Abstract
Background
Self‐monitoring of blood pressure (SMBP) improves blood pressure (BP) outcomes at 12‐months, but information is lacking on how SMBP affects hypertension care processes and longer‐term BP outcomes.
Methods and Results
We pooled individual participant data from 4 randomized clinical trials of SMBP in the United Kingdom (combined n=2590) with varying intensities of support. Multivariable random effects regression was used to estimate the probability of antihypertensive intensification at 12 months for usual care versus SMBP. Using these data, we simulated 5‐year BP control rates using a validated mathematical model. Trial participants were mostly older adults (mean age 66.6 years, SD 9.5), male (53.9%), and predominantly white (95.6%); mean baseline BP was 151.8/85.0 mm Hg. Compared with usual care, the likelihood of antihypertensive intensification increased with both SMBP with feedback to patient or provider alone (odds ratio 1.8, 95% CI 1.2–2.6) and with telemonitoring or self‐management (3.3, 2.5–4.2). Over 5 years, we estimated 33.4% BP control (<140/90 mm Hg) with usual care (95% uncertainty interval 27.7%–39.4%). One year of SMBP with feedback to patient or provider alone achieved 33.9% (28.3%–40.3%) BP control and SMBP with telemonitoring or self‐management 39.0% (33.1%–45.2%) over 5 years. If SMBP interventions and associated BP control processes were extended to 5 years, BP control increased to 52.4% (45.4%–59.8 %) and 72.1% (66.5%–77.6%), respectively.
Conclusions
One year of SMBP plus telemonitoring or self‐management increases the likelihood of antihypertensive intensification and could improve BP control rates at 5 years; continuing SMBP for 5 years could further improve BP control.
Keywords: blood pressure, hypertension, self‐monitoring of blood pressure, simulation modeling
Subject Categories: Hypertension, Primary Prevention, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- BPCM
Blood Pressure Control Model
- SMBP
self‐monitoring of blood pressure
- TASMINH
Telemonitoring And Self‐Management in the Control of Hypertension study
Clinical Perspective
What Is New?
We used individual participant data from 4 randomized clinical trials of self‐monitoring of blood pressure (SMBP) with varying levels of support (eg, feedback to patient or provider alone, telemonitoring or self‐management) to examine the changes in the processes of hypertension care (eg, visit frequency, treatment intensification) that led to observed 1‐year blood pressure improvements.
We used a validated mathematical model to project the 5‐year blood pressure outcomes resulting from 1 year of SMBP with varying levels of support based on the observed changes to hypertension care processes at 1 year.
What Are the Clinical Implications?
We found that, compared with usual care, increased levels of support for SMBP significantly increased the likelihood of antihypertensive intensification after an uncontrolled blood pressure measurement.
Compared with usual care, we projected significant improvements in blood pressure control at 5 years after 1 year of SMBP with telemonitoring or self‐management; increasing the duration of SMBP to 5 years further improved blood pressure control.
SMBP with increased levels of support, including telemonitoring or self‐management, increases appropriate intensification of therapy and may be an effective way to improve long‐term blood pressure outcomes.
There is growing evidence that self‐monitoring of blood pressure (SMBP) with guided support beyond usual primary care improves BP control. 1 , 2 The TASMINH (Telemonitoring And Self‐Management in the Control of Hypertension) trials demonstrated that SMBP plus support, including telemonitoring (remotely monitoring patients using communication technology) and self‐management (self‐titration of medications), significantly improves BP control compared with usual primary care. 3 , 4 , 5 , 6 The effect of the short‐term BP control in these trials has been extrapolated in cost‐effectiveness models, which suggest that the SMBP interventions are likely to be cost‐effective provided that the BP effects are maintained for at least 3 years. 7 , 8 , 9 However, these models make assumptions about how SMBP affects clinical care processes (ie, antihypertensive intensification, time between visits) that result in BP lowering, which may not reflect persistent effects of these components on longer‐term BP control.
Simulation models are an efficient way to extrapolate observations from short‐term clinical trials to project longer‐term outcomes and thereby inform clinical guidelines and treatment decisions. 10 , 11 , 12 The BP Control Model (BPCM) is a validated computer simulation model that accurately predicts long‐term BP outcomes driven by 5 essential clinical care processes: (1) time between clinic visits, (2) accuracy of BP measurements, (3) probability antihypertensive medications are intensified when BP is uncontrolled, (4) patient adherence to prescribed antihypertensive medications, and (5) expected BP reduction when antihypertensive treatment is intensified (dose increase or new medication added). 13 , 14
We sought to examine the impact of SMBP with varying levels of support on (1) processes of hypertension care (ie, antihypertensive regimen intensification, frequency of provider encounters) and (2) 5‐year BP and hypertension control outcomes. To accomplish this, we estimated hypertension clinical care process measures using pooled individual participant data from the TASMINH trials and, after entering these data into the BPCM, simulated expected long‐term BP and hypertension outcomes expected from usual care versus SMBP strategies. We then varied the modeled assumptions about how hypertension clinical care processes would be sustained over a 5‐year period.
METHODS
The TASMINH data used in the Phase 1 analysis may be available to researchers for independent analysis subject to data governance permissions and submission of an approved statistical analysis plan. The BPCM and key inputs used in the Phase 2 analysis are available to interested researchers upon reasonable request. Interested researchers can submit a 1‐ to 2‐page research proposal and collaboration plan to Dr. Bellows (BPCM) and are requested to contact Dr. McManus to discuss access requirements (TASMINH data).
Phase 1: Effect of SMBP on Processes of Hypertension Care and BP Outcomes
TASMINH Trials
We pooled individual participant data from 4 TASMINH trials: TASMINH (N=440), TASMINH2 (N=527), TASMIN‐SR (N=450), and TASMINH4 (N=1173). 1 , 4 , 6 , 8 Participants included in the TASMINH studies had uncontrolled hypertension at baseline and were recruited from primary care clinics in the United Kingdom (detailed descriptions, including eligibility criteria, in Table S1). Participants were randomized to receive either usual care alone or usual care with SMBP and support that varied according to the TASMINH trial design (Table 1).
Table 1.
Summary of the Original TASMINH Trial SMBP Interventions
| Study | SMBP Level 2 and Description of Intervention* |
|---|---|
| TASMINH 6 | Level 1—In‐clinic SMBP: Patients performed SMBP in the clinic once each month and were given cards with BP goals and when to seek medical appointment |
| TASMINH4 4 |
Level 2—Home SMBP: Patients performed SMBP at home 2 times per day, received instructions when to contact physician, and sent BP readings to provider through the mail Level 3—Home SMBP+telemonitoring: In addition to Level 2 home SMBP, telemonitoring service included patients sending BP readings to provider via text, alerted patients to contact office for very high or low BP readings, sent reminders if too few readings sent, and sent readings to general practitionerr office |
| TASMINH2 1 and TASMIN‐SR 8 | Level 3—Home SMBP+self‐titration: Patients performed SMBP at home 2 times per day and given a color‐coded system to rate BP measurements. If BP was “above target” for ≥2 consecutive months, patients could self‐titrate according to predetermined schedule |
BP indicates blood pressure; SMBP, self‐monitoring of BP; TASMIN‐SR, Targets and Self‐Management for the control of blood pressure in Stroke and at Risk groups; and TASMINH, Telemonitoring And Self‐Management in the Control of Hypertension.
All trials examined patients with uncontrolled BP in UK primary care settings. Usual care without SMBP was the comparator in each trial. No SMBP interventions included regular one‐to‐one contact with provider for BP management.
At each study visit, BP was measured in the office using automated cuffs (Omron 705CP or BP TRU BPM 100 or 200). The primary outcome in each study was mean systolic BP (SBP) change from baseline with the SMBP intervention compared with usual care at 12 months. Additionally, each study collected data, antihypertensive regimen changes, and healthcare utilization (number of physician visits) for both trial arms.
All TASMINH studies contributing data received full ethical approval from an independent National Research Ethics Committee and all participants provided written informed consent. Only anonymized data were used in the analyses described here.
SMBP Interventions
The comparator arm in all of the TASMINH studies was usual primary care received at the participants' clinic with follow‐up frequency at the discretion of their physician. 1 , 4 , 6 , 8 We classified the 4 TASMINH SMBP trial interventions into 3 levels of support, with degree of support increasing with each level (Table 1). 2 , 15 Level 1 consisted of monthly SMBP physically located in the patients' clinic and educational materials provided at the start of the trial without ongoing physician contact. 6 Level 2 consisted of monthly home SMBP, with instructions indicating when to contact the primary physician's office. 4 Level 3 consisted of monthly home SMBP with telemonitoring (patients sent BP readings to provider and received feedback via SMS text) and/or a prespecified BP management plan, which directed the patient to self‐titrate antihypertensive medications when indicated. 1 , 4 , 8
Outcomes
The primary outcome of the Phase 1 analysis was the association between SMBP (by level of intervention) and the hypertension clinical care processes (ie, physician visits, nonphysician visits, and antihypertensive regimen intensifications) and BP outcomes (SBP and diastolic BP [DBP] changes from baseline) at 12 months. We defined physician and nonphysician visits separately as the total number of in‐person visits during the 12‐month follow‐up. In Phase 1, treatment intensification was defined as the addition of at least 1 new medication class.
Statistical Analysis
To determine the impact of SMBP interventions on the processes of hypertension care, we performed random effects regression analyses including the individual TASMINH study as a random effect. We estimated the probability of treatment intensification at 12 months by level of intervention (with usual care as the reference category), controlling for key components in the BPCM: baseline age, sex, and baseline SBP and DBP, number of antihypertensive medications at baseline, number of physician visits, nonphysician visits, and the number of visits with a controlled SBP and DBP. We used random effects generalized least squares linear regression models to predict mean cumulative number of physician and nonphysician office visits after 12 months of follow‐up for each SMBP intervention level and controlled for age, sex, and baseline SBP and DBP.
To estimate the impact of SMBP interventions on changes in SBP and DBP at 12 months for each SMBP intervention level, we used random effects generalized least squares regression, again with the individual TASMINH study as a random effect. These models adjusted for key characteristics and events used in the BPCM: number of physician visits, number of nonphysician visits, number of antihypertensive medications at baseline, number of antihypertensive treatment regimen intensifications, age, sex, and baseline SBP and DBP. All analyses were performed using STATA version 14.1 (StataCorp LP, College Station, TX).
Phase 2: Simulating the Effect of SMBP Interventions on Long‐Term BP Control Outcomes
BPCM Overview
The BPCM is an individual patient (ie, microsimulation) model that simulates the weekly processes of hypertension management under usual primary care and can be used to simulate BP management interventions (Figure S1). 13 , 14 Every week, the model determines if the patient had an office‐based visit with a physician. At each office visit, the model estimates the patient's measured BP and, when uncontrolled, if the physician intensifies the patient's antihypertensive medication regimen. The model simulates treatment intensification by first by increasing the dose of an existing antihypertensive medication, then subsequently adding a new antihypertensive medication. Finally, patients may become nonadherent to (ie, permanently discontinue) antihypertensive medications each week. For this analysis, we adapted the existing BPCM to include the pill‐taking execution component of adherence (percentage of doses missed) and the impact it has on expected BP reduction, regression to the mean, and simulate SMBP support levels 1 to 3 (Data S1).
The BPCM has been shown to use hypertension care processes to accurately predict 5 to 10‐year SBP, DBP, and BP control rates when compared with the large US‐based observational Multi‐Ethnic Study of Atherosclerosis (MESA), the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), and the Valsartan Antihypertensive Long‐term Use Evaluation Trial (VALUE). 13 , 16 , 17 , 18 Inputs for the BPCM were derived from published literature and national sources (Table S2).
Simulated Population
The BPCM was designed using participants from the 2007–2014 National Health and Nutrition Examination Survey (NHANES). Similar to prior analyses, we created a population of NHANES participants with uncontrolled BP matching the characteristics of the pooled TASMINH studies (Data S1). 1 , 4 , 6 , 8
Model Adaptations
To simulate the impact each SMBP support level had on the processes of hypertension care, we calibrated 2 key processes of hypertension care among US‐based BPCM inputs to match the Phase 1 regression model predictions from the UK‐based TASMINH studies. We first calibrated the frequency of physician visits and then the probability of adding at least one new antihypertensive class at 1 year. We did not match antihypertensive medication adherence as this was not collected by all of the TASMINH studies and rather used existing model inputs (see Model Calibration and Validation). 13 The expected blood pressure reduction from antihypertensive medications was derived from meta‐analyses (Data S1, Table S3). 19 , 20
Outcomes
Our primary outcome was simulated BP control rate (defined based on TASMINH trial thresholds as <140/90 mm Hg without diabetes mellitus or chronic kidney disease; <130/80 mm Hg with diabetes mellitus or chronic kidney disease) over 5 years. Secondary outcomes were mean SBP change, DBP change, and number of physician visits after 5 years.
Model Calibration and Validation
We validated the mean 12‐month SBP and DBP changes predicted by the BPCM against the mean SBP and DBP regression estimates from the TASMINH studies described in Phase 1. For each simulated patient, we used the Phase 1 regression equations predicting changes in processes of care because of the level of intervention to determine their expected SBP and DBP changes and captured their BPCM simulated changes. As SMBP interventions may improve adherence, 21 we calibrated the US‐based adherence parameters (Tables S3 and S4) of the model until mean SBP and DBP changes were within ±2.5 mm Hg of the regression‐based expected mean changes at 12 months for each SMBP support level based on opinion of clinically significant BP changes informed by hypertension management experience.
Statistical Analysis
We simulated 1000 probabilistic iterations of 1000 hypothetical TASMINH trial patients (frequency matched to the pooled TASMINH studies) to compare BP reductions under each of the 4 different SMBP support levels to usual primary care over 5 years. For each probabilistic iteration, the model randomly selected model parameters from prespecified distributions. We defined the 95% uncertainty intervals (95% UI) as the 2.5th to 97.5th percentiles from the 1000 probabilistic iterations.
In the base‐case analysis, we assumed that the SMBP intervention was implemented for 1 year, followed by return to usual care afterward. We also assumed that after SMBP intervention ended, the effect of SMBP on adherence would gradually decrease over time at a constant rate until it was no different than usual care by the end of 5 years (ie, 4 years after the end of the SMBP intervention). In the first of 2 alternative scenarios, we assumed that SMBP interventions were implemented for all 5 years (5‐year SMBP intervention); thus, the impact on hypertension control processes and subsequent effect on BP was sustained for the entire 5 years. In the second scenario, we assumed that SMBP interventions were implemented for only 1 year, but the effect on patient medication adherence was sustained for 5 years. In a 2‐way sensitivity analysis, we examined the effect of simultaneously changing the duration of SMBP interventions (from 1–4 years) and the time until adherence returned to usual care values (from 0–4 years). In another alternative scenario, we assumed no impact of SMBP on adherence over the entire time horizon. All Phase 2 analyses were performed using TreeAge Pro 2019 (TreeAge Software, Inc, Williamstown, MA) and R (R version 3.3.2, Vienna, Austria).
Results
Phase 1: Effect of SMBP on Processes of Hypertension Care and BP Outcomes
Pooled TASMINH Population
TASMINH participants were mostly older adults (mean [SD] age 66.6 [9.5] years), male (53.9%), and largely white (95.6%). BP was assessed at baseline and after 6 and 12 months of follow‐up. Mean baseline SBP was 151.8 (14.2) mm Hg; mean baseline DBP was 85.0 (9.8) mm Hg.
Antihypertensive Medication Intensification
After controlling for covariates in the pooled TASMINH studies, compared with usual care, SMBP interventions with more support, as opposed to self‐monitoring alone, were associated with an increased likelihood of antihypertensive medication intensification by 12 months (Level 2 odds ratio [OR], 1.8; 95% CI, 1.2–2.6; Level 3 OR, 3.3; 95% CI, 2.5–4.2; Table 2). However, Level 1 SMBP interventions (SMBP measured at clinic) were not associated with an increased likelihood of medication intensification compared with usual care (OR, 0.7; 95% CI, 0.4–1.2). The odds of medication intensification were increased with each additional physician visit (OR 1.4; 95% CI, 1.3–1.6) or nonphysician visit (OR, 1.3; 95% CI, 1.2–1.4) during follow up.
Table 2.
Association Between SMBP Intervention Support and Odds of Regimen Intensification During 12‐Month Follow‐Up
| Variable | Odds Ratio | 95% CI | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| Support of intervention (REF: usual care) | |||
| Level 1 | 0.70 | 0.41 | 1.20 |
| Level 2 | 1.80 | 1.20 | 2.60 |
| Level 3 | 3.20 | 2.53 | 4.17 |
Model adjusted for number of physician visits, number of nonphysician visits, number of visits with BP controlled, age, sex, number of physician consultations, and baseline BP. Included 2266 patients from 4 studies. Analysis was a random effects logistic regression with study as a random effect. SMBP indicates self‐monitoring of BP.
Physician and Nonphysician Visits
There was no apparent trend in the association between SMBP support level and number of office visits. (Table 3 and Table S5). SMBP Level 1 was associated with a small increase in physician office visits (0.7; 95% CI, 0.4–1.0) compared with usual care; however, Level 2 was associated with a decrease (−1.2; 95% CI, −1.5 to −1.0) and Level 3 was not significantly different than usual care (−0.0; 95% CI, −0.2 to 0.2). Similarly, compared with usual care, Level 1 interventions were associated with a small increase in nonphysician visits (0.4; 95% CI, 0.2–0.6), whereas both Level 2 (−0.8; 95% CI, −0.9 to −0.6) and Level 3 (−0.4; 95% CI, −0.5 to −0.3) were associated with small decreases.
Table 3.
Association Between SMBP Intervention Support With Number of Physician Visits During 12‐Month Follow‐Up
| Variable | Beta Coefficient | 95% CI | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| Support of intervention (REF: usual care) | |||
| Level 1 | 0.70 | 0.37 | 1.04 |
| Level 2 | −1.24 | −1.47 | −1.00 |
| Level 3 | −0.03 | −0.22 | 0.15 |
Model adjusted for age, sex, number of antihypertensive medications at baseline, and baseline BP. Included 2438 patients from 4 studies. Analysis was a random effects generalized least squares regression with study as a random effect. SMBP indicates self‐monitoring of BP.
SBP and DBP Changes
Compared with the SBP change with usual care at 12 months (mean: −9.5 mm Hg), the adjusted SBP was 3.5 mm Hg higher (95% CI, 0.9–6.0) for SMBP Level 1, 3.8 mm Hg lower (95% CI, −5.8 to −1.8) for Level 2, and 5.4 mm Hg lower (95% CI, −6.9 to −3.8) for Level 3 interventions (Tables S6 and S7). Similarly, compared with the DBP change with usual care at 12 months (mean: −4.7 mm Hg), the adjusted DBP was no different for Level 1 (0.2 mm Hg; 95% CI, −1.3 to 1.3) but was significantly lower for both Level 2 (−1.5 mm Hg 95% CI, −2.5 to −0.4) and Level 3 (−1.5 mm Hg; 95% CI, −2.2 to −0.7) interventions (Tables S6 and S8).
Phase 2: Simulating Long‐Term BP Control Outcomes
BPCM Calibration and Validation
The simulated population was similar to the pooled TASMINH population at baseline; mean age was 65.8 years (95% UI, 65.2–66.4), 53.9% (95% UI, 50.7–56.7%) were male, mean baseline SBP was 152.0 mm Hg (95% UI, 151.4–152.7), and mean DBP was 84.3 mm Hg (95% UI, 83.8–84.9) (Table S9). The calibrated BPCM accurately reproduced the regression analysis results in Phase 1. After calibration, all of the mean values for number of physician visits, antihypertensive medication intensification, SBP change, and DBP change at 12 months predicted by the BPCM in Phase 2 were within the prespecified validation ranges (Table S10).
Simulated Long‐Term BP Outcomes
According to the BPCM, usual care would result in a mean SBP of 140.8 mm Hg (95% UI, 139.3–142.3), mean DBP of 77.5 mm Hg (95% UI, 76.7–78.3), and 33.4% BP control after 5 years (95% UI, 27.7–39.4%; Figure 1 and Table S11). In the base‐case, in which all BP processes returned to usual care values at 12 months, 5‐year BP control rates ended up similar to usual care with Level 1 (33.0% [95% UI, 27.7–39.4%]) and Level 2 (33.9% [95% UI, 28.3–40.3%]), and were improved with Level 3 (39.0% [95% UI, 33.1–45.2%]). In the first scenario in which SBPM‐related BP process improvements persisted for all 5 years, 5‐year BP control rates increased to 52.4% in Level 2 (95% UI, 45.4–59.8%) and to 72.1% in Level 3 (95% UI, 66.5–77.6%). In the second scenario that assumed adherence behavior was sustained for 5 years while other processes of care returned to usual care values, SMBP Levels 2 and 3 had BP control rates of 49.5% (95% UI, 43.7–56.0%) and 54.9% (95% UI, 49.0–61.3%), respectively.
Figure 1.
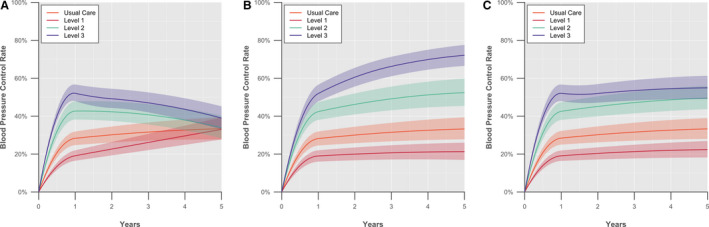
Long‐term simulated blood pressure control rates for SMBP interventions. (A) One year of SMBP followed by return to usual care; (B) 5 years of SMBP; (C) 1 year of SMBP with sustained adherence. The figure shows how blood pressure (BP) control changes over time when patients (A) return to usual care after 1 year of SMBP with various levels of support, (B) SMBP and the associated changes in hypertension care processes continues for 5 years, and (C) return to usual care after 1 year of SMBP but adherence behavior is sustained for 5 years. BP control is defined as BP <130/80 mm Hg with diabetes mellitus or chronic kidney disease and <140/90 mm Hg without chronic kidney disease or diabetes mellitus. The solid lines represent the mean BP control rate and the shaded areas the 95% uncertainty interval (2.5th to 97.5th percentiles); both derived from 1000 probabilistic iterations. SMBP levels are defined as SMBP in clinic (Level 1), home SMBP with feedback when requested by patient (Level 2), and SMBP with telemonitoring or self‐management (Level 3). SMBP indicates self‐monitoring of blood pressure.
Sensitivity Analysis
BP control rates were sensitive to both the duration of SMBP interventions and the time until adherence returned to usual care values in the 2‐way sensitivity analysis (Figure 2). Prolonging the duration of SMBP or time until adherence returned to usual care values improved BP outcomes at 5 years. In the scenario analysis where we assumed no effect of SMBP on adherence, only Level 3 continued to result in improved BP control rates compared with usual care. At 5 years, Level 3 resulted in 36.7% BP control with only 1 year of SMBP and 53.5% BP control with 5 years of SMBP.
Figure 2.
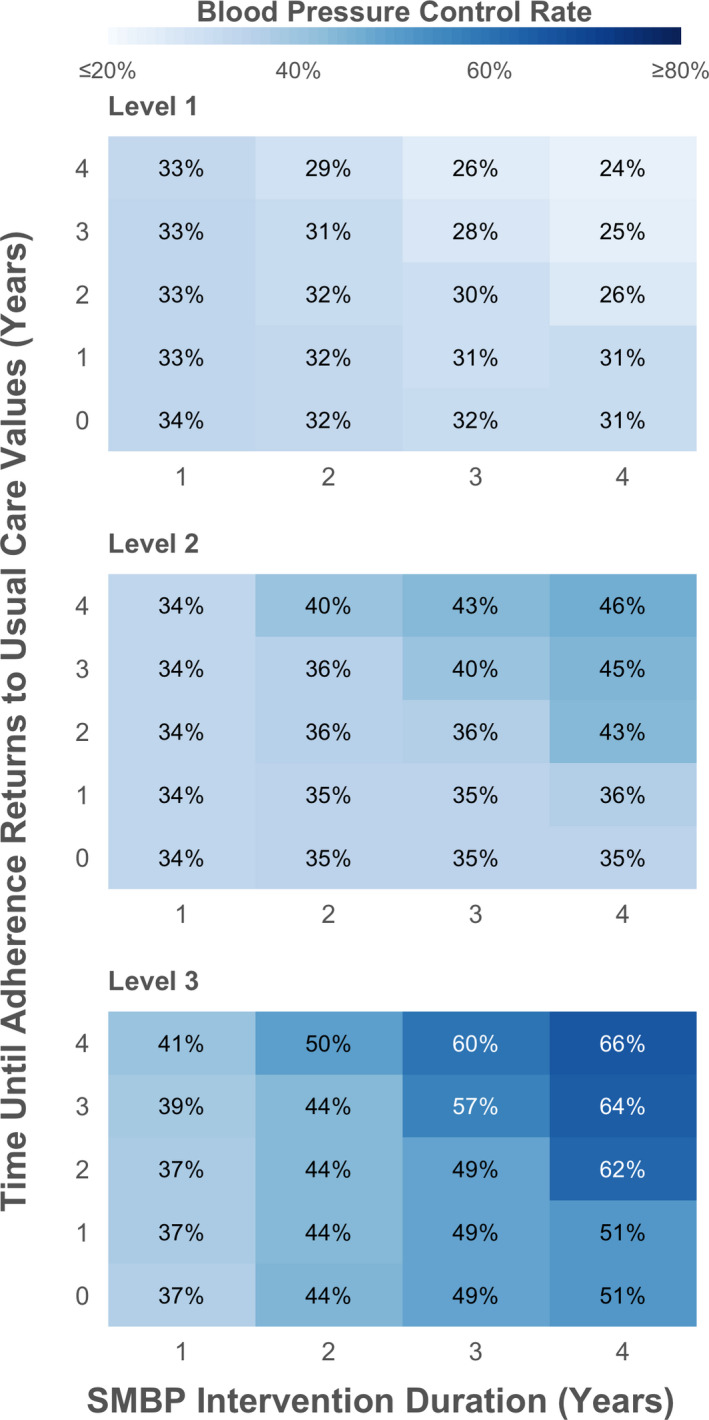
Five‐year blood pressure control rates when varying SMBP duration and time period over which treatment adherence returns to usual care. The figure shows the blood pressure (BP) control rate at 5 years when simultaneously varying the duration of SMBP from 1 to 5 years (x‐axis) and how long it takes the impact of SMBP on adherence to return to usual care estimates (y‐axis) in the BP Control Model. SMBP levels are defined as SMBP in clinic (Level 1), home SMBP with feedback when requested by patient (Level 2), and SMBP with telemonitoring or self‐management (Level 3). BP Control defined as BP <130/80 mm Hg with diabetes mellitus or chronic kidney disease and <140/90 mm Hg without chronic kidney disease or diabetes mellitus. SMBP indicates self‐monitoring of BP.
DISCUSSION
In this analysis based on pooled, individual participant data from 4 published SMBP trials (TAMSINH trials), we found that strategies with more support (Level 2 or 3) increase the probability of clinically indicated antihypertensive intensification, whereas self‐monitoring alone (Level 1) does not differ from usual care. Using a mathematical model, we projected strategies with support may lead to substantial increases in hypertension control at 5 years. These data suggest that SMBP with cointerventions is an effective way to improve long‐term blood pressure control by reducing clinical inertia around treatment intensification.
Prior studies have examined the impact of SMBP on adherence and clinical inertia, but to our knowledge this is the first to quantify the effect of SMBP on processes of routine hypertension care (ie, antihypertensive intensification, time between visits) over an extended time period. 21 , 22 , 23 , 24 The BP reductions projected by the BPCM in our study are consistent with observed findings from the few prior studies that examined the impact of SMBP on BP outcomes beyond 1 year. 24 , 25 , 26 However, none reported outcomes beyond 2 years. A trial of a tailored behavioral telephone intervention paired with SMBP found a nearly 4‐mm Hg SBP reduction over 24 months compared with usual care. 24 Compared with usual care at 24 months, our model projected SBP reductions of 2.8 and 4.9 mm Hg with Level 2 and 3 SMBP interventions, respectively.
Meta‐analyses have confirmed that SMBP accompanied by patient support consistently improves BP and the magnitude of this effect is directly associated with the level of the support. 2 , 27 , 28 Several individual studies in US populations randomizing participants to SMBP with varying levels of support also demonstrate the observed improvements in BP control in the TASMINH studies. 29 , 30 , 31 Despite this evidence base, barriers to integrating SMBP into usual clinical practice remain. 32 , 33 , 34 In the United States and the United Kingdom, about 18% to 33% of adults have used some form of SMBP, but the level of support provided, if any, is unclear. 35 , 36 It is also unknown how effectively the BP information is communicated back to providers so that BP treatment may be intensified when BP is uncontrolled. In a qualitative study in the United Kingdom, patients using SMBP tended not to discuss their experience or BP results with their primary care providers, 37 and providers have indicated they would like more patient involvement in hypertension care, though their clinical workflow is not always structured to handle these tasks outside of usual care. 38 As previously demonstrated, without additional support or a cointervention, SMBP alone has little impact on BP outcomes. 2 , 27 , 28 This notion is confirmed in the differential rates of BP control by intervention level in the current study.
Lack of communication between patient and clinical team regarding BP measurement outside of usual care and high rates of clinical inertia may be mitigated by supported SMBP. Our results show that increased support of SMBP (Level 2 or 3) significantly reduces clinical inertia, an important barrier to achieving high rates of BP control. Self‐titration, included in the Level 3 SMBP intervention in our study, may be a viable strategy to support SMBP, reduce clinical inertia, and improve BP outcomes. 39 , 40 Our findings support prior analyses that BP self‐management, including self‐titration, may be a cost‐effective way to significantly improve BP control. 7 , 8 , 9 Our projections also show that healthcare providers should consider continuing SMBP interventions beyond 1 year to sustain improvements in BP control with supported SMBP.
Limitations
There are a few limitations to note when interpreting the results of our analyses. First, our covariate selection process for Phase 1 was restricted to those related to the processes of hypertension management that may be simulated in BPCM. There may be other important confounders or interaction terms we did not consider. Second, because antihypertensive adherence was not measured in all of the TASMINH studies and the association of SMBP support level with antihypertensive adherence is unknown, we manually calibrated the effect of SMBP on antihypertensive adherence in the BPCM. However, our calibrated estimates were similar to previously published ranges of observed antihypertensive adherence in SMBP trials. 21 Additionally, the BPCM assumes that processes of hypertension care are independent of one another and it does not account for interactions that may exist (eg, physicians may be less likely to intensify medications in patients with poor adherence). The first TASMINH study found a slightly increased BP for Level 1 interventions compared with usual care, though other studies have demonstrated small, but not always statistically significant, decreases in BP for similar interventions. 2 , 6 In our analysis, Level 1 interventions were associated with lower adherence rates, which is perhaps reflective of individuals discontinuing antihypertensive medications based on measured BP, which may be subject to improper measurement technique and lead to an apparent worsening of BP control. Additionally, we did not explicitly model adverse medication events. However, our adherence rates were derived from literature‐based estimates of antihypertensive discontinuation for any reason. Lastly, limited long‐term data are available regarding the duration of BP changes after return to usual care and, to our knowledge, no data examine long‐term (up to 5 years) sustained SMBP interventions. 41 , 42 However, our base‐case approach, in which patients returned to usual care after the 12‐month SMBP intervention, resulted in a similar difference in SBP between Level 3 SMBP and usual care to a study that examined SBP outcomes 54 months after a 12‐month telemonitoring with pharmacist management intervention. At 18 months, the difference in SBP for Level 3 SMBP versus usual care was −5.5 (−6.0 to −4.9) in our analysis, which is comparable to the −6.6 (−10.7 to −2.5) for the intervention versus usual care in the published study. At 54 months, the difference in SBP was −2.3 (−2.8 to −1.7) in our simulation compared with −2.5 (−6.3 to 1.2) in the published study. 42
Conclusions
In conclusion, our pooled analysis of individual participant data found that supported SMBP increased the likelihood of antihypertensive medication intensification over 12 months. Over 5 years, we projected that supported SMBP would significantly improve BP control compared with usual care. Our results underscore the importance of reducing clinical inertia in hypertension and that SMBP may be viable way to improve long‐term BP outcomes.
Sources of Funding
Dr Bryant is supported by T32 HP 10260 from the Health Resources and Services Administration. Dr Sheppard receives funding from the Wellcome Trust/Royal Society via a Sir Henry Dale Fellowship (ref: 211182/Z/18/Z). He also receives funding from the National Institute for Health Research (NIHR) School for Primary Care Research and the NIHR Oxford Biomedical Research Centre at Oxford Health NHS Foundation Trust. Dr Fontil is supported by K23 HL136899 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Dr Moran is supported by R01 HL130500‐01A1 and R01 HL139837 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Dr McManus is an NIHR Senior Investigator and receives support from the NIHR Thames Valley ARC and NIHR School for Primary Care Research. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Dr Bellows is supported by K01 HL140170 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Disclosures
Dr McManus has received BP monitors for research from Omron. The remaining authors have no disclosures to report.
Supporting information
Acknowledgments
This work would not have been possible without the help of the patients and investigators from the TASMINH trials and NHANES.
(J Am Heart Assoc. 2020;9:e016174 DOI: 10.1161/JAHA.120.016174.)
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Bray EP, Holder R, Mant J, McManus RJ. Does self‐monitoring reduce blood pressure? Meta‐analysis with meta‐regression of randomized controlled trials. Ann Med. 2010;42:371–386. [DOI] [PubMed] [Google Scholar]
- 2. Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Earle K, George J, Godwin M, Green BB, et al. Self‐monitoring of blood pressure in hypertension: a systematic review and individual patient data meta‐analysis. PLoS Med. 2017;14:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, Kaambwa B, Banting M, Bryan S, Little P, et al. Telemonitoring and self‐management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163–172. [DOI] [PubMed] [Google Scholar]
- 4. McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, Bradburn P, Farmer A, Grant S, et al. Efficacy of self‐monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, et al. Effect of self‐monitoring and medication self‐titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN‐SR randomized clinical trial. JAMA. 2014;312:799–808. [DOI] [PubMed] [Google Scholar]
- 6. McManus RJ, Mant J, Roalfe A, Oakes RA, Bryan S, Pattison HM, Hobbs FD. Targets and self monitoring in hypertension: randomised controlled trial and cost effectiveness analysis. BMJ. 2005;331:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Penaloza‐Ramos MC, Jowett S, Mant J, Schwartz C, Bray EP, Sayeed Haque M, Richard Hobbs FD, Little P, Bryan S, Williams B, et al. Cost‐effectiveness of self‐management of blood pressure in hypertensive patients over 70 years with suboptimal control and established cardiovascular disease or additional cardiovascular risk diseases (TASMIN‐SR). Eur J Prev Cardiol. 2016;23:902–912. [DOI] [PubMed] [Google Scholar]
- 8. Kaambwa B, Bryan S, Jowett S, Mant J, Bray EP, Hobbs FD, Holder R, Jones MI, Little P, Williams B, et al. Telemonitoring and self‐management in the control of hypertension (TASMINH2): a cost‐effectiveness analysis. Eur J Prev Cardiol. 2014;21:1517–1530. [DOI] [PubMed] [Google Scholar]
- 9. Monahan M, Jowett S, Nickless A, Franssen M, Grant S, Greenfield S, Hobbs FDR, Hodgkinson J, Mant J, McManus RJ. Cost‐effectiveness of telemonitoring and self‐monitoring of blood pressure for antihypertensive titration in primary care (TASMINH4). Hypertension. 2019;73:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abraham JM. Using microsimulation models to inform U.S. health policy making. Health Serv Res. 2013;48:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith BT, Smith PM, Harper S, Manuel DG, Mustard CA. Reducing social inequalities in health: the role of simulation modelling in chronic disease epidemiology to evaluate the impact of population health interventions. J Epidemiol Community Health. 2014;68:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al.; Measures AATFoP and Guidelines AATFoP . ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 13. Bellows BK, Ruiz‐Negron N, Bibbins‐Domingo K, King JB, Pletcher MJ, Moran AE, Fontil V. Clinic‐based strategies to reach United States million hearts 2022 blood pressure control goals. Circ Cardiovasc Qual Outcomes. 2019;12:e005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontil V, Bibbins‐Domingo K, Kazi DS, Sidney S, Coxson PG, Khanna R, Victor RG, Pletcher MJ. Simulating strategies for improving control of hypertension among patients with usual source of care in the United States: the blood pressure control model. J Gen Intern Med. 2015;30:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Godwin M, Green B, Hebert P, Hobbs FDR, et al. Individual patient data meta‐analysis of self‐monitoring of blood pressure (BP‐SMART): a protocol. BMJ Open. 2015;5:e008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 17. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 18. Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, Laragh J, Schork MA, Hua TA, Amerena J, et al. The Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006;48:385–391. [DOI] [PubMed] [Google Scholar]
- 19. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fletcher BR, Hartmann‐Boyce J, Hinton L, McManus RJ. The effect of self‐monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta‐analysis. Am J Hypertens. 2015;28:1209–1221. [DOI] [PubMed] [Google Scholar]
- 22. Bosworth HB, Olsen MK, Neary A, Orr M, Grubber J, Svetkey L, Adams M, Oddone EZ. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns. 2008;70:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barton AB, Okorodudu DE, Bosworth HB, Crowley MJ. Clinical inertia in a randomized trial of telemedicine‐based chronic disease management: lessons learned. Telemed J E Health. 2018;24:742–748. [DOI] [PubMed] [Google Scholar]
- 24. Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, et al. Two self‐management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009;151:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hebert PL, Sisk JE, Tuzzio L, Casabianca JM, Pogue VA, Wang JJ, Chen Y, Cowles C, McLaughlin MA. Nurse‐led disease management for hypertension control in a diverse urban community: a randomized trial. J Gen Intern Med. 2012;27:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milosavljevic A, Aspden T, Harrison J. Community pharmacist‐led interventions and their impact on patients' medication adherence and other health outcomes: a systematic review. Int J Pharm Pract. 2018;26:387–397. [DOI] [PubMed] [Google Scholar]
- 27. Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, Cooper LA, He J. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta‐analysis. Ann Intern Med. 2018;168:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self‐measured blood pressure monitoring in the management of hypertension: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:185–194. [DOI] [PubMed] [Google Scholar]
- 29. Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, Kerby TJ, Klotzle KJ, Maciosek MV, Michels RD, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, Gentry PW, Rose C, Van Houtven C, Wang V, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–1180. [DOI] [PubMed] [Google Scholar]
- 31. Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liyanage‐Don N, Fung D, Phillips E, Kronish IM. Implementing home blood pressure monitoring into clinical practice. Curr Hypertens Rep. 2019;21:14. [DOI] [PubMed] [Google Scholar]
- 33. Carter EJ, Moise N, Alcantara C, Sullivan AM, Kronish IM. Patient barriers and facilitators to ambulatory and home blood pressure monitoring: a qualitative study. Am J Hypertens. 2018;31:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kronish IM, Kent S, Moise N, Shimbo D, Safford MM, Kynerd RE, O'Beirne R, Sullivan A, Muntner P. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens. 2017;11:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostchega Y, Zhang G, Kit BK, Nwankwo T. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011–2014. Am J Hypertens. 2017;30:1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baral‐Grant S, Haque MS, Nouwen A, Greenfield SM, McManus RJ. Self‐monitoring of blood pressure in hypertension: a UK primary care survey. Int J Hypertens. 2012;2012:582068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grant S, Greenfield SM, Nouwen A, McManus RJ. Improving management and effectiveness of home blood pressure monitoring: a qualitative UK primary care study. Br J Gen Pract. 2015;65:e776–e783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones MI, Greenfield SM, Bray EP, Hobbs FR, Holder R, Little P, Mant J, Williams B, McManus RJ. Patient self‐monitoring of blood pressure and self‐titration of medication in primary care: the TASMINH2 trial qualitative study of health professionals' experiences. Br J Gen Pract. 2013;63:e378–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz CL, Seyed‐Safi A, Haque S, Bray EP, Greenfield S, Hobbs FDR, Little P, Mant J, Williams B, McManus RJ. Do patients actually do what we ask: patient fidelity and persistence to the Targets and Self‐Management for the Control of Blood Pressure in Stroke and at Risk Groups blood pressure self‐management intervention. J Hypertens. 2018;36:1753–1761. [DOI] [PubMed] [Google Scholar]
- 40. Jones MI, Greenfield SM, Bray EP, Baral‐Grant S, Hobbs FD, Holder R, Little P, Mant J, Virdee SK, Williams B, et al. Patients' experiences of self‐monitoring blood pressure and self‐titration of medication: the TASMINH2 trial qualitative study. Br J Gen Pract. 2012;62:e135–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maciejewski ML, Bosworth HB, Olsen MK, Smith VA, Edelman D, Powers BJ, Kaufman MA, Oddone EZ, Jackson GL. Do the benefits of participation in a hypertension self‐management trial persist after patients resume usual care? Circ Cardiovasc Qual Outcomes. 2014;7:269–275. [DOI] [PubMed] [Google Scholar]
- 42. Margolis KL, Asche SE, Dehmer SP, Bergdall AR, Green BB, Sperl‐Hillen JM, Nyboer RA, Pawloski PA, Maciosek MV, Trower NK, et al. Long‐term outcomes of the effects of home blood pressure telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension: follow‐up of a cluster randomized clinical trial. JAMA Netw Open. 2018;1:e181617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 44. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iskedjian M, Einarson TR, MacKeigan LD, Shear N, Addis A, Mittmann N, Ilersich AL. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta‐analysis. Clin Ther. 2002;24:302–316. [DOI] [PubMed] [Google Scholar]
- 46. Lowy A, Munk VC, Ong SH, Burnier M, Vrijens B, Tousset EP, Urquhart J. Effects on blood pressure and cardiovascular risk of variations in patients' adherence to prescribed antihypertensive drugs: role of duration of drug action. Int J Clin Pract. 2011;65:41–53. [DOI] [PubMed] [Google Scholar]
- 47. Salam A, Atkins E, Sundstrom J, Hirakawa Y, Ettehad D, Emdin C, Neal B, Woodward M, Chalmers J, Berge E, et al.; A Blood Pressure Lowering Treatment Trialists C . Effects of blood pressure lowering on cardiovascular events, in the context of regression to the mean: a systematic review of randomized trials. J Hypertens. 2019;37:16–23. [DOI] [PubMed] [Google Scholar]
- 48. Poisot T. The digitize package: extracting numerical data from scatterplots. R J. 2011;3:25–26. [Google Scholar]
- 49. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 50. Sheppard JP, Stevens R, Gill P, Martin U, Godwin M, Hanley J, Heneghan C, Hobbs FD, Mant J, McKinstry B, et al. Predicting Out‐of‐Office Blood Pressure in the Clinic (PROOF‐BP): derivation and validation of a tool to improve the accuracy of blood pressure measurement in clinical practice. Hypertension. 2016;67:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheppard JP, Martin U, Gill P, Stevens R, Hobbs FR, Mant J, Godwin M, Hanley J, McKinstry B, Myers M, et al. Prospective external validation of the Predicting Out‐of‐OFfice Blood Pressure (PROOF‐BP) strategy for triaging ambulatory monitoring in the diagnosis and management of hypertension: observational cohort study. BMJ. 2018;361:k2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kerr EA, Zikmund‐Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717–727. [DOI] [PubMed] [Google Scholar]
- 53. Selby JV, Uratsu CS, Fireman B, Schmittdiel JA, Peng T, Rodondi N, Karter AJ, Kerr EA. Treatment intensification and risk factor control: toward more clinically relevant quality measures. Med Care. 2009;47:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Desai N, Madhavankutty Saraswathy V, Hunter K, McFadden C. Prevalence of true therapeutic inertia in blood pressure control in an academic chronic kidney disease clinic. J Clin Hypertens (Greenwich). 2013;15:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010;56:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bolen SD, Samuels TA, Yeh HC, Marinopoulos SS, McGuire M, Abuid M, Brancati FL. Failure to intensify antihypertensive treatment by primary care providers: a cohort study in adults with diabetes mellitus and hypertension. J Gen Intern Med. 2008;23:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Franklin SS, Wt G, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. [DOI] [PubMed] [Google Scholar]
- 58. Kronish IM, Lynch AI, Oparil S, Whittle J, Davis BR, Simpson LM, Krousel‐Wood M, Cushman WC, Chang TI, Muntner P. The association between antihypertensive medication nonadherence and visit‐to‐visit variability of blood pressure: findings from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Hypertension. 2016;68:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


