Abstract
Background
Although US recent data suggest that mitral regurgitation (MR) is severely undertreated and carries a poor outcome, population‐based views on outcome and management are limited. We aimed to define the current treatment standards, clinical outcomes, and costs related to MR at the nationwide level.
Methods and Results
In total, 107 412 patients with MR were admitted in France in 2014 to 2015. Within 1 year, 8% were operated and 92% were conservatively managed and constituted our study population (68% primary MR and 32% secondary MR). The mean age was 77±15 years; most patients presented with comorbidities. In‐hospital and 1‐year mortality rates were 4.1% and 14.3%, respectively. Readmissions were common (63% at least once and 37% readmitted ≥2 times). Rates of 1‐year mortality or all‐cause readmission and 1‐year mortality or heart failure readmission were 67% and 34%, respectively, and increased with age, Charlson index, heart failure at admission, and secondary MR etiology; however, the event rate remained notably high in the primary MR subset (64% and 28%, respectively). The mean costs of hospital admissions and of readmissions were 5345±6432 and 10 080±10 847 euros, respectively.
Conclusions
At the nationwide level, MR was a common reason for admission and affected an elderly population with frequent comorbidities. Less than 10% of patients underwent a valve intervention. All subsets of patients who were conservatively managed incurred high mortality and readmissions rates, and MR represented a major societal burden with an extrapolated annual cost of 350 to 550 million euros (390–615 million US dollars). New strategies to improve the management and outcomes of patients with both primary and secondary MR are critical and warranted.
Keywords: mitral valve regurgitation, outcomes, management, cost
Subject Categories: Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- HF
heart failure
- ICD‐10
International Classification of Diseases, Tenth Revision
- MR
mitral regurgitation
- PMR
primary mitral regurgitation
- PMSI
Programme de Médicalisation des Systèmes d’Information
- SMR
secondary mitral regurgitation
- VHD
valvular heart disease
Clinical Perspective
What Is New?
Large and comprehensive contemporary data assessing the management and outcome of patients with mitral regurgitation (MR) at the nationwide level and the societal burden of the disease are currently lacking.
We identified 107 412 MR admissions in France in 2014 to 2015. MR affected an elderly population with frequent comorbidities; <10% underwent a valve intervention, and all subsets incurred high mortality and readmission rates.
MR represented an extrapolated annual cost between 350 and 550 million euros (390–615 million US dollars).
What Are the Clinical Implications?
Strategies to improve MR overall management and outcomes are urgently needed.
Epidemiological studies have shown that the incidence and prevalence of valvular heart disease (VHD) are high and increase as the population ages, creating the next epidemic. 1 , 2 The only curative treatment for many forms of VHD is the performance of a valve intervention, either surgically or through a transcatheter approach, which has increased dramatically in the past decade. 3 , 4 , 5 , 6 , 7 , 8 , 9
Mitral regurgitation (MR) is the most common valve disease in Western countries. 1 MR etiologies are divided into primary MR (PMR), led by degenerative etiologies with leaflet abnormalities, and secondary MR (SMR), in which the valve components are structurally normal. 10 Numerous reports, usually retrospective or single‐center studies, present results of surgical and, more recently, transcatheter interventions for patients with MR. In contrast with the burden of the disease inferred from epidemiological studies, the low number of MR interventions suggests a marked undertreatment of patients with MR. In the Euro Heart Survey, a substantial number of patients were denied any intervention. 11 However, the Euro Heart Survey was a brief cross‐sectional analysis of patients referred to cardiology centers and was conducted >15 years ago. In a recent community‐based study, MR was associated with excess mortality and morbidity including heart failure (HF), but only a minority of individuals were offered mitral valve intervention. 12 Taken together, these studies suggest that patients referred for a valve intervention constitute only the visible part of the iceberg and neglect the vast majority of patients with MR. Large and comprehensive contemporary data assessing the management and outcome of patients with MR at the nationwide level and the societal burden of the disease are currently lacking.
The Programme de Médicalisation des Systèmes d’Information (PMSI) is a national database collecting information on all consecutive patients admitted to public and private hospitals in France, and participation is mandatory for all French healthcare institutions. The PMSI offers the unique opportunity to define the current treatment standards, clinical outcomes, and costs related to MR at the nationwide level.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design
All healthcare institutions in France are mandated to transfer information regarding their activity into the PMSI data set. The PMSI dataset includes information about the patient (age and sex), the hospital, the admission (date of admission, date of discharge, and mode of discharge), pathologies, procedures, and in‐hospital outcome. 13 Primary and secondary diagnoses are coded using the International Classification of Diseases, Tenth Revision (ICD‐10). Procedures are coded using a French standardized classification. 14
We collected all admissions in France from both public and private hospitals in 2014 and 2015 for patients aged ≥30 years with MR as a primary or secondary discharge code (I340 and/ or I341). We excluded patients with codes related to congenital diseases, infective endocarditis, and rheumatic valve disease. Patients who underwent an intervention related to an exclusion condition such as pulmonary valve surgery, mitral commissurotomy, or interatrial septal closure were also excluded. According to ICD‐10 codes and associated diseases, the MR population was divided into those with PMR or SMR. In the PMR group, patients had to be free from ischemic or dilated cardiomyopathy, history of coronary disease, myocardial infarction, and coronary artery bypass grafting or other cardiac surgery. In contrast, patients with SMR had to have at least one of the above‐mentioned associated conditions. The Charlson index was used to assess patient comorbidities. 15 Centers were classified as public or private. Ethics approval was not required because all data were anonymized.
Outcome
In‐hospital mortality was defined as death occurring during the same hospital stay. Length of stay (total and intensive care unit) was calculated as the time between admission and discharge and expressed in days. From the PMSI database, we also collected in‐hospital death, all‐cause readmissions, and readmissions for HF that occurred during the following year. Expected 1‐year mortality rates according to age and sex provided by the Institut National de la Statistique et des etudes Economiques (INSEE, https://www.insee.fr/fr/statistiques/3311422?sommaire=3311425) were used for comparison.
Cost Analysis
Cost analysis was restricted to public hospitals and analyzed from the payer perspective based on reimbursement provided to hospitals. For each admission, a reimbursement fee (groupement homogène de séjour [GHS]) is established based on patients’ diseases and comorbidities. These fees do not include specific fees for intensive care unit admissions and devices that were added to the GHS. The same calculation was performed for readmissions. Total costs were extrapolated to the entire population assuming similar cost in public and private hospitals.
Statistical Analysis
Continuous variables were expressed as mean±SD or median (interquartile range [IQR; 25%–75%]), and categorical variables were expressed as number of patients (percentage). Differences between groups were calculated with the use of the χ2 test for categorical variables and the Student t test or Wilcoxon/Kruskall‐Wallis tests for continuous variables, as appropriate. We performed a multiple logistic regression analysis to evaluate the impact of age, HF at presentation, Charlson index, and MR etiology on in‐hospital mortality, 1‐year mortality, 1‐year death or all‐cause readmission, and 1‐year death or HF readmission. All tests were 2‐sided and were performed using JMP v9.0 (SAS Institute). P<0.05 was considered statistically significant.
Results
Population
In 2014 and 2015, 107 412 patients with MR were admitted in 1238 public and private hospitals in France. Characteristics of the population are presented in Table 1. Among the 107 412 patients, 8676 (8%) were operated within 1 year of the considered index admission in the present study, and 98 736 (92%) were conservatively managed. The latter group constituted our study population (Figure 1), with 67 095 patients (68%) presenting with PMR and 31 641 (32%) with SMR. The mean age of the conservatively managed subset was 77±15 years, and 55% were female; 50% were aged ≥80 years, and 12% were ≥90 years. Most patients presented with comorbidities; the mean Charlson index was 2.39±2.87 (median, 2; IQR, 1–3) and was ≥2 in 53% of the population (18% with an index of 2 and 35% with an index ≥3; Figure 2). Overall, 39% (38 281 patients) presented with HF.
Table 1.
Characteristics and Outcome of the Population Overall and in the Subsets Referred for Surgery and Conservatively Managed, Overall and According to PMR or SMR Etiology
| Overall | Surgery | Conservative Management | ||||
|---|---|---|---|---|---|---|
| (N = 107 412) | (n = 8676) |
Overall (n = 98 736) |
PMR (n = 67 095) |
SMR (n = 31 641) |
P Value, PMR vs SMR | |
| Age | 76±13 | 67±12 | 77±15 | 77±15 | 76±12 | <0.0001 |
| Female sex | 57 130 (53) | 3285 (38) | 53 845 (55) | 41 407 (61) | 12 798 (40) | <0.0001 |
| Previous cardiac surgery | 13 964 (13) | 1004 (12) | 12 960 (13) | … | 12 960 (40) | NA |
| Ischemic cardiomyopathy | 19 788 (18) | 945 (11) | 18 843 (19) | … | 18 843 (59) | NA |
| Congestive HF | 40 909 (38) | 2628 (30) | 32 281 (39) | 20 952 (31) | 17 329 (55) | <0.0001 |
| Charlson index | 2.30±2.81 | 1.24±1.80 | 2.39±2.87 | 1.95±2.80 | 3.31±2.79 | <0.0001 |
| Charlson index ≥2 | 55 213 (51) | 2471 (28) | 52 742 (53) | 30 125 (41) | 25 088 (75) | <0.0001 |
| Charlson index ≥3 | 36 194 (34) | 1338 (15) | 34 856 (35) | 18 022 (27) | 16 834 (53) | <0.0001 |
| Public hospital | 76 149 (71) | 5656 (65) | 70 493 (71) | 47 051 (70) | 23 442 (74) | <0.0001 |
| Length of stay, total | … | … | 7 (3–12) | 6 (3–11) | 7 (4–13) | <0.0001 |
| Length of stay, intensive care unit | … | … | 0 (0–1) | 0 (0–0) | 0 (0–3) | <0.0001 |
| In‐hospital mortality | … | … | 4083 (4.1) | 2383 (3.6) | 1700 (5.4) | <0.0001 |
| 1‐y mortality | … | … | 14 140 (14.3) | 8502 (12.8) | 5638 (17.8) | <0.0001 |
| 1‐y all‐cause readmission | … | … | 61 871 (68) | 8502 (12.8) | 21 742 (69) | <0.0001 |
| Number of all‐cause readmissions | … | … | 2.4±1.7 | 2.3±1.6 | 2.6±1.8 | <0.0001 |
| ≥2 all‐cause readmissions | … | … | 37 003 (37) | 22 905 (34) | 14 098 (45) | <0.0001 |
| 1‐y readmission for HF | … | … | 25 427 (26) | 13 890 (21) | 11 537 (36) | <0.0001 |
| Number of readmissions for HF | … | … | 0.5±1.0 | 0.4±0.9 | 0.7±1.2 | <0.0001 |
| ≥2 readmissions for HF | … | … | 10 774 (11) | 5449 (8) | 5325 (17) | <0.0001 |
| 1‐y mortality/all‐cause readmissions | … | … | 65 937 (67) | 42 506 (64) | 23 431 (74) | <0.0001 |
| 1‐y mortality/heart failure readmission | … | … | 33 275 (34) | 18 940 (28) | 14 335 (45) | <0.0001 |
| Cost in euros of the first admission* | … | … | 5345±6432 | 4835±5199 | 6370±8282 | <0.0001 |
| Cost in euros of all readmissions* | … | … | 10 080±10 847 | 9389±10 201 | 11 303±11 804 | <0.0001 |
Values are mean±SD, number of patients (percentage), or median (interquartile range). HF indicates heart failure; PMR, primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Analysis restricted to the 70 493 patients admitted in public hospitals.
Figure 1. Flow chart of the population according to management and etiology of the mitral regurgitation.
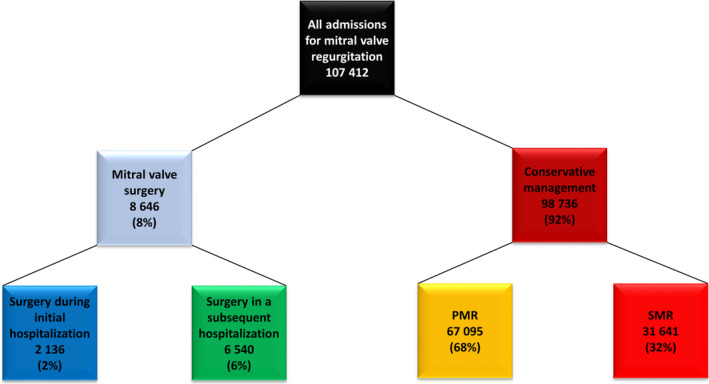
PMR indicates primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Figure 2. Charlson index distribution overall and according to the etiology (PMR or SMR) of the regurgitation in the 98 736 conservatively managed patients.
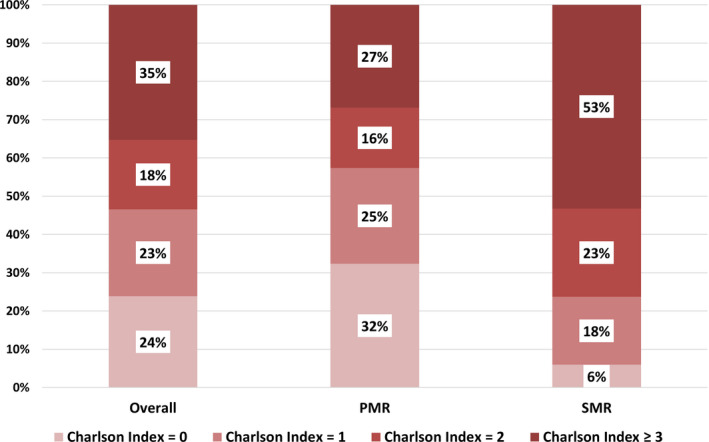
PMR indicates primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Compared with the patients who were conservatively managed, the 8676 patients who underwent a mitral valve intervention were younger (67±12 versus 77±15 years, P<0.0001), less frequently female (38% versus 55%, P<0.0001), and presented less often with a history of cardiac surgery (11.6% versus 13.1%, P<0.0001), ischemic cardiomyopathy (11% versus 19%, P<0.0001), or congestive HF (30% versus 39%, P<0.0001; Table 1). The mean Charlson index was markedly lower (1.24±1.80 [median, 1; IQR, 0–2] versus 2.39±2.87 [median, 2; IQR, 1–3]; P<0.0001). MR etiology was PMR in 7003 patients (81%) and SMR in 1673 (19%). Surgery was performed during the same hospital stay in 2136 patients and during a readmission in 6540 patients. A mitral intervention was performed within 1 year in 9% of patients with PMR and 5% of patients with SMR.
Mortality and Readmission Rates
Mortality and readmission rates are presented in Table 1. In‐hospital and 1‐year mortality rates were 4.1% and 14.3%, respectively. Readmissions were common; 63% of the population was readmitted at least once, and 37% were readmitted ≥2 times. The mean number of readmissions per patient among those readmitted at least once was 2.4±1.7 (median, 2; IQR, 1–3). Overall, 41% of readmissions were related to HF. The rates of 1‐year mortality or all‐cause readmission and 1‐year mortality or HF readmission were 67% and 34%, respectively (Figure 3).
Figure 3. One‐year event rates (all‐cause mortality, all‐cause mortality or readmission, and all‐cause mortality or readmission for HF) overall and according to the etiology (PMR or SMR) of the regurgitation.
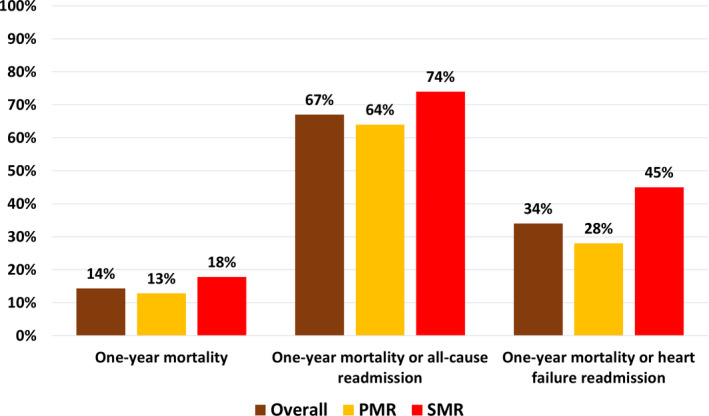
HF indicates heart failure; PMR, primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Mortality and readmissions rates increased with age (Figure 4) and Charlson index (Figure 5), and very high event rates were observed in the highest categories. Patients who presented with HF had higher rates of 1‐year mortality (21.7% versus 9.6%, P<0.0001), mortality or all‐cause readmission (75.1% versus 61.5%, P<0.0001), and 1‐year mortality or HF readmission (55.0% versus 20.2%, P<0.0001) than those who were free of HF at presentation (Table 2). Observed 1‐year mortality rates according to age and sex were markedly higher than expected (Table 3).
Figure 4.
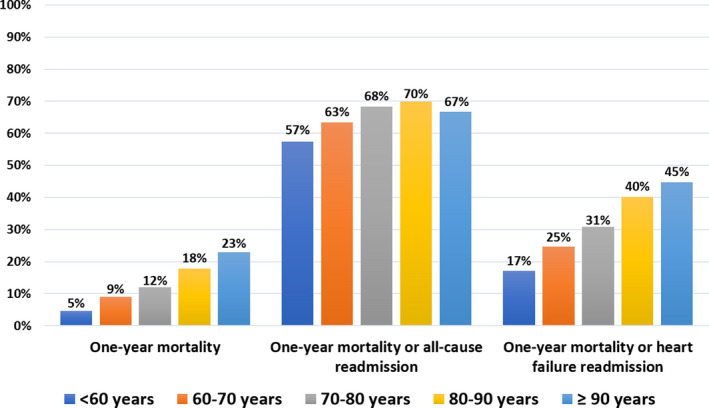
One‐year event rates (all‐cause mortality, all‐cause mortality or readmission, and all‐cause mortality or readmission for heart failure) according to age categories
Figure 5.
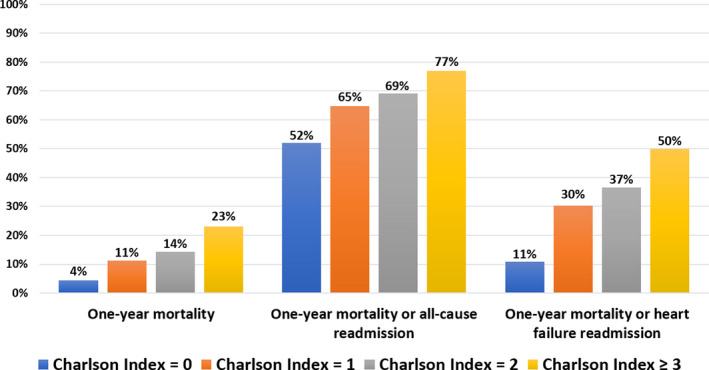
One‐year event rates (all‐cause mortality, all‐cause mortality or readmission, and all‐cause mortality or readmission for heart failure) according to Charlson index
Table 2.
Event Rates in Percentage Overall and in the Subsets of Patients with PMR and SMR According to the Presence of HF at Presentation
| In‐Hospital Mortality | 1‐Year Mortality | 1‐Year Mortality or Readmissions All‐Cause | 1‐Year Mortality or HF Readmission | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF − | HF + | P Value | HF − | HF + | P Value | HF − | HF + | P Value | HF − | HF + | P Value | |
| Overall | 2.5 | 6.7 | <0.0001 | 9.6 | 21.7 | <0.0001 | 61.5 | 75.1 | <0.0001 | 20.2 | 55.0 | <0.0001 |
| PMR | 2.2 | 6.5 | <0.0001 | 8.8 | 21.2 | <0.0001 | 59.0 | 73.0 | <0.0001 | 17.5 | 51.9 | <0.0001 |
| SMR | 3.5 | 6.9 | <0.0001 | 12.2 | 22.4 | <0.0001 | 69.8 | 77.6 | <0.0001 | 29.1 | 58.7 | <0.0001 |
HF indicates heart failure; PMR, primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Table 3.
Observed and Predicted 1‐Year Mortality Rates According to Age and Sex, From the Institut National de la Statistique et des etudes Economiques (INSEE)
| Age Category | Male | Female | ||
|---|---|---|---|---|
| Observed (%) | Expected (%) | Observed (%) | Expected (%) | |
| Mean age, y* | 15.0 | 2.3 | 12.6 | 1.9 |
| <60 | 4.8 | 1.0 | 3.6 | 0.4 |
| 60–70 | 9.8 | 1.3 | 6.2 | 0.6 |
| 70–80 | 14.1 | 2.7 | 9.2 | 1.4 |
| 80–90 | 21.3 | 8.3 | 15.4 | 5.2 |
| ≥90 | 27.4 | 24.7 | 21.1 | 19.8 |
Mean age of the 98 736 conservatively managed patients was 73 y in men and 78 y in women.
As shown in Table 1, patients with SMR were of similar age but more frequently male, presented with higher Charlson index, and more often presented with HF than patients with PMR (all P<0.0001). Event rates were significantly higher in the SMR subset than the PMR subset (all P<0.0001). However, event rates were high even in the PMR subset (Figure 3). As in the overall population, patients with HF had higher event rates than patients who were free of HF at presentation both in the PMR and SMR subsets (all P<0.0001; Table 2). Although statistically different, event rates in PMR and SMR patients presenting with HF were of similar magnitude (21% versus 22% for 1‐year mortality, 73% versus 78% for 1‐year mortality or all‐cause readmission, and 52% versus 59% for 1‐year mortality or HF readmission). In multivariate analysis, age, Charlson index, HF at presentation, and SMR etiology were independently associated with in‐hospital mortality, 1‐year mortality or all‐cause readmission, and 1‐year mortality or HF readmission (all P<0.0001; Table S1).
Cost Analysis
Cost analysis was performed for the 70 493 conservatively managed patients admitted in public hospitals (47 051 PMR [67%] and 23 442 SMR [33%]). The mean cost of hospital (index) admission was 5345±6432 euros (6215±7227 US dollars; median, 3819 euros; IQR, 2589–5893 euros). Among these 70 493 patients, 41 023 were readmitted at least once (mean number of readmissions, 2.3±1.6; median, 2; IQR, 1–3). The mean cumulative cost of all readmissions following the index admission was 10 080±10 847 euros (median, 6482 euros; IQR, 3110–13 237 euros). The total annual cost in public hospitals was 395 million euros including initial hospitalization (188 million euros) and all‐cause readmissions (207 million euros). When only HF readmissions were considered, the total annual cost was 257 million euros. Extrapolating cost calculation to the entire population, total annual costs were 553 million for all‐cause admissions and 359 millions when only HF readmissions were considered. As shown in Table 1, costs of the first hospital stay and readmissions were higher for SMR than for PMR (both P<0.0001). SMR accounted for 40% of the first admission costs and 41% of all readmissions costs. Patients with HF accounted for 46% of first admissions costs and 50% of readmission costs. Distribution of costs according to MR etiology and presence of HF is presented in Figure 6.
Figure 6. Distribution of the costs for first admission (A) and readmissions (B), according to the etiology the regurgitation (PMR in orange and SMR in red) and presence (HF+, hatched) or absence (HF–, solid color) of HF at presentation.
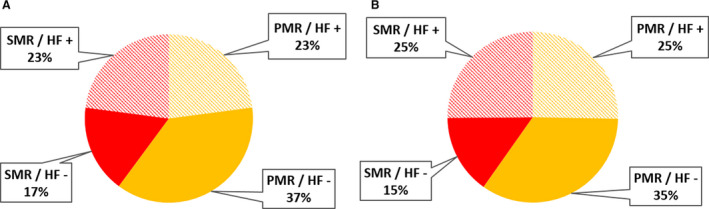
. HF indicates heart failure; PMR, primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Discussion
In this study, we report the presentation, management, and outcomes of all consecutive patients with MR admitted in France in the contemporary era. The main results can be summarized as follows. First, MR was a common reason for admission and affected an elderly population with frequent comorbidities. Second, <10% of patients admitted with MR underwent a valve intervention (even in the subset of PMR), and the vast majority were conservatively managed. Third, MR was associated with high mortality and readmissions rates that increased with age, Charlson index, HF at admission, and secondary etiology of MR, but high event rates were observed in all subsets. Finally, MR represented a major societal burden, with an extrapolated annual cost between 350 and 550 million euros (390–615 million US dollars).
Profile of the Population With Mitral Regurgitation
Unbiased information on the clinical presentation of patients with significant MR are scarce. To the best of our knowledge, our study is of the first to collect all patients with significant MR admitted at a nationwide level in the contemporary era. Other nationwide studies have analyzed the incidence of different types of valvular disease but did not report management. 16 In the present study, conservatively managed patients were on average 10 years older than those referred for an intervention (77 versus 67 years). A younger age of patients referred for surgery was also observed in the Society of Thoracic Surgeons (STS) database and is consistent across the literature 17 , 18 , 19 . Patients with MR enrolled in echocardiographic registries, 20 patients with SMR enrolled in the CSTN (Cardiothoracic Surgical Trials Network) trials, 21 , 22 , 23 patients enrolled in transcatheter randomized controlled trials, 6 , 9 or patients in prospective cohorts 24 are also usually markedly younger. Interestingly, half of the patients with MR admitted in French hospitals in 2014 to 2015 were octogenarians and 12% were nonagenarians. Consequently, most patients with MR presented with a high burden of comorbidities, in both the PMR and SMR subsets, and compared with patients referred for an intervention, the Charlson index was 2‐fold higher in conservatively managed patients. The difference in the burden of comorbidities was even more striking in the subset of patients with SMR, in which three‐quarters of the population presented with a Charlson index of ≥2. Our nationwide data clearly show that patients referred for surgery are a highly selected population, not representative of the overall population of patients with MR, both in the PMR and SMR subgroups.
Prognosis and Management of Patients With MR
The present study capturing all consecutive admissions in France clearly shows the poor prognosis of MR patients who are conservatively managed. One‐year mortality was 14%, and readmission rates (all‐cause or HF) were high. One‐year observed mortality rates were markedly higher than expected in every age and sex category (except, not surprisingly, in nonagenarians). Approximately two‐thirds of patients were readmitted with HF accounting for almost 40% of all readmissions. These rates were even higher among older patients or those with a high burden of comorbidities, who represented the majority of the population. The poor prognosis of patients with SMR has been reported previously, and SMR is a major prognostic factor in patients with ischemic or dilated cardiomyopathy. 25 , 26 Similar high event rates were observed in 2 recent randomized controlled trials comparing the Mitraclip system with optimal medical therapy in 1000 patients with SMR. 6 , 9 In our study, even PMR was associated with high event rates, especially in patients with HF at presentation. In this subset, event rates were of similar magnitude between PMR and SMR. Our study confirms, at the population level, the poor outcome of patients with SMR and extends these findings to the patients with PMR, showing that the prognosis of patients with MR is poor overall when conservatively managed.
Despite this dismal prognosis, the vast majority of patients were not treated with the only available curative option for MR, a valve intervention, particularly for PMR. Optimal management of patients with MR requires accurate screening for the disease, appropriate follow‐up and timely intervention, and individualized therapeutic decision‐making. Several recent studies have shown low awareness of VHD among both the general population and the medical community. Auscultation is rarely performed during routine visits to family practitioners, precluding screening and identification of patients with VHD. 27 However, in the present study, MR was clearly identified and coded in the discharge summary. Nevertheless, <10% of patients admitted with significant MR underwent a valve intervention within 1 year, leaving the vast majority of the population untreated. The rate of intervention was remarkably low for both PMR and SMR. These low rates of interventions are even more striking considering that all individuals were inpatients, admitted to French hospitals with relatively easy access to cardiologists. Although we acknowledge that the appropriateness of management cannot be inferred from the current database, the low rate of intervention suggests undertreatment of patients with MR, especially those who presented with HF. Such undertreatment has also been described at the community level. 12 Among Olmsted County (MN, USA) residents, <15% of patients with MR and a class I or IIa indication for intervention were referred for a valve intervention despite the identification of disease on echocardiography and easy access to a high‐quality medical center offering all types of mitral valve interventions. Our population‐wide data show coherently in Europe and the United States that the vast majority of patients with MR are conservatively managed. The gap between the recommendations of scientific societies and real‐life practice may be related to insufficient knowledge of VHD evaluation and management or poor compliance with guidelines by medical caregivers, 28 , 29 as shown in a recent survey highlighting an important and substantial opportunity to improve outcomes of patients with MR. 30
Societal Burden and Clinical Implications
There were ≈27 million yearly admissions in France during the study period including 1 470 000 that were related to cardiovascular diseases. When admissions and readmissions were added (130 000 yearly admissions), the number of MR‐related admissions accounted for 0.5% of all admissions and 9% of cardiovascular admissions. The incidence and mortality rates of MR are similar to those for breast cancer in France, with 58 459 new cases and 12 146 deaths in 2018 (Institut de Veille Sanitaire, https://www.santepubliquefrance.fr/maladies‐et‐traumatismes/cancers/cancer‐du‐sein). Despite the dismal prognosis observed in the current study, <10% of patients underwent a valve intervention, and MR was responsible for a major societal burden in terms of both healthcare organization and costs. One‐sixth of patients with MR died, and two‐thirds were readmitted within 1 year. We estimated that MR is associated with an annual cost between 350 and 550 million euros (390–615 million US dollars) when considering only in‐hospital costs of conservatively managed patients. Our results highlight the critical need to develop strategies to improve the overall management and outcomes of patients with both PMR and SMR. Dedicated programs to raise awareness of VHD in the population, screening programs allowing earlier recognition of the disease, and education of care providers regarding clinical guidelines 28 , 29 should be implemented to reduce the number of patients referred at an advanced disease stage and to facilitate optimal management of these patients. In line with the suboptimal surgical results observed at the nationwide level (submitted), implementation of dedicated referral pathways and Heart Valve Centers of Excellence may improve evaluation and management of patients with MR. The rapid and intense development of transcatheter mitral therapies may increase referral, offer curative therapies to patients who otherwise were not considered candidates for valve intervention, and improve outcomes of patients with MR.
Strengths and Limitations
The present study deserves several comments. First, variables such as New York Heart Association functional class, left ventricular ejection fraction, and creatinine were not available in the PMSI database, and the assessment of comorbidity burden relied on the Charlson index (no surgical risk score could be calculated). Left ventricular ejection fraction is a major prognostic factor in both PMR and SMR and is expected to be lower in conservatively managed patients than in those referred for an intervention. However, we were able to capture all consecutive patients with MR admitted in France during the study period. Second, the database has no information regarding MR severity or grading. However, MR was deemed severe enough by the treating physicians to code the diagnosis in the discharge summary. Nevertheless, we cannot exclude that the same degree of MR (ie, moderate) may have been coded differently for PMR and SMR. Third, MR classification as PMR and SMR did not rely on a centralized assessment but rather on a specific algorithm we developed. A similar methodology has been used previously, 17 and noteworthy MR etiology was not available in up to one‐third of patients in the STS database. 18 , 19 , 31 Importantly, high mortality and event rates were observed regardless of MR etiology, emphasizing the importance of our findings. The PMR/SMR ratio may be at odds with prior publications, but in a recent multicenter study, PMR accounted for 55% of all moderate or severe cases, whereas SMR represented only 30% (15% had mixed disease). 20 Fourth, only deaths occurring in the hospital (at admission or during follow‐up) and HF readmissions are captured by the PMSI and thus were available for this study. Therefore, it is likely that death and HF rates might have been underestimated, but the magnitude of this underestimation could not be determined. Fifth, the cause of death was not recorded, and accountability of MR for both death and readmissions is only hypothetical. The cause of death is often complex to ascertain, and the inclusion of MR as a diagnosis code strongly suggests that MR was thought to have played a role in the patient’s admission and complications. Sixth, the appropriateness of the management strategy could not be evaluated. For some patients, surgery may not have been considered because the patients were at too high a risk because of comorbidities or the mitral valve anatomy (degree of calcification), but the low rate of intervention is concerning and strongly suggests marked undertreatment of patients with MR. Although uncertainties regarding the benefit of surgery in patients with SMR may explain, at least in part, the low rate of intervention in this subset, similar low rates were observed in PMR, for which clear recommendations for intervention are available. Regardless of the appropriateness of the surgical decision, this study highlights the dismal prognosis and unmet need of the MR population. Seventh, the vast majority of mitral valve interventions in 2014 to 2015 were surgeries; transcatheter therapies had restricted access and were seldom performed. Finally, the direct costs could not be calculated from the PMSI database, and the payer perspective was adopted. However, these costs represents the economic burden faced by the country.
Conclusions
In this large contemporary nationwide database, MR was a common reason for admission and affected an elderly population with frequent comorbidities, highlighting that surgical series are not representative of the overall MR population. Less than 10% of patients underwent a valve intervention, and the vast majority of patients with both PMR and SMR were conservatively managed. All subsets of conservatively managed patients incurred high mortality and readmission rates regardless of MR etiology, and MR represented a major societal burden, with an extrapolated annual cost between 350 and 550 million euros (390–615 million US dollars). These finding highlight the critical need to develop strategies to improve the overall management and outcomes of patients with both PMR and SMR.
Sources of Funding
This study was funded through a research contract between International Health Market Trends and Edwards Lifesciences. The statistical methodology and analyses were independently performed by David Messika‐Zeitoun.
Disclosures
David Messika‐Zeitoun is a consultant for Edwards Lifesciences, Mardil and Cardiawave and receives research grants from Edwards Lifesciences and Abbott vascular. Pascal Candolfi is an Edwards Lifesciences employee. Alec Vahanian has received speaker’s fees from Edwards Lifesciences and Abbott Vascular and is consultant for Cardiawave. Patrick Verta is an Edwards Lifesciences employee. Ted E. Feldman is an Edwards Lifesciences employee. Bernard Iung has received consultant fees from Edwards Lifesciences and speaker’s fees from Boehringer Ingelheim and Novartis. Maurice Enriquez‐Sarano has received research grants from Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc. 2020;9:e016086 DOI: 10.1161/JAHA.120.016086.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson‐Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, et al. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 4. Messika‐Zeitoun D, Nickenig G, Latib A, Kuck KH, Baldus S, Schueler R, La Canna G, Agricola E, Kreidel F, Huntgeburth M, et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur Heart J. 2019;40:466–472. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen V, Michel M, Eltchaninoff H, Gilard M, Dindorf C, Iung B, Mossialos E, Cribier A, Vahanian A, Chevreul K, et al. Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol. 2018;71:1614–1627. [DOI] [PubMed] [Google Scholar]
- 6. Obadia JF, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 7. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 8. Sorajja P, Mack M, Vemulapalli S, Holmes DR Jr, Stebbins A, Kar S, Lim DS, Thourani V, McCarthy P, Kapadia S, et al. Initial experience with commercial transcatheter mitral valve repair in the United States. J Am Coll Cardiol. 2016;67:1129–1140. [DOI] [PubMed] [Google Scholar]
- 9. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 10. Enriquez‐Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. [DOI] [PubMed] [Google Scholar]
- 11. Mirabel M, Iung B, Baron G, Messika‐Zeitoun D, Detaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. [DOI] [PubMed] [Google Scholar]
- 12. Dziadzko V, Clavel MA, Dziadzko M, Medina‐Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez‐Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PMSI . Programme de médicalisation des systèmes d’informations (PMSI). http://www.drees.sante.gouv.fr/redressements‐du‐programme‐de‐medicalisation‐des‐systemes‐d‐informations‐pmsi, 2012.
- 14. CCAM . Classification commune des actes médicaux(CCAM). http://www.ameli.fr/accueil‐de‐la‐ccam/trouver‐un‐acte/index.php. 2012.
- 15. Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16. Andell P, Li X, Martinsson A, Andersson C, Stagmo M, Zoller B, Sundquist K, Smith JG. Epidemiology of valvular heart disease in a Swedish nationwide hospital‐based register study. Heart. 2017;103:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chikwe J, Toyoda N, Anyanwu AC, Itagaki S, Egorova NN, Boateng P, El‐Eshmawi A, Adams DH. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. 2017;69:2397–2406. [DOI] [PubMed] [Google Scholar]
- 18. Gammie JS, Chikwe J, Badhwar V, Thibault DP, Vemulapalli S, Thourani VH, Gillinov M, Adams DH, Rankin JS, Ghoreishi M, et al. Isolated mitral valve surgery: The society of thoracic surgeons adult cardiac surgery database analysis. Ann Thorac Surg. 2018;106:716–727. [DOI] [PubMed] [Google Scholar]
- 19. Rankin JS, Grau‐Sepulveda M, Shahian DM, Gillinov AM, Suri R, Gammie JS, Bolling SF, McCarthy PM, Thourani VH, Ad N, et al. The impact of mitral disease etiology on operative mortality after mitral valve operations. Ann Thorac Surg. 2018;106:1406–1413. [DOI] [PubMed] [Google Scholar]
- 20. Monteagudo Ruiz JM, Galderisi M, Buonauro A, Badano L, Aruta P, Swaans MJ, Sanchis L, Saraste A, Monaghan M, Theodoropoulos KC, et al. Overview of mitral regurgitation in Europe: results from the European Registry of mitral regurgitation (EuMiClip). Eur Heart J Cardiovasc Imaging. 2018;19:503–507. [DOI] [PubMed] [Google Scholar]
- 21. Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, et al. Mitral‐valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2013;370:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, et al. Two‐year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2015;374:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartko PE, Arfsten H, Heitzinger G, Pavo N, Toma A, Strunk G, Hengstenberg C, Hulsmann M, Goliasch G. A unifying concept for the quantitative assessment of secondary mitral regurgitation. J Am Coll Cardiol. 2019;73:2506–2517. [DOI] [PubMed] [Google Scholar]
- 25. Grigioni F, Detaint D, Avierinos JF, Scott C, Tajik J, Enriquez‐Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol. 2005;45:260–267. [DOI] [PubMed] [Google Scholar]
- 26. Grigioni F, Enriquez‐Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long‐term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. [DOI] [PubMed] [Google Scholar]
- 27. Gaede L, Di Bartolomeo R, van der Kley F, Elsasser A, Iung B, Mollmann H. Aortic valve stenosis: what do people know? A heart valve disease awareness survey of over 8,800 people aged 60 or over. EuroIntervention. 2016;12:883–889. [DOI] [PubMed] [Google Scholar]
- 28. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017;38:2739–2791.28886619 [Google Scholar]
- 29. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 30. Iung B, Delgado V, Lazure P, Murray S, Sirnes PA, Rosenhek R, Price S, Metra M, Carrera C, De Bonis M, et al. Educational needs and application of guidelines in the management of patients with mitral regurgitation. A European mixed‐methods study. Eur Heart J. 2018;39:1295–1303. [DOI] [PubMed] [Google Scholar]
- 31. Badhwar V, Rankin JS, He X, Jacobs JP, Gammie JS, Furnary AP, Fazzalari FL, Han J, O'Brien SM, Shahian DM. The society of thoracic surgeons mitral repair/replacement composite score: a report of the society of thoracic surgeons quality measurement task force. Ann Thorac Surg. 2016;101:2265–2271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


