Abstract
Background
Few studies have investigated optimal revascularization strategies in non–ST‐segment–elevation myocardial infarction with multivessel disease. We investigated 3‐year clinical outcomes according to revascularization strategy in patients with non–ST‐segment–elevation myocardial infarction and multivessel disease.
Methods and Results
This retrospective, observational, multicenter study included patients with non–ST‐segment–elevation myocardial infarction and multivessel disease without cardiogenic shock. Data were analyzed at 3 years according to the percutaneous coronary intervention strategy: culprit‐only revascularization (COR), 1‐stage multivessel revascularization (MVR), and multistage MVR. The primary outcome was major adverse cardiac events (MACE: a composite of all‐cause death, nonfatal spontaneous myocardial infarction, or any repeat revascularization). The COR group had a higher risk of MACE than those involving other strategies (COR versus 1‐stage MVR; hazard ratio, 0.65; 95% CI, 0.54–0.77; P<0.001; and COR versus multistage MVR; hazard ratio, 0.74; 95% CI, 0.57–0.97; P=0.027). There was no significant difference in the incidence of MACE between 1‐stage and multistage MVR (hazard ratio, 1.14; 95% CI, 0.86–1.51; P=0.355). The results were consistent after multivariate regression, propensity score matching, inverse probability weighting, and Bayesian proportional hazards modeling. In subgroup analyses stratified by the Global Registry of Acute Coronary Events score, 1‐stage MVR lowered the risk of MACE compared with multistage MVR in low‐to‐intermediate risk patients but not in patients at high risk.
Conclusions
MVR reduced 3‐year MACE in patients with non–ST‐segment–elevation myocardial infarction and multivessel disease compared with COR. However, 1‐stage MVR was not superior to multistage MVR for reducing MACE except in low‐to‐intermediate risk patients.
Keywords: multivessel coronary artery disease, myocardial infarction, percutaneous coronary intervention
Subject Categories: Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- COR
culprit‐only revascularization
- GRACE
Global Registry of Acute Coronary Events
- HR
hazard ratio
- KAMIR‐NIH
Korea Acute Myocardial Infarction Registry‐National Institutes of Health
- MACE
major adverse cardiac events
- MI
myocardial infarction
- MVD
multivessel coronary artery disease
- MVR
multivessel revascularization
- NSTEMI
non–ST‐segment–elevation myocardial infarction
- PCI
percutaneous coronary intervention
- STEMI
ST–segment–elevation myocardial infarction
Clinical Perspective
What Is New?
Previous studies have shown the benefit of multivessel revascularization in patients with non–ST‐segment–elevation myocardial infarction and multivessel coronary artery disease. However, few studies have focused on staged percutaneous coronary intervention strategies in these patients.
Although 1‐stage multivessel revascularization was not superior to multistage multivessel revascularization for reducing major adverse cardiac events, it was associated with a lower rate of major adverse cardiac events in low‐to‐intermediate risk patients but not in patients at high risk.
What Are the Clinical Implications?
Our results provide information for optimal timing of staged percutaneous coronary intervention non‐infarct‐related artery stenosis using the large nationwide registry data.
Additional clinical studies, including randomized trials, are needed to determine the optimal timing of staged percutaneous coronary intervention for non‐infarct‐related artery stenosis.
Many patients with non–ST‐segment–elevation myocardial infarction (NSTEMI) have multivessel coronary artery disease (MVD), which is associated with poor clinical outcomes. 1 , 2 In cases of hemodynamically stable ST‐segment–elevation myocardial infarction (STEMI) and MVD, many studies demonstrated the superiority of complete revascularization by both 1‐stage and multistage procedures compared with culprit‐only revascularization (COR). 3 , 4 , 5 , 6 , 7 The 2017 European Society of Cardiology guidelines for STEMI recommend routine revascularization for nonculprit lesions before hospital discharge in patients without cardiogenic shock. 8 However, there have been few studies of revascularization strategy in patients with NSTEMI and MVD. Only 1 randomized controlled trial, the SMILE (Impact of One Stage Compared With Multistaged PCI Complete Revascularization on Clinical Outcome in Multivessel NSTEMI Patients) trial, compared 1‐stage and multistage multivessel revascularization (MVR) in these patients. 9 Although the results of most studies analyzing interventional strategies in patients with NSTEMI and MVD showed superior results of MVR compared with COR, 10 , 11 , 12 they did not provide information about staged revascularization. One‐stage MVR was associated with better clinical outcomes compared with multistage MVR in the SMILE trial, whereas 1‐stage and multistage MVR had similar incidences of adverse outcomes in large registry data. 9 , 13 Although the 2018 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines for myocardial revascularization recommend complete 1‐stage revascularization in NSTEMI and MVD, it emphasizes individualization based on clinical status and comorbidities, as well as disease severity. 14
Therefore, we compared the long‐term clinical outcomes among COR, 1‐stage MVR, and multistage MVR in hemodynamically stable patients with NSTEMI and MVD using a Korean multicenter registry.
Methods
Study Protocols and Patient Selection
We used data from the prospective, multicenter, web‐based KAMIR‐NIH (Korea Acute Myocardial Infarction Registry‐National Institutes of Health), which includes patients from 20 major cardiovascular centers admitted between November 2011 and December 2015. The data that support the findings of this study are available from the corresponding author upon reasonable request. The details of the study protocols have been published previously. 15 The study protocols were approved by the ethics committees at each participating center, and all followed the principles of the Declaration of Helsinki (institutional review board approval number: CNUH‐2011‐172). All patients provided written informed consent to participate in the registry.
Among 13 104 patients with acute myocardial infarction (MI), we analyzed 2872 patients with NSTEMI and MVD (Figure 1). The exclusion criteria were STEMI, patients who did not receive percutaneous coronary intervention (PCI), cardiogenic shock, single‐vessel disease, failed PCI, and loss to follow‐up. The patients were divided into 3 groups according to the PCI strategy: COR was defined as culprit‐only PCI (N=1294), 1‐stage MVR was defined as the simultaneous treatment of culprit and nonculprit arteries during index PCI (N=1244), and multistage MVR was defined as PCI for the culprit artery followed by staged PCI for the nonculprit artery during initial hospitalization (N=334).
Figure 1. Study flowchart.
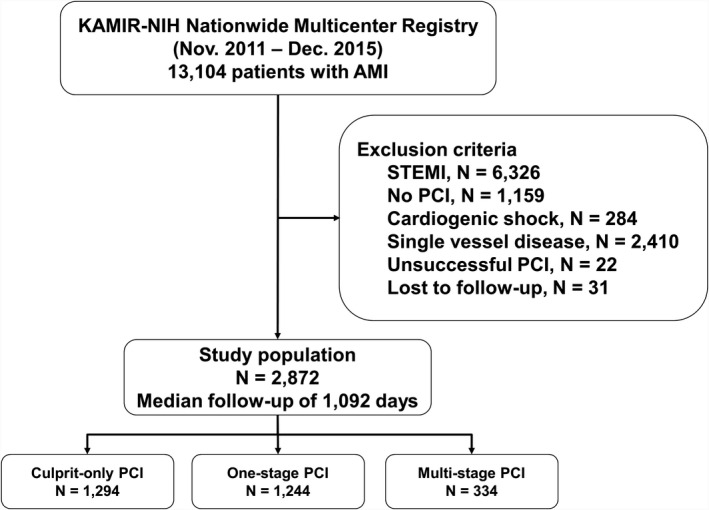
AMI indicates acute myocardial infarction; KAMIR‐NIH, Korea Acute Myocardial Infarction Registry‐National Institutes of Health; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
The diagnosis of NSTEMI was based on the criteria for a fourth universal definition of MI. 16 MVD was defined as having additional ≥70% diameter stenosis in at least 1 major non‐infarct‐related artery or ≥50% diameter stenosis in the left main coronary artery. We assessed the clinical and diagnostic parameters of all subjects. Coronary angiography was performed through the femoral or radial artery. After PCI, dual antiplatelet therapy was prescribed daily as a maintenance dose. Angiographic data were obtained visually by PCI operators at the investigative site. Patients were managed according to current guidelines. 14 , 17 Stent type, diameter, and length and the choice of therapeutic strategy, including the use of medication, a glycoprotein IIb/IIIa inhibitor, thrombus aspiration, intravascular imaging, or hemodynamic support devices, were left to the discretion of the operator. Successful PCI was defined as final residual stenosis <30% with thrombolysis in myocardial infarction grade 3 blood flow.
Study Outcomes
The primary outcome was major adverse cardiac events (MACE: a composite of all‐cause mortality, nonfatal spontaneous MI, or any repeat revascularization). The secondary outcome was all‐cause mortality, cardiac mortality, nonfatal spontaneous MI, any repeat revascularization, nontarget vessel revascularization repeat PCI, definite or probable stent thrombosis, and all‐cause death or nonfatal spontaneous MI during 3 years of clinical follow‐up. All deaths were considered cardiac deaths unless there was a definite noncardiac cause. Nonfatal spontaneous MI was defined as the development of recurrent angina symptoms accompanied by changes in the 12‐lead electrocardiogram or increased levels of cardiac‐specific biomarkers. Repeat revascularization was defined as the need for clinically driven revascularization that occurred after discharge from the index hospitalization, according to Academic Research Consortium definitions.
Statistical Analysis
Continuous variables are presented as means±SD or as medians with interquartile ranges and were compared by the unpaired t test, the Mann–Whitney rank‐sum test or 1‐way analysis of variance. Discrete variables are expressed as counts with percentages and were compared using Pearson's chi‐square test or Fisher's exact test. We prepared Kaplan‐Meier curves of the primary and secondary outcomes according to the interventional strategy. As differences in baseline characteristics could significantly affect outcomes, sensitivity analyses were performed to adjust for confounding factors as much as possible. First, a multivariate Cox regression model was used for each of the above cutoffs, with covariates that had P<0.05 on univariate analysis. Second, we performed propensity score matching between groups. The percent standardized mean difference after propensity score matching was within 10% across nearly all matched covariates, demonstrating a successful balance between the groups (Tables S1 through S6). Third, for inverse probability weighting adjustment, the inverse of the propensity score was assessed by calculating the percent standardized mean differences in the covariate used to generate the propensity score. The values after inverse probability weighting adjustment were within ±10% across all matched covariates, demonstrating successful balance between the groups (Figures S1 through S3). Fourth, we performed Bayesian modeling, with internal validation data as an additional sensitivity analysis to assess the effects of unmeasured confounders on the summary estimates. The Bayesian estimators were adjusted by combining internal validation and study data for unmeasured confounding factors, as described previously. 18 In Bayesian analysis, the hazard ratio (HR) and 95% CI were calculated by Cox regression. All analyses were 2 tailed, and P<0.05 was taken to indicate significance. Comparisons of primary outcome among the different interventional strategies were stratified by the GRACE (Global Registry of Acute Coronary Events) score, and the interaction between the treatment effect and these covariates was assessed using a Cox regression model. 19
All statistical analyses were performed using the R statistical package (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria. https://www.R‐project.org).
Results
Baseline Characteristics
Three years of follow‐up were completed for all patients; the median follow‐up duration was 1092 days. The patients' baseline clinical characteristics, and lesion‐ and procedure‐related profiles are described in Tables 1 and 2. The patients in the multistage MVR group were mostly male. They also had a shorter door‐to‐balloon time and lower left ventricular ejection fraction. The COR group had a higher rate of a history of PCI. There were no significant differences in other atherosclerotic risk factors among the groups except for a lower rate of current smokers in the 1‐stage MVR group. The enzymatic infarction size by peak level of troponin I was larger in the multistage MVR group. With regard to medications at discharge, patients in the multistage MVR group had a slightly lower rate of treatment with aspirin (99.9% versus 100% versus 99.4%; P=0.010) and a slightly higher rate of treatment with renin‐angiotensin‐aldosterone system inhibitors. Other evidence‐based medications for MI had similar rates of prescription among the 3 groups. The proportion of patients with the left main coronary artery as the culprit vessel was 6.7% in the 1‐stage MVR group. The prevalence of American College of Cardiology/American Heart Association‐defined complex lesions, long lesions, and triple vessel disease was higher in the multistage MVR group. Patients in the COR group received second‐generation drug‐eluting stents less frequently than those in the MVR group (87.9% versus 91.6% versus 92.8%; P=0.022). The multistage MVR group had lower baseline thrombolysis in myocardial infarction flow in the culprit artery and a lower rate of imaging‐guided PCI. In the multistage MVR group, the median interval between the index and second‐stage PCI was 5.0 (4.0–7.0) days. The length of hospital stay was longer in the multistage MVR group (median 4.0 versus 4.0 versus 7.0 days; P<0.001). There were no significant differences in in‐hospital complication rates among the groups.
Table 1.
Baseline Clinical Characteristics
|
Culprit‐Only PCI (N=1294) |
One‐Stage MVR (N=1244) |
Multistage MVR (N=334) |
P Value | |
|---|---|---|---|---|
| Age, y | 66.4±12.0 | 66.1±11.5 | 64.9±11.7 | 0.111 |
| Male | 938 (72.5%) | 847 (68.1%) | 254 (76.0%) | 0.005 |
| Body mass index | 23.9±3.3 | 24.1±3.5 | 23.9±3.4 | 0.182 |
| Killip class 3 | 143 (11.1%) | 120 (9.6%) | 37 (11.1%) | 0.473 |
| GRACE score ≥140 | 462 (35.7%) | 429 (34.5%) | 125 (37.4%) | 0.575 |
| Process of care index | ||||
| Symptom‐to‐door time, h | 46.1±143.1 | 45.6±114.8 | 49.3±121.3 | 0.896 |
| Door‐to‐balloon time, min | 27.0±55.1 | 27.1±47.9 | 19.3±33.0 | 0.028 |
| Past medical history | ||||
| Hypertension | 755 (58.3%) | 738 (59.3%) | 190 (56.9%) | 0.702 |
| Diabetes mellitus | 459 (35.5%) | 436 (35.0%) | 111 (33.2%) | 0.747 |
| Dyslipidemia | 151 (11.7%) | 157 (12.6%) | 38 (11.4%) | 0.704 |
| Current smoker | 449 (34.7%) | 367 (29.5%) | 126 (37.7%) | 0.003 |
| Previous history of myocardial infarction | 119 (9.2%) | 92 (7.4%) | 23 (6.9%) | 0.169 |
| Previous history of PCI | 113 (8.7%) | 77 (6.2%) | 14 (4.2%) | 0.004 |
| Previous history of CVA | 114 (8.8%) | 102 (8.2%) | 26 (7.8%) | 0.776 |
| LVEF, % | 52.6±11.2 | 53.7±11.0 | 51.2±11.7 | 0.001 |
| Laboratory findings | ||||
| eGFR, mL/min per 1.73 m2 | 83.3±38.8 | 85.5±38.9 | 85.0±31.7 | 0.350 |
| Peak level of troponin I, ng/mL | 24.2±50.6 | 21.1±69.2 | 30.6±53.0 | 0.032 |
| Peak level of CK‐MB, ng/mL | 61.9±115.1 | 56.8±173.7 | 73.5±93.8 | 0.153 |
| Medications at discharge | ||||
| Aspirin | 1293 (99.9%) | 1244 (100.0%) | 332 (99.4%) | 0.100 |
| P2Y12 inhibitor | 1287 (99.5%) | 1241 (99.8%) | 332 (99.4%) | 0.434 |
| Ticagrelor | 266 (20.6%) | 263 (21.1%) | 92 (27.5%) | |
| Prasugrel | 85 (6.6%) | 117 (9.4%) | 43 (12.9%) | |
| Clopidogrel | 937 (72.4%) | 861 (69.2%) | 199 (59.6%) | |
| ACEI or ARB | 1058 (81.8%) | 1003 (80.6%) | 296 (88.6%) | 0.003 |
| Beta‐blocker | 1104 (85.3%) | 1072 (86.2%) | 287 (85.9%) | 0.823 |
| Statin | 1216 (94.0%) | 1181 (94.9%) | 319 (95.5%) | 0.407 |
Values are mean±SD, median (interquartile range), or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐II receptor blocker; CK‐MB, creatine kinase‐myocardial band; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; LVEF, left ventricular ejection fraction; MVR, multivessel revascularization; and PCI, percutaneous coronary intervention.
Table 2.
Coronary Angiographic and Procedural Characteristics
|
Culprit‐Only PCI (N=1294) |
One‐Stage MVR (N=1244) |
Multistage MVR (N=334) |
P Value | |
|---|---|---|---|---|
| Culprit lesion profiles | ||||
| Culprit vessel | <0.001 | |||
| Left main coronary artery | 62 (4.8%) | 83 (6.7%) | 7 (2.1%) | |
| Left anterior descending artery | 484 (37.4%) | 449 (36.1%) | 104 (31.1%) | |
| Left circumflex artery | 346 (26.7%) | 365 (29.3%) | 68 (20.4%) | |
| Right coronary artery | 402 (31.1%) | 347 (27.9%) | 155 (46.4%) | |
| ACC/AHA B2/C lesion | 1098 (84.9%) | 1054 (84.7%) | 306 (91.6%) | 0.004 |
| Small vessel* | 485 (37.5%) | 467 (37.5%) | 103 (30.8%) | 0.059 |
| Long lesion † | 577 (44.6%) | 559 (44.9%) | 177 (53.0%) | 0.017 |
| Overall‐lesion profiles | ||||
| Left main disease | 123 (9.5%) | 156 (12.5%) | 30 (9.0%) | 0.026 |
| Triple vessel disease | 428 (33.1%) | 456 (36.7%) | 185 (55.4%) | <0.001 |
| Procedural characteristics | ||||
| Transradial approach | 609 (47.1%) | 600 (48.2%) | 180 (53.9%) | 0.213 |
| Use of glycoprotein IIb/IIIa inhibitor | 88 (6.8%) | 84 (6.8%) | 46 (13.8%) | <0.001 |
| Thrombus aspiration | 154 (11.9%) | 80 (6.4%) | 65 (19.5%) | <0.001 |
| IRA treatment | 0.022 | |||
| Bare metal stent | 37 (2.9%) | 23 (1.8%) | 5 (1.5%) | |
| First‐generation DES | 15 (1.2%) | 8 (0.6%) | 4 (1.2%) | |
| Second‐generation DES | 1137 (87.9%) | 1140 (91.6%) | 310 (92.8%) | |
| Plain balloon angioplasty | 105 (8.1%) | 73 (5.9%) | 15 (4.5%) | |
| Total number of stents | 1.2±0.6 | 2.3±0.9 | 2.6±1.1 | <0.001 |
| Pre‐PCI TIMI flow 0–1 in culprit lesion | 525 (40.6%) | 441 (35.5%) | 174 (52.1%) | <0.001 |
| IVUS guided PCI | 337 (26.0%) | 326 (26.2%) | 59 (17.7%) | 0.004 |
| OCT guided PCI | 33 (2.6%) | 38 (3.1%) | 0 | 0.006 |
| Hemodynamic support device | ||||
| IABP | 5 (0.4%) | 10 (0.8%) | 0 | 0.128 |
| ECMO | 1 (0.1%) | 0 | 0 | 0.543 |
| Interval between index and second stage PCI, d | 5.0 (4.0–7.0) | |||
| Complete revascularization | 922 (74.1%) | 207 (62.0%) | ||
| Complications during hospitalization | ||||
| Heart failure | 51 (3.9%) | 48 (3.9%) | 21 (6.3%) | 0.122 |
| Atrioventricular block | 10 (0.8%) | 6 (0.5%) | 4 (1.2%) | 0.342 |
| Ventricular tachycardia | 14 (1.1%) | 6 (0.5%) | 6 (1.8%) | 0.053 |
| Ventricular fibrillation | 4 (0.3%) | 3 (0.2%) | 2 (0.6%) | 0.583 |
| Length of hospital stay, d | 4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 7.0 (6.0–9.0) | <0.001 |
Values are mean±SD, median (interquartile range), or n (%). ACC/AHA indicates American College of Cardiology/American Heart Association; DES, drug‐eluting stent; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; IRA, infarct‐related artery; IVUS, intravascular ultrasound; MVR, multivessel revascularization; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; and TIMI, thrombolysis in myocardial infarction.
Small vessel: reference diameter ≤2.75 mm.
Long lesion: length ≥28 mm.
Clinical Outcomes According to Treatment Strategy
At 3 years, the patients in the COR group had a higher risk of MACE (COR versus 1‐stage MVR; 25.0% versus 17.1%; HR, 0.63; 95% CI, 0.53–0.75; P<0.001; and COR versus multistage MVR; 25.0% versus 19.5%; HR, 0.73; 95% CI, 0.56–0.96; P=0.027), mainly driven by a significantly higher risk of all and nontarget vessel revascularization repeat PCI. The risk of all‐cause death was also significantly higher in the COR group compared with 1‐stage MVR (Figures 2 and 3).
Figure 2. Cumulative incidence of MACE and all‐cause death.
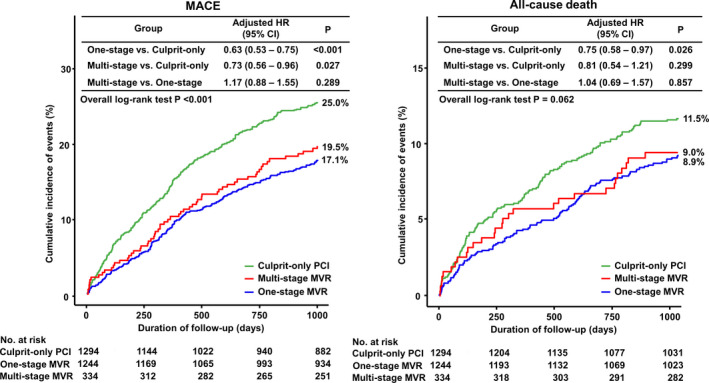
HR indicates hazard ratio; MACE, major adverse cardiac events; MVR, multivessel revascularization; and PCI, percutaneous coronary intervention.
Figure 3. Cumulative incidence of cardiac death, nonfatal spontaneous MI, all repeat revascularization and non‐TVR repeat PCI.
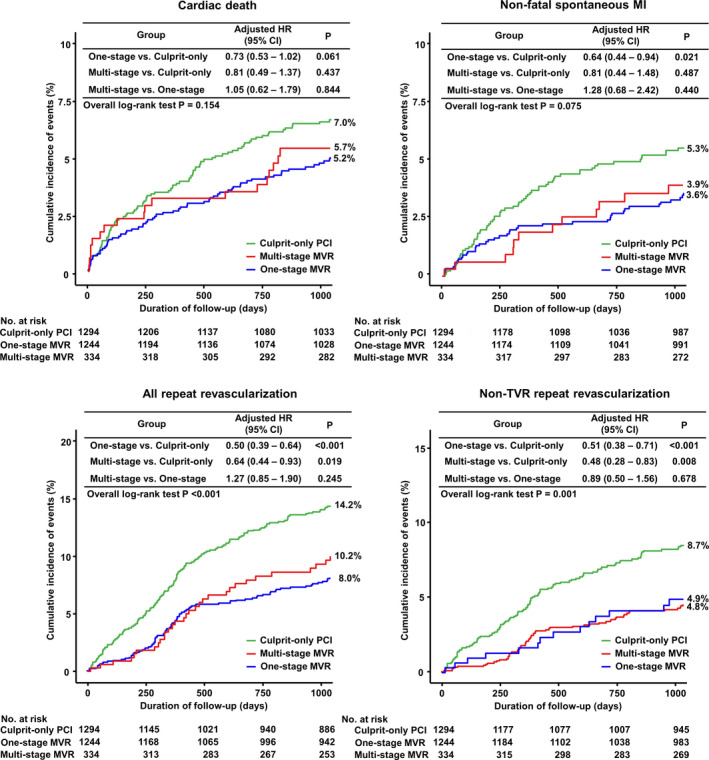
HR indicates hazard ration; MI, myocardial infarction; MVR, multivessel revascularization; PCI, percutaneous coronary intervention; and TVR, target‐vessel revascularization.
Sensitivity analyses using multivariate Cox regression, propensity score matching, inverse probability weighting, and Bayesian modeling showed a significantly higher risk of MACE in the COR group than in the 1‐stage and multistage MVR groups, and a lower risk of all‐cause death in the 1‐stage MVR group than in the COR group. There were no significant differences in MACE or all‐cause death between the 1‐stage and multistage MVR groups (Table 3).
Table 3.
Comparison of 3‐year Clinical Outcomes According to Interventional Strategy
|
Culprit‐Only PCI (N=1294) |
Multistage MVR (N=334) |
Unadjusted | Adjusted | PS‐Matched | IPW‐Adjusted | Bayesian Model | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| All‐cause death | 149 (11.5%) | 30 (9.0%) | 0.76 (0.52–1.13) | 0.81 (0.54–1.21) | 0.73 (0.48–1.10) | 0.89 (0.54–1.48) | 0.73 (0.52–1.09) |
| Cardiac death | 90 (7.0%) | 19 (5.7%) | 0.80 (0.49–1.31) | 0.82 (0.49–1.37) | 0.74 (0.44–1.25) | 0.87 (0.45–1.71) | 0.84 (0.55–1.31) |
| Nonfatal spontaneous MI | 69 (5.3%) | 13 (3.9%) | 0.71 (0.39–1.28) | 0.80 (0.44–1.48) | 0.72 (0.39–1.36) | 0.66 (0.31–1.39) | 0.78 (0.41–1.26) |
| Any repeat revascularization | 184 (14.2%) | 34 (10.2%) | 0.67 (0.47–0.97) | 0.64 (0.44–0.93) | 0.66 (0.45–0.97) | 0.61 (0.39–0.94) | 0.69 (0.48–0.93) |
| Non‐TVR repeat PCI | 112 (8.7%) | 16 (4.8%) | 0.53 (0.31–0.89) | 0.48 (0.28–0.83) | 0.52 (0.30–0.90) | 0.48 (0.26–0.89) | 0.51 (0.32–0.86) |
| Definite/probable ST | 5 (0.4%) | 2 (0.6%) | 1.52 (0.29–7.82) | 1.81 (0.31–10.4) | 2.14 (0.30–15.2) | 1.73 (0.11–26.1) | 2.09 (0.58–4.71) |
| All‐cause death or MI | 198 (15.3%) | 41 (12.3%) | 0.78 (0.56–1.01) | 0.85 (0.60–1.20) | 0.76 (0.53–1.08) | 0.81 (0.52–1.26) | 0.80 (0.60–1.01) |
| MACE* | 323 (25.0%) | 65 (19.5%) | 0.74 (0.57–0.97) | 0.73 (0.56–0.96) | 0.71 (0.54–0.94) | 0.71 (0.51–0.99) | 0.73 (0.56–0.98) |
|
One‐Stage MVR (N=1244) |
Multistage MVR (N=334) |
Unadjusted | Adjusted | PS‐Matched | IPW‐Adjusted | Bayesian Model | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| All‐cause death | 111 (8.9%) | 30 (9.0%) | 1.01 (0.67–1.50) | 1.04 (0.69–1.57) | 0.99 (0.65–1.50) | 1.01 (0.61–1.67) | 1.01 (0.72–1.77) |
| Cardiac death | 65 (5.2%) | 19 (5.7%) | 1.08 (0.65–1.81) | 1.05 (0.62–1.79) | 0.99 (0.59–1.67) | 1.07 (0.55–2.05) | 1.24 (0.82–1.74) |
| Nonfatal spontaneous MI | 45 (3.6%) | 13 (3.9%) | 1.07 (0.58–1.98) | 1.28 (0.68–2.42) | 0.91 (0.49–1.70) | 1.32 (0.58–3.03) | 1.13 (0.82–1.66) |
| Any repeat revascularization | 99 (8.0%) | 34 (10.2%) | 1.28 (0.87–1.89) | 1.27 (0.85–1.90) | 1.19 (0.80–1.77) | 1.47 (0.87–2.49) | 1.30 (0.86–1.99) |
| Non‐TVR repeat PCI | 61 (4.9%) | 16 (4.8%) | 0.97 (0.56–1.69) | 0.89 (0.50–1.56) | 0.89 (0.51–1.56) | 1.05 (0.52–2.13) | 0.83 (0.57–1.46) |
| Definite/probable ST | 7 (0.6%) | 2 (0.6%) | 1.06 (0.22–5.09) | 1.01 (0.20–5.06) | 0.99 (0.20–4.89) | 1.30 (0.15–11.4) | 2.57 (1.39–7.43) |
| All‐cause death or MI | 147 (11.8%) | 41 (12.3%) | 1.03 (0.73–1.46) | 1.13 (0.79–1.61) | 0.98 (0.69–1.39) | 1.08 (0.69–1.68) | 1.01 (0.76–1.48) |
| MACE* | 213 (17.1%) | 65 (19.5%) | 1.14 (0.86–1.51) | 1.17 (0.88–1.55) | 1.08 (0.81–1.44) | 1.23 (0.85–1.76) | 1.13 (0.89–1.47) |
|
Culprit‐Only PCI (N=1294) |
One‐Stage MVR (N=1244) |
Unadjusted | Adjusted | PS‐Matched | IPW‐Adjusted | Bayesian Model | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| All‐cause death | 149 (11.5%) | 111 (8.9%) | 0.76 (0.59–0.97) | 0.75 (0.58–0.97) | 0.76 (0.60–0.98) | 0.77 (0.61–0.99) | 0.73 (0.60–0.92) |
| Cardiac death | 90 (7.0%) | 565 (5.2%) | 0.74 (0.53–1.01) | 0.73 (0.53–1.02) | 0.73 (0.53–1.01) | 0.79 (0.56–1.11) | 0.73 (0.54–1.09) |
| Nonfatal spontaneous MI | 69 (5.3%) | 45 (3.6%) | 0.66 (0.45–0.96) | 0.64 (0.44–0.94) | 0.67 (0.47–0.99) | 0.63 (0.43–0.93) | 0.71 (0.53–0.88) |
| Any repeat revascularization | 184 (14.2%) | 99 (8.0%) | 0.53 (0.41–0.67) | 0.50 (0.39–0.64) | 0.53 (0.42–0.68) | 0.49 (0.38–0.62) | 0.50 (0.36–0.63) |
| Non‐TVR repeat PCI | 112 (8.7%) | 61 (4.9%) | 0.54 (0.40–0.74) | 0.51 (0.37–0.71) | 0.56 (0.41–0.76) | 0.49 (0.36–0.66) | 0.62 (0.44–0.92) |
| Definite/probable ST | 5 (0.4%) | 7 (0.6%) | 1.43 (0.45–4.51) | 1.48 (0.47–4.71) | 1.47 (0.47–4.64) | 2.09 (0.55–7.94) | 2.30 (1.72–4.72) |
| All‐cause death or MI | 198 (15.3%) | 147 (11.8%) | 0.75 (0.61–0.93) | 0.74 (0.60–0.92) | 0.76 (0.61–0.94) | 0.76 (0.61–0.95) | 0.75 (0.62–0.91) |
| MACE* | 323 (25.0%) | 213 (17.1%) | 0.75 (0.54–0.77) | 0.63 (0.53–0.75) | 0.65 (0.55–0.78) | 0.63 (0.53–0.75) | 0.65 (0.55–0.77) |
Values are n (%) unless otherwise indicated. HR indicates hazard ratio; IPW, inverse probability weight; IRA, infarct‐related artery; MACE, major adverse cardiovascular event; MI, myocardial infarction; MVR, multivessel revascularization; PCI, percutaneous coronary intervention; PS, propensity score; ST, stent thrombosis; and TVR, target‐vessel revascularization.
MACE was defined as a composite of all‐cause death, nonfatal spontaneous myocardial infarction, or any repeat revascularization.
Independent Predictors of MACE and All‐Cause Death
A multivariate Cox proportional hazard model identified independent predictors of the primary and secondary outcomes (Table 4). The multivessel (1‐stage and multistage) MVR group was associated with lower incidences of all‐cause death (HR, 0.742; 95% CI, 0.578–0.951; P=0.019) and MACE (HR, 0.643; 95% CI, 0.540–0.765; P<0.001) than COR at 3 years.
Table 4.
Independent Predictors of Clinical Outcomes at 3 Years
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| All‐cause death | |||
| Multivessel (1‐ and multistage) MVR* | 0.742 | 0.578–0.951 | 0.019 |
| Multistage MVR* | 0.771 | 0.519–1.144 | 0.197 |
| Age> 60 y | 3.569 | 2.372–5.370 | <0.001 |
| Killip class ≥3 | 1.859 | 1.409–2.453 | <0.001 |
| eGFR <60 mL/min per 1.73 m2 | 2.619 | 2.052–3.342 | <0.001 |
| LVEF <50% | 2.196 | 1.706–2.825 | <0.001 |
| Left main disease | 1.605 | 1.101–2.339 | 0.014 |
| Previous history of MI | 1.518 | 1.097–2.100 | 0.012 |
| MACE | |||
| Multivessel (1‐ and multistage) MVR* | 0.643 | 0.540–0.765 | <0.001 |
| Multistage MVR* | 0.747 | 0.571–0.975 | 0.032 |
| Age >60 y | 1.426 | 1.169–1.737 | <0.001 |
| Killip class ≥3 | 1.613 | 1.295–2.008 | <0.001 |
| eGFR <60 mL/min per 1.73 m2 | 1.960 | 1.644–2.335 | <0.001 |
| LVEF <50% | 1.355 | 1.138–1.611 | 0.001 |
| Left main disease | 1.654 | 1.272–2.150 | <0.001 |
| Previous history of MI | 1.453 | 1.139–1.853 | 0.003 |
Hazard rations and their 95% CIs were calculated using multivariate Cox regression analysis. eGFR indicates estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MI, myocardial infarction; and MVR, multivessel revascularization.
Reference is a culprit‐only percutaneous coronary intervention.
Subgroup Analyses
In subgroup analyses stratified by GRACE score, 1‐stage and multistage MVR reduced MACE in both high‐risk (GRACE score ≥140) patients and low‐to‐intermediate risk (GRACE score <140) patients. However, there was no significant difference in the incidence of MACE between the 1‐stage and multistage MVR groups in high‐risk patients. Interestingly, 1‐stage MVR was associated with a lower risk of MACE compared with the multistage MVR (HR for multistage MVR, 1.55; 95% CI, 1.06–2.27; P=0.023) and COR groups (HR, 0.56; 95% CI, 0.44–0.72; P<0.001) in low‐to‐intermediate risk patients (Figures 4 and 5).
Figure 4. Cumulative incidence of MACE according to interventional strategies stratified by GRACE score.
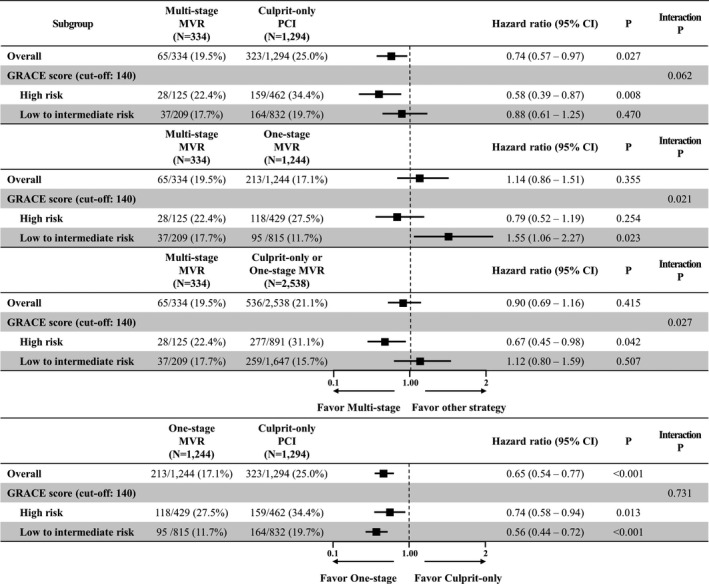
GRACE indicates Global Registry of Acute Coronary Events; HR, hazard ratio; MACE, major adverse cardiac events; MVR, multivessel revascularization; and PCI, percutaneous coronary intervention.
Figure 5. Subgroup analysis for MACE stratified by GRACE score.
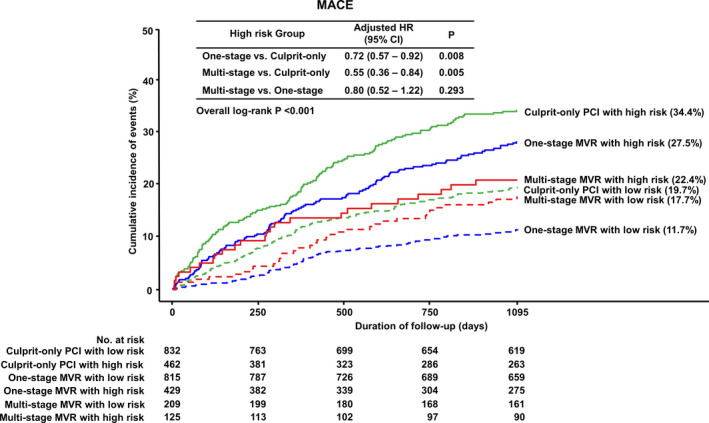
GRACE indicates Global Registry of Acute Coronary Events; HR, hazard ratio; MACE, major adverse cardiac events; MVR, multivessel revascularization; and PCI, percutaneous coronary intervention.
DISCUSSION
The present study compared 3 years of clinical outcomes among different treatment strategies in patients with NSTEMI and MVD using data from a nationwide, multicenter, prospective registry. As the main findings, we found that the multivessel MVR strategy (1‐stage and multistage MVR) was associated with significantly lower incidences of MACE and all‐cause death than the COR strategy. In addition, there were no significant differences in the incidences of any of the primary or secondary outcomes between the 1‐stage and multistage MVR groups. However, subgroup analyses stratified by GRACE score revealed a significantly lower risk of MACE in 1‐stage MVR in low‐to‐intermediate risk patients (GRACE score <140) compared with multistage MVR but not in high‐risk patients (GRACE score ≥140).
MVD is common in NSTEMI patients, and it adversely affects clinical outcomes. 1 , 2 However, there have been few studies of the impacts of different interventional strategies in these patients. Earlier studies using the registry or single‐center data have reported significant benefits of MVR compared with COR. Shishehbor et al showed that MVR was significantly associated with a lower revascularization rate, but not hard clinical end points, compared with COR. 10 In addition, a Korean study using the KAMIR registry reported that MVR was associated with a 42% reduction in all‐cause death or nonfatal MI and a 56% reduction in nontarget vessel revascularization repeat PCI during a 1‐year follow‐up period. 11 In hemodynamically stable patients with STEMI and MVD, complete revascularization is more beneficial than COR. 3 , 4 , 5 , 6 , 7 Complete revascularization also appears to be useful in patients with NSTEMI with MVD. A recently published large‐scale study using British Cardiac Intervention Society data showed that 1‐stage complete revascularization was superior to COR in terms of long‐term mortality. 12 However, the impact of staged PCI in patients with NSTEMI and MVD is uncertain. Data from 1 registry showed comparable 3‐year mortality rates between 1‐stage and multistage MVR in NSTEMI and MVD. 13 To our knowledge, only 1 randomized controlled trial has been performed about this issue. The SMILE trial compared 1‐stage and multistage MVR, and all patients in the multistage MVR group received staged PCI at 3 to 7 days after index PCI. 9 The results indicated that 1‐stage MVR was superior in terms of lower 1‐year composite outcomes, mainly because of lower target vessel revascularization and lower mortality rates, compared with multistage MVR. Based on these results, the 2018 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines for myocardial revascularization recommend complete 1‐stage MVR over multistage MVR in NSTEMI and MVD. However, the guidelines emphasize the importance of individualization of interventional strategy based on clinical status and comorbidities, as well as disease severity. 14 That is, various subgroup analyses are needed to define an optimal interventional strategy for these patients. Our study investigated which strategy (1‐stage or multistage MVR) was more beneficial and which patients could be integrated to determine the revascularization approach.
In the present study, there was a significantly lower risk of MACE and all‐cause death at 3 years in the multivessel PCI (1‐stage and multistage MVR) group compared with the COR group. These findings are consistent with other studies indicating the superiority of MVR over COR. However, both 1‐stage and multistage MVR had comparable incidences of primary and secondary outcomes on analyses using various statistical methods. The results are different from those of the SMILE trial; that large registry data study comparing 1‐stage and multistage MVR also investigated only mortality. 13 The patients enrolled in the SMILE trial were at low risk, but 35.9% of all patients in the present study were at high risk (GRACE score ≥140).
A potentially important finding of the present study is that subgroup analyses stratified by GRACE score showed a significantly lower risk of MACE for 1‐stage MVR in patients at low‐to‐intermediate risk (GRACE score <140) but not in patients at high risk (GRACE score ≥140). The main reasons for the differences in interventional strategy between STEMI and NSTEMI are heterogeneity and more comorbidities in NSTEMI. Therefore, we stratified the study patients into 2 groups by GRACE risk score to reflect their clinical status. Although the incidence of MACE in multistage MVR was not significantly different from that in 1‐stage MVR, it showed a lower trend of MACE in high‐risk patients (22.4% versus 27.5%). Furthermore, the Kaplan‐Meier curve showed definite separation among the 3 interventional strategies in high‐risk patients (Figure 4). The main reason for the insufficient statistical power of this analysis was the small number of patients with multistage MVR. In the present study, a low proportion (11.6% of all patients) of patients received multistage MVR. In South Korea, patients with NSTEMI and MVD should be revascularized for both culprit and nonculprit arteries simultaneously because of the national insurance system, and in special cases with NSTEMI and MVD, such as patients with renal insufficiency or the use of large amounts of contrast media, which is covered by national insurance. In aforementioned registry data, 1‐stage MVR was also associated with a higher short‐term mortality despite of lower long‐term mortality compared with COR. 12 A large‐scale randomized trial is needed to confirm this issue.
This study has some limitations. First, we used observational registry data. Therefore, selection bias was inevitable. However, we attempted to perform various sensitivity analyses to adjust for measured or unmeasured confounders of different clinical characteristics between the groups. Second, we conducted an angiographic assessment of non‐infarct‐related artery stenosis. Although there was insufficient evidence, fractional flow‐guided and imaging‐guided PCI are useful to assess the nonculprit artery. Third, we did not collect data on procedure‐related risks, including procedure time, radiation dose, total amount of contrast dye, and incidence of contrast media‐induced nephropathy.
In conclusion, MVR reduced 3‐year MACE in patients with NSTEMI and MVD compared with COR. Although 1‐stage MVR was not superior to multistage MVR for reducing MACE, it was associated with fewer MACE in patients at low‐to‐intermediate risk but not in patients at high risk. These findings were based on retrospective studies; thus, additional large‐scale randomized trials are required for verification.
Sources of Funding
This study was supported by a grant of the Bio & Medical Technology Development Program of the National Research Foundation of Korea funded by the Korean Government, Ministry of Science, ICT and Future Planning (2017M3A9E8023020).
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S3
Optimal Revascularization Strategy in Non–ST-Segment–Elevation Myocardial Infarction With Multivessel Coronary Artery Disease: Culprit-Only Versus One-Stage Versus Multistage Revascularization. (J Am Heart Assoc. 2020;9:e016575 DOI: 10.1161/JAHA.120.016575.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016575
For Sources of Funding and Disclosures, see page 12.
References
- 1. Venkitachalam L, Kip KE, Selzer F, Wilensky RL, Slater J, Mulukutla SR, Marroquin OC, Block PC, Williams DO, Kelsey SF. Twenty‐year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institutes‐sponsored, multicenter 1985–1986 PTCA and 1007–2006 dynamic registries. Circ Cardiovasc Interv. 2009;2:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corpus RA, House JA, Marso SP, Grantham JA, Huber KC Jr, Laster SB, Johnson WL, Daniels WC, Barth CW, Giorgi LV, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493–500. [DOI] [PubMed] [Google Scholar]
- 3. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 4. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, et al. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engstrom T, Kelbaek H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 6. Smits PC, Abdel‐Wahab M, Neumann FJ, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, et al. Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 7. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, Lopez‐Sendon J, Faxon DP, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 8. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 9. Sardella G, Luscisano L, Garbo R, Pennacchi M, Cavallo E, Stio RE, Calcagno S, Ugo F, Boccuzzi G, Fedele F, et al. Single‐staged compared with multi‐staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67:264–272. [DOI] [PubMed] [Google Scholar]
- 10. Shishehbor MH, Lauer MS, Singh IM, Chew DP, Karha J, Brener SJ, Moliterno DJ, Ellis SG, Topol EJ, Bhatt DL. Is unstable angina or non‐ST‐segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culprit‐only stenting? J Am Coll Cardiol. 2007;49:849–854. [DOI] [PubMed] [Google Scholar]
- 11. Kim MC, Jeong MH, Ahn Y, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, et al. What is optimal revascularization strategy in patients with multivessel coronary artery disease in non‐ST‐elevation myocardial infarction? Multivessel or culprit‐only revascularization. Int J Cardiol. 2011;153:148–153. [DOI] [PubMed] [Google Scholar]
- 12. Rathod KS, Koganti S, Jain AK, Astroulakis Z, Lim P, Rakhit R, Kalra SS, Dalby MC, O’Mahony C, Malik IS, et al. Complete versus culprit‐only lesion intervention in patients with acute coronary syndromes. J Am Coll Cardiol. 2018;72:1989–1999. [DOI] [PubMed] [Google Scholar]
- 13. Hannan EL, Samadashvili Z, Walford G, Jacobs AK, Stamato NJ, Venditti FJ, Holmes DR Jr, Sharma S, King SB III. Staged versus one‐time complete revascularization with percutaneous coronary intervention for multivessel coronary artery disease patients without ST‐elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:12–20. [DOI] [PubMed] [Google Scholar]
- 14. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 15. Kim JH, Chae SC, Oh DJ, Kim HS, Kim YJ, Ahn Y, Cho MC, Kim CJ, Yoon JH, Park HY, et al. Multicenter Cohort Study of Acute Myocardial Infarction in Korea‐interim analysis of the Korea Acute Myocardial Infarction Registry‐National Institutes of Health Registry. Circ J. 2016;80:1427–1436. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 17. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 18. McCandless LC, Gustafson P, Levy A. Bayesian sensitivity analysis for unmeasured confounding in observational studies. Stat Med. 2007;26:2331–2347. [DOI] [PubMed] [Google Scholar]
- 19. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA Jr, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S3


