Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) constitutes half of hospitalized heart failure cases and is commonly associated with obesity. The role of natriuretic peptide levels in hospitalized obese patients with HFpEF, however, is not well defined. We sought to evaluate change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels by obesity category and related clinical outcomes in patients with HFpEF hospitalized for acute heart failure.
Methods and Results
A total of 89 patients with HFpEF hospitalized with acute decompensated heart failure were stratified into 3 obesity categories: nonobese (body mass index [BMI] <30.0 kg/m2, 19%), obese (BMI 30.0–39.9 kg/m2, 29%), and severely obese (BMI ≥40.0 kg/m2, 52%), and compared for percent change in NT‐proBNP during hospitalization and clinical outcomes. Clinical characteristics were compared between patients with normal NT‐proBNP (≤125 pg/mL) and elevated NT‐proBNP. Admission NT‐proBNP was inversely related to BMI category (nonobese, 2607 pg/mL [interquartile range, IQR: 2112–5703]; obese, 1725 pg/mL [IQR: 889–3900]; and severely obese, 770.5 pg/mL [IQR: 128–1268]; P<0.01). Severely obese patients had the largest percent change in NT‐proBNP with diuresis (−64.8% [95% CI, −85.4 to −38.9] versus obese −40.4% [95% CI, −74.3 to −12.0] versus nonobese −46.9% [95% CI, −57.8 to −37.4]; P=0.03). Nonobese and obese patients had significantly worse 1‐year survival compared with severely obese patients (63% versus 76% versus 95%, respectively; P<0.01). Patients with normal NT‐proBNP (13%) were younger, with higher BMI, less atrial fibrillation, and less structural heart disease than those with elevated NT‐proBNP.
Conclusions
In hospitalized patients with HFpEF, NT‐proBNP was inversely related to BMI with the largest decrease in NT‐proBNP seen in the highest obesity category. These findings have implications for the role of NT‐proBNP in the diagnosis and assessment of treatment response in obese patients with HFpEF.
Keywords: diuresis, heart failure with preserved ejection fraction, natriuretic peptides, NT‐proBNP, obesity
Subject Categories: Heart Failure, Metabolic Syndrome, Biomarkers
Nonstandard Abbreviations and Acronyms
- 6MWT
6‐minute walk test
- ACC/AHA/HFSA
American College of Cardiology/American Heart Association/Heart Failure Society of America
- BMI
body mass index
- BNP
brain natriuretic peptide
- COACH
Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- I‐PRESERVE
Irbesartan in Heart Failure With Preserved Ejection Fraction
- IQR
interquartile range
- NP
natriuretic peptide
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- ROPA‐DOP
Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine
Clinical Perspective
What Is New?
This is the first study of change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) during hospitalization for acute heart failure (HF) in an obese heart failure with preserved ejection fraction (HFpEF) cohort.
We found that the largest percent change in NT‐proBNP with decongestion occurred in the highest obesity category, and that after adjustment for clinically relevant variables, there remained a large change in NT‐proBNP during hospitalization across all obesity categories.
Thirteen percent of HFpEF patients presenting with acute HF had a normal NT‐proBNP and an overall similar presentation to those with an elevated NT‐proBNP.
What Are the Clinical Implications?
The diagnosis and treatment of acute HF in obese HFpEF patients can be challenging due to the presence of multiple co‐morbidities and challenging physical examination.
Our findings show that HFpEF patients hospitalized with acute HF, across all body mass index categories and irrespective of admission NT‐proBNP, had a decline in NT‐proBNP with treatment for acute HF, and that NT‐proBNP may have clinical utility in both diagnosis and assessment of decongestion in an obese HFpEF population.
With a more in‐depth understanding of natriuretic peptide levels in obese HFpEF, a body mass index‐calibrated natriuretic peptide scale is likely warranted for the diagnosis and management of acute HF in obese HFpEF.
Hospitalizations for patients who have heart failure (HF) with preserved ejection fraction (HFpEF) are increasing relative to HF with reduced ejection fraction (HFrEF) and presently constitute approximately half of all HF hospitalizations.1 Following hospitalization, patients with HFpEF have similar adverse outcomes compared to patients with HFrEF, highlighting the major need for defining successful management strategies and therapeutic targets.1, 2, 3 Natriuretic peptides (NPs), including brain natriuretic peptide (BNP) and NT‐proBNP (N‐terminal pro‐B‐type NP), have been shown to be independently associated with morbidity and mortality in HF.4, 5, 6 The latest American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) Guidelines for the Management of Heart Failure recommend checking NP levels for diagnostic evaluation of acute HF and for post‐hospital discharge prognostication of patients with chronic HF.7 However, the role of NP levels in the evaluation of acute decompensated HFpEF is less well understood.
Complicating the interpretation of NP levels in HFpEF is the fact that obesity is increasingly prevalent as a comorbidity and risk factor in this population and the inverse relationship between obesity and NP levels is well established.8, 9, 10 Furthermore, although obesity increases the risk for development of HF, it is associated with a protective effect on clinical outcomes in established HF, a phenomenon termed the obesity paradox.11 Although few studies have reported on BNP or NT‐proBNP levels in HFpEF patients with acute HF,12, 13, 14, 15 to our knowledge no study to date has reported the change in NP levels in HFpEF patients hospitalized with acute HF, and in particular, the change in NP levels according to body mass index (BMI). In this study, we sought to evaluate the percent change in NT‐proBNP from admission to discharge and clinical outcomes by obesity category in patients with HFpEF hospitalized with acute HF. We further compared clinical characteristics and outcomes of patients with HFpEF who had normal admission NT‐proBNP levels (≤125 pg/mL) to patients with elevated admission NT‐proBNP levels.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. We analyzed data from the ROPA‐DOP (Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine) trial.16 The ROPA‐DOP trial design and methodology have been previously described.16 The ROPA‐DOP trial was a single‐center, randomized, single‐blinded inpatient treatment study of patients with HFpEF hospitalized with acute HF (n=90) comparing intravenous diuretic strategies with and without the addition of low‐dose dopamine. The diagnosis of acute HF was based on the presence of at least 1 symptom (dyspnea, orthopnea, or edema) and 1 sign of HF (rales, jugular venous distension, positive hepatojugular reflux, peripheral edema, ascites, or pulmonary vascular congestion on chest radiography). Patients were included if they had an established left ventricular ejection fraction ≥50% within 12 months of admission without an interval change to suggest a decline in cardiac function. Patients with a systolic blood pressure <90 mm Hg, estimated glomerular filtration rate (as determined by the Chronic Kidney Disease Epidemiology Collaboration threshold) ≤15 mL/min per 1.73 m2, recent acute coronary syndrome, or other primary cause of their cardiomyopathy (myocarditis, hypertrophic cardiomyopathy, severe valvular disease, infiltrative cardiomyopathy, congenital heart disease, constrictive cardiomyopathy), and those with severe pulmonary hypertension were excluded.17 All participants provided informed consent, and the study was approved by the Johns Hopkins INSTITUTIONAL REVIEW BOARD.
Participants underwent baseline assessment within 24 hours of admission to the hospital at the time of enrollment into the clinical trial. Baseline assessments included history and physical examination, laboratory testing including NT‐proBNP, 6‐minute walk test (6MWT), and global assessment of symptoms and dyspnea using a visual analog scale. NT‐proBNP collected from baseline assessment (referred to as admission NT‐proBNP) and from within 12 hours of hospital discharge (referred to as discharge NT‐proBNP) were used for this study. BMI was calculated using height and weight measured at hospital admission and before the start of any diuretic treatment. Worsening renal function was defined as a rise in serum creatinine ≥0.3 mg/dL at 72 hours.
Participants were stratified into 3 obesity categories based on BMI: nonobese: BMI <30.0 kg/m2; obese (inclusive of class I and II obesity): BMI 30.0 to 39.9 kg/m2; and severely obese (inclusive of class III obesity): BMI ≥40.0 kg/m2. The obesity categories were defined based on the traditional World Health Organization definitions of obesity because of the high prevalence of severe obesity in our cohort.18 The primary end point was the percent change in the absolute value of NT‐proBNP from admission to discharge in each obesity class. Analyses of the primary outcome were performed in participants with complete NT‐proBNP data on admission and discharge. Secondary end points included changes in other markers of decongestion (change in weight, net volume of diuresis, and 6MWT from admission to discharge), and clinical outcomes including development of worsening renal function at 72 hours, hospital length of stay, 30‐day all‐cause readmission, and 1‐year survival within each obesity category. A Kaplan–Meier survival analysis was performed to evaluate all‐cause mortality by obesity category.
The lowest level of detection for NT‐proBNP at our institutional laboratory is 20 pg/mL. Since the primary focus of this analysis was to examine trends in NT‐proBNP, 3 patients with undetectable NT‐proBNP levels on admission were not included in the analysis for percent change from admission to discharge. These patients are included in the reporting of the median admission and discharge NT‐proBNP levels. One patient developed septic shock necessitating upgrade to a higher level of care and significant fluid resuscitation, therefore this patient was excluded from the analyses.
Baseline characteristics were compared between obesity categories using chi‐square test for categorical variables and Kruskal–Wallis test for continuous variables. Absolute admission and discharge NT‐proBNP levels between the 3 obesity groups were compared by Kruskal–Wallis test. NT‐proBNP was log‐transformed to aid in the visual comparison of change from admission to discharge and between the BMI categories for Figure 1. Univariate linear regression analyses with clinical characteristics known to be associated with NT‐proBNP level, including age, sex, history of hypertension, diabetes mellitus, atrial fibrillation, and estimated glomerular filtration rate, were performed. A multivariable linear regression model that included obesity class and covariates found to be significantly associated with the primary outcome in univariate analyses was then constructed. Absolute measurements (non–log‐transformed) of NT‐proBNP were used in all regression models incorporating NT‐proBNP as an outcome or variable and in calculations of percent change in NT‐proBNP. In all regression models, obesity category was treated as a factor variable, as the relationship between different obesity groups and any outcome was not assumed to be proportional.
Figure 1. Change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) by obesity category in HFpEF patients hospitalized with acute heart failure.
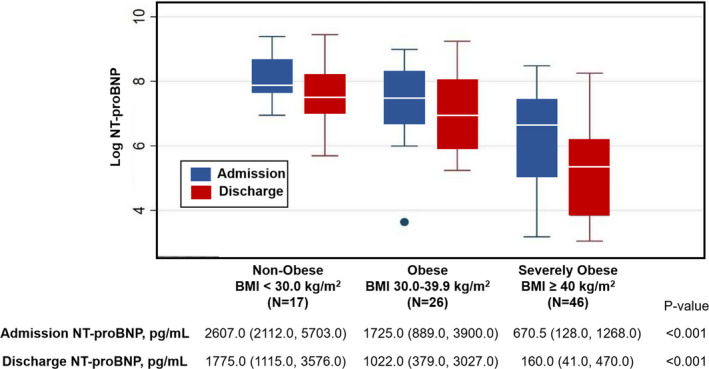
Box plot of log NT‐proBNP on admission and discharge and table of absolute median values (interquartile range) by different obesity categories. BMI indicates body mass index.
The associations between obesity category and characteristics including age, estimated glomerular filtration rate, atrial fibrillation, admission NT‐proBNP, 6MWT distance, and hospital length of stay with mortality were first explored using univariate logistic regression. The 6MWT completed closest to discharge was used for each patient. Multivariable logistic regression analysis was performed including all covariates from the univariate analyses with a threshold P value <0.05.
A further exploratory analysis was performed to characterize participants with a normal admission NT‐proBNP, defined as ≤125 pg/mL,19 compared with those with an elevated admission NT‐proBNP (>125 pg/mL). Baseline characteristics, including demographics, echocardiographic parameters, and clinical outcomes, including hospital course, functional outcomes, and rehospitalization, were compared between these 2 groups. Categorical outcomes were compared with the chi‐square test, while continuous outcome variables were compared using either a Student t test or Wilcoxon rank sum test, as appropriate, based on data distribution. All analyses were performed using Stata 15.0 (StataCorp LLC).
Results
Of the 90 patients enrolled in the ROPA‐DOP trial between October 2013 and December 2016, 88 (98%) and 71 (79%) patients had complete NT‐proBNP data at admission and discharge, respectively. The baseline characteristics of the entire cohort have been previously described.16 The mean age of the study cohort was 66±13 years, 68% were women, and 62% were black. The mean BMI was 40.8±12.9 kg/m2 and median admission NT‐proBNP was 1257 pg/mL (interquartile range [IQR], 367–2656).
Baseline Characteristics by Obesity Category
Over half of the cohort (52%) were classified as severely obese (BMI ≥40 kg/m2), 39% as obese (BMI 30.0–39.9 kg/m2), and 19% as nonobese (BMI <30.0 kg/m2). The baseline characteristics by obesity category are shown in Table 1. Severely obese participants were significantly younger than obese and nonobese participants (severely obese mean age 61±12 years versus obese 70±11 years versus nonobese 73±14 years, P<0.001), with a higher burden of atrial fibrillation in the nonobese participants (59%) compared with the obese (31%) and severely obese (28%, P=0.068) participants, although this was not statistically significant. Sodium was significantly lower in nonobese participants compared with obese and severely obese participants. In comparing echocardiographic characteristics, left ventricular mass index was higher in nonobese (median left ventricular mass index 110.0 g/m2 [IQR, 96.4–130.1]) versus obese 96.4 g/m2 [IQR, 88.3–139.2]) and severely obese patients 85.2 g/m2 [IQR, 69.8–102.3]; P=0.029).
Table 1.
Baseline Characteristics by Obesity Category in a Hospitalized HFpEF Cohort
| Obesity Category | P Value | |||
|---|---|---|---|---|
| Nonobese (BMI <30 kg/m2), n=17 | Obese (BMI 30–39.9 kg/m2), n=26 | Severely Obese (BMI ≥40 kg/m2), n=46 | ||
| Age, y | 73±14 | 70±11 | 61±12 | <0.001 |
| Men, No. (%) | 7 (41) | 11 (42) | 10 (22) | 0.120 |
| Black, No. (%) | 11 (65) | 13 (50) | 31 (67) | 0.390 |
| Hypertension, No. (%) | 16 (94) | 25 (96) | 43 (93) | 0.890 |
| Diabetes mellitus, No. (%) | 7 (41) | 16 (62) | 29 (63) | 0.270 |
| History of atrial fibrillation, No. (%) | 10 (59) | 8 (31) | 13 (28) | 0.068 |
| History of CAD, No. (%) | 6 (35) | 9 (35) | 12 (26) | 0.660 |
| SBP at enrollment, mm Hg | 122 (107–138) | 130 (113–160) | 136 (121–152) | 0.180 |
| Clinical signs/symptoms | ||||
| Edema, No. (%) | 16 (94) | 24 (92) | 44 (96) | 0.840 |
| Orthopnea, No. (%) | 9 (53) | 18 (69) | 34 (74) | 0.280 |
| Jugular venous distension, No. (%) | 16 (94) | 25 (96) | 44 (96) | 0.950 |
| Rales, No. (%) | 12 (71) | 20 (77) | 27 (59) | 0.270 |
| Outpatient median furosemide dose equivalent, mg/d | 40.0 (20.0–320.0) | 80.0 (80.0–160.0) | 180.0 (40.0–400.0) | 0.260 |
| Sodium, mmol/L | 139 (136–140) | 142 (139–143) | 140 (139–142) | 0.023 |
| Blood urea nitrogen, mmol/L | 24 (15–34) | 23 (17–33) | 20 (14–38) | 0.780 |
| Creatinine, mg/dL | 1.5 (0.8–1.8) | 1.4 (1.1–1.9) | 1.1 (0.8–1.5) | 0.088 |
| eGFR, mL/min per 1.73 m2 | 47.5 (34.0–79.0) | 44.8 (39.1–65.0) | 70.2 (42.1–92.9) | 0.060 |
| Plasma cystatin C, mg/L | 1.7 (1.1–2.1) | 1.6 (1.4–1.9) | 1.3 (1.1–2.0) | 0.530 |
| Echocardiographic parameters | ||||
| LVEF, % | 61.5 (7.0) | 60.2 (10.1) | 63.8 (6.8) | 0.170 |
| LV end‐diastolic diameter, cm | 4.5 (0.8) | 4.8 (0.9) | 4.8 (0.5) | 0.320 |
| IVS diastolic thickness, cm | 1.2 (0.3) | 1.4 (0.3) | 1.3 (0.3) | 0.280 |
| LVPW diastolic thickness, cm | 1.2 (0.3) | 1.2 (0.2) | 1.1 (0.2) | 0.650 |
| LV mass index, g/m2 | 110.0 (96.4–130.1) | 96.4 (88.3–139.2) | 85.2 (69.8–102.3) | 0.029 |
| LA diameter, cm | 4.3 (0.8) | 4.5 (1.1) | 4.0 (0.8) | 0.096 |
| E/A | 1.4 (1.0–1.8) | 1.2 (0.9–1.9) | 1.2 (0.9–1.4) | 0.540 |
| E/e′ (average) | 20.9 (14.0) | 18.5 (9.7) | 16.1 (5.8) | 0.230 |
| Tricuspid regurgitant peak velocity, cm/s | 314.6 (52.3) | 272.6 (89.9) | 278.6 (44.6) | 0.130 |
| RVSP, mmHg | 54.4 (15.2) | 45.8 (15.2) | 45.0 (12.9) | 0.110 |
Values are expressed as mean±SD or median (interquartile range) unless otherwise indicated. BMI indicates body mass index; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; IVS, intraventricular septum; LA, left atrium, LV, left ventricle; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall; RVSP, right ventricular systolic pressure; and SBP, systolic blood pressure.
Change in NT‐proBNP by Obesity Category During Hospitalization
Admission NT‐proBNP was elevated across all obesity categories, with an inverse relationship between NT‐proBNP and BMI (nonobese: median NT‐proBNP 2607 pg/mL [IQR, 2112–5703]; obese: 1725 pg/mL [IQR, 889–3900]; and severely obese: 670.5 pg/mL [IQR, 128–1268]; P<0.001, Figure 1). All obesity categories demonstrated a decrease in NT‐proBNP from admission to discharge, with discharge NT‐proBNP levels also inversely related to BMI (P<0.001, Figure 1). There was a significant difference in percent change in NT‐proBNP across all obesity categories with severely obese participants having the largest percent change in NT‐proBNP from admission to discharge (severely obese, –64.8% [95% CI, −85.4 to −38.9]; obese, −40.4% [95% CI, −74.3 to −12.0]; and nonobese, −46.9% [95% CI, −57.8 to −37.4]; P=0.025, Table 2). Univariate and multivariable linear regression analysis showed no significant difference in percent change NT‐proBNP in obese or severely obese categories compared with nonobese after adjustment for age and estimated glomerular filtration rate (Table S1). There was no significant association between percent change in NT‐proBNP and odds of 30‐day rehospitalization, 1‐year mortality, or changes in markers of functional status including change in 6MWT, area under the curve of global well‐being score, or change in dyspnea score from admission to discharge across obesity categories.
Table 2.
Changes in Markers of Decongestion From Admission to Discharge and Clinical Outcomes by Obesity Category in Hospitalized HFpEF
| Obesity Category | P Value | |||
|---|---|---|---|---|
| Nonobese | Obese | Severely Obese | ||
| Markers of decongestion | ||||
| Percent change in absolute NT‐proBNP from admission to discharge | −46.9 (−57.8 to −37.4) | −40.4 (−74.3 to −12.0) | −64.8 (−85.4 to −38.9) | 0.025 |
| Weight change at discharge, kg | −5.3 (−7.0 to −2.6) | −3.2 (−6.6 to −1.5) | −2.8 (−7.3 to −1.0) | 0.310 |
| Net diuresis at discharge, mL | −7140.0 (−8653.0 to −3155.0) | −5418.0 (−8397.0 to −2522.0) | −6392.0(−10,662.0 to −3928.0) | 0.490 |
| 6MWT at discharge, m | 54.7 (29.3 to 142.6) | 121.9 (50.0 to 205.7) | 80.8 (30.5 to 166.7) | 0.750 |
| Clinical outcomes | ||||
| Development of WRF at 72 h | 2 (12) | 7 (27) | 10 (22) | 0.490 |
| Hospital length of stay, d | 7 (5–8) | 7 (5–10) | 7 (4–10) | 0.840 |
| All‐cause 30‐d readmission, No. (%) | 5 (29) | 4 (16) | 13 (28) | 0.470 |
| 1‐y Survival, No. (%) | 10 (63) | 19 (76) | 42 (95) | 0.005 |
Values are expressed as median (interquartile range) unless otherwise indicated. 6MWT indicates 6‐minute walk test; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and WRF, worsening renal function.
Hospital Course and Clinical Outcomes by Obesity Category
There were no differences in other markers of decongestion from admission to discharge or in clinical outcomes including worsening renal function at 72 hours, hospital length of stay, or 30‐day readmission rates across obesity categories (Table 2). Nonobese and obese patients had significantly worse survival at 1 year compared with severely obese patients (63% versus 76% versus 95%, respectively; P=0.005) (Table 2). Cumulative survival probability at 18 months was also significantly different between the obesity categories (P=0.002, Figure 2). In multivariable logistic regression analysis, there remained a significantly higher risk of mortality for both nonobese and obese patients compared with severely obese patients after adjusting for age and 6MWT distance (nonobese odds ratio, 11.8; 95% CI, 1.71–81.2 [P=0.012] and obese odds ratio, 7.75; 95% CI, 1.20–50.2 [P=0.032]) (Table 3).
Figure 2. Kaplan–Meier survival curves of hospitalized HFpEF by body mass index (BMI) category.
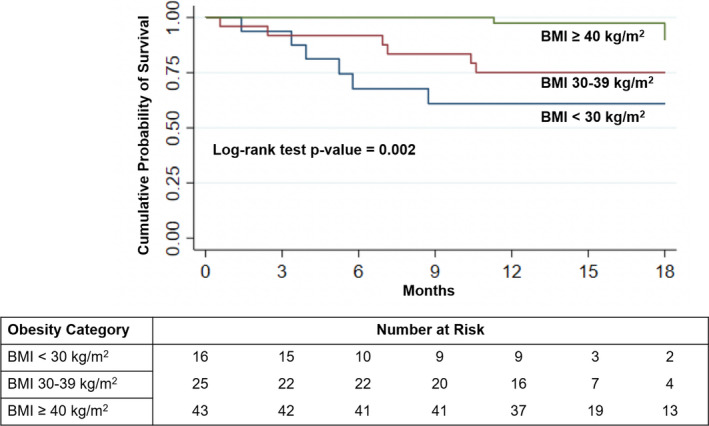
.
Table 3.
Unadjusted and Adjusted Odds of Mortality by Obesity Category
| Univariate Logistic Regression | Multivariable Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
| BMI Category, kg/m2 | ||||||
| Severely obese (≥40) | Reference | Reference | Reference | Reference | Reference | Reference |
| Obese (30–39.9) | 6.63 | 1.22–35.9 | 0.028 | 7.75 | 1.20–50.2 | 0.032 |
| Nonobese (<30) | 12.6 | 2.2–71.9 | 0.004 | 11.8 | 1.71–81.2 | 0.012 |
| Age, per 10 y | 1.84 | 1.11–3.06 | 0.019 | 1.08 | 0.61–1.91 | 0.790 |
| 6MWT, per 10 m | 0.84 | 0.73–0.96 | 0.012 | 0.83 | 0.71–0.97 | 0.018 |
| Hospital length of stay, per d | 0.97 | 0.85–1.10 | 0.588 | … | … | … |
| eGFR at presentation, per 10 mL/min | 0.99 | 0.81–1.21 | 0.919 | … | … | … |
| Atrial fibrillation | 2.58 | 0.78–8.53 | 0.121 | … | … | … |
| Absolute NT‐proBNP on admission, per 100 pg/mL | 1.01 | 0.99–1.03 | 0.058 | … | … | … |
Note: Model including variables with P<0.05 performed significantly better than P value thresholds of 0.1 or 0.2 (P value for likelihood ratio test was 0.73 including variables with P<0.2 compared with final model and P value for likelihood ratio test was 0.43 for model including variables with P<0.1 compared with the final model). 6MWT indicates 6‐minute walk test; BMI, body mass index; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and OR, odds ratio.
Normal Versus Elevated Admission NT‐proBNP in Hospitalized HFpEF
Normal NT‐proBNP (≤125 pg/mL) was seen in 12 (13%) of our participants on admission. Participants with normal NT‐proBNP were significantly younger, more obese, less likely to have atrial fibrillation, and taking a higher average outpatient diuretic dose compared with those who had an elevated NT‐proBNP; however, no differences were seen in clinical presentation compared with those who had elevated NT‐proBNP (Table 4). Participants with an elevated admission NT‐proBNP had significantly worse cardiac remodeling and diastolic dysfunction by echocardiography, including greater left ventricular mass index, increased left ventricular posterior wall thickness, larger left atrial diameter, higher E/e’, and higher E/A (Table 4). Patients with normal NT‐proBNP had improved 6MWT distance at discharge compared to those with elevated NT‐proBNP; however, there were no differences in clinical outcomes between the groups including net volume of diuresis, weight change from admission to discharge, 30‐day readmissoin, or survival at 1 year (Table 4).
Table 4.
Clinical Characteristics and Echocardiographic Parameters of Hospitalized Patients With HFpEF Who Had Normal Admission NT‐proBNP Level (≤125 pg/mL) vs Elevated NT‐proBNP Level
| NT‐proBNP | P Value | ||
|---|---|---|---|
| ≤125 pg/mL (n=12) | >125 pg/mL (n=77) | ||
| Age, mean (SD), y | 53.9 (8.3) | 67.9 (12.5) | <0.001 |
| BMI, mean (SD), kg/m2 | 50.0 (9.9) | 39.1 (12.7) | 0.006 |
| Women, No. (%) | 10 (83) | 51 (66) | 0.240 |
| Black , No. (%) | 24 (56) | 46 (60) | 0.580 |
| Hypertension, No. (%) | 10 (83 | 74 (96) | 0.074 |
| Diabetes mellitus, No. (%) | 23 (53) | 29 (63) | 0.210 |
| History of atrial fibrillation, No. (%) | 0 (0) | 31 (40) | 0.006 |
| NYHA functional class, No. (%) | 0.200 | ||
| II | 3 (25) | 11 (14) | |
| III | 9 (75) | 51 (55) | |
| IV | 0 (0) | 15 (19) | |
| Edema, No. (%) | 12 (100) | 72 (94) | 0.360 |
| Orthopnea, No. (%) | 10 (83) | 51 (66) | 0.240 |
| Jugular venous distension, No. (%) | 11 (92) | 74 (96) | 0.490 |
| Rales, No. (%) | 8 (67) | 51 (66) | 0.980 |
| Outpatient median furosemide equivalent dose, mg/d | 400.0 (260.0–400.0) | 80.0 (40.0–200.0) | 0.030 |
| Echocardiographic parameters | |||
| LVEF, % | 65 (60.0–67.5) | 65 (55.0–65.0) | 0.430 |
| E/E′ | 11.9 (10.0–13.2) | 17.5 (11.9–22.0) | 0.005 |
| E/A | 1.1 (0.8–1.2) | 1.2 (0.9–1.9) | 0.037 |
| LV mass index, g/m2 | 73.6 (65.9–85.3) | 98.3 (81.7–126.0) | 0.002 |
| IVS diastolic thickness, cm | 1.1 (0.9–1.3) | 1.2 (1.1–1.6) | 0.061 |
| LVPW diastolic thickness | 1.0 (0.8–1.2) | 1.2 (1.1–1.3) | 0.008 |
| LA diameter, cm | 4.3 (3.1–3.8) | 4.4 (3.8–4.9) | <0.001 |
| RVSP, mm Hg | 35 (32.5–40.9) | 48 (39.0–58.0) | 0.073 |
| Markers of decongestion | |||
| Net fluid loss, mL | −5127 (−6485.5 to 2434.5) | −6804 (−9729.0 to −3377.0) | 0.088 |
| Change in weight, kg | −2.0 (−4.2 to −1.0) | −3.9 (−7.3 to −1.5) | 0.100 |
| 6MWT at discharge | 169.3 (122.0 to 260.0) | 79 (26.8 to 152.4) | 0.040 |
| Clinical course | |||
| Development of WRF at 72 h, No. (%) | 2 (17) | 17 (22) | 0.670 |
| Hospital length of stay | 6.5 (3.5–7.0) | 7.0 (5.0–10.0) | 0.240 |
| 30‐d Readmission, No. (%) | 3 (25) | 19 (25) | 1.000 |
| 1‐y Survival, No. (%) | 12 (100) | 59 (81) | 0.090 |
Values are median (interquartile range) unless otherwise indicated. 6MWT indicates 6‐minute walk test; BMI, body mass index; IVS, interventricular septum; LA, left atrium; LV, left ventricular; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RVSP, right ventricular systolic pressure; and WRF, worsening renal function.
Discussion
Morbidity and mortality following hospitalization for acute decompensated HFpEF has been shown to be comparable to HFrEF in population‐based studies; however, the diagnostic and prognostic significance of NP levels are less well understood in HFpEF.20 Even less well understood is the role of NPs in an increasingly obese HFpEF population seen in real‐world clinical practice today. Furthermore, patients with HFpEF who have normal BNP or NT‐proBNP levels are often excluded from clinical trial enrollment, yet may present with signs and symptoms of HF no different from their counterparts with elevated NP levels.
In a morbidly obese HFpEF cohort hospitalized with acute HF, we found NT‐proBNP on admission to be inversely associated with BMI, though elevated across all obesity categories. The largest percent decrease in NT‐proBNP was seen in the highest obesity category with treatment for acute HF. After adjustment for relevant clinical variables, there was no significant difference in percent change NT‐proBNP across all obesity categories. Nonobese and obese participants had significantly worse 1‐year survival compared with severely obese participants, which has previously been described in HFpEF.21 Patients with HFpEF who had a normal NT‐proBNP in our study had a similar burden of HF, response to diuretic therapy, and clinical outcomes compared with those who had an elevated NT‐proBNP. To our knowledge, this is the first study to examine NT‐proBNP as it corresponds to clinical course in an obese, hospitalized HFpEF cohort marked by a high burden of comorbidities.
NPs in Obese Patients With HFpEF
It is established that NP levels are generally lower in patients with HFpEF compared with HFrEF and may have decreased sensitivity in the diagnosis of HF.22, 23 Despite this, BNP and NT‐proBNP still have prognostic value in HFpEF.24, 25 In a sub hoc analysis from COACH (Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure), baseline BNP levels were followed in patients with HFrEF and HFpEF. The average BNP was significantly lower in patients with an ejection fraction >50%; however, BNP remained a strong predictor of outcomes.25 Obesity is a prominent comorbidity in HFpEF and has been increasingly recognized as a distinct HFpEF phenotype, associated with high morbidity and adverse outcomes.10 The clinical diagnosis of acute HF in obese patients with HFpEF can be challenging given the overlapping symptoms on presentation related to multiple comorbid conditions. Furthermore, volume assessment on presentation and throughout the treatment course in hospitalized obese patients with HFpEF is often challenging. Although the inverse relationship between NP levels and obesity is well‐known,8, 9, 10 we found the median NT‐proBNP level to be markedly elevated even in our most obese patients (BMI ≥40 kg/m2). This has potential implications for the utility of NT‐proBNP in the specific scenario of diagnosing acute HF in obese patients with HFpEF, an otherwise challenging clinical scenario. The findings also support the development of a BMI‐adjusted NP scale for use in clinical practice.
Change in NT‐proBNP With Diuresis by Obesity Category
Although the percent change in NP level with HF treatment has been extensively studied in HFrEF, there are comparatively fewer studies in HFpEF.14, 26, 27, 28 Current ACC/AHA/HFSA guidelines recommend checking an admission (class I, level of evidence A) and predischarge (class IIa, level of evidence B) BNP or NT‐proBNP for HF prognostication with no established role for targeting a certain threshold value or relative change during HF hospitalization.7 The literature on NP levels in acute HFpEF is limited. There are 3 studies to date investigating BNP or NT‐proBNP levels in HFpEF patients with acute HF demonstrating the inverse relationship between NP level and BMI; lower NP levels in patients with HFpEF compared with those with HFrEF; and the prognostic impact of an elevated NP level during hospitalization.12, 13, 14 Our study is the first to report the change in NP level with treatment for acute HF in an obese HFpEF patient population.
We found that, unexpectedly, the highest obesity group, which had the relatively lowest admission and discharge NT‐proBNP levels, had the greatest percent decrease in NT‐proBNP with acute HF treatment. This has potential implications for both clinical practice and for clinical trials. In clinical practice, there may be utility to assess a change in NT‐proBNP as a marker of decongestion even in severely obese patients with HFpEF. In HFpEF clinical trials, both obesity and low NT‐proBNP are often exclusion criteria with the concern that the presence of 1 or both may not represent “true” clinical HFpEF. Our study suggests that in fact the most obese patients with HFpEF may be the most responsive to diuretic therapy and potentially other NP pathway‐targeting therapies. Our findings that in adjusted analysis all obesity categories demonstrated a similar percent change in NT‐proBNP would again suggest that morbidly obese patients with HFpEF respond to HF treatment as much as their lower BMI counterparts.
Survival Post‐Hospitalization in Obese Patients With HFpEF
The relationship between survival and obesity in HF has been explored in both HFrEF and HFpEF. A subanalysis from the I‐PRESERVE (Irbesartan in Heart Failure With Preserved Ejection Fraction) trial demonstrated that the highest rates of adverse outcomes, including death or cardiovascular hospitalizations, were seen in the extreme BMI categories after adjustment for multiple risk variables.29 A similar U‐shaped relationship was described in 2 separate meta‐analyses that included both patients with HFrEF and those with HFpEF.21, 30 Interestingly, we did not observe a U‐shaped relationship between BMI and survival; however, our cohort was overall morbidly obese, with a relatively small percentage who were nonobese. We also did not observe significant differences in baseline comorbidities across different BMI categories, which has been proposed to explain the U‐shaped relationship seen in larger clinical studies. Ultimately, the mechanistic and prognostic associations between obesity, NP levels, and survival in HFpEF warrants further investigation.
Normal Natriuretic Peptide Levels in HFpEF
Prior studies have shown that patients with HFpEF can have a normal BNP level despite clinical evidence of HF.24, 31 In the 13% of our patients who presented with a normal NT‐proBNP level on hospital admission, there was no difference in clinical presentation or treatment course compared with those with an elevated NT‐proBNP level. There was also no difference in clinical outcomes, although prior studies have shown worse clinical outcomes in patients with HFpEF who have an elevated BNP level compared with those who have a normal BNP level.24 The low NP levels in our patients can likely be explained by younger age, higher BMI, lower rates of atrial fibrillation, and relatively less structural heart disease compared with patients with HFpEF who have elevated NT‐proBNP level. Our finding of a large percent decrease in NT‐proBNP with acute HF management irrespective of the admission NT‐proBNP level supports a mechanistic role for change in left ventricular end‐diastolic pressure contributing to NT‐proBNP levels in acute decompensated HFpEF. These findings would suggest that relative (or change) in NP levels may aid in the assessment of response to therapies in patients with acute decompensated HFpEF. Further understanding of NP levels (normal and elevated) in HFpEF and response to acute HF therapies in larger clinical trials is warranted.
Limitations
The results of this study should be interpreted in the context of several limitations. Our study was underpowered to detect differences in clinical events between obesity categories, as this was a secondary analysis of a clinical trial. Inpatient diuretic dosing was not available from the original clinical trial because of a change in the electronic medical record system at our institution during the course of the clinical trial. Although dosage information was not included as a clinical variable in our study, there was no significant difference in fluid loss between the different diuretic categories (intermittent furosemide versus continuous infusion groups). Additionally, we defined obesity categories by BMI cutoffs and did not include measures of central adiposity including waist circumference and waist‐to‐hip ratio. Finally, BMI values were used based on admission weight, which may be subject to inherent inaccuracies attributable to discrepancies in hospital scales and weight fluctuations during the hospitalization course with treatment for acute HF.
Conclusions
HFpEF is an increasingly challenging condition to treat in real‐world practice, with a high prevalence of obesity in this patient population. In a morbidly obese HFpEF cohort hospitalized for acute HF, we found that NT‐proBNP was inversely associated with BMI, and that the highest obesity category saw the greatest percent decrease in NT‐proBNP with diuresis. Severely obese patients had improved survival at 1 year following hospitalization compared with those who were less obese and nonobese. Finally, 13% of our cohort had a normal NT‐proBNP level on hospital admission, yet had a similar clinical presentation, hospitalization course, and outcomes postdischarge compared with patients with elevated NT‐proBNP. Our findings have implications for the role and interpretation of NT‐proBNP in patients with acute decompensated HFpEF, particularly in obese patients, from diagnosis to assessment of therapeutic response. Future studies are warranted to understand the role of NPs in this population and for the development of BMI‐calibrated NP scales for use in the diagnosis and management of acute decompensated HFpEF.
Sources of Funding
None.
Disclosures
K. Sharma has grant funding from an American Heart Association Go Red for Women network grant (AHA #16SFRN28780016), Dallas, TX, and a Johns Hopkins University Clinician Scientist Award. K. Sharma is a consultant and advisory board member for Novartis and receives honoraria. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e015738 DOI: 10.1161/JAHA.119.015738)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015738
For Sources of Funding and Disclosures, see page 10.
References
- 1. Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ, Rosamond WD. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014). Circulation. 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49:1943–1950. [DOI] [PubMed] [Google Scholar]
- 5. Zairis MN, Tsiaousis GZ, Georgilas AT, Makrygiannis SS, Adamopoulou EN, Handanis SM, Batika PC, Prekates AA, Velissaris D, Kouris NT, et al Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int J Cardiol. 2010;141:284–290. [DOI] [PubMed] [Google Scholar]
- 6. Cheng V, Kazanagra R, Garcia A, Lenert L, Krishnaswamy P, Gardetto N, Clopton P, Maisel A. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37:386–391. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 8. Krauser DG, Lloyd‐Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, Chen A, Tung R, Januzzi JL. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149:744–750. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. [DOI] [PubMed] [Google Scholar]
- 10. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–279. [DOI] [PubMed] [Google Scholar]
- 12. Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, Jneid H, Agarwal S, Chang PP, Loehr L, et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: the Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol. 2017;233:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stavrakis S, Pakala A, Thadani U, Thomas J, Chaudhry MA. Obesity, brain natriuretic peptide levels and mortality in patients hospitalized with heart failure and preserved left ventricular systolic function. Am J Med Sci. 2013;345:211–217. [DOI] [PubMed] [Google Scholar]
- 14. Salah K, Stienen S, Pinto YM, Eurlings LW, Metra M, Bayes‐Genis A, Verdiani V, Tijssen JG, Kok WE. Prognosis and NT‐proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart. 2019;105:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckley LF, Canada JM, Del Buono MG, Carbone S, Trankle CR, Billingsley H, Kadariya D, Arena R, Van Tassell BW, Abbate A. Low NT‐proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction: HFpEF and very low NT‐proBNP levels. ESC Heart Fail. 2018;5:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma K, Vaishnav J, Kalathiya R, Hu JR, Miller J, Shah N, Hill T, Sharp M, Tsao A, Alexander KM, et al Randomized evaluation of heart failure with preserved ejection fraction patients with acute heart failure and dopamine. JACC Heart Fail. 2018;6:859–870. Available at: https://linkinghub.elsevier.com/retrieve/pii/S2213177918302865. Accessed August 30, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Castro AF, Feldman HI, Kusek JW, Eggers P, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization ed . Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 19. Anon . proBNP Method Sheet/Package Insert. Roche Diagnostics USA, Indianapolis, IN. Available at: https://usdiagnostics.roche.com/en/documentation.html#/c/MS/n/KITPARAM1200MAT04842464160/pb. Accessed August 1, 2018.
- 20. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction. J Am Coll Cardiol. 2017;24140. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, Cleland JGF. Body mass index and all‐cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose–response meta‐analysis. Clin Res Cardiol. 2019;108:119–132. [DOI] [PubMed] [Google Scholar]
- 22. Maisel AS, Hollander JE, Abraham WT, Westheim A, Kazanegra R. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;37:161–167. [DOI] [PubMed] [Google Scholar]
- 23. O'Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, Januzzi JL. The effects of ejection fraction on N‐terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Cardiac Fail. 2005;11:S9–S14. [DOI] [PubMed] [Google Scholar]
- 24. Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiadi M. Prevalence, clinical phenotype, and outcomes associated with normal B‐type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JGP, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- 26. Karlström P, Alehagen U, Boman K, Dahlström U; on behalf of the UPSTEP‐study group . Brain natriuretic peptide‐guided treatment does not improve morbidity and mortality in extensively treated patients with chronic heart failure: responders to treatment have a significantly better outcome. Eur J Heart Fail. 2011;13:1096–1103. [DOI] [PubMed] [Google Scholar]
- 27. Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, Hamid AK, Nicholis MG, Richards MA. N‐terminal Pro–B‐type natriuretic peptide‐guided treatment for chronic heart failure. J Am Coll Cardiol. 2009;55:53–60. [DOI] [PubMed] [Google Scholar]
- 28. Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, Yamuti S, Wagner B, Pacher R. N‐terminal Pro–B‐type natriuretic peptide‐guided, intensive patient management in addition to multidisciplinary care in chronic heart failure. J Am Coll Cardiol. 2010;55:645–653. [DOI] [PubMed] [Google Scholar]
- 29. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massi BM, Body Carson PE. Mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the irbesartan in heart failure with Preserved Ejection Fraction (I‐PRESERVE) Trial. Circ Heart Fail. 2011;4:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Padwal R, McAlister FA, McMurray JJV, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta‐analysis of individual patient data. Int J Obes. 2014;38:1110–1114. [DOI] [PubMed] [Google Scholar]
- 31. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure. J Am Coll Cardiol. 2006;47:742–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


