjective evaluation. P<0.10 was considered statistically significant.18 In case of a significant publication bias, the Duval and Tweedie trim‐and‐fill method was used to allow for the estimation of an adjusted effect size.19 To be as conservative as possible, the random‐effect method was used to take into account the heterogeneity among included studies.
Meta‐Regression Analyses
We hypothesized that differences among included studies may be affected by demographic variables (mean age and male sex) and clinical data (body mass index, diabetes mellitus, and baseline LDL‐C level). To assess the possible effect of such variables in explaining different results observed across studies, we planned to perform meta‐regression analyses after implementing regression models with efficacy and safety outcomes as dependent variables (y) and the above mentioned covariates as independent variables (x). This analysis was performed with Comprehensive Meta‐Analysis (Version 2).
Results
After excluding duplicate results, the search retrieved 50 articles. Of these studies, 40 were excluded because they were off the topic after scanning the title and/or the abstract, because they were reviews/comments/case reports or they lacked data of interest. Three studies20, 21, 22 were excluded after full‐length article evaluation because of reporting on dosages of BA other than 180‐mg once daily (Figure S1).
Overall, 7 RCTs23, 24, 25, 26, 27, 28, 29 enrolling 2767 BA‐treated patients and 1469 controls were included in the final analysis, with a mean study duration of 25 weeks. A total of 3 studies23, 24, 26 included patients with high cardiovascular risk (atherosclerotic cardiovascular disease or multiple vascular risk factors), heterozygous familial hypercholesterolemia, or both receiving stable doses of maximally tolerated statin therapy alone or in combination with other lipid‐lowering therapies. Atherosclerotic cardiovascular disease included a history of acute myocardial infarction, silent myocardial infarction, unstable angina, coronary revascularization procedure, clinically significant coronary heart disease, symptomatic peripheral arterial disease, or cerebrovascular atherosclerotic disease. The presence of multiple vascular risk factors was defined as diabetes mellitus plus 1 other risk factor or 3 vascular risk factors from the following list: age (men ≥45 years, women ≥55 years), family history of coronary disease, smoking, hypertension, or low HDL‐C, or coronary calcium score above the 95th percentile for the patient's age, sex, and race/ethnicity. Fasting LDL‐C required at randomization was ≥70 mg/dL for Goldberg et al23 and Ray et al,26 whereas for Ballantyne et al,24 it was ≥100 mg/dL for patients with atherosclerotic cardiovascular disease or multiple vascular risk factors or ≥130 mg/dL for patients with multiple cardiovascular risk factors while receiving stable maximally tolerated statin therapy.
Two studies25, 27 enrolled patients with statin intolerance receiving no statin, low‐dose statin, or maximally tolerated statin therapy. Fasting LDL‐C required at randomization was ≥100 mg/dL for Ballantyne et al,27 whereas for Laufs et al,25 it was ≥100 mg/dL for patients with atherosclerotic cardiovascular disease or ≥130 mg/dL for primary cardiovascular prevention patients.
Two studies28, 29 enrolled patients with hypercholesterolemia on maximally tolerated statin therapy, with a required LDL‐C of 115 to 220 mg/dL for Ballantyne et al29and 130 to 220 mg/dL for Thompson et al.28 The study by Ballantyne et al24provided separate data for patients receiving BA and those receiving BA plus ezetimibe. The 2 populations were analyzed as separate data sets.
All the 7 studies were randomized controlled trials, and major characteristics of study populations are shown in Table 1 and Table S2. Changes in triglycerides and high‐density lipoprotein were only reported by 2 phase 2 studies,28, 29 and were expressed as median values for triglycerides. Thus, no meta‐analytic analysis was performed for these 2 outcomes.
Table 1.
Characteristics of Included Studies
| Study | Population | No. of Patients | Study Duration, wk | Age, y | Male sex | LDL‐C | BMI | Diabetes Mellitus | |
|---|---|---|---|---|---|---|---|---|---|
|
Ray 2019 Harmony26 |
CAD and/or FH |
BA Control |
1487 742 |
52 |
65.8 66.8 |
1099 529 |
103.6 102.3 |
29.7 29.4 |
425 212 |
|
Goldberg 2019 Wisdom23 |
CAD and/or FH |
BA Control |
522 257 |
52 |
64.1 64.4 |
328 168 |
119 122 |
30 30.6 |
155 81 |
| Ballantyne 2019 a24 | CAD and/or FH and/or multiple VRFs |
BA Control |
88 41 |
12 |
65.2 65.6 |
45 33 |
147 153 |
30.6 30.5 |
62 24 |
| Ballantyne 2019 b24 | CAD and/or FH and/or multiple VRFs |
BA Control |
86a 86a |
12 |
63 64.4 |
50 52 |
152 147 |
31.2 30.4 |
49 61 |
| Ballantyne 201629 | Hypercholesterolemic |
BA Control |
45 45 |
12 |
57 56 |
14 23 |
134 131 |
30 31 |
N.R. |
| Thompson 201628 | Hypercholesterolemic |
BA Control |
100 99a |
12 |
59 60 |
49 48 |
166 165 |
31 30 |
N.R. |
|
Ballantyne 2018 Tranquility27 |
Statin‐intolerant patients |
BA Control |
181 88 |
12 |
63.8 63.7 |
72 32 |
129.9 123 |
29.5 30.5 |
111 51 |
|
Laufs 2019 Serenity25 |
Statin‐intolerant patients |
BA Control |
234 111 |
24 |
65.2 65.1 |
101 50 |
158.5 155.6 |
30.1 30.6 |
63 26 |
BA indicates bempedoic acid; BMI, body mass index; CAD, coronary artery disease; FH, familial hypercholesterolemia; LDL‐C, low‐density lipoprotein cholesterol; N.R., not reported; and VRF, vascular risk factor.
Patients receiving ezetimibe.
Efficacy Outcomes
The 7 studies included in the analysis23, 24, 25, 26, 27, 28, 29 showed a more significant reduction in LDL‐C after 12 weeks of treatment with BA compared with control treatment (MD, −17.5%; 95% CI, −22.9% to −12.0%; P<0.001; Figure 1). Heterogeneity among these studies was statistically significant (I2=80.3%; P<0.001), and no reduction in the overall heterogeneity was found after excluding one study at time. Two studies enrolling high‐risk patients24, 26 showed that an LDL‐C target <70 mg/dL was achieved by 30.3% of BA‐treated patients and 8.6% of controls (OR, 4.65; 95% CI, 3.6–6.0; P<0.001; I2=0%; P=0.631).
Figure 1.
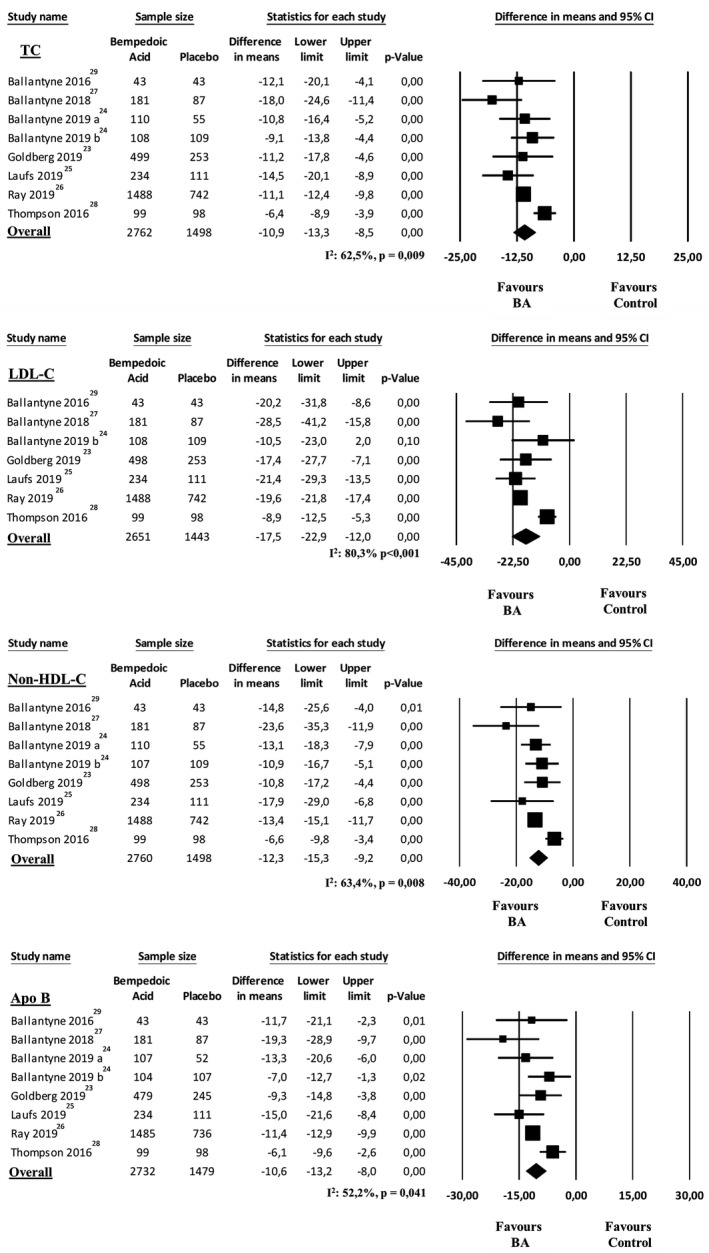
Changes in total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), non–high‐density lipoprotein cholesterol (non–HDL‐C), and apolipoprotein B (ApoB) after 12 weeks of treatment with bempedoic acid compared with control treatment.
In parallel, we observed a more significant reduction of TC (MD, −10.9%; 95% CI, −13.3% to −8.5%; P<0.001; I2=62.5%; P=0.009; Figure 1), non–HDL‐C (MD, −12.3%; 95% CI, −15.3% to −9.20%; P<0.001; I2=63.4%; P=0.008; Figure 1), and ApoB (MD, −10.6%; 95% CI, −13.2% to −8.02%; P<0.001; I2=52.2%; P=0.041; Figure 1) levels in BA‐treated patients compared with control treatment group.
Levels of hs‐CRP were significantly reduced by treatment with BA compared with control treatment (MD, −13.2%; 95% CI, −16.7% to −9.79%; P<0.001; I2=69.0%; P= 0.002; Figure S2).
All results were confirmed when separately analyzing studies on patients with high cardiovascular risk, studies on statin‐intolerant patients, and studies on patients with hypercholesterolemia on maximally tolerated statin therapy (Table 2).
Table 2.
Subgroup Analyses
| Population | TC | LDL‐C | Non–HDL‐C | ApoB | hs‐CRP | |
|---|---|---|---|---|---|---|
| Hypercholesterolemic | MD, % | −7.9 | −13.1 | −9.0 | −7.1 | −9.0 |
| 95% CI, % | −12.9 to −3.0 | −23.8 to −2.4 | −16.3 to −1.7 | −11.3 to −2.9 | −16.3 to −1.7 | |
| P value | 0.002 | 0.016 | 0.016 | 0.001 | 0.016 | |
| Statin intolerant | MD, % | −16.0 | −23.4 | −20.6 | −16.4 | −19.7 |
| 95% CI, % | −20.3 to −11.7 | −30.1 to −16.7 | −28.6 to −12.5 | −21.8 to −10.9 | −25.2 to −14.3 | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| High cardiovascular risk | MD, % | −11.0 | −19.0 | −13.1 | −11.1 | −13.2 |
| 95% CI, % | −12.1 to −9.8 | −21.7 to −16.4 | −14.6 to −11.6 | −12.5 to −9.7 | −14.8 to −11.7 | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Changes in TC, LDL‐C, non–HDL‐C, ApoB, and hs‐CRP after 12 weeks of treatment with bempedoic acid compared with control treatment group, separately analyzing patients with high cardiovascular risk, statin‐intolerant patients, and patients with hypercholesterolemia on maximally tolerated statin therapy.
ApoB indicates apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MD, mean difference; and TC, total cholesterol.
Changes in lipid profile and hs‐CRP observed after 12 weeks of treatment with BA were also confirmed at 24 and 52 weeks (Figure 2).
Figure 2.
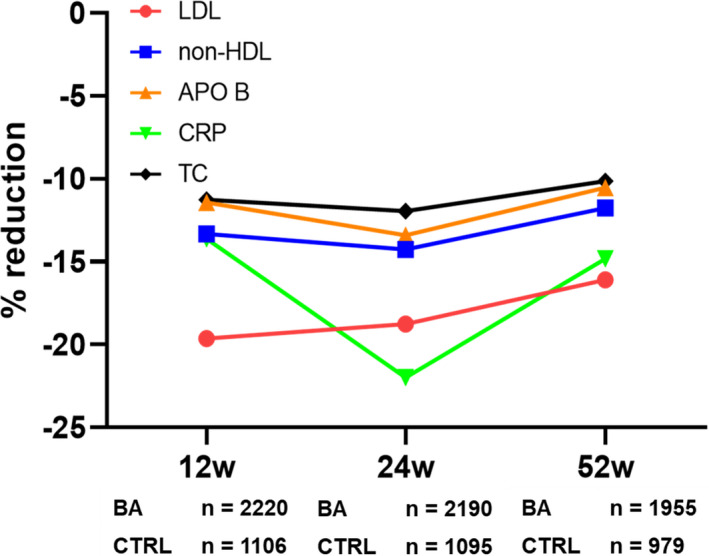
Changes in total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), non–high‐density lipoprotein cholesterol (non–HDL‐C), apolipoprotein B (ApoB), and hs‐CRP (high‐sensitivity C‐reactive protein) at different time points during treatment with bempedoic acid (BA) or control treatment (CTRL). 12w indicates 12 weeks of treatment; 24 w, 24 weeks of treatment; and 52 w, 52 weeks of treatment.
Meta‐regression models (Table 3) showed that an increasing age was associated with a more significant difference in TC, LDL‐C, non–HDL‐C, ApoB, and hs‐CRP reduction between BA‐treated patients and controls, whereas a higher prevalence of male sex only impacted on difference in LDL‐C.
Table 3.
Meta‐Regression Analyses
| Outcome | Covariates | |||||
|---|---|---|---|---|---|---|
| Age | Male Sex | BMI | Diabetes Mellitus | Baseline LDL | ||
| TC | Z value | −2.82 | −0.95 | 2.30 | −0.22 | 2.48 |
| P value | 0.005 | 0.343 | 0.021 | 0.822 | 0.013 | |
| LDL‐C | Z value | −4.41 | −2.67 | 4.32 | 0.13 | 4.41 |
| P value | <0.001 | 0.007 | <0.001 | 0.894 | <0.001 | |
| Non–HDL‐C | Z value | −3.28 | −1.51 | 2.62 | 0.08 | 3.00 |
| P value | 0.001 | 0.131 | 0.009 | 0.935 | 0.003 | |
| ApoB | Z value | −2.42 | −1.13 | 2.12 | 0.32 | 2.01 |
| P value | 0.015 | 0.259 | 0.033 | 0.746 | 0.044 | |
| hs‐CRP | Z value | −3.23 | −0.87 | 2.46 | −0.96 | 2.56 |
| P value | 0.001 | 0.385 | 0.014 | 0.338 | 0.011 | |
Impact of age, male sex, BMI, diabetes mellitus, and baseline LDL‐C on the difference in reduction of TC, LDL‐C, non–HDL‐C, ApoB, and hs‐CRP between patients receiving bempedoic acid and control treatment group. ApoB indicates apolipoprotein B; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; LDL‐C, LDL cholesterol; and TC, total cholesterol.
An increasing body mass index and higher baseline LDL‐C values were associated with a lower difference in TC, LDL‐C, non–HDL‐C, ApoB, and hs‐CRP reduction between BA‐treated patients and controls. No effect of diabetes mellitus on any outcome was observed.
Visual inspection of funnel plots suggested the absence of publication bias and of small‐study effect for all efficacy outcomes considered (Figure S3), confirmed by the Egger test (P always >0.10).
Safety Outcomes
As reported in Figure S4, the 7 studies included23, 24, 25, 26, 27, 28, 29 showed a similar rate of any adverse events (OR, 1.086; 95% CI, 0.943–1.251; P=0.253; I2=0%; P=0.495), serious adverse events (OR, 1.065; 95% CI, 0.874–1.299; P=0.532; I2=0%; P=0.892), and muscle‐related adverse events (OR, 1.139; 95% CI, 0.851–1.524; P=0.381; I2=15.4%; P=0.313) between BA‐treated patients and controls, whereas the rate of treatment discontinuation caused by adverse effect was higher in BA‐treated patients than in controls (OR, 1.393; 95% CI, 1.107–1.753; P=0.005; I2=0%; P=0.591). However, the result seems to be driven by only one study and, after excluding the study by Ray et al,26 the difference was no longer significant (OR, 1.22; 95% CI, 0.878–1.688; P=0.237; I2=0%; P=0.638). A total of 3 studies23, 26, 27 showed a lower incidence of new‐onset diabetes mellitus in BA‐treated patients than in controls (OR, 0.691; 95% CI, 0.493–0.969; P=0.032; I2=0%; P=0.454). On the other hand, patients receiving BA showed a significant increase in uric acid compared with controls (MD, 0.7 mg/dL; 95% CI, 0.5–0.9 mg/dL; P<0.01; I2=77.6%; P=0.004) and a higher rate of gout flare (OR, 3.2; 95% CI, 0.1.2–8.2; P=0.002; I2=0%; P=0.792).
Meta‐regression analyses (Table S3) showed that an increasing age was associated with changes in uric acid (Z value, 3.40; P<0.001) and had a trend toward a higher rate of muscle‐related adverse effects (Z value, 1.84; P=0.065) and drug discontinuation because of adverse effects (Z value, 1.92; P=0.053). We also found a significant association of male sex with muscle‐related adverse events (Z value, 2.05; P=0.041). All the other meta‐regression analyses did not show any significant impact of clinical and demographic variables on the safety outcomes.
Visual inspection of funnel plots suggested the presence of a marginally significant publication bias and of small‐study effect, confirmed by the Egger test (P=0.09) only for the outcome of any adverse event. Results were adjusted by means of the Duval and Tweedie trim‐and‐fill method, and the absence of difference between BA and control treatment was confirmed (Figure S5). Visual inspection of funnel plots suggested the absence of publication bias and of small‐study effect for all the other safety outcomes considered (Figure S6), confirmed by the Egger test (P always >0.10).
Discussion
In the present meta‐analysis on phase 2 and phase 3 RCTs, we evaluated safety and efficacy of BA in patients with hypercholesterolemia. The previous meta‐analysis available on this topic only included phase 2 studies on BA given at heterogeneous dosages, often other than 180‐mg once daily.
Data from 7 RCTs included showed a more significant reduction in LDL‐C, TC, non–HDL‐C, and ApoB in 2767 subjects receiving BA compared with 1469 subjects receiving standard treatment.
Overall, after 12 weeks of treatment with BA, we observed a 11% to 12% reduction in TC, non–high‐density lipoprotein, and ApoB, accompanied by an 18% reduction in LDL‐C. These results are intriguing, also considering that they are obtained on top of maximally tolerated statin treatment.
In addition, extending these findings, a 13% reduction in hs‐CRP was found in BA arm compared with standard treatment. Given the recognized role of hs‐CRP in prediction of cardiovascular event,30 this finding supports a positive effect of BA on overall cardiovascular risk profile.
As to safety, we observed no significant difference between standard treatment and BA in any adverse events, serious adverse events, and muscle‐related adverse events, whereas a 39% higher rate of discontinuation of treatment attributable to adverse effects was found for BA compared with standard treatment. However, this result is mainly driven by one study and, after excluding the study by Ray et al,26 the difference was no longer significant. A further interesting result is that, in the frame of a meta‐regression analysis, we found a trend toward statistical significance for the association between an increasing age and an increased rate of muscle‐related adverse effects and drug discontinuation because of adverse effects. This might suggest a concomitant presence of some codiseases or compliance problems associated with aging and potentially contributing to adverse effects and drug discontinuation. In addition, patients receiving BA showed a modest but significant increase in uric acid, with a 3‐fold increased rate of gout flare and related disabling symptoms compared with control treatment. This effect may be attributable to a potential competition between uric acid and the glucuronide metabolite of BA for the same renal transporter(s).25 Overall, these effects should be investigated in further ad hoc designed studies.
On the other hand, it is noteworthy to highlight that BA was associated with an ≈30% lower incidence of new‐onset diabetes mellitus compared with standard treatment. Although needing to be confirmed in further studies, this finding is supported by a pathophysiological point of view by the mechanism of action of BA. Indeed, by inhibiting adenosine triphosphate–citrate lyase, besides suppressing cholesterol synthesis and triggering upregulation of low‐density lipoprotein receptor expression in the liver, BA modifies fatty acid metabolism and gluconeogenesis.13 Indeed, BA, by activating AMP‐activated protein kinase, determines an inhibitory phosphorylation of acetyl‐CoA carboxylase that, in turn, leads to inhibition of sterol and fatty acid synthesis, increase in mitochondrial long‐chain fatty acid oxidation, and improvement of glucose metabolism.31, 32 This might suggest a potential ancillary effect of BA in patients with atherogenic hypercholesterolemia.
There are some differences in study population characteristics of studies included in the analysis. Three studies23, 24, 26 enrolled patients with high cardiovascular risk and/or heterozygous familial hypercholesterolemia receiving stable doses of maximally tolerated statin therapy alone or in combination with other lipid‐lowering therapies; 2 studies25, 27 enrolled patients with statin intolerance receiving no statin, low‐dose statin, or maximally tolerated statin therapy; and 2 studies28, 29 enrolled patients with hypercholesterolemia on maximally tolerated statin therapy.
We performed a subgroup analysis to evaluate differences in efficacy of BA in different settings, and we, interestingly, found that in both high‐risk patients and statin‐intolerant subjects, BA determined an ≈20% reduction in LDL‐C. In contrast, a somehow higher efficacy in non–HDL‐C, ApoB, and hs‐CRP reduction was observed in statin‐intolerant patients compared with high‐risk patients. This is likely caused by the lack of an adequate treatment in the large majority of statin‐intolerant patients, thus making BA treatment proportionally more efficacious.
More in detail, the 2 studies specifically enrolling patients with statin intolerance25, 27 suggested that BA with or without ezetimibe may be a valuable therapeutic option for patients unable to tolerate statins because of adverse effects. By a clinical point of view, this is of great relevance considering that statin intolerance has been linked to a lower likelihood of achieving LDL‐C goals, increased risk for nonfatal cardiovascular events with related disability, and higher healthcare costs.33, 34
Furthermore, on the basis of obtained results, BA can be considered also as an intriguing option in high‐risk patients. Several lines of data5 suggest that despite adequate lipid‐lowering treatment, many patients fail to achieve target LDL‐C and remain at elevated cardiovascular risk. This is significant mainly in patients with high LDL‐C levels (familial hypercholesterolemia) and in those requiring low LDL‐C targets (previous vascular events or multiple vascular risk factors).1, 11 Data from the Voyager study5 showed that, despite a treatment with high‐intensity statins, patients with high LDL‐C at baseline fail to achieve an LDL‐C target <100 and <70 mg/dL in 25% to 30% and 70% to 80% of cases, respectively. Moreover, only 22% of patients with familial hypercholesterolemia taking lipid‐lowering treatments reached the therapeutic target of LDL‐C <100 mg/dL.35 This therapeutic concern is even more stringent considering most recent guidelines suggesting a further reduction in LDL‐C target levels.36 This evidence suggests the need for further therapeutic options on top of standard treatments. Although in the past years proprotein convertase subtilisin/kexin type 9 inhibitors have been licensed for use in hypercholesterolemic patients and demonstrated a high efficacy rate (≈60% LDL‐C reduction),37 not all patients have criteria for eligibility to this treatment and, in some cases, problems with compliance to a subcutaneous treatment are reported. On this hand, BA can be considered a valuable therapeutic option with a good safety and efficacy profile. Indeed, a separate analysis on 2 studies enrolling high‐risk patients24, 26 showed that the addition of BA on top of maximally tolerated statin therapy, with or without other lipid‐lowering therapies, leads to an achievement of an LDL‐C target <70 mg/dL in ≈30% of cases. Moreover, although the LDL‐C reduction is less significant compared with proprotein convertase subtilisin/kexin type 9 inhibitors, BA is characterized by an oral formulation and has anticipated lower costs than the monoclonal antibody inhibitors.
Some potential limitations of our study need to be discussed. First of all, the relatively small number of individuals studied to date in different studies (≈3000 patients) and short‐term exposure to BA (≈25 weeks’ mean study duration) can potentially limit relevance of our results, suggesting the need of data on long‐term exposure to BA.
Moreover, studies included in our meta‐analysis have different inclusion and exclusion criteria, and most of patients included in the analysis had concomitant cardiovascular risk factors. As a result, heterogeneity among studies is usually high for efficacy outcomes. With the aim to address potential sources of heterogeneity, we performed meta‐regression analyses that consistently showed that an increasing age was associated with a higher effect of BA on LDL‐C, TC, non–HDL‐C, ApoB, and hs‐CRP reduction, whereas a higher prevalence of male sex only impacted on difference in LDL‐C. On the contrary, an increasing body mass index and higher baseline LDL‐C values were associated with a lower effect of BA on LDL‐C, non–HDL‐C, ApoB, and hs‐CRP reduction. All results were entirely independent of the presence of diabetes mellitus. Overall, these data could be useful to identify criteria potentially predicting response to treatment with BA. However, because meta‐analysis is performed on aggregate data and some missing information is present in each study, the meta‐regression approach allowed for the adjustment for some, but not all, potential confounders. Thus, ad hoc designed studies are needed to address this issue.
Furthermore, although it was not possible to conclusively ascertain sources of heterogeneity, the presence of publication bias has been excluded for all efficacy outcomes and for most of the safety outcomes. When present (analysis on any adverse event), results were adjusted by means of the Duval and Tweedie trim‐and‐fill analysis and entirely confirmed.
In conclusion, while waiting for data on a larger number of individuals with a long‐term exposure to BA and for results of the ongoing trial evaluating the impact of BA treatment on hypercholesterolemia‐related clinical outcomes and complications, such as coronary and peripheral artery disease (CLEAR [Cholesterol Lowering via Bempedoic Acid, an ACL‐Inhibiting Regimen] Outcomes, NCT02993406), results of the present meta‐analysis of RCTs showed that BA is a safe and effective lipid‐lowering agent in hypercholesterolemic patients and may be a good treatment alternative for both patients with statin intolerance and those with high cardiovascular risk.
Disclosures
None.
Supporting information
Tables S1–S3 Figures S1‐S6
Acknowledgments
Author contributions: Drs M.N.D. Di Minno and A. Di Minno conceived and designed the study, performed statistical analysis, interpreted results, and drafted the manuscript. Drs Lupoli, Calcaterra, Poggio, and Ambrosino reviewed literature data, interpreted results, drafted the manuscript, and performed critical revisions. Drs Iannuzzo, Spadarella, and Forte acquired clinical data and drafted the manuscript. All authors read and approved the final version of the manuscript.
(J Am Heart Assoc. 2020;9:e016262 DOI: 10.1161/JAHA.119.016262.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 2. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 3. Hamm LF, Wenger NK, Arena R, Forman DE, Lavie CJ, Miller TD, Thomas RJ. Cardiac rehabilitation and cardiovascular disability: role in assessment and improving functional capacity: a position statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2013;33:1–11. [DOI] [PubMed] [Google Scholar]
- 4. Kuo HK, Al Snih S, Kuo YF, Raji MA. Chronic inflammation, albuminuria, and functional disability in older adults with cardiovascular disease: the national health and nutrition examination survey, 1999‐2008. Atherosclerosis. 2012;222:502–508. [DOI] [PubMed] [Google Scholar]
- 5. Nicholls SJ, Brandrup‐Wognsen G, Palmer M, Barter PJ. Meta‐analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from voyager). Am J Cardiol. 2010;105:69–76. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni‐Berthold I, Lopez‐Miranda J, Schiele F, et al. Clinical efficacy and safety of achieving very low LDL‐cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the fourier trial. Lancet. 2017;390:1962–1971. [DOI] [PubMed] [Google Scholar]
- 7. Arca M, Ansell D, Averna M, Fanelli F, Gorcyca K, Iorga SR, Maggioni AP, Paizis G, Tomic R, Catapano AL. Statin utilization and lipid goal attainment in high or very‐high cardiovascular risk patients: insights from Italian general practice. Atherosclerosis. 2018;271:120–127. [DOI] [PubMed] [Google Scholar]
- 8. Ferrieres J, Gorcyca K, Iorga SR, Ansell D, Steen DL. Lipid‐lowering therapy and goal achievement in high‐risk patients from French general practice. Clin Ther. 2018;40(1484–1495):e1422. [DOI] [PubMed] [Google Scholar]
- 9. Menzin J, Aggarwal J, Boatman B, Yu J, Stern K, Harrison DJ, Patel JG. Ezetimibe use and LDL‐C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm. 2017;23:1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Razek O, Cermakova L, Armani H, Lee T, Francis GA, Mancini GBJ, Frohlich J, Brunham LR. Attainment of recommended lipid targets in patients with familial hypercholesterolemia: real‐world experience with PCSK9 inhibitors. Can J Cardiol. 2018;34:1004–1009. [DOI] [PubMed] [Google Scholar]
- 11. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 12. Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, Cramer CT, Spahr MA, Brant AF, Houghton JL, et al. AMP‐activated protein kinase and ATP‐citrate lyase are two distinct molecular targets for etc.‐1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54:134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruscica M, Banach M, Sahebkar A, Corsini A, Sirtori CR. Etc.‐1002 (bempedoic acid) for the management of hyperlipidemia: from preclinical studies to phase 3 trials. Expert Opin Pharmacother. 2019;20:791–803. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Luo S, Gan X, He C, Huang R. Safety and efficacy of etc.‐1002 in hypercholesterolaemic patients: a meta‐analysis of randomised controlled trials. Kardiologia Polska. 2019;77:207–216. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ. 2001;323:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 20. Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, Newton RS. Use of etc.‐1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015;9:295–304. [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez MJ, Rosenberg NL, Macdougall DE, Hanselman JC, Margulies JR, Strange P, Milad MA, McBride SJ, Newton RS. Efficacy and safety of etc.‐1002, a novel investigational low‐density lipoprotein‐cholesterol‐lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–683. [DOI] [PubMed] [Google Scholar]
- 22. Ballantyne CM, Davidson MH, Macdougall DE, Bays HE, Dicarlo LA, Rosenberg NL, Margulies J, Newton RS. Efficacy and safety of a novel dual modulator of adenosine triphosphate‐citrate lyase and adenosine monophosphate‐activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. J Am Coll Cardiol. 2013;62:1154–1162. [DOI] [PubMed] [Google Scholar]
- 23. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low‐density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the clear wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, Stroes ES, MacDougall D, Zhao X, Catapano AL. Bempedoic acid plus ezetimibe fixed‐dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, Kelly S, Stroes ESG. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8:e011662. doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 27. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, Leiter LA. Efficacy and safety of bempedoic acid added to ezetimibe in statin‐intolerant patients with hypercholesterolemia: a randomized, placebo‐controlled study. Atherosclerosis. 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 28. Thompson PD, MacDougall DE, Newton RS, Margulies JR, Hanselman JC, Orloff DG, McKenney JM, Ballantyne CM. Treatment with etc.‐1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol. 2016;10:556–567. [DOI] [PubMed] [Google Scholar]
- 29. Ballantyne CM, McKenney JM, MacDougall DE, Margulies JR, Robinson PL, Hanselman JC, Lalwani ND. Effect of etc.‐1002 on serum low‐density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol. 2016;117:1928–1933. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, Wan Q, He R, Wang Z. Hs‐CRP and all‐cause, cardiovascular, and cancer mortality risk: a meta‐analysis. Atherosclerosis. 2017;259:75–82. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg R. Targeting low‐density lipoprotein and dysmetabolism in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikolic D, Mikhailidis DP, Davidson MH, Rizzo M, Banach M. Etc.‐1002: a future option for lipid disorders? Atherosclerosis. 2014;237:705–710. [DOI] [PubMed] [Google Scholar]
- 33. Graham JH, Sanchez RJ, Saseen JJ, Mallya UG, Panaccio MP, Evans MA. Clinical and economic consequences of statin intolerance in the United States: results from an integrated health system. J Clin Lipidol. 2017;11(70–79):e71. [DOI] [PubMed] [Google Scholar]
- 34. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, Chen L, Huang L, Dent R, Kent ST, et al. Statin intolerance and risk of coronary heart events and all‐cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–1395. [DOI] [PubMed] [Google Scholar]
- 35. Huijgen R, Kindt I, Verhoeven SB, Sijbrands EJ, Vissers MN, Kastelein JJ, Hutten BA. Two years after molecular diagnosis of familial hypercholesterolemia: majority on cholesterol‐lowering treatment but a minority reaches treatment goal. PLoS ONE. 2010;5:e9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 37. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3 Figures S1‐S6


