Abstract
Background
Data on the association of systolic and diastolic blood pressure with the structure and function of failing hearts with preserved ejection fraction (EF) are sparse.
Methods and Results
This analysis included 935 patients with heart failure (49.4% women; mean age, 69.9 years) with preserved EF (≥45%) enrolled in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial before initiation of randomized therapy. Left ventricular (LV) structure (dimensions, wall thickness, and mass index), diastolic function (left atrial volume index, transmitral blood flow, and mitral annular velocities), and systolic function (EF and longitudinal strain) were assessed echocardiographically. In multivariable‐adjusted analyses, association sizes expressed per 1‐SD (14.8–mm Hg) increment in systolic blood pressure were 0.020 cm (P=0.003) and 0.018 cm (P=0.004) for LV septal and posterior wall thickness, respectively, and 2.42 mg/m2 (P=0.018) for LV mass index. The corresponding associations with diastolic blood pressure were nonsignificant (P≥0.067). In similarly adjusted analyses, the association sizes expressed per 1‐SD (10.7–mm Hg) increment in diastolic blood pressure were −0.15 for E/A (P<0.001), −0.76 for E/e′ (P=0.006), and −0.62% for EF (P=0.024). These findings were consistent, if models including systolic blood pressure were additionally adjusted for diastolic blood pressure and vice versa, albeit that the relation of EF with diastolic blood pressure weakened (−0.54%; P=0.10).
Conclusions
In diastolic heart failure, LV wall thickness and LV mass index increased with higher systolic blood pressure, but not with higher diastolic blood pressure, whereas functional measures reflecting diastolic LV function decreased with higher diastolic blood pressure, independent of systolic blood pressure. These observations highlight the importance of controlling both systolic and diastolic blood pressure as modifiable risk factors to reduce the risk of LV remodeling and diastolic LV dysfunction.
Keywords: blood pressure, diastolic heart failure, echocardiography, hypertension, left ventricle
Subject Categories: Heart Failure, High Blood Pressure, Echocardiography
Nonstandard Abbreviation and Acronyms
- e′ peak
peak early diastolic mitral annular tissue velocity
- E
peak early diastolic transmitral flow velocity
- EF
ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- LV
left ventricular
Clinical Perspective
What Is New?
In patients with heart failure with preserved ejection fraction, low diastolic blood pressure is a forerunner of adverse cardiovascular events.
However, to our knowledge, no previous study described how left ventricular structure and function in patients with heart failure with preserved ejection fraction are related to systolic and diastolic blood pressure.
What Are the Clinical Implications?
Our current study highlights the importance of controlling both systolic and diastolic blood pressure as modifiable risk factors to reduce the risk of left ventricular remodeling and diastolic left ventricular dysfunction in patients with heart failure with preserved ejection fraction or at risk of heart failure.
Overtreatment with antihypertensive drugs to reduce left ventricular afterload and to improve the ejection fraction should be balanced against the risk of excessively lowering diastolic blood pressure, exposing the myocardium to ischemia and further functional deterioration.
Hypertension is the most important modifiable cardiovascular risk factor, as documented in numerous population studies,1 patient cohorts,2, 3 and randomized clinical trials.4 More than half a century ago, the Framingham investigators established that higher blood pressure increases cardiovascular complications.5 Diastolic blood pressure drives the cardiovascular risk in young and middle‐aged adults, whereas in older people, cardiovascular complications are more closely associated with the pulsatile components of blood pressure, as exemplified by systolic blood pressure or pulse pressure.6, 7 Systolic hypertension increases the afterload against which the left ventricle (LV) has to operate.8 Diastolic blood pressure sustains blood flow through the cardiac capillary network.9, 10 An excessively low diastolic blood pressure leads to reduced coronary blood flow9 and subclinical myocardial damage.10 Although the aforementioned observations are firmly established in populations and hypertensive patients,1, 2, 3, 4, 5, 6, 7 in patients with heart failure with preserved ejection fraction (HFpEF), the risk associated with systolic and diastolic blood pressure might be different. HFpEF, also known as diastolic heart failure, represents ≈50% of all heart failure cases.11 In HFpEF, low diastolic blood pressure is a forerunner of adverse cardiovascular events.12, 13 Shah and colleagues demonstrated that the echocardiographic phenotype of HFpEF is heterogeneous, but did not report on the association of the echocardiographic traits with blood pressure.14 Furthermore, using as key words in title or abstract “blood pressure” AND “heart failure” combined with one of the following search terms, “echocardiograph*” OR “left ventricul*” OR “ejection fraction,” a literature search did not reveal any previous study describing how LV structure and function in patients with HFpEF are related to systolic and diastolic blood pressure. We addressed this issue by analyzing the echocardiographic data obtained at baseline in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial (NCT00094302).15
Methods
Study Population
The TOPCAT Trial was an international, multicenter, randomized, double‐blind, placebo‐controlled trial.15 The study was designed to investigate whether spironolactone improved clinical outcomes in patients with HFpEF compared with placebo. The TOPCAT Trial complied with the Declaration of Helsinki16 and received ethical clearance. All patients signed informed consent before randomization. To obtain access to the TOPCAT Trial data, we first registered at the website of the Biologic Specimen and Data Repository Information Coordinating Center of National Heart, Lung, and Blood Institute (https://biolincc.nhlbi.nih.gov/). Next, we submitted a request for accessing the TOPCAT Trial data along with a protocol for the intended post hoc analysis and the approval by the ethics committee of the First Affiliated Hospital, Sun Yat‐Sen University, Guangzhou, China. After we signed a Research Materials Distribution Agreement, National Heart, Lung, and Blood Institute transferred anonymized data. The requests to access the data set should be sent to the National Heart, Lung, and Blood Institute.
At 233 sites in 6 countries, 3445 patients with HFpEF were randomly assigned to spironolactone or placebo. Eligible patients were aged ≥50 years, had ≥1 sign, and had at least 1 symptom of heart failure with an ejection fraction (EF) not lower than 45%, controlled systolic blood pressure (defined as a systolic blood pressure of <140 or <160 mm Hg if the patient was on ≥3 antihypertensive drugs), and a serum potassium concentration level of <5.0 mmol/L. Of 3445 randomized patients with HFpEF, 935 (27.1%) underwent echocardiography before the initiation of randomized treatment14 and were available for statistical analysis in the current study.
Echocardiographic Measurement
At 27 of 270 TOPCAT Trial study sites, patients consented to participate in the echocardiographic substudy, which was performed according to the recommendations of the American Society of Echocardiography, as previously described.14, 17 Dedicated analysts read all study echocardiograms at the core laboratory at the Brigham and Women's Hospital, Boston, MA. The readers were blinded to clinical information and randomized assignment. Of the 935 analyzable imaging studies, complete 2‐dimensional and Doppler data were available in 553 (59%), with all Doppler measures missing in 181 (19%) and tissue Doppler only missing in an additional 147 (16%) patients. Among the 78% of participants with Doppler measures, 76% were in sinus rhythm. Each measure was performed by the same analyst for all study participants. Intraobserver variability, performed in 60 studies, was as follows: wall thickness: coefficient of variation, 12%; bias, 0.02±0.1 cm; LV end‐diastolic volume: coefficient of variation, 12%; bias, 1.6±10.5 mL; LV end‐systolic volume: coefficient of variation, 18%; bias, 2.6±5.9 mL; LV EF: coefficient of variation, 6.6%; bias, 2.0±4.3%; peak early diastolic mitral annular tissue velocity (e′): coefficient of variation, 7.0%; bias, 0.1±0.4 cm/s; peak early diastolic transmitral flow velocity (E)/e′ ratio: coefficient of variation, 11%; bias, 0.2±1.2.14, 17
In this study, we statistically analyzed LV structure, including LV dimensions, wall thickness, and mass index; diastolic function, including left atrial volume index, transmitral blood flow, and mitral annular tissue velocities; and systolic function, including EF and longitudinal strain.
In short, LV endocardial borders were manually traced at end diastole and end systole in the apical 4‐ and 2‐chamber views, and LV volumes were derived according to the modified biplane Simpson rule.18 In cases where the Simpson method could not be used because of missing or poor‐quality apical views, the EF was calculated using the Teicholz method.19 Given the low prevalence of regional wall motion abnormalities, LV mass was calculated by the American Society of Echocardiography recommended formula for estimation of LV mass from LV linear dimensions and indexed to body surface area.
Left atrial volume indexed to body surface area was assessed by the biplane area‐length method from apical 2‐ and 4‐chamber views at end systole.18 The e′ value was measured from the septal and lateral sites of the mitral annulus. Mitral inflow velocity was assessed by pulsed wave Doppler from the apical 4‐chamber view, by positioning the sample volume at the tip of the mitral leaflets. E/e′ ratio was calculated as E wave divided by e′. LV longitudinal strain was assessed by 2‐dimensional speckle‐tracking echocardiography.
Other Measurements
Patients who participated in the TOPCAT Trial underwent a detailed baseline evaluation. Blood pressure was measured manually in 75.6% of participants and by automated techniques in 24.4%. Body mass index was weight in kilograms divided by the height squared in meters. Study nurses also administered a standardized questionnaire inquiring into each participant's medical history, smoking habits, and intake of medications.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute Inc, Cary, NC), maintenance level 5. We applied the Kolmogorov‐Smirnov test for assessing the normality of distributions. For between‐group comparison of means and proportions, we applied the large‐sample z‐test and Fisher exact test, respectively. For ease of interpretation, we used the absolute value of the longitudinal strain measurements, which were all negative. Significance was a 2‐sided α level of ≤0.05.
In unadjusted and multivariable‐adjusted linear regression analyses, we expressed the association sizes of the echocardiographic indexes with blood pressure for a 1‐SD increment in systolic or diastolic blood pressure. In multivariable‐adjusted analyses, in line with previous TOPCAT Trial publications,14, 17 we accounted for sex, age, ethnicity, body mass index, heart rate, current smoking, dyslipidemia, diabetes mellitus, use of antihypertensive medications by drug class (ie, diuretics, β blockers, inhibitors of the renin‐angiotensin system [angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers], and calcium channel blockers), and intake of aspirin, lipid‐lowering drugs, other cardiovascular medications, and antidiabetic agents. Missing values in the independent variables body mass index (n=2) and blood pressure (n=1) were replaced by their respective means. Missing values in the dependent variables (LV structure and function) were not imputed. In unadjusted and multivariable‐adjusted models that included both systolic and diastolic blood pressure, we computed the variance inflation factor to assess to what extent parameter estimates for systolic and diastolic blood pressure levels were affected by collinearity. We examined whether the association of the echocardiographic indexes with blood pressure differed by subgroups by introducing the interactions of either systolic or diastolic blood pressure with sex, ethnicity, and age.
Results
Characteristics of Participants
The 935 patients with HFpEF included 462 (49.4%) women and were predominantly white (82.4%). Mean±SD values in all patients were 69.9±9.7 years for age, 32.6±7.3 kg/m2 for body mass index, and 128.1±14.8/73.6±10.7 mm Hg for systolic/diastolic blood pressure.
Table 11 lists the characteristics of participants by median of systolic or diastolic blood pressure. Patients in the high compared with the low systolic blood pressure group were more likely to be women, had higher prevalence of hypertension, and more frequently used inhibitors of the renin system and calcium channel blockers. Participants in the high compared with the low diastolic blood pressure group were more likely to be white and had a lower prevalence of diabetes mellitus and dyslipidemia; they used fewer antidiabetic drugs, β blockers, and diuretics, but more inhibitors of the renin system (P≤0.045).
Table 1.
Characteristics of Participants by Median of Systolic and Diastolic Blood Pressure
| Characteristics | Systolic Blood Pressure | Diastolic Blood Pressure | ||||
|---|---|---|---|---|---|---|
| <130 mm Hg | ≥130 mm Hg | P Value | <75 mm Hg | ≥75 mm Hg | P Value | |
| No. (%) with characteristic | 426 (45.6) | 509 (54.4) | 460 (49.2) | 475 (50.8) | ||
| Women | 191 (44.8) | 271 (53.2) | 0.010 | 225 (48.9) | 237 (49.9) | 0.76 |
| Race | ||||||
| White | 358 (84.0) | 412 (80.9) | 0.22 | 367 (79.8) | 403 (84.8) | 0.042 |
| Black | 56 (13.2) | 71 (14.0) | 0.72 | 71 (15.4) | 56 (11.8) | 0.10 |
| Others | 12 (2.8) | 26 (5.1) | 0.025 | 22 (4.8) | 16 (3.4) | 0.52 |
| Current smoking | 45 (10.6) | 36 (7.1) | 0.060 | 32 (7.0) | 49 (10.3) | 0.070 |
| NYHA class III or IV | 153 (36.2) | 190 (37.3) | 0.72 | 179 (39.1) | 164 (34.6) | 0.16 |
| Hypertension | 370 (86.8) | 484 (95.3) | <0.001 | 413 (90.0) | 441 (92.8) | 0.12 |
| Diabetes mellitus | 158 (37.1) | 215 (42.3) | 0.10 | 212 (46.2) | 161 (33.9) | <0.001 |
| Dyslipidemia | 294 (69.0) | 345 (67.9) | 0.72 | 336 (73.2) | 303 (63.8) | 0.002 |
| eGFR <60 mL/min per 1.73 m2 | 175 (41.1) | 225 (44.2) | 0.34 | 229 (49.8) | 171 (36.0) | <0.001 |
| Medications | ||||||
| β Blockers | 347 (81.5) | 394 (77.4) | 0.13 | 377 (82.0) | 364 (76.6) | 0.045 |
| Diuretic | 351 (82.4) | 429 (84.3) | 0.44 | 397 (86.3) | 383 (80.6) | 0.020 |
| Inhibitors of the renin system | 321 (75.4) | 435 (85.5) | <0.001 | 350 (76.1) | 406 (85.5) | <0.001 |
| Calcium channel blocker | 129 (30.3) | 230 (45.2) | <0.001 | 179 (38.9) | 180 (37.9) | 0.75 |
| Antidiabetic agent | 143 (33.6) | 191 (37.5) | 0.21 | 200 (43.5) | 134 (28.2) | <0.001 |
| Other cardiovascular medication | 406 (95.3) | 465 (91.4) | 0.017 | 441 (95.9) | 430 (90.5) | 0.001 |
| Mean±SD of characteristic | ||||||
| Age, y | 70.1±9.9 | 69.7±9.5 | 0.54 | 72.3±9.5 | 67.6±9.3 | <0.001 |
| Body mass index, kg/m2 | 32.5±7.5 | 32.7±7.2 | 0.72 | 33.1±8.1 | 32.1±6.5 | 0.034 |
| Systolic blood pressure, mm Hg | 115.3±9.4 | 138.9±8.5 | <0.001 | 122.0±15.4 | 134.0±11.4 | <0.001 |
| Diastolic blood pressure, mm Hg | 68.4±9.9 | 77.8±9.5 | <0.001 | 64.5±6.7 | 82.3±5.1 | <0.001 |
| Heart rate, beats/min | 68.9±11.7 | 69.1±11.1 | 0.78 | 67.4±11.5 | 70.6±11.0 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 66.6±24.2 | 66.2±19.2 | 0.78 | 62.8±20.4 | 70.0±22.2 | <0.001 |
eGFR was calculated according to the 4‐component MDRD (Modification of Diet in Renal Disease) study prediction equation. Inhibitors of the renin‐angiotensin system include angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers. P values denote the significance of the between‐group differences. eGFR indicates estimated glomerular filtration rate (estimated from serum creatinine); and NYHA, New York Heart Association.
Women compared with men had smaller (P<0.001) LV end‐diastolic and end‐systolic volumes, septal and posterior wall thickness, and LV mass index, but higher (P≤0.002) EF and longitudinal strain (Table 22).
Table 2.
Baseline Cardiac Structure and Function
| Characteristics | Women | Men | All | |||
|---|---|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | No. | Mean±SD | |
| LV structure | ||||||
| LV end‐diastolic volume index, mL/m2 | 429 | 46.2±14.3 | 433 | 53.6±15.7‡ | 862 | 49.9±15.5 |
| LV end‐systolic volume index, mL/m2 | 429 | 18.4±8.4 | 433 | 23.0±10.5‡ | 862 | 20.7±9.8 |
| LV end‐diastolic dimension, cm | 431 | 4.61±0.51 | 447 | 4.99±0.58‡ | 878 | 4.80±0.58 |
| LV end‐systolic dimension, cm | 431 | 3.19±0.44 | 447 | 3.54±0.52‡ | 878 | 3.36±0.51 |
| Septal wall thickness, cm | 431 | 1.14±0.19 | 447 | 1.26±0.21‡ | 878 | 1.20±0.21 |
| Posterior wall thickness, cm | 431 | 1.11±0.18 | 446 | 1.21±0.20‡ | 877 | 1.16±0.20 |
| LV mass index, mg/m2 | 429 | 102.8±29.2 | 446 | 116.3±30.6‡ | 875 | 109.7±30.7 |
| Relative wall thickness | 431 | 0.49±0.10 | 446 | 0.49±0.11 | 877 | 0.49±0.10 |
| LV diastolic function | ||||||
| E/A ratio | 301 | 1.20±0.68 | 249 | 1.29±0.68 | 550 | 1.24±0.68 |
| TDI e′ (lateral), cm/s | 269 | 7.93±3.36 | 234 | 8.53±3.1* | 503 | 8.21±3.3 |
| TDI e′ (septal), cm/s | 257 | 5.95±2.32 | 254 | 6.31±2.2 | 511 | 6.13±2.2 |
| E/e′ (lateral) | 267 | 12.3±6.1 | 226 | 11.3±5.6 | 493 | 11.8±5.9 |
| E/e′ (septal) | 254 | 15.9±6.7 | 245 | 15.4±7.0 | 499 | 15.6±6.8 |
| Left atrial volume index, mL/m2 | 420 | 29.6±12.1 | 414 | 29.9±12.9 | 834 | 29.8±12.5 |
| LV systolic function | ||||||
| Ejection fraction, % | 462 | 60.6±7.3 | 473 | 58.0±8.2‡ | 935 | 59.3±7.9 |
| Longitudinal strain, % | 240 | 16.0±3.5 | 207 | 15.0±3.4† | 447 | 15.6±3.5 |
Longitudinal strain is a negative value, but for ease of interpretation, the absolute value was reported. e′ indicates peak early diastolic mitral annular tissue velocity; E, peak early diastolic transmitral flow velocity; E/A ratio, the ratio of peak early (E) to late (A) diastolic velocities; LV, left ventricular; and TDI, tissue Doppler imaging.
Significance of the sex difference: *P≤0.05, † P≤0.01, and ‡ P≤0.001.
Association of LV Structure With Blood Pressure
In unadjusted analyses (Table 33), the septal and posterior wall thickness and the LV mass index increased with systolic blood pressure. The corresponding associations with diastolic blood pressure were nonsignificant (P≥0.47). Adjustment for diastolic blood pressure did not remove the significance of the associations with systolic blood pressure.
Table 3.
Cardiac Structure and Function in Relation to Blood Pressure at Baseline
| Blood Pressure Model | Models Including SBP or DBP | Models Including SBP and DBP | ||
|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |
| Unadjusted | ||||
| Septal wall thickness, cm | 0.022 (0.008 to 0.036)† | 0.005 (−0.009 to 0.019) | 0.026 (0.010 to 0.042)† | −0.009 (−0.025 to 0.008) |
| Posterior wall thickness, cm | 0.020 (0.007 to 0.033)† | 0.003 (−0.010 to 0.016) | 0.025 (0.010 to 0.040)† | −0.009 (−0.024 to 0.006) |
| LV mass index, mg/m2 | 2.46 (0.45 to 4.46)* | 0.021 (−2.00 to 2.05) | 3.33 (0.99 to 5.66)† | −1.70 (−4.06 to 0.65) |
| Relative wall thickness | 0.007 (0.0004 to 0.014)* | 0.004 (−0.003 to 0.010) | 0.007 (−0.0007 to 0.015) | −0.00001 (−0.008 to 0.008) |
| E/A ratio | −0.056 (−0.11 to −0.0002)* | −0.16 (−0.22 to −0.11)‡ | 0.026 (−0.035 to 0.088) | −0.18 (−0.24 to −0.11)‡ |
| TDI e′, cm/s | −0.11 (−0.32 to 0.11) | 0.003 (−0.22 to 0.23) | −0.14 (−0.39 to 0.10) | 0.076 (−0.18 to 0.33) |
| E/e′ | −0.009 (−0.50 to 0.48) | −1.10 (−1.61 to −0.60)‡ | 0.65 (0.11 to 1.20)* | −1.44 (−2.01 to −0.86)‡ |
| LA volume index, mL/m2 | −0.78 (−1.62 to 0.058) | −1.05 (−1.90 to −0.19)* | −0.35 (−1.32 to 0.62) | −0.86 (−1.86 to 0.13) |
| Ejection fraction, % | −0.13 (−0.64 to 0.38) | −0.93 (−1.43 to −0.43)‡ | 0.48 (−0.11 to 1.07) | −1.18 (−1.77 to −0.59)‡ |
| Longitudinal strain, % | 0.20 (−0.12 to 0.52) | −0.32 (−0.64 to 0.0004)* | 0.48 (0.12 to 0.86)† | −0.57 (−0.94 to −0.20)† |
| Adjusted | ||||
| Septal wall thickness, cm | 0.020 (0.007 to 0.033)† | 0.013 (−0.0009 to 0.027) | 0.019 (0.003 to 0.035)* | 0.002 (−0.015 to 0.019) |
| Posterior wall thickness, cm | 0.018 (0.006 to 0.030)† | 0.012 (−0.001 to 0.025) | 0.017 (0.002 to 0.032)* | 0.002 (−0.014 to 0.018) |
| LV mass index, mg/m2 | 2.42 (0.41 to 4.43)* | 1.03 (−1.11 to 3.16) | 2.71 (0.30 to 5.12)* | −0.56 (−3.12 to 2.00) |
| Relative wall thickness | 0.005 (−0.001 to 0.012) | 0.007 (−0.0002 to 0.014) | 0.002 (−0.006 to 0.011) | 0.006 (−0.003 to 0.014) |
| E/A ratio | −0.069 (−0.13 to −0.013)* | −0.15 (−0.21 to −0.091)‡ | 0.006 (−0.059 to 0.071) | −0.15 (−0.22 to −0.083)‡ |
| TDI e′, cm/s | −0.090 (−0.31 to 0.13) | −0.060 (−0.31 to 0.19) | −0.086 (−0.35 to 0.18) | −0.010 (−0.30 to 0.28) |
| E/e′ | −0.17 (−0.67 to 0.32) | −0.76 (−1.30 to −0.22)† | 0.27 (−0.31 to 0.85) | −0.91 (−1.55 to −0.28)† |
| LA volume index, mL/m2 | −0.62 (−1.46 to 0.22) | −0.038 (−0.94 to 0.86) | −0.86 (−1.87 to 0.14) | 0.47 (−0.61 to 1.55) |
| Ejection fraction, % | −0.41 (−0.92 to 0.098) | −0.62 (−1.16 to −0.081)* | −0.13 (−0.74 to 0.48) | −0.54 (−1.19 to 0.10) |
| Longitudinal strain, % | 0.051 (−0.28 to 0.38) | −0.32 (−0.66 to 0.024) | 0.30 (−0.090 to 0.68) | −0.48 (−0.88 to −0.079)* |
Effect sizes (95% CIs) express the changes in the echocardiographic traits associated with a 1‐SD increase in SBP and DBP. Adjusted estimates account for sex, age, ethnicity, body mass index, heart rate, current smoking, dyslipidemia, diabetes mellitus, use of antihypertensive medications by drug class (ie, diuretics, β blockers, inhibitors of the renin‐angiotensin system, and calcium channel blockers), and intake of aspirin, lipid‐lowering drugs, other cardiovascular medications, and antidiabetic agents. In all models, the variance inflation factor for collinearity between SBP and DBP was ≤1.72. Longitudinal strain is a negative value, but for ease of interpretation, longitudinal strain was expressed as an absolute value. DBP indicates diastolic blood pressure; e′, peak early diastolic mitral annular tissue velocity; E, peak early diastolic transmitral flow velocity; E/A ratio, the ratio of peak early (E) to late (A) diastolic velocities; LA, left atrial; LV, left ventricular; SBP, systolic blood pressure; and TDI, tissue Doppler imaging.
Significance of the associations: *P≤0.05, † P≤0.01, and ‡ P≤0.001.
With adjustments applied for sex, age, ethnicity, body mass index, heart rate, current smoking, dyslipidemia, diabetes mellitus, use of antihypertensive medications by drug class, and intake of aspirin, lipid‐lowering drugs, other cardiovascular medications, and antidiabetic agents, the association sizes with systolic blood pressure were 0.020 cm (P=0.003) and 0.018 cm (P=0.004) for septal and posterior thickness, respectively, and 2.42 mg/m2 (P=0.018) for LV mass index. Additional adjustment for diastolic blood pressure produced confirmatory results (Figure).
Figure 1. Multivariable‐adjusted associations of the left ventricular (LV) mass index (A) and E wave/peak early diastolic tissue velocity (E/e′) (B) with systolic (SBP) and diastolic (DBP) blood pressure in patients with heart failure with preserved ejection fraction.
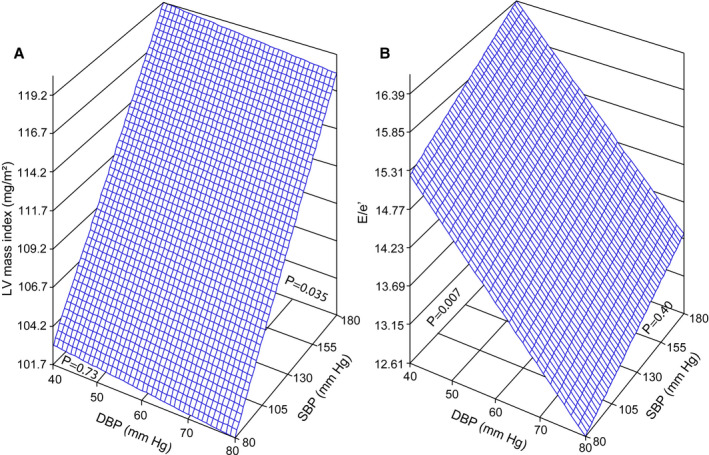
The plane shows the independent associations of LV mass index and E/e′ with SBP and DBP. The plotted plane was standardized to the mean distribution in the whole study patients of sex, age, ethnicity, body mass index, heart rate, current smoking, dyslipidemia, diabetes mellitus, use of antihypertensive medications by drug class (ie, diuretics, β blockers, inhibitors of the renin‐angiotensin system, and calcium channel blockers), and intake of aspirin, lipid‐lowering drugs, other cardiovascular medications, and antidiabetic agents.
Association of LV Function With Blood Pressure
In unadjusted analyses (Table 33), E/A, E/e′, and left atrial volume index decreased with higher diastolic blood pressure with association sizes per 1‐SD (10.7–mm Hg) increment in diastolic blood pressure. Conversely, the EF and longitudinal strain decreased with higher diastolic blood pressure. Adjustment for systolic blood pressure did not remove the significance of the associations with diastolic blood pressure, except for left atrial volume index (P=0.088).
In multivariable‐adjusted models, the association sizes with diastolic blood pressure were −0.15 for E/A (P<0.001), −0.76 for E/e′ (P=0.006), and −0.62% for EF (P=0.024). With additional adjustment for systolic blood pressure, the corresponding association sizes were −0.15 for E/A (P<0.001), −0.91 for E/e′ (P=0.005), and −0.54% for EF (P=0.10). In all models including both systolic and diastolic blood pressure, the variance inflation factor was ≤1.72.
Sensitivity Analysis
In multivariable‐adjusted models relating diastolic dysfunction to diastolic blood pressure, we additionally adjusted for longitudinal strain, which produced confirmatory results. The same was true when models relating longitudinal strain to diastolic blood pressure were additionally adjusted for E/e′.
Sensitivity analyses of LV structure and function related to blood pressure in various subgroups delineated by sex (Tables S1 and S2), ethnicity (whites versus nonwhites; Table S3), and median of age (Table S4) generated confirmatory results. Introducing an interaction term of systolic or diastolic blood pressure with sex (P≥0.25), ethnicity (P≥0.051), or age (P≥0.053) into multivariable‐adjusted models relating indexes of LV structure and diastolic dysfunction to blood pressure produced results similar to those reported in Table 33. However, the interaction of ethnicity was significant for septal (P≤0.025) and posterior thickness (P≤0.003) with systolic and diastolic blood pressure and for E/A (P=0.043) with diastolic blood pressure. The interaction of age was significant for septal (P=0.002) and posterior thickness (P<0.001), LV mass index (P<0.001), and E/e′ (P=0.024) with systolic blood pressure and for left atrial volume index (P=0.046) with diastolic blood pressure.
Discussion
In the current study, we examined the association of LV structure and function with systolic and diastolic blood pressure in patients with HFpEF. The key findings of our study can be summarized as follows: (1) in patient with HFpEF, even with multiple adjustments applied, LV wall thickness and LV mass index increased with higher systolic blood pressure, independent of diastolic blood pressure; and (2) the functional measures reflecting LV diastolic function were inversely associated with higher diastolic blood pressure, independent of systolic blood pressure.
The literature describes several pathophysiological mechanisms potentially underlying the differential association of cardiac structure and function with systolic and diastolic blood pressure.20 First, the pathophysiological characteristics of HFpEF include not only diastolic function but also impaired ventricular‐vascular coupling and an excessive peripheral vasodilation.21 A low systolic blood pressure in HFpEF is part of the syndrome and a hallmark indicative of a more severely ill patient population. The same applies to the low stroke volume, which may reflect concentric LV remodeling and LV hypertrophy, resulting in a disproportionally small and stiff LV. Antihypertensive therapy may reduce arterial and ventricular stiffness, enhance ventricular‐arterial coupling, and improve systolic and diastolic LV function.22 Furthermore, a low diastolic blood pressure can result in decreased coronary perfusion pressure, leading to further myocardial damage and worsening LV dysfunction.10
Our current findings might explain previously reported outcome studies relating adverse health outcomes to systolic or diastolic blood pressure. In TOPCAT Trial patients, there was no association between adverse health outcomes and systolic blood pressure at enrollment.23 In contrast, in 10 535 patients with heart failure and reduced EF enrolled in the Medicare‐linked OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry, a systolic blood pressure of <130 mm Hg predicted all‐cause mortality and rehospitalization for any cause or for heart failure.24 The hazard ratios amounted to 1.32 (95% CI, 1.15–1.53), 1.11 (95% CI, 1.01–1.23), and 1.24 (95% CI, 1.09–1.42), respectively.24 Among 3471 TOPCAT Trial patients followed up for 3.0 years, 881 experienced a primary outcome event.12 Compared with patients with a diastolic blood pressure of 80 to 89 mm Hg, the adjusted hazard ratios in patients with diastolic blood pressure <60 mm Hg were 1.65 (95% CI, 1.29–2.11) for the primary outcome and 1.89 (95% CI, 1.37–2.61) for all‐cause mortality.12 The association between hospitalization for heart failure and diastolic blood pressure was linear, whereas the association of death and cardiovascular death was nonlinear, with a greater risk of death if diastolic blood pressure was <60 mm Hg or ≥90 mm Hg.13 In line with these findings, our current findings demonstrated that the functional measures reflecting LV diastolic function were inversely associated with higher diastolic blood pressure. Furthermore, patients with HFpEF usually have clinical or subclinical LV systolic dysfunction, as assessed with LV global strain.25 In multivariable‐adjusted models relating diastolic dysfunction to diastolic blood pressure, we additionally adjusted for longitudinal strain, which produced confirmatory results. The same was true when models relating longitudinal strain to diastolic blood pressure were additionally adjusted for E/e′. Of note, increased afterload is well known to reduce longitudinal strain in the general population25, 26 and in individuals with stage A subclinical heart failure.25 Our study also indicates that higher diastolic blood pressure was associated with a decline in longitudinal strain in patients with preserved systolic function.
Similar mechanisms as currently described might also be at play in the early stages of LV dysfunction. Indeed, heart failure is a progressive condition that begins with risk factors for LV dysfunction (eg, hypertension), proceeds to asymptomatic changes in cardiac structure (eg, LV hypertrophy) and function (eg, impaired LV relaxation), and then evolves into clinically overt heart failure, disability, and death.27 The 5‐year mortality rate of symptomatic heart failure is ≈60%.28 Diastolic heart failure is characterized by slow LV relaxation, increased LV stiffness, increased interstitial deposition of collagen, and modified extracellular matrix proteins.21 Diastolic heart failure accounts for 40% to 50% of all heart failure cases and has a prognosis as ominous as systolic heart failure.21 In randomly recruited European population samples, the frequency of asymptomatic echocardiographically diagnosed diastolic LV dysfunction (early stage) is as high as 27%,29, 30 with a 5‐year progression rate of 10%,31 resulting in 22.5 hospitalization days per 1000 citizens (http://www.ehnheart.org; 2017). Over a 5‐ to 8‐year horizon, both diastolic32 and systolic33 LV dysfunction predict the incidence of cardiovascular complications. Along similar lines, electrocardiographic34 and echocardiographic35 LV hypertrophy predict fatal and nonfatal cardiovascular outcomes.
Strengths and Limitations
To the best of our knowledge, our study is the first to report on the association of cardiac structure and function with both systolic and diastolic levels in patients with HFpEF. We checked whether our multivariable‐adjusted models including both blood pressure components were vulnerable to problems caused by collinearity. However, the variance inflation factor between systolic and diastolic blood pressure levels did not exceed 1.72. On the other hand, our study must also be interpreted within the context of its limitations. First, the present study had a cross‐sectional design, which precludes direct causal inference. Second, not all patients randomized into the TOPCAT Trial underwent echocardiography at baseline. Compared with TOPCAT Trial participants not included in the echocardiographic study, those included differed in some baseline characteristics, which, although relatively minor, may limit the generalizability of these findings.12, 13 Patients with a baseline echocardiogram were on average 1.80 years older (P<0.001) and had a slightly higher body mass index (0.71 kg/m2; P=0.009). However, participants with and without baseline echocardiogram had a similar heart rate and included proportionally a similar number of women, hypertensive patients, and smokers (P≥0.093). Third, we acknowledge that the main aim of the TOPCAT Trial was not to determine the role of blood pressure in patients with HFpEF and that future studies including clinical trials of blood pressure targets in HFpEF may be warranted. Finally, as the current analysis was retrospective, the number of echocardiographic traits available for analysis differed across the study population, with fewer measurements being available for cardiac function than structure. However, body mass index was similar in patients with and without missing echocardiographic traits, suggesting that missingness was not related to the obesity of the patients.
Perspective
From a clinical point of view, our current study highlights the importance of controlling both systolic and diastolic blood pressure as modifiable risk factors to reduce the risk of LV remodeling and diastolic LV dysfunction in patients at risk of diastolic LV dysfunction or with overt HFpEF. Overtreatment with antihypertensive drugs to reduce LV afterload and to improve the EF should be balanced against the risk of excessively lowering diastolic blood pressure, exposing the myocardium to ischemia and further functional deterioration.10
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
Acknowledgments
The authors thank TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial investigators for conducting this trial and making these data available.
(J Am Heart Assoc. 2020;9:e016009 DOI: 10.1161/JAHA.119.016009.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.016009
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Yugang Dong, Email: liuch75@mail.sysu.edu.cn.
Chen Liu, Email: dongxg@mail.sysu.edu.cn.
References
- 1. Li Y, Staessen JA, Sheng CS, Huang QF, O'Rourke M, Wang JG. Age dependency of peripheral and central systolic pressures: cross‐sectional and longitudinal observations in a Chinese population. Hypertens Res. 2012;115–122. [DOI] [PubMed] [Google Scholar]
- 2. Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;2407–2415. [DOI] [PubMed] [Google Scholar]
- 3. Sheppard JP, Tucker KL, Davison WJ, Stevens R, Aekplakorn W, Bosworth HB, Bove A, Earle K, Godwin M, Green BB, et al. Self‐monitoring of blood pressure in patients with hypertension‐related multi‐morbidity: systematic review and individual patient data meta‐analysis. Am J Hypertens. 2019;243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;957–967. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Chest. 1969;43–51. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Björklund‐Bodegård K, Ohkubo T, Jeppesen JL, et al. Ambulatory hypertension subtypes and 24‐hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Staessen JA, Wang JG. Association of target organ damage with 24‐hour systolic and diastolic blood pressures and hypertension subtypes in untreated Chinese. Hypertension. 2014;222–228. [DOI] [PubMed] [Google Scholar]
- 8. Cauwenberghs N, Knez J, D′hooge J, Thijs L, Yang WY, Wei FF, Zhang ZY, Staessen JA, Kuznetsova T. Longitudinal changes in LV structure and diastolic function in relation to arterial properties in general population. JACC Cardiovasc Imaging. 2017;1307–1316. [DOI] [PubMed] [Google Scholar]
- 9. Polese A, De Cesare N, Montorsi P, Fabbiocchi F, Guazzi M, Loaldi A, Guazzi MD. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation. 1991;845–853. [DOI] [PubMed] [Google Scholar]
- 10. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;263–271. [DOI] [PubMed] [Google Scholar]
- 12. Tsujimoto T, Kajio H. Low diastolic blood pressure and adverse outcomes in heart failure with preserved ejection fraction. Int J Cardiol. 2018;69–74. [DOI] [PubMed] [Google Scholar]
- 13. Sandesara PB, O'Neal WT, Kelli HM, Topel M, Samman‐Tahhan A, Sperling LS. Diastolic blood pressure and adverse outcomes in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) Trial. J Am Heart Assoc. 2018;e007475 DOI: 10.1161/JAHA.117.007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;1383–1392. [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;2191–2194. [DOI] [PubMed] [Google Scholar]
- 17. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography guidelines and standards Commitee and the Chamber Quantification Writing group, developed in conjunction with the European Association of Echocardiography, a brand of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;7–11. [DOI] [PubMed] [Google Scholar]
- 20. Leite S, Rodrigues S, Tavares‐Silva M, Oliveira‐Pinto J, Alaa M, Abdellatif M, Fontoura D, Falcão‐Pires I, Gillebert TC, Leite‐Moreira AF, et al. Afterload‐induced diastolic dysfunction contributes to high filling pressures in experimental heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2015;H1648–H1654. [DOI] [PubMed] [Google Scholar]
- 21. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam CS, Shah AM, Borlaug BA, Cheng S, Verma A, Izzo J, Oparil S, Aurigemma GP, Thomas JD, Zile MR, et al. Effect of antihypertensive therapy on ventricular‐arterial mechanics, coupling, and efficiency. Eur Heart J. 2013;676–683. [DOI] [PubMed] [Google Scholar]
- 23. Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail. 2018;483–490. [DOI] [PubMed] [Google Scholar]
- 24. Arundel C, Lam PH, Gill GS, Patel S, Panjrath G, Faselis C, White M, Morgan CJ, Allman RM, Aronow WS, et al. Systolic blood pressure and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;3054–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuznetsova T, Nijs E, Cauwenberghs N, Knez J, Thijs L, Haddad F, Yang WY, Kerkhof PL, Voigt JU, Staessen JA. Temporal changes in left ventricular longitudinal strain in general population: clinical correlates and impact on cardiac remodeling. Echocardiography. 2019;458–468. [DOI] [PubMed] [Google Scholar]
- 27. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;e154–e235. [DOI] [PubMed] [Google Scholar]
- 28. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, et al. How to diagnose heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;2539–2550. [DOI] [PubMed] [Google Scholar]
- 29. Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;105–112. [DOI] [PubMed] [Google Scholar]
- 30. Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, Loster M, Thijs L, Jin Y, Malyutina S, et al. Prevalence of diastolic left ventricular dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovasc Ultrasound. 2012;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuznetsova T, Thijs L, Knez J, Cauwenberghs N, Petit T, Gu YM, Zhang Z, Staessen JA. Longitudinal changes in left ventricular diastolic dysfunction in a general population. Circ Cardiovasc Imaging. 2015;e002882. [DOI] [PubMed] [Google Scholar]
- 32. Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, Staessen JA. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;e000789 DOI: 10.1161/JAHA.114.000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuznetsova T, Cauwenberghs N, Knez J, Yang WY, Herbots L, D'hooge J, Haddad F, Thijs L, Voigt JU, Staessen JA. Additive prognostic value of left ventricular systolic dysfunction in a population‐based cohort. Circ Cardiovasc Imaging. 2016;e004661. [DOI] [PubMed] [Google Scholar]
- 34. Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram: prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;89–105. [DOI] [PubMed] [Google Scholar]
- 35. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;1561–1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


