Abstract
Background
Cardiac features diverge in Asians; however, it is not known how these differences relate to embolic stroke of unknown source (ESUS) in Southeast Asian and Eastern Mediterranean regions.
Methods and Results
A retrospective analysis of prospectively collected acute ischemic stroke data from 2014 to 2018 was performed. Stroke subtypes were noncardioembolic stroke (large‐vessel and small‐vessel disease; n=1348), cardioembolic stroke (n=532), and ESUS (n=656). Subtypes were compared by demographic, clinical, and echocardiographic factors. In multivariate logistic regression, patients with ESUS in comparison with noncardioembolic stroke were twice as likely to have left ventricular diastolic dysfunction (P=0.001), 3 times the odds of global hypokinesia (P=0.001), and >7 times the odds of left ventricular wall motion abnormalities (P=0.001). In the second model comparing ESUS with cardioembolic stroke, patients with ESUS were 3 times more likely to have left ventricular wall motion abnormalities (P=0.001) and 1.5 times more likely to have left ventricular diastolic dysfunction grade I (P=0.009), and 3 times more likely to have left ventricular diastolic dysfunction grades II and III (P=0.009), whereas age (P=0.001) and left atrial volume index (P=0.004) showed an inverse relation with ESUS. ESUS in patients ≥61 years old had higher levels of traditional risk factors such as coronary artery disease, but the coronary artery disease was not significantly different in ESUS age groups (P=0.80) despite higher left ventricular wall motion abnormalities (P=0.001).
Conclusions
Patients with ESUS and noncardioembolic stroke were younger than patients with cardioembolic stroke. While a third of the patients with ESUS >45 years old had coronary artery disease, it was unrecognized or underreported in the older ESUS age group (≥61 years old). In patients with ESUS from Southeast Asia and Eastern Mediterranean regions, left ventricular wall motion abnormalities and left ventricular diastolic dysfunction were related to ESUS.
Keywords: cardiac wall motion abnormalities, embolic stroke, ESUS, left atrial volume index
Subject Categories: Cardiovascular Disease, Epidemiology, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- aOR
adjusted odds ratio
- CAD
coronary artery disease
- CES
cardioembolic stroke
- DM
diabetes mellitus
- EF
ejection fraction
- HF
heart failure
- LAVI
left atrial volume index
- LVDD
left ventricular diastolic dysfunction
- LVWMAs
left ventricular wall motion abnormalities
- MI
myocardial infarction
- NCES
noncardioembolic stroke
Clinical Perspective
What Is New?
Embolic stroke of undetermined source (ESUS) is an important global public health issue; cardiac features diverge in Asians, and increasing evidence from across the world shows that ESUS risk and clinical profiles differ on the basis of ethnicity.
Two major studies, the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) and RE‐SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source), reported data on 12 522 ESUS patients with paucity of ESUS from northern Africa, the Middle East and many parts of Asia, representing over one third of the world's population; our study is the first to provide data on ESUS from West Asia, South Asia, and North Africa showing that the burden of ESUS in these areas is greater than other global estimates and that ESUS occurs at a much younger age.
Our results indicate that cardiac wall motion abnormalities and left ventricular diastolic dysfunction are an important factor in ESUS development in South Asians, implying that underlying cardiac wall motion abnormalities may be a cause of embolism in ESUS; coronary artery disease and myocardial infarction are more common, at a younger age, in these ethnic groups.
What Are the Clinical Implications?
The risk factor and clinical profiles of patients with ESUS may differ from area to area across the world, influencing the global variations in etiology, occurrence, and outcome of ESUS.
The Southeast Asian and Eastern Mediterranean regions of the World Health Organization mostly comprise Asian low‐income countries. Four Southeast Asian countries (India, Pakistan, Bangladesh, and Sri Lanka) constitute 25% of the world's population and contribute nearly 60% of the global cardiovascular disease burden and up to 40% of global stroke deaths. 1 , 2 The stroke‐related risk factors relatively specific to Asia include stroke at a younger age, premature atherosclerosis, higher prevalence of intracranial atherosclerosis, pre–diabetes mellitus (DM), and new‐onset DM compared with Whites. 2 , 3 , 4 While coronary artery disease (CAD) has declined in developed countries over the past 40 years, rates have doubled in the South Asian immigrant population. 5 CAD studies including South Asian immigrants living in Europe and the United States report a higher prevalence, predisposition to, and more extensive CAD at a younger age 6 and a higher proportional mortality rate compared with non‐Hispanic Whites. 7 The CVDNOR (Cardiovascular Disease in Norway) project showed that acute myocardial infarction (MI) and stroke rates were highest in South Asians. 8 Furthermore, in the SHARE (Study of Health Assessment and Risk in Ethnic Groups), South Asian ethnic origin was an independent risk factor for cardiovascular disease. 9
Embolic stroke of unknown source (ESUS) accounts for 16% to 32% of ischemic strokes. 10 Whereas ESUSs are suspected to be of cardiac origin (particularly covert atrial fibrillation [AF]), South Asian ethnicity is associated with a lower risk of AF despite a higher prevalence of established risk factors. 11 This is especially interesting since NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) and RE‐SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source) trials with over 13 000 patients did not show the superiority of anticoagulation over aspirin suggesting that undetected paroxysmal AF is not a major cause of recurrent stroke. Recent publications highlight a wide range of potential cardiac embolic sources associated with ESUS, such as atrial fibrosis without AF, left atrial enlargement, unrecognized MI with myocardial scar, left ventricular wall motion abnormalities (LVWMAs), patent foramen ovale, and elevated cardiac troponin levels. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Studies have shown that atrial and ventricular features diverge in South Asians compared with the non‐South Asians. 20 However, there is a paucity of data on how left heart factors relate to ESUS from the 2 aforementioned World Health Organization regions.
The objective of this study, therefore, was to identify left heart factors that are associated with patients with ESUS in a multiethnic Asian and North African cohort from member countries of World Health Organization's Southeast Asian and Eastern Mediterranean regions.
Methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Hamad Medical Corporation, Qatar. The study did not require a consent process because of study design (retrospective).
The data were prospectively collected at a tertiary referral center with well‐established comprehensive stroke service accredited by Joint Commission International. The stroke service includes acute stroke diagnostic, stroke units, vascular interventional services, vascular neurological surgery, and rehabilitation services. An acute stroke team provides a rapid assessment service 24 hours a day, 7 days a week. The data that support the findings of this study are available from the corresponding author upon reasonable request.
All patients ≥18 years old with acute stroke admitted from January 1, 2014, through December 31, 2018, were included in the study. Exclusion criteria (Figure) consisted of malignancy, hypercoagulable state, incomplete workup, missing data, and ESUS with intracranial or nonstenotic (<50%) carotid atherosclerotic plaques ipsilateral to the ESUS event. The stroke subtypes were classified according to TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria by stroke neurologists. 21 Data included age, sex, hypertension, pre‐DM and DM (defined according to the American Diabetes Association criteria), 22 dyslipidemia, smoking, CAD (defined by a history of MI or typical angina, a positive diagnostic test [stress test, coronary angiography], or appropriate treatment). Admission National Institute of Health Stroke Scale score and CHA₂DS₂VASc score were also recorded. Vascular imaging included craniocervical magnetic resonance angiography, computed tomography angiography, or digital subtraction angiography. Holter monitoring was performed for at least 24 to 48 hours in patients with nonlacunar stroke within 5 days of symptoms (except for patients with known AF), and 2‐dimensional transthoracic echocardiography and additional transesophageal echocardiography (at the discretion of treating physicians) was performed up to 4 days after admission.
Figure 1. Flowchart with inclusion and exclusion criteria.
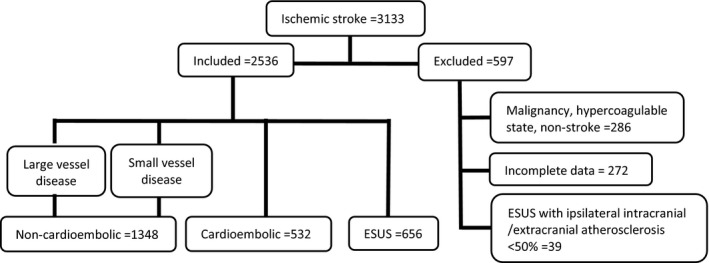
ESUS indicates embolic stroke of undetermined source.
Echocardiography
Transthoracic echocardiography was performed according to the American Society of Echocardiography guidelines 23 by specialists blinded to the other variables and independent of the study team. The following atrial and ventricular variables were recorded:
Left atrium and valves: Left atrial volume index (LAVI, expressed as mL/m2), left atrial appendage stasis/spontaneous echo densities, patent foramen ovale/atrial septal aneurysm, mitral and aortic annular calcification, valvular strands, valvular vegetations, and aortic root diameter.
Left ventricle: Left ventricular ejection fraction (EF) as a percentage, LVWMA (scored on a 17‐segment model) with wall motion score index also calculated. Left ventricular mass indexed to height2.7 to obtain LV mass index LVMI g/m2.7. 24 Regional wall thickness with a value of >0.42 used to indicate abnormal wall thickness. 25 Left heart failure (HF) classified according to EF: HF with reduced EF, HF with medium‐range EF, or HF with preserved EF 26 and left ventricular diastolic dysfunction (LVDD) categorized as mild (grade I), moderate (grade II), and severe (grade III). 27
Holter monitoring: Two categories of atrial premature beats were studied: runs of premature atrial contractions defined as atrial premature beat runs of >20 beats (>20 consecutive atrial premature beats) and the number of isolated atrial premature beats within 24 hours divided into the following groups: >0 to <100, ≥100 to 499, 500 to 999, 1000 to 1499, and ≥1500. 28
Statistical Analysis
Descriptive statistics in the form of mean and SD were calculated for all interval variables (eg, age, hemoglobin A1c, LAVI mL/m2), with frequency and percentages calculated for each categorical variable in the study. Data were explored after grouping according to stroke subtype (noncardioembolic strokes [NCES], cardioembolic strokes [CES], and ESUS) and by age (arbitrarily divided into 3 groups, ≤45 years old, 46–60 years old, and >60 years old) for patients with ESUS. One‐way ANOVA with post hoc analysis (Bonferroni) were performed for all interval variables, whereas chi‐square tests were used to test for associations between categorical variables. Two multivariate logistic regressions were performed: First, ESUS was compared with NCES; and second, ESUS was evaluated in relation to patients with CES. P<0.05 (2‐tailed) were considered significant for all statistics. SPSS version 22.0 (SPSS Inc., Chicago, IL) was used for all analysis.
Results
A total of 2536 stroke patients were included in the final analysis after excluding 597 patients that did not meet inclusion criteria (Figure). Within the final cohort, the stroke subtypes were 1348 (53.2%) NCES (large‐vessel and small‐vessel disease), 532 (21.0%) CES, and 656 (25.8%) ESUS. The baseline demographics, clinical characteristics, and atrial and ventricular factors measured are listed in Table 1. The mean age of patients with ESUS was similar to NCES but significantly younger than CES (P=0.001). For stroke risk factors, there was a higher prevalence of DM in CES, while smoking was more frequent in ESUS (P=0.01) (Table 1).
Table 1.
Comparison Between Noncardioembolic Strokes, Cardioembolic Strokes, and ESUS
| Factors |
Noncardioembolic (Large‐Vessel and Small‐Vessel Disease) (1348/2536) 53.2% |
Cardioembolic Stroke (532/2536) 20.9% |
ESUS (656/2536) 25.9% |
P Value |
|---|---|---|---|---|
| Age, y | 55.6±12.7 | 60.5±14.1* | 56.4±13.7 † | 0.001 |
| Female, n (%) | 232 (17.2) | 176 (33.1) | 114 (17.4) | 0.001 |
| Male, n (%) | 1116 (82.8) | 355 (66.9) | 542 (82.6) | |
| Diabetes mellitus, n (%) | 776 (57.6) | 376 (70.7) | 369 (56.3) | 0.001 |
| Pre—diabetes mellitus, n (%) | 278 (20.6) | 69 (13) | 127 (19.4) | 0.001 |
| Hemoglobin A1c % | 6.4±1.5 | 6.4±1.3 | 6.5±1.5 | 0.38 |
| Hypertension, n (%) | 1082 (80.3) | 426 (80.1) | 531 (80.9) | 0.92 |
| Metabolic syndrome, n (%) | 184 (13.6) | 76 (14.3) | 80 (12.2) | 0.53 |
| Smoking, n (%) | 166 (12.5) | 62 (11.8) | 106 (16.6) | 0.01 |
| Dyslipidemia, n (%) | 951 (70.5) | 346 (85) | 455 (69.5) | 0.06 |
| Coronary artery disease, n (%) | 432 (32) | 141 (26.5) | 200 (30.5) | 0.06 |
| LAVI, mL/m2 | 23.5±10 | 30.1±16.5* | 25.6±12.1 † , ‡ | 0.001 |
| PFO/AS aneurysm, n (%) | 22 (1.6) | 15 (2.8) | 8 (1.2) | 0.10 |
| Spontaneous echo contrast, n (%) | 9 (0.7) | 4 (0.8) | 8 (1.2) | 0.47 |
| Mitral calcification, n (%) | 326 (25.1) | 186 (36.5) | 187 (28.8) | 0.001 |
| Aortic sclerosis, n (%) | 487 (37.0) | 213 (40.6) | 248 (38.0) | 0.36 |
| Aortic root diameter | 3.1±0.5 | 30±0.6 | 3.1±0.6 † | 0.10 |
| EF% | 53.5±6.8 | 50.8±9.2* | 49.6±10 † , ‡ | 0.001 |
| LVWMA, n (%) | 91 (7.0) | 66 (12.9) | 187 (28.7) | 0.001 |
| Global hypokinesis, n (%) | 42 (3.2) | 47 (9.2) | 66 (10.1) | 0.001 |
| WMSI | 1±0.2 | 1.1±0.2* | 1.2±0.3 † , ‡ | 0.001 |
| LVMI g/m2.7 | 37.7±31 | 35.0±39.5 | 41.2±36.1 † , ‡ | 0.01 |
| RWT | 0.4±0.5 | 0.3±0.5* | 0.4±0.5 | 0.09 |
| Heart geometry, n (%) | ||||
| Concentric remodeling | 156 (13.6) | 57 (15.4) | 82 (14.2) | 0.10 |
| Eccentric LV hypertrophy | 126 (11.0) | 32 (8.6) | 68 (11.8) | |
| Concentric LV hypertrophy | 133 (11.6) | 53 (14.3) | 72 (12.5 | |
| LVDD, n (%) | ||||
| Grade I | 620 (55.6) | 201 (45.5) | 293 (54.5) | 0.001 |
| LVDD Grade II+III | 25 (2.2) | 15 (3.4) | 31 (5.8) | |
| Congestive heart failure, n (%) | ||||
| HFrEF | 49 (3.6) | 58 (10.9) | 86 (13.1) | 0.001 |
| HFmrEF | 20 (1.5) | 21 (3.9) | 25 (3.8) | |
| HFpEF | 117 (8.7) | 95 (17.9) | 58 (8.8) | |
| Holter, n (%) | ||||
| APB run >20 beats | 17 (1.3) | 12 (2.3) | 14 (2.1) | 0.19 |
| APB/24 h | 0.49 | |||
| >0 to <100 | 271 (68.6) | 122 (66.3) | 133 (67.2) | |
| ≥100 to 499 | 102 (25.8) | 43 (23.4) | 48 (24.2) | |
| 500 to 999 | 4 (1.0) | 2 (1.1) | 1 (0.5) | |
| 1000 to 1499 | 1 (0.3 | 2 (1.1%) | 3 (1.5) | |
| ≥1500 | 17 (4.3) | 15 (8.2) | 13 (6.6) | |
| Admission NIHSS | 4.6±4.7 | 5.3±6.4* | 5.7±5.4 ‡ | 0.001 |
| CHA2DS2VASc | 4.1±1.1 | 4.5±1.2* | 4.3±1.2 † , ‡ | 0.001 |
APB indicates atrial premature beat; AS, atrial septal aneurysm; EF, ejection fraction; ESUS, embolic stroke of unknown source; HFmrEF, heart failure with medium‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LAVI, left atrial volume index; LV, left ventricular; LVMI, left ventricular mass index; LVWMA, left ventricular wall motion abnormalities; NIHSS, National Institute of Health Stroke Scale; PFO, patent foramen ovale; RWT, regional wall thickness; and WMSI, wall motion score index.
Noncardioembolic stroke with cardioembolic.
Cardioembolic stroke with ESUS (P≤0.05).
Noncardioembolic stroke with ESUS.
Stroke Subtypes and Left Atrium
LAVI was largest in CES (30.1±16.5 mL/m2) followed by ESUS (25.6±12.1 mL/m2) and NCES (P=0.001). Mitral valve calcification was observed more often in CES than ESUS and NCES (P=0.001). No difference was seen for patent foramen ovale and atrial septal aneurysm (P=0.10), valvular strands (P=0.23), and aortic arch atheroma (P=0.20) among the 3 stroke groups. Valvular vegetations were reported in 5 patients with CES, and no significant difference in supraventricular arrhythmias was found among the 3 groups (Table 1).
Stroke Subtypes and Left Ventricle
ESUS had the lowest EF (49.6±10%) followed by CES (50.8±9.2%) and NCES (53.5±6.8%) (P=0.001). LVWMAs were more frequent (28.7% versus 12.9% versus 7.0%) with higher wall motion score index in ESUS compared with CES and NCES (P=0.001). LVMI was not different between NCES and CES but significantly higher in ESUS (P=0.01) with no divergence noted in heart geometry (P=0.10). The percentage of patients with HF with reduced EF was highest in ESUS followed by CES and least in NCES (P=0.001). While grade I LVDD was similar in NCES and ESUS but lower in CES, grade II‐III LVDD was more common in ESUS alone (P=0.001). Admission National Institute of Health Stroke Scale was higher in ESUS compared with other groups, while the CHA2DS2VASC scores were higher in CES (Table 1).
In the first multivariate logistic regression (Table 2), patients with ESUS in comparison with patients with NCES were twice as likely to have LVDD (adjusted odds ratio [aOR], 2.15; 95% CI, 1.07–4.31; P=0.001), over 3 times the odds of global hypokinesia (aOR, 3.72; 95% CI, 2.0–7.0; P=0.001), and more than 7 times the odds of LVWMAs (aOR, 7.14; 95% CI, 4.44–10.90; P=0.001) after adjustment for age, DM, smoking, mitral calcification, EF, LVMI, and CHA2DS2VASC score. In the second model (Table 3), patients with ESUS in comparison with patients with CES were 3 times more likely to have LVWMAs (aOR, 3.10; 95% CI, 2.06–4.63; P=0.001), LVDD grade I (aOR, 1.50; 95% CI, 1.12–2.03; P=0.009) and grades II and III (aOR, 2.90; 95% CI, 1.30–6.32; P=0.009), whereas age (aOR, 0.98; 95% CI, 0.97–0.99; P=0.001), DM (aOR, 0.59; 95% CI, 0.42–0.81; P=0.001), and LAVI (aOR, 0.98; 95% CI, 0.97–0.99; P=0.004) showed an inverse relation with ESUS after adjusting for age, hypertension, DM, dyslipidemia, smoking, CAD, HF (HF with reduced EF, HF with medium‐range EF and HF with preserved EF), LVMI, and global hypokinesia.
Table 2.
Multivariate Logistic Analysis for ESUS in Comparison With Noncardioembolic Stroke
| ESUS in Comparison With Noncardioembolic Stroke | ||
|---|---|---|
| Variable | aOR (95% CI) | P Value |
| Age | 0.988 (0.97–0.99) | 0.04 |
| LAVI | 1.00 (0.99–1.02) | 0.38 |
| EF | 1.00 (0.98–1.02) | 0.89 |
| LVMI | 1.00 (0.99–1.00) | 0.19 |
| CHA2DS2VASC | 1.04 (0.90–1.22) | 0.53 |
| Diabetes mellitus | 0.83 (0.62–1.12) | 0.24 |
| Smoking | 0.85 (0.58–1.25) | 0.42 |
| Mitral calcification | 1.26 (0.90–1.77) | 0.18 |
| LVDD grade I DD | 0.97 (0.96–0.98) | 0.073 |
| LVDD grade II+III DD | 2.15 (1.07–4.31) | 0.001 |
| LVWMAs | 7.14 (4.44–10.87) | 0.001 |
| Global hypokinesia | 3.72 (1.98–7) | 0.001 |
aOR indicates adjusted odds ratio; EF, ejection fraction; ESUS, embolic stroke of unknown source; LAVI, left atrial volume; LVDD, left ventricular diastolic dysfunction; LVDD, left ventricular diastolic dysfunction; LVMI, left ventricular mass index; and LVWMA, left ventricular wall motion abnormalities.
Table 3.
Multivariate Logistic Analysis for ESUS in Comparison With Cardioembolic Stroke
| ESUS in Comparison With Cardioembolic Stroke | ||
|---|---|---|
| Variable | aOR (95% CI) | P Value |
| Age | 0.98 (0.97–0.99) | 0.001 |
| Hypertension | 0.81 (0.55–1.19) | 0.29 |
| Diabetes mellitus | 0.59 (0.42–0.81) | 0.001 |
| Dyslipidemia | 1.30 (0.93–1.81) | 0.12 |
| Smoking | 0.92 (0.58–1.45) | 0.71 |
| CAD | 1.001 (0.72–1.39) | 0.99 |
| LAVI | 0.98 (0.97–0.99) | 0.004 |
| LVDD grade I | 1.50 (1.12–2.03) | 0.009 |
| LVDD grades II+III | 2.90 (1.30–6.32) | 0.009 |
| HFrEF | 0.70 (0.43–1.17) | 0.18 |
| HFmrEF | 0.70 (0.39–1.26) | 0.24 |
| HFpEF | 1.12 (0.47–2.65) | 0.81 |
| LVMI | 1.006 (1.00–1.01) | 0.77 |
| LVWMAs | 3.13 (1.95–5.01) | 0.001 |
| Global hypokinesia | 1.36 (0.73–2.53) | 0.34 |
aOR indicates adjusted odds ratio; CAD, coronary artery disease; HFmEF, HF with medium‐range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; LAVI, left atrial volume; LVDD, left ventricular diastolic dysfunction; LVMI, left ventricular mass index; and LVWMAs, left ventricular wall motion abnormalities.
ESUS Subgroup Comparison by Age
Univariate analysis was performed according to age categories (≤45, 46–60, and ≥61 years old) in patients with ESUS. Patients with ESUS ≥61 years old had more DM, hypertension, LAVI, mitral calcification, and LVWMAs and higher wall motion score index and CHA2DS2VASC. Dyslipidemia, smoking, and LVDD were more frequent in the 46 to 60 years age group. LVWMAs were observed in 12.2% of the patients ≤45 years old. EF showed a decline with increasing age (P=0.001). Patent foramen ovale (P=0.54) and atrial septal aneurysm (P=0.13) were not significantly different among the age groups. LAVI was significantly associated with LVDD grades (P=0.001) in reference to age category ≤45 (0.21), 46 to 60 (P=0.03), and >61 (P=0.04). No association was found between LVDD and heart geometry (P=0.77) with age ≤45 (0.96), 46 to 60 (P=0.99), and ≥61 (P=0.44). CAD was not significantly different in ESUS age groups (P=0.80) despite higher LVWMAs (P=0.001) in the older age groups (Table 4).
Table 4.
ESUS Subgroup Comparison by Age
| Factors | ≤45 y (139/656) | 46 to 60 y (280/656) | ≥61 y (237/656) | P Value |
|---|---|---|---|---|
| Female, n (%) | 16 (11.5) | 28 (10.0) | 70 (29.5) | 0.001 |
| Male, n (%) | 123 (88.5) | 252 (90.0) | 167 (70.5) | |
| Diabetes mellitus, n (%) | 48 (34.5) | 159 (56.8) | 162 (68.4) | 0.001 |
| Pre–diabetes mellitus, n (%) | 37 (26.6) | 56 (20.0) | 34 (14.3) | 0.01 |
| Hemoglobin A1c% | 5.9±1.2 | 6.6±1.6* | 6.6±1.5 † | 0.001 |
| Hypertension, n (%) | 102 (73.4) | 229 (81.8) | 200 (84.4) | 0.03 |
| Metabolic syndrome, n (%) | 19 (13.7) | 32 (11.4) | 29 (12.2) | 0.80 |
| Smoking, n (%) | 15 (11.0) | 60 (22.1) | 31 (13.5) | 0.005 |
| Dyslipidemia, n (%) | 85 (61.6) | 212 (75.7) | 158 (66.7) | 0.01 |
| Coronary artery disease, n (%) | 45 (32.4) | 86 (30.7) | 69 (29.1) | 0.8 |
| LAVI, mL/m2 | 22.7±9.4 | 24.3±11.6 | 29.3±13.6 † , ‡ | 0.001 |
| Mitral calcification, n (%) | 2 (1.1) | 52 (27.8) | 133 (71.1) | 0.001 |
| Aortic root diameter | 3.1±0.6 | 3.1±0.6 | 3.1±0.5 | 0.96 |
| EF% | 52.6±8.4 | 49.5±9.8* | 48±10.8 † | 0.001 |
| LVWMAs, n (%) | 17 (12.2) | 83 (29.6) | 87 (36.7) | 0.001 |
| Global hypokinesia, n (%) | 13 (9.4) | 28 (10.0 | 25 (10.5) | 0.93 |
| WMSI | 1.0±0.2 | 1.1±0.4* | 1.2±0.4 † | 0.001 |
| LVMI g/m2.7 | 38.2±24.2 | 40.5±41.8 | 43.6±34.5 | 0.34 |
| RWT | 0.36±0.2 | 0.37±0.5 | 0.42±0.6 | 0.58 |
| Heart geometry, n (%) | ||||
| Concentric remodeling | 16 (13.2) | 36 (14.6) | 30 (14.3) | 0.13 |
| Eccentric LVH | 18 (14.9) | 20 (8.1) | 30 (14.3) | |
| Concentric LVH | 16 (13.2) | 39 (15.8) | 17 (23.6) | |
| LVDD, n (%) | ||||
| Grade I | 45 (15.4) | 134 (45.7) | 114 (38.9) | 0.01 |
| LVDD Grades II+III | 5 (16.1) | 16 (51.6) | 10 (32.3) | |
| APB/24 h, n (%) | ||||
| >0 to <100 | 29 (65.9) | 64 (77.1) | 40 (56.3) | 0.12 |
| ≥100 to 499 | 13 (29.5) | 15 (18.1) | 20 (28.2) | |
| 500 to 999 | 0 | 0 | 1 (1.4) | |
| 1000 to 1499 | 1 (2.3) | 0 | 2 (2.8) | |
| >1500 | 1 (2.3) | 4 (4.8) | 8 (11.3) | |
| CHA2DS2‐VASC | 3.6±1 | 4±0.9* | 5.1±1.1 † , ‡ | 0.001 |
APB indicates atrial premature beats; EF, ejection fraction; LAVI, left atrial volume index; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; LVWMAs, left ventricular wall motion abnormalities; RWT, regional wall thickness; and WMSI, wall motion score index.
≤45 years with 46 to 50 years.
≤45 years with ≥61 years.
46 to 50 years with ≥61 years.
Discussion
The present study shows that patients with ESUS were younger in comparison with CES and not NCES, with higher frequency of LVWMAs and LVDD in comparison with NCE and CE strokes. LAVI was higher in patients with CES followed by patients with ESUS and NCES, and it was inversely related to ESUS when compared with CES in multivariate analysis. LVMI was higher in patients with ESUS compared with NCE and CES groups without any significant difference in left ventricular remodeling. Surprisingly, there was no significant difference in the frequency of CAD among the ESUS age groups, despite higher frequency of LVWMAs in the older groups.
The mean age of our ESUS cohort was 10 years younger compared with the NAVIGATE ESUS and RE‐SPECT ESUS, trials with twice the percentage of patients <60 years old, compared with all NAVIGATE ESUS ethnic subgroups. 29 , 30 A third of patients with ESUS had CAD, while the frequency of DM, hypertension, and dyslipidemia was higher than reported in the 2 above randomized trials and the ESUS Global Registry. 10 , 29 , 30 Although ESUS subgroups showed no difference in the CAD, higher frequency of LVWMAs in older groups point to either unrecognized and or underreported CAD in the older age groups. Of particular concern was the frequency of CAD in patients with ESUS <45 years old (32.4%), 12% having evidence of previous MI (LVWMA), 32% EF <52% and ventricular hypertrophy/remodeling present in 21%. Our data support previously reported MI rates of 23% in patients of South Asian origin under the age of 40. 31 In the NAVIGATE ESUS trial, 4% of East Asians had CAD, a finding shared by the CVDNOR study, highlighting the ethnic inequalities within Asia and showing that South Asians have the highest risk of acute MI while East Asians have the lowest risk. 8 , 29
LAVI in our cohort was higher in patients with ESUS than patients with NCES but less than patients with CES. When age was taken into consideration, 15% of the patients with ESUS <45 years old had increased LAVI. Recent evidence supports the association between left atrial enlargement and recurrent embolic stroke independent of confounders, including AF. 32 The presence of atrial cardiopathy in the Asian ESUS population could serve as a risk‐stratifying marker for stroke recurrence. In a subgroup of the NAVIGATE ESUS trial with moderate to severe left atrial enlargement, anticoagulation showed a reduction in stroke recurrence. 33 Whether anticoagulant therapy reduces stroke recurrence in patients with atrial cardiopathy is being tested in the ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke) trial. 34
A high burden of supraventricular tachyarrhythmias also indicates an abnormal atrial substrate and is strongly associated with incident AF and increased risk of stroke independent of diagnosed AF 31 but our data do not support these findings. Patients with ESUS in the current study were younger, with atrial cardiopathy without any significant difference in supraventricular tachyarrhythmias between stroke subtypes (NCES, CES, and ESUS), suggesting that the younger Asian ESUS cohort may have a lower risk of AF.
To our knowledge, there is no data on the implications of left ventricular pathology in patients with ESUS from Asia. The MESA (Multi‐Ethnic Study of Atherosclerosis) and LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) studies have reported the association of left ventricular hypertrophy and left ventricular geometry with an increased risk of stroke. 35 Our data show higher LVMI in patients with ESUS compared with NCE and CE stroke but the pattern of cardiac remodeling showed no difference. Although LVMI increased with age in ESUS, overall no difference was found in the pattern of remodeling, and it was only concentric left ventricular hypertrophy that increased with age without any association with stroke type. LVMI and cardiac geometry may reflect the severity and chronicity of vascular risk factors, and the regression of LVMI with antihypertension medication may confer protection for ESUS independent of blood pressure reduction. 36
LVWMA is another manifestation of left ventriclular systolic dysfunction encountered in 5% of patients without known CAD, 37 which confers a greater risk for any cardiovascular event including stroke. 38 The frequency of LVWMAs in our ESUS cohort was 28.7%, in comparison with 4% reported in the ESUS Global Registry. 10 The difference is most likely due to the prevalence of CAD and MI in our younger Asian cohort. Although there is some ambiguity regarding the potential embolic role of LVWMAs in stroke, 14 , 39 our data show a significant and independent association of LVWMAs with ESUS, which remained significant even in the ESUS age subgroups. The current ESUS cohort had lower EF and more HF with reduced EF compared with NCES and CES, and EF decreased further in ESUS according to age subgroups.
The current data show that LVDD was more frequent in patients with ESUS compared with patients with NCES and CES. Consistent with previous publications, we found that LVDD had a significant association with LAVI. 40 LVDD leads to structural and functional changes of the left atrium, which is a predictor of stroke recurrence and correlates with the new onset of AF. Therefore, LVDD might be a useful marker of atrial myopathy in Asian patients with ESUS and could be helpful in discriminating stroke mechanisms without detected AF.
Multiple studies have reported a higher prevalence of nonstenosing atherosclerotic plaque ipsilateral to ESUS that could be a coexisting potential source of embolism, 41 , 42 making it difficult to determine the exact mechanism of stroke. To avoid this, we excluded patients with ESUS with nonstenotic (<50%) intracranial or extracranial vessel atherosclerosis, ipsilateral to the ESUS event. In our data, the minor embolic sources and aortic arch atheroma were not more prevalent in ESUS than NCE and CE strokes. Moreover, the prevalence of extracranial carotid and aortic arch atherosclerotic disease is less in Asians compared with Whites. 43 Although prospective studies have shown that mitral annular calcification increases the risk of stroke, 44 we did not observe such an association despite elevated mitral calcification in ESUS age subgroups (46–60 and ≥61 years old).
Our study has the limitations of a retrospective analysis of prospectively collected data such as collection bias, registration bias, and unregistered confounding factors. Moreover, the number of females was less than males because the expatriate population was mostly men. Despite these limitations, our findings provide the only detailed data from regions where there is a dearth of literature about ESUS.
In conclusion, patients with ESUS from Southeast Asia and Western Mediterranean World Health Organization regions were younger, with a higher frequency of risk factors. The relative mean young age of patients with ESUS was further compounded by the increased incidence of CAD in those <45 years old. The involvement of potential embolic left atrial enlargement and the risk factors for developing AF were present at a much younger age. LVSWMAs and LVDD were a significant cardioembolic risk factor in patients with ESUS from Southeast Asia and Eastern Mediterranean regions. Findings from this study could guide future research and clinical practice and improve outcomes for this at‐risk population with the highest stroke mortality.
Sources of Funding
Open Access funding provided by the Qatar National Library.
Disclosures
None.
Acknowledgments
The authors acknowledge the valuable support of Ms. Reny Francis, Laxmi Kumari Ojha, Nosheen Mir, Deborah M. Morgan, Hiba Banday, Sujatha Joseph, and Mark Santos from the Neuroscience Institute, Hamad Medical Corporation, for providing support with data collection and management.
(J Am Heart Assoc. 2020;9:e016534 DOI: 10.1161/JAHA.120.016534.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol. 2014;10:135–143. [DOI] [PubMed] [Google Scholar]
- 3. Tan C‐T. Neurology in Asia. Neurology. 2015;84:623–625. [DOI] [PubMed] [Google Scholar]
- 4. Gujral UP, Pradeepa R, Weber MB, Narayan KMV, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations: type 2 diabetes in South Asians. Ann N Y Acad Sci. 2013;1281:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. [DOI] [PubMed] [Google Scholar]
- 6. Kanaya AM, Kandula NR, Ewing SK, Herrington D, Liu K, Blaha MJ, Srivastava S, Dave SS, Budoff MJ. Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis. 2014;234:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hastings KG, Jose PO, Kapphahn KI, Frank ATH, Goldstein BA, Thompson CA, Eggleston K, Cullen MR, Palaniappan LP. Leading causes of death among Asian American subgroups (2003–2011). PLoS One. 2015;10:e0124341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabanal KS, Selmer RM, Igland J, Tell GS, Meyer HE. Ethnic inequalities in acute myocardial infarction and stroke rates in Norway 1994–2009: a nationwide cohort study (CVDNOR). BMC Public Health. 2015;15:1994–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet. 2000;356:279–284. [DOI] [PubMed] [Google Scholar]
- 10. Perera KS, Vanassche T, Bosch J, Giruparajah M, Swaminathan B, Mattina KR, Berkowitz SD, Arauz A, O’Donnell MJ, Ameriso SF, et al. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke. 2016;11:526–533. [DOI] [PubMed] [Google Scholar]
- 11. Gillott RG, Willan K, Kain K, Sivananthan UM, South TMH. Asian ethnicity is associated with a lower prevalence of atrial fibrillation despite greater prevalence of established risk factors: a population‐based study in Bradford Metropolitan District. Europace. 2017;19:356–363. [DOI] [PubMed] [Google Scholar]
- 12. Tandon K, Tirschwell D, Longstreth WT, Smith B, Akoum N. Embolic stroke of undetermined source correlates to atrial fibrosis without atrial fibrillation. Neurology. 2019;93:e381–e387. [DOI] [PubMed] [Google Scholar]
- 13. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MSV. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi J‐Y, Cha J, Jung J‐M, Seo W‐K, Oh K, Cho K‐H, Yu S. Left ventricular wall motion abnormalities are associated with stroke recurrence. Neurology. 2017;88:586–594. [DOI] [PubMed] [Google Scholar]
- 15. Tsivgoulis G, Katsanos AH, Köhrmann M, Caso V, Lemmens R, Tsioufis K, Paraskevas GP, Bornstein NM, Schellinger PD, Alexandrov AV, et al. Embolic strokes of undetermined source: theoretical construct or useful clinical tool? Ther Adv Neurol Disord. 2019;12:175628641985138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event‐free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. [DOI] [PubMed] [Google Scholar]
- 17. Kamran S, Akhtar N, George P, Singh R, Imam Y, Salam A, Babu B, Burke P, Own A, Vattoth S, et al. Embolic pattern of stroke associated with cardiac wall motion abnormalities; narrowing the embolic stroke of undetermined source category. J Stroke Cerebrovasc Dis. 2020;29:104509. [DOI] [PubMed] [Google Scholar]
- 18. Katsanos AH, Bhole R, Frogoudaki A, Giannopoulos S, Goyal N, Vrettou A‐R, Ikonomidis I, Paraskevaidis I, Pappas K, Parissis J, et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology. 2016;87:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merkler AE, Gialdini G, Murthy SB, Salehi Omran S, Moya A, Lerario MP, Chong J, Okin PM, Weinsaft JW, Safford MM, et al. Association between troponin levels and embolic stroke of undetermined source. J Am Heart Assoc. 2017;6:e005905 DOI: 10.1161/JAHA.117.005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poppe KK, Doughty RN, Gardin JM, Hobbs FDR, McMurray JJV, Nagueh SF, Senior R, Thomas L, Whalley GA, Aune E, et al. Ethnic‐specific normative reference values for echocardiographic LA and LV size, LV mass, and systolic function. JACC Cardiovasc Imaging. 2015;8:656–665. [DOI] [PubMed] [Google Scholar]
- 21. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S13–S27. [DOI] [PubMed] [Google Scholar]
- 23. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 24. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 25. Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. [DOI] [PubMed] [Google Scholar]
- 26. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 27. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 28. Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, Thorpe KE, Aviv R, Boyle K, Blakely J, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936–941. [DOI] [PubMed] [Google Scholar]
- 29. Kasner SE, Lavados P, Sharma M, Wang Y, Wang Y, Dávalos A, Shamalov N, Cunha L, Lindgren A, Mikulik R, et al. Characterization of patients with embolic strokes of undetermined source in the NAVIGATE ESUS randomized trial. J Stroke Cerebrovasc Dis. 2018;27:1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diener H‐C, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 31. Chaikhouni A, Chouhan L, Pomposiello C, Banna A, Mahrous F, Thomas G, Abu Al‐Hassan N, Khalifa S, Jaddan A, Bsata MW, et al. Myocardial infarction in Qatar: the first 2515 patients. Clin Cardiol. 1993;16:227–230. [DOI] [PubMed] [Google Scholar]
- 32. Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. [DOI] [PubMed] [Google Scholar]
- 33. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, Haeusler KG, Mikulik R, Kasner SE, Toni D, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 2019;76:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamel H, Longstreth W, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, et al. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verdecchia P, Angeli F, Gattobigio R, Sardone M, Pede S, Reboldi G. Regression of left ventricular hypertrophy and prevention of stroke in hypertensive subjects. Am J Hypertens. 2006;19:493–499. [DOI] [PubMed] [Google Scholar]
- 37. Cicala S, de Simone G, Wachtell K, Gerdts E, Boman K, Nieminen MS, Dahlöf B, Devereux RB. Clinical impact of “in‐treatment” wall motion abnormalities in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hypertens. 2008;26:806–812. [DOI] [PubMed] [Google Scholar]
- 38. Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, et al. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;57:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramasamy S, Yaghi S, Salehi Omran S, Lerario MP, Devereux R, Okin PM, Gupta A, Navi BB, Kamel H, Merkler AE. Association between left ventricular ejection fraction, wall motion abnormality, and embolic stroke of undetermined source. J Am Heart Assoc. 2019;8:e011593 DOI: 10.1161/JAHA.118.011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seo J‐Y, Lee KB, Lee J‐G, Kim J‐S, Roh H, Ahn M‐Y, Park BW, Hyon MS. Implication of left ventricular diastolic dysfunction in cryptogenic ischemic stroke. Stroke. 2014;45:2757–2761. [DOI] [PubMed] [Google Scholar]
- 41. Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, Bamberg F, Linn J, Reiser M, Yuan C, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. [DOI] [PubMed] [Google Scholar]
- 42. Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the approach to embolic stroke of undetermined source: a review. JAMA Neurol. 2019;76:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–655. [DOI] [PubMed] [Google Scholar]
- 44. Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET, Best LG, Resnick HE, Roman MJ, Devereux RB. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36:2533–2537. [DOI] [PubMed] [Google Scholar]


