Abstract
Background
It has been reported that liver stiffness assessed by transient elastography are correlated with right atrial pressure, which is associated with worse outcome in patients with heart failure (HF). We aimed to clarify clinical implications of hepatic hemodynamic evaluation (liver congestion and hypoperfusion) by abdominal ultrasonography in patients with HF.
Methods and Results
We performed abdominal ultrasonography, right‐heart catheterization, and echocardiography, then followed up for cardiac events such as cardiac death or worsening HF in patients with HF. Regarding liver congestion, liver stiffness assessed by shear wave elastography (SWE) of the liver was significantly correlated with right atrial pressure determined by right‐heart catheterization (R=0.343; P<0.01), right atrial end‐systolic area, and inferior vena cava diameter determined by echocardiography. Regarding liver hypoperfusion, peak systolic velocity (PSV) of the celiac artery was correlated with cardiac index determined by right‐heart catheterization (R=0.291; P<0.001) and tricuspid annular plane systolic excursion determined by echocardiography. According to the Kaplan–Meier analysis, HF patients with high SWE and low PSV had the highest cardiac event rate (log‐rank P=0.033). In the Cox proportional hazard analysis, high SWE and low PSV were associated with high cardiac event rate (high SWE: hazard ratio [HR], 2.039; 95% CI, 1.131–4.290; low PSV: HR, 2.211; 95% CI, 1.199–4.449), and the combination of high SWE and low PSV was a predictor of cardiac events (HR, 4.811; 95% CI, 1.562–14.818).
Conclusions
Intrahepatic congestion and hypoperfusion determined by abdominal ultrasonography (liver SWE and celiac PSV) are associated with adverse prognosis in patients with HF.
Keywords: hemodynamics, liver, liver congestion, liver function test, liver perfusion, prognosis, right‐heart catheterization
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- BNP
B‐type natriuretic peptide
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- LFTs
liver function tests
- LS
liver stiffness
- LVEF
left ventricular ejection fraction
- PSV
peak systolic velocity
- RAP
right atrial pressure
- RHC
right‐heart catheterization
- SWE
shear wave elastography
Clinical Perspective
What Is New?
Intrahepatic congestion and hypoperfusion can be determined by abdominal ultrasonography.
What Are the Clinical Implications?
Intrahepatic congestion and hypoperfusion are associated with adverse prognosis.
Heart failure (HF) is a widespread and serious problem that has been reported in many countries. 1 Nohria‐Stevenson profiles demonstrated the clinical importance of assessments of perfusion (“cold” versus “warm”), as well as congestion (“wet” versus “dry”). 2 , 3 The abdominal compartment, which includes the liver, splanchnic vasculature, gut, and so on, has recently been investigated to determine whether it significantly contributes to a deranged cardiac function as well as multiple organ function in patients with HF. 4 , 5 HF causes liver dysfunction with a combination of reduced arterial perfusion and passive congestion, and this association is called “cardiohepatic interaction.” 6 , 7 , 8 , 9
With regard to liver function tests (LFTs), liver dysfunction, such as the elevation of serum bilirubin, ALP, gamma‐glutamyl transferase, AST, and ALT, frequently occurs in HF related to reduced arterial perfusion and passive congestion, and is associated with disease severity and prognosis. 5 , 7 , 10 , 11 , 12 Liver congestion attributable to increased central venous pressure might directly contribute to a state of impaired natriuresis. 5 , 13 In addition, elevated central venous pressure and right atrial pressure (RAP) may contribute to cholestatic abnormalities (elevated bilirubin, ALP, gamma‐glutamyl transferase), as well as impairment of both hepatocyte function and liver reserve in patients with HF. 6 , 7 , 8
With regard to image testing, evaluation of chronic liver disease based on liver stiffness (LS) assessed by transient elastography has attracted growing interest in the field of clinical hepatology. LS is recently calculated on the basis of shear wave velocity measurements, and is used as a noninvasive method to assess liver fibrosis. It has been reported that LS is highly reflective of right‐sided filling pressure, and might be a marker of liver congestion in patients with HF. 14 , 15 , 16 , 17 Congestive hepatopathy 6 , 7 attributable to HF causes functional abnormalities of the liver, 8 and increased LS indicates adverse prognosis. 14 , 15 , 16 , 18 , 19 , 20 , 21 However, associations between low liver perfusion and LFTs or prognosis have not been investigated in previous studies. 14 , 15 , 16 , 18 , 19 , 20 , 21 Clinical implications of a possible marker of liver perfusion (peak systolic velocity [PSV] of celiac artery), as well as congestion (liver shear wave elastography [SWE]) determined by abdominal ultrasonography in patients with HF have not been examined.
Thus, in the current study, we aimed to clarify (1) the relationships between hemodynamics (right‐heart catheter) and the parameters of liver SWE and celiac PSV, as well as LFTs; (2) the associations between the parameters of liver SWE, celiac PSV, and those of echocardiography; and (3) the prognostic impacts of liver SWE and celiac PSV on patients with HF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects and Study Protocol
The patient flow is described in Figure 1. This was a prospective observational study of 379 decompensated patients with HF who had undergone abdominal ultrasonography and were discharged from Fukushima Medical University Hospital between April 2018 and March 2019. Diagnosis of decompensated HF was made by each patient's attending cardiologist on the basis of the established HF guidelines. 22 , 23 , 24 Blood samples, abdominal ultrasonography, and echocardiography were obtained at hospital discharge. All patients underwent testing for hepatitis B surface antigen and hepatitis C antibodies, and their medical histories were checked for chronic liver disease (cirrhosis, hepatic tumors, bile duct disease, etc) and alcohol abuse (≥30 g/day for men, ≥20 g/day for women). Patients with the above‐mentioned liver diseases, alcohol abuse, acute coronary syndrome, advanced cancer, or were undergoing dialysis, were excluded. Finally, 350 patients were enrolled. Of these 350 patients, right‐heart catheterization (RHC) was partly performed within 3 days of abdominal ultrasonography. Because of lack or poor image of specific data (RHC, SWE, and PSV), 171 were used for the RHC analysis, 342 were used for the SWE analysis, 321 were used for the PSV analysis, and 313 were used for a combination of SWE and PSV analysis. Patients were divided on the basis of the median levels of liver SWE or celiac PSV: (1) the low‐SWE group (SWE <1.31 m/s; n=171) or high‐SWE group (SWE ≥1.31 m/s; n=171), and (2) low‐PSV group (PSV <65 m/s; n=158) or high‐PSV group (celiac PSV ≥65 cm/s; n=163).
Figure 1. Patient flow chart.
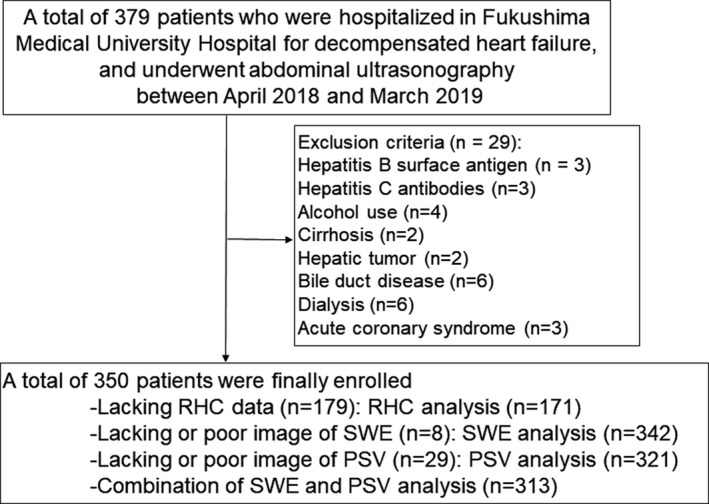
PSV indicates peak systolic velocity; RHC, right‐heart catheterization; and SWE, shear wave elastography.
First, we compared the clinical features and results from laboratory tests and echocardiography between the 2 groups. In addition, we performed a correlation analysis of interaction between levels of both liver SWE or celiac PSV and parameters of laboratory tests and echocardiography. Second, the patients were followed up until August 2019 for cardiac events as composites of cardiac death or unplanned rehospitalization for HF treatment. For patients who experienced ≥2 events, only the first event was included in the analysis. Since these patients visited patient's referring hospital monthly or bimonthly, we were able to follow up on all patients. Status and dates of death were obtained from the patient's medical records. Those administering the survey were blind to the analyses, and written informed consent was obtained from all study subjects. The study protocol was approved by the Ethics Committee of Fukushima Medical University, and was carried out in accordance with the principles outlined in the Declaration of Helsinki. Reporting of the study conforms to Strengthening the Reporting of Observational Studies in Epidemiology along with references to Strengthening the Reporting of Observational Studies in Epidemiology and the Enhancing the Quality and Transparency of Health Research guidelines. 25
Hypertension was defined as the recent use of antihypertensive drugs, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Dyslipidemia was defined as the recent use of cholesterol‐lowering drugs, a triglyceride value of ≥150 mg/dL, a low‐density lipoprotein cholesterol value of ≥140 mg/dL, or a high‐density lipoprotein cholesterol value of <40 mg/dL. Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 cm2, 26 and anemia was defined as hemoglobin levels of <12.0 g/dL in women and <13.0 g/dL in men. 1 Atrial fibrillation was identified by an ECG performed during hospitalization or from medical records, including past history.
Abdominal Ultrasonography
Abdominal ultrasonography was performed with the patients in a stable condition without changes in medications at hospital discharge, by 2 experienced sonographers (S.I., with 15 years of experience in abdominal ultrasonography, and M.M., with 23 years of experience), who were blinded to all clinical data, before discharge using an ultrasound system (Aplio i800, Canon Medical Systems, Tochigj, Japan) equipped with a wideband convex i8CX1 multifrequency probe (central frequency, 4.0 MHz; range, 1.8–6.4 MHz). Patients fasted for at least 12 hours before the abdominal ultrasonography. Images were obtained from patients in the supine or decubitus position through the subcostal, transverse, oblique, sagittal, and intercostal planes. 27 As shown in Figure 2, we measured liver SWE and celiac PSV. SWE examination was performed in the right liver lobe through the intercostal space to obtain a proper sonic window. The tip of the probe transducer was placed on the skin of the patient between the rib bones and at the level of the right lobe of the liver. The detection site was fixed at 1.0 to 2.0 cm beneath the right liver capsule, away from the intrahepatic vessels and the gallbladder. 28 The convex probe was kept still for 1 second during acquisition in one‐shot mode, and participants were requested to hold their breath during acquisition. 29 When the measurement area was located, the operator initiated the SWE sequence measurements that targeted the region of interest. A sample box ≈2×2 cm was placed on a gray‐scale image at least 1 cm beneath the Glisson capsule to avoid reverberation artifacts. 29 The median value from 7 measurements of SWE performed at the depths ranging from 25 to 65 mm was defined as the liver SWE (m/s). 28 Since selective depiction of the common hepatic artery was technically difficult, we evaluated the celiac artery. PSV of the celiac artery was defined as celiac PSV (cm/s). The intraobserver variability (standard deviation of the differences/average value) of SWE was 7±2%, whereas that of PSV was 8±2%.
Figure 2. Abdominal ultrasonography.
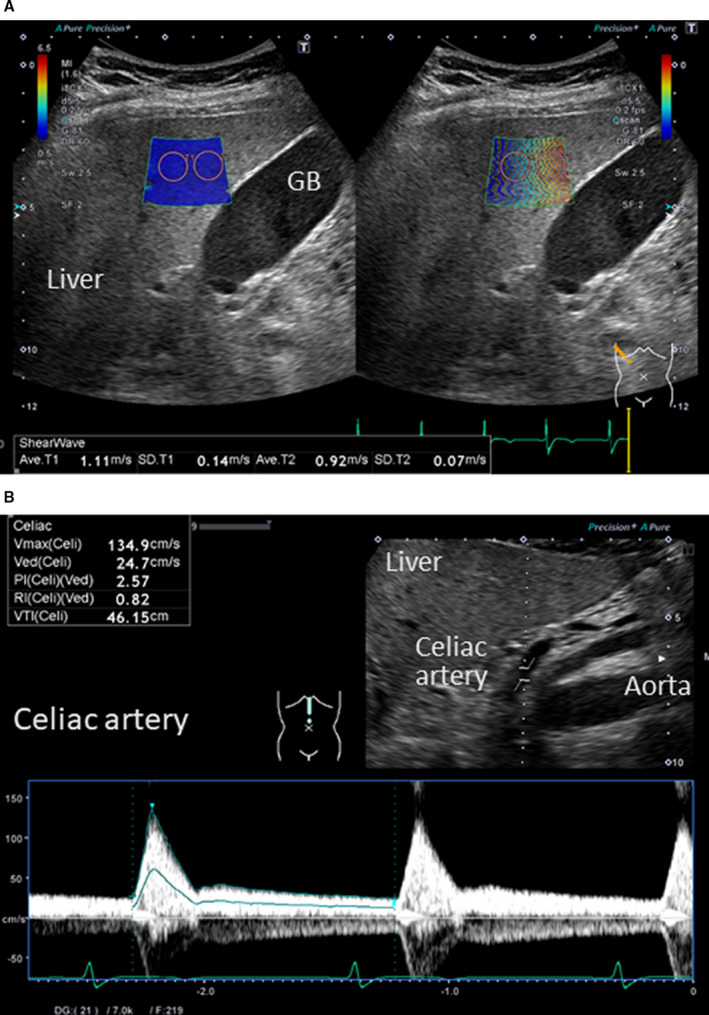
A, Liver‐SWE (m/s), (B) Celiac‐PSV (cm/s). GB indicates gallbladder; PSV, peak systolic velocity; and SWE, shear wave elastography.
Echocardiography
Echocardiography was performed by experienced echocardiographers using standard techniques as previously reported. 30 The echocardiographic parameters included left ventricular ejection fraction (LVEF), left atrial volume, early transmitral flow velocity to mitral annular velocity ratio (mitral valve E/e′), right atrium and ventricle areas, right ventricular fractional area change, inferior vena cava diameter, tricuspid regurgitation pressure gradient, tissue Doppler‐derived tricuspid lateral annular systolic velocity (tricuspid valve S′), and tricuspid annular plane systolic excursion. 31 The LVEF was calculated using Simpson's method. The right ventricular fractional area change, defined as (end diastolic area–end‐systolic area)/end‐diastolic area×100, was a measure of right ventricular systolic function. 31 All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc, Mountain View, CA, USA).
RHCs and Hemodynamic Measurements
Of 350 patients, RHC was partly performed based on remedial judgment of the attending physician in 171 patients. RHC were performed within 3 days of abdominal ultrasonography, with the patients in a stable condition without changes in medications including doses, similar to setting of abdominal ultrasonography. All right‐heart catheterizations were performed with the patients in a stable condition, in a resting supine position under fluoroscopic guidance, at room air, and at rest for more than 30 minutes after catheter placement. Mean RAP and cardiac output were measured using a 7F Swan‐Ganz catheter (Edwards Lifesciences, Irvine, CA, USA). Cardiac output was calculated based on the direct Fick method. 32
Measurement of Laboratory Data
The BNP (B‐type natriuretic peptide) levels were measured using a specific immunoradiometric assay (Shionoria BNP kit; Shionogi, Osaka, Japan). The serum 7S domain of collagen type IV, which indicates liver fibrosis, was measured by radioimmunoassay (type IV collagen 7S kit; SCETI MEDICAL LABO K.K., Tokyo, Japan). 32 , 33 These assays were blindly performed by experienced laboratory technicians.
Statistical Analysis
Normally distributed data are presented as mean±SD, and nonnormally distributed data are presented as median and interquartile range. The categorical variables are expressed as numbers and percentages, and the chi‐square test was used for their comparisons. Parametric variables were compared using Student t test, and nonparametric variables were compared using the Mann–Whitney U test. Correlations between both SWE and celiac PSV and the parameters of laboratory data, echocardiography, or RHC were assessed using Pearson's correlation analysis for parametric variables and Spearman's correlation analysis for nonparametric variables. Kaplan–Meier analysis was used with a log‐rank test to assess cardiac event rates as composites of cardiac death or unplanned rehospitalization for HF treatment. These curves helped in identifying nonproportionality patterns in hazard function such as convergence (difference in risk between the groups decreases with time), divergence, or crossing of the curves. In addition, a Schoenfeld test for the violation of proportional hazards, which can be used to assess the correlation between scaled residuals and time, was also conducted. Cox proportional hazard analyses were used to evaluate SWE and PSV levels as a predictor of cardiac events. A P value of <0.05 was considered statistically significant for all comparisons. These analyses were performed using a statistical software package (SPSS ver. 24.0; IBM, Armonk, NY).
Results
Correlation analysis with mean RAP, cardiac index and LFTs, liver SWE, and celiac PSV are presented in Table 1. There were significant positive correlations between total bilirubin, gamma‐glutamyl transferase, 7S domain of collagen type IV, SWE and mean RAP, as well as significant positive correlations between total protein, albumin, cholinesterase, PSV, and cardiac index, suggesting that SWE is a marker of central venous pressure, and PSV is a marker of liver perfusion.
Table 1.
Correlation Analyses With RAP, Cardiac Index, and Other Variables (n=171)
| r | P Value | |
|---|---|---|
| Right atrium pressure, mm Hg | ||
| Laboratory data | ||
| Total protein, g/dL | −0.077 | 0.316 |
| Albumin, g/dL | −0.047 | 0.539 |
| Total bilirubin, mg/dL | 0.168 | 0.028 |
| Direct bilirubin, mg/dL | 0.136 | 0.075 |
| Aspartate aminotransferase, U/L | 0.097 | 0.208 |
| Alanine aminotransferase, U/L | 0.121 | 0.113 |
| Alkaline phosphatase, U/L | 0.130 | 0.089 |
| Gamma‐glutamyl transferase, U/L | 0.211 | 0.005 |
| Cholinesterase, U/L | 0.001 | 0.992 |
| P4NP 7S, ng/mL | 0.231 | 0.002 |
| Abdominal ultrasonography | ||
| Liver SWE, m/s | 0.343 | <0.001 |
| Celiac PSV, cm/s | 0.053 | 0.504 |
| Cardiac index, L/min per m2 | ||
| Laboratory data | ||
| Total protein, g/dL | 0.187 | 0.014 |
| Albumin, g/dL | 0.294 | <0.001 |
| Total bilirubin, mg/dL | −0.173 | 0.024 |
| Direct bilirubin, mg/dL | −0.168 | 0.028 |
| Aspartate aminotransferase, U/L | −0.089 | 0.247 |
| Alanine aminotransferase, U/L | −0.048 | 0.537 |
| Alkaline phosphatase, U/L | −0.046 | 0.553 |
| Gamma‐glutamyl transferase, U/L | −0.054 | 0.486 |
| Cholinesterase, U/L | 0.271 | <0.001 |
| P4NP 7S, ng/mL | −0.154 | 0.074 |
| Abdominal ultrasonography | ||
| Liver SWE, m/s | −0.060 | 0.442 |
| Celiac PSV, cm/s | 0.291 | <0.001 |
P4NP 7S indicates 7S domain of collagen type IV; PSV, peak systolic velocity; RAP, right atrial pressure; and SWE, shear wave elastography.
Comparisons of patients with low and high SWE groups are summarized in Table 2. The high SWE group were older, had a higher body mass index, and New York Heart Association class, higher prevalence of AF, chronic kidney disease and anemia, higher levels of direct bilirubin, alkaline phosphatase, gamma‐glutamyl transferase, 7S domain of collagen type IV and creatinine, lower levels of hemoglobin, albumin, cholinesterase and eGFR, higher levels of left atrial volume, mitral valve E/e′, right atrial end‐systolic area, and inferior vena cava diameter. In addition, there were significant associations between SWE and age, body mass index, levels of albumin, direct bilirubin, ALP, gamma‐glutamyl transferase, 7S domain of collagen type IV, log BNP, eGFR, left atrial volume, mitral valve E/e′, right atrial end‐systolic area, and inferior vena cava diameter. These results suggest that elevated SWE indicates liver congestion, and underlying increased central venous pressure or right‐heart volume overload.
Table 2.
Comparisons of Patient Characteristics (N=342)
| SWE Group (Low vs High) | Correlation With SWE | ||||
|---|---|---|---|---|---|
|
Low SWE (SWE <1.31, n=171) |
High SWE (1.31 ≤ SWE, n=171) |
P Value | Correlation Coefficient | P Value | |
| SWE, m/s | 1.22±0.14 | 1.49±0.23 | <0.001 | … | … |
| Demographics | |||||
| Age, y | 65.6±12.3 | 69.3±12.7 | 0.006 | 0.198 | <0.001 |
| Male sex (n, %) | 104 (60.8) | 108 (63.2) | 0.656 | −0.010 | 0.849 |
| Body mass index, kg/m2 | 22.9±3.7 | 24.1±4.3 | 0.005 | 0.136 | 0.012 |
| Systolic BP, mm Hg | 118.2±17.8 | 121.0±18.2 | 0.153 | −0.011 | 0.845 |
| Diastolic BP, mm Hg | 69.1±12.1 | 68.4±13.0 | 0.630 | −0.096 | 0.076 |
| Heart rate, bpm | 71.5±14.1 | 69.3±13.8 | 0.142 | −0.102 | 0.059 |
| NYHA class III or IV | 5 (2.9) | 17 (9.9) | 0.008 | 0.066 | 0.222 |
| Etiology ischemic/myopathy/valvular/a arrhythmia/pulmonary/congenital/others | 52 (30.4)/45 (26.3)/33 (19.3)/18 (10.5)/18 (10.5)/3 (1.8)/2 (1.2) | 45 (26.3)/39 (22.8)/46 (26.9)/22 (12.9)/13 (7.6)/4 (2.3)/2 (1.2) | 0.620 | … | … |
| Comorbidities | |||||
| CAD, n (%) | 47 (27.5) | 49 (28.7) | 0.810 | … | … |
| Atrial fibrillation, n (%) | 46 (26.9) | 74 (43.3) | 0.002 | … | … |
| Hypertension, n, (%) | 96 (56.1) | 112 (65.5) | 0.076 | … | … |
| Dyslipidemia, n (%) | 127 (74.3) | 115 (67.3) | 0.154 | … | … |
| Diabetes mellitus, n (%) | 48 (28.1) | 62 (36.3) | 0.105 | … | … |
| CKD, n (%) | 79 (46.2) | 103 (60.2) | 0.009 | … | … |
| Anemia, n (%) | 67 (39.2) | 90 (52.6) | 0.013 | … | … |
| Laboratory data | |||||
| Total protein, g/dL | 7.0±0.7 | 6.9±0.8 | 0.424 | −0.001 | 0.984 |
| Albumin, g/dL | 4.0±0.5 | 3.9±0.6 | 0.008 | −0.114 | 0.035 |
| Total bilirubin, mg/dL | 0.9±0.8 | 0.9±0.4 | 0.612 | 0.066 | 0.220 |
| Direct bilirubin, mg/dL | 0.1±0.0 | 0.1±0.1 | 0.026 | 0.343 | <0.001 |
| Aspartate aminotransferase, U/L | 24.8±12.3 | 27.2±15.7 | 0.121 | 0.084 | 0.118 |
| Alanine aminotransferase, U/L | 23.3±17.9 | 26.7±17.3 | 0.293 | 0.045 | 0.411 |
| Alkaline phosphatase, U/L | 239.8±71.0 | 260.2±112.0 | 0.045 | 0.137 | 0.011 |
| Gamma‐glutamyl transferase, U/L | 43.9±42.5 | 63.5±74.3 | 0.003 | 0.224 | <0.001 |
| Cholinesterase, U/L | 303.2±82.6 | 270.9±108.1 | 0.002 | −0.206 | <0.001 |
| P4NP 7S, ng/mL | 5.3±1.6 | 6.2±2.2 | <0.001 | 0.344 | <0.001 |
| Log BNP, pg/mL | 1.9±0.7 | 2.0±0.6 | 0.169 | 0.140 | 0.009 |
| Creatinine, mg/dL | 1.0±0.5 | 1.2±0.9 | 0.049 | 0.106 | 0.050 |
| eGFR, mL/min per 1.73 cm2 | 59.3±18.1 | 55.1±19.3 | 0.037 | −0.201 | <0.001 |
| Sodium, mEq/L | 139.7±2.9 | 139.7±2.6 | 0.871 | −0.021 | 0.697 |
| Echocardiography | |||||
| LVEF, % | 55.1±15.8 | 54.4±15.7 | 0.858 | 0.017 | 0.756 |
| Left atrial volume, mL | 77.8±41.0 | 96.7±55.5 | 0.001 | 0.299 | <0.001 |
| Mitral valve E/e’ | 12.7±8.3 | 14.8±8.5 | 0.029 | 0.248 | <0.001 |
| RA end‐systolic area, cm2 | 16.6±6.6 | 20.0±7.5 | 0.006 | 0.293 | <0.001 |
| RV area diastole, cm2 | 19.5±7.8 | 21.7±7.7 | 0.154 | 0.151 | 0.116 |
| RV area systole, cm2 | 12.4±6.6 | 13.8±7.0 | 0.303 | 0.096 | 0.320 |
| RV fractional area change, % | 38.1±11.3 | 38.6±11.7 | 0.808 | 0.030 | 0.717 |
| Inferior vena cava diameter, mm | 14.7±3.6 | 16.1±4.8 | 0.004 | 0.203 | <0.001 |
| TRPG, mm Hg | 25.3±12.2 | 26.8±12.8 | 0.350 | 0.098 | 0.108 |
| Tricuspid valve S’, cm | 10.9±3.6 | 10.2±3.3 | 0.161 | −0.096 | 0.173 |
| TAPSE | 18.7±4.3 | 18.4±4.9 | 0.607 | −0.059 | 0.352 |
BNP indicates B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; P4NP 7S, 7S domain of collagen type IV; RA, right atrial; RV, right ventricular; SWE, shear wave elastography; TAPSE, tricuspid annular plane systolic excursion; and TRPG, tricuspid regurgitation pressure gradient.
Comparisons of the low and high PSV groups are summarized in Table 3. The low‐PSV group was older; had a lower body mass index, higher prevalence of New York Heart Association class III or IV, female sex, chronic kidney disease and anemia; had higher levels of log BNP and lower levels of total protein, cholinesterase, eGFR; and sodium; and had higher levels of mitral valve E/e′ and lower levels of tricuspid annular plane systolic excursion. In addition, there were significant associations between PSV and age, body mass index, levels of total protein, eGFR, LVEF, mitral valve E/e′, tricuspid valve S′ and tricuspid annular plane systolic excursion. These results suggest that decreased PSV indicates liver hypoperfusion, underlying low cardiac output, and leads to protein synthesis decrease.
Table 3.
Comparisons of Patient Characteristics (N=321)
| Celiac PSV Group (Low vs High) | Correlation With SWE | ||||
|---|---|---|---|---|---|
|
Low PSV (PSV <65, n=158) |
High PSV (65 ≤ PSV, n=163) |
P Value | Correlation Coefficient | P Value | |
| PSV, cm/s | 51.7±9.8 | 84.7±17.9 | <0.001 | … | … |
| Demographics | |||||
| Age, y | 69.9±11.2 | 65.6±12.9 | 0.001 | −0.166 | 0.003 |
| Male sex (n, %) | 84 (53.2) | 110 (67.5) | 0.009 | 0.118 | 0.035 |
| Body mass index, kg/m2 | 23.0±3.6 | 24.1±4.3 | 0.008 | 0.156 | 0.005 |
| Systolic BP, mm Hg | 119.3±19.0 | 120.0±17.3 | 0.746 | 0.024 | 0.665 |
| Diastolic BP, mm Hg | 68.4±12.8 | 68.8±12.6 | 0.757 | 0.028 | 0.625 |
| Heart rate, bpm | 69.3±13.2 | 70.9±14.6 | 0.310 | 0.104 | 0.064 |
| NYHA class III or IV | 15 (9.5) | 6 (3.7) | 0.035 | … | … |
| Etiology ischemic/myopathy/valvular/a arrhythmia/pulmonary/congenital/others | 48 (30.4)/30 (19.0)/38 (24.1)/22 (13.9)/14 (8.9)/3 (1.9)/3 (1.9) | 46 (28.2)/47 (28.8)/29 (17.8)/17 (10.4)/19 (11.7)/4 (2.5)/1 (0.6) | 0.280 | … | … |
| Comorbidities | |||||
| CAD, n (%) | 52 (32.9) | 39 (23.9) | 0.074 | … | … |
| Atrial fibrillation, n (%) | 50 (31.6) | 59 (36.2) | 0.389 | … | … |
| Hypertension, n (%) | 99 (62.7) | 94 (57.7) | 0.361 | … | … |
| Dyslipidemia, n (%) | 108 (68.4) | 118 (72.4) | 0.428 | … | … |
| Diabetes mellitus, n (%) | 49 (31.0) | 54 (33.1) | 0.685 | … | … |
| CKD, n (%) | 97 (61.4) | 74 (45.4) | 0.004 | … | … |
| Anemia, n (%) | 81 (51.3) | 62 (38.0) | 0.017 | … | … |
| Laboratory data | |||||
| Total protein, g/dL | 6.8±0.7 | 7.0±0.7 | 0.022 | 0.157 | 0.046 |
| Albumin, g/dL | 3.9±0.6 | 4.0±0.5 | 0.218 | 0.043 | 0.442 |
| Total bilirubin, mg/dL | 0.9±0.9 | 0.9±0.4 | 0.361 | −0.101 | 0.073 |
| Direct bilirubin, mg/dL | 0.1±0.1 | 0.1±0.0 | 0.075 | −0.108 | 0.055 |
| Aspartate aminotransferase, U/L | 25.7±12.3 | 25.8±15.9 | 0.940 | −0.005 | 0.930 |
| Alanine aminotransferase, U/L | 25.3±18.1 | 24.7±18.5 | 0.858 | 0.003 | 0.953 |
| Alkaline phosphatase, U/L | 247.9±110.9 | 247.8±73.0 | 0.991 | −0.049 | 0.388 |
| Gamma‐glutamyl transferase, U/L | 53.6±65.2 | 49.9±45.8 | 0.564 | −0.021 | 0.713 |
| Cholinesterase, U/L | 271.2±87.9 | 298.4±102.0 | 0.012 | 0.082 | 0.148 |
| P4NP 7S, ng/mL | 5.8±2.0 | 5.6±1.9 | 0.332 | −0.059 | 0.305 |
| Log BNP, pg/mL | 2.0±0.6 | 1.9±0.6 | 0.028 | −0.106 | 0.058 |
| Creatinine, mg/dL | 1.2±0.9 | 1.0±0.7 | 0.141 | −0.057 | 0.310 |
| eGFR, mL/min per 1.73 cm2 | 54.6±20.9 | 59.9±17.5 | 0.015 | 0.143 | 0.011 |
| Sodium, mEq/L | 139.4±3.0 | 140.0±2.6 | 0.034 | 0.090 | 0.107 |
| Echocardiography | |||||
| LVEF, % | 53.6±16.5 | 55.5±14.3 | 0.261 | 0.206 | <0.001 |
| Left atrial volume, mL | 87.2±52.7 | 85.6±45.7 | 0.787 | −0.060 | 0.298 |
| Mitral valve E/e′ | 15.1±9.7 | 12.5±7.0 | 0.008 | −0.215 | <0.001 |
| RA end‐systolic area, cm2 | 19.0±8.1 | 18.4±6.5 | 0.666 | 0.026 | 0.773 |
| RV area diastole, cm2 | 20.0±8.4 | 20.9±7.2 | 0.551 | 0.133 | 0.180 |
| RV area systole, cm2 | 13.2±7.7 | 12.5±5.5 | 0.583 | 0.089 | 0.370 |
| RV fractional area change, % | 37.7±12.3 | 40.1±10.7 | 0.225 | −0.067 | 0.434 |
| Inferior vena cava diameter, mm | 15.5±4.6 | 15.4±3.6 | 0.877 | 0.039 | 0.488 |
| TRPG, mm Hg | 27.5±13.9 | 24.9±11.7 | 0.105 | −0.070 | 0.266 |
| Tricuspid valve S′, cm | 10.0±3.2 | 10.8±3.7 | 0.099 | 0.201 | 0.004 |
| TAPSE | 17.8±4.8 | 19.1±4.5 | 0.034 | 0.162 | 0.013 |
BNP indicates B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; P4NP 7S, 7S domain of collagen type IV; PSV, peak systolic velocity; RA, right atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; and TRPG, tricuspid regurgitation pressure gradient.
During the follow‐up period (mean, 231 days; range, 12–523 days), 40 cardiac events including 6 cardiac deaths and 34 worsening HF occurred. As shown in Figure 3, in the Kaplan–Meier analysis, cardiac event rates were significantly higher in the high‐SWE group than in the low‐SWE group (log‐rank P=0.024), as well as in the low‐PSV group than in the high‐PSV group (log‐rank P=0.022). In addition, we further classified patients into four subsets in consideration of Nohria‐Stevenson clinical profiles 2 : subset 1, low SWE and high PSV (warm‐dry); subset 2, high SWE and high PSV (warm‐wet); subset 3, low SWE and low PSV (cold‐dry); and subset 4, high SWE and low PSV (cold‐wet). HF patients with high SWE and low PSV had the highest cardiac event rate (Figure 4; log‐rank P=0.033), followed by high‐SWE and high‐PSV group, low‐SWE and low‐PSV group, and low‐SWE and high‐PSV group. In the Cox proportional hazard analysis (Table 4), high SWE and low PSV were associated with high cardiac event rates (high SWE: hazard ratio [HR], 2.039; 95% CI, 1.131–4.290; low PSV: HR, 2.211; 95% CI, 1.199–4.449), and the combination of high SWE and low PSV was a predictor of cardiac events in patients with HF (HR, 4.811; 95% CI, 1.562–14.818). As limited number of events (40 events), we selected only 4 confounding factors (age, sex, BNP, and LVEF) in the multivariable adjusted Cox proportional hazard analyses. These results suggest that the combination of SWE and PSV might be a useful tool for evaluating hepatic hemodynamic subsets like in the Nohria‐Stevenson classification (Figure 5).
Figure 3. Kaplan–Meier analysis for cardiac event rates stratified by (A) liver‐SWE or (B) celiac‐PSV levels.
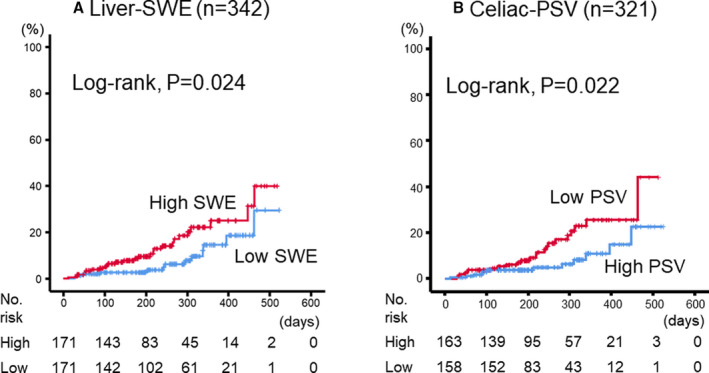
PSV indicates peak systolic velocity; and SWE, shear wave elastography.
Figure 4. Kaplan–Meier analysis for cardiac event rates stratified by combination of liver SWE and celiac PSV levels.

PSV indicates peak systolic velocity; and SWE, shear wave elastography.
Table 4.
Cox Proportional Hazard Model of Cardiac Events (Factors in Parameters of Liver Ultrasonography)
| Cardiac Events | HR | 95% CI | P Value |
|---|---|---|---|
| Liver SWE group | |||
| SWE (high vs low) | 2.039 | 1.131–4.290 | 0.013 |
| SWE (high vs low)* | 2.113 | 1.069–4.060 | 0.033 |
| Celiac PSV group | |||
| PSV (low vs high) | 2.211 | 1.199–4.449 | 0.010 |
| PSV (low vs high)* | 2.046 | 1.029–4.147 | 0.041 |
| Combination of liver SWE and celiac PSV group | |||
| Subset 1 (low SWE and high PSV) | Reference | … | … |
| Subset 2 (high SWE and high PSV) | 2.789 | 0.938–9.279 | 0.059 |
| Subset 3 (low SWE and low PSV) | 3.088 | 1.118–9.850 | 0.030 |
| Subset 3 (low SWE and low PSV)* | 2.430 | 1.045–8.410 | 0.045 |
| Subset 4 (high SWE and low PSV) | 4.811 | 1.562–14.818 | 0.006 |
| Subset 4 (high SWE and low PSV)* | 4.153 | 1.476–13.043 | 0.018 |
HR indicates hazard ratio; PSV, peak systolic velocity; and SWE, shear wave elastography.
Adjusted for age, sex, B‐type natriuretic peptide, and left ventricular ejection fraction.
Figure 5. Cardiac event rates stratified by combination of liver‐SWE and celiac‐PSV levels.
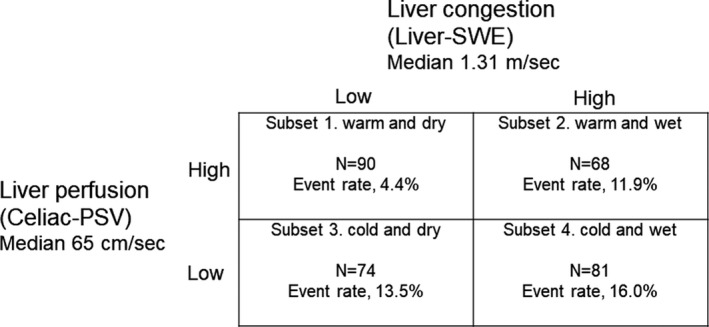
PSV indicates peak systolic velocity; and SWE, shear wave elastography.
Discussion
To the best of our knowledge, the present study is the first to report that the associations between parameters of not only liver congestion (right‐sided HF), but also liver hypoperfusion (left‐sided HF) determined by abdominal ultrasonography and LFTs, RHC, and echocardiography, as well as its prognostic impact in HF.
Cardiohepatic interaction has been reported previously. 6 , 9 Systemic venous congestion rises to neurohormonal activation (eg, renin‐angiotensin system), decreases plasma natriuretic peptide, 34 , 35 leads to HF progression, may contribute to worsening multiple organ failure, 20 , 36 , 37 and results in adverse prognosis. 38 HF causes increased right‐sided pressure overload, and leads to LS increase. 20 , 39 , 40 Liver transient elastography showed increased LS at admission attributable to HF and an improvement at discharge. 18 Increased central venous pressure causes hepatocyte atrophy and perisinusoidal edema in the liver. 6 , 8 Increased pressure within the hepatic sinusoid favors bile duct damage by disrupting endothelial cells and the interhepatocytic tight junctions that separate the extravascular space from the bile canaliculus. Further, stagnant flow favors thrombosis within sinusoids, hepatic venules, and portal tracts, thereby contributing to liver fibrosis. 7 , 41 Centrilobular liver cell necrosis can extend to peripheral areas if HF persists and worsens and is followed by the deposition and spread of connective tissue bridging one central vein to another, ultimately leading to liver cirrhosis. 7 Concordant with these findings, increased right‐sided pressure (RAP) in the current study was associated with higher levels of total bilirubin, gamma‐glutamyl transferase, and SWE. In addition, high SWE was associated with right and left volume overload (RAP, left atrial volume, mitral E/e′, right atrial end‐systolic area, and inferior vena cava diameter) and high cardiac event rates.
On the other hand, although HF consists of not only congestion (right‐sided HF), but also reduced arterial flow called hypoxic hepatopathy, liver hypoperfusion (left‐sided HF) determined by image testing has not yet been reported. Hypoxia has been reported to cause centrilobular necrosis and sinusoidal damage in the liver, and leads to impaired transaminase clearance. 42 , 43 In the present study, decreased cardiac output was associated with celiac hypoperfusion, and lower levels of nutrients (total protein, albumin, cholinesterase), which are established predictors of patients with HF. 44 Furthermore, low PSV was associated with impaired right and left systolic function (cardiac index, LVEF, tricuspid valve S′, tricuspid annular plane systolic excursion) and high cardiac event rate. The current study is the first to report the associations between impaired liver perfusion and impaired nutrients, cardiac function, LFTs, and prognosis. In addition, the combination of SWE and PSV might be a useful tool for evaluating abdominal hemodynamic subsets like in the Nohria‐Stevenson clinical profiles. 2
Study Limitations
The current study has several limitations. First, as a prospective cohort study of a single center with a relatively small number of patients and short follow‐up period, the study may be somewhat underpowered. However, our sample size was larger than those of previous studies. 14 , 15 , 16 , 18 , 19 , 20 , 21 Second, although patients with HF with documented liver disease were excluded, we were unable to completely exclude the presence of liver diseases, which may affect hepatic ultrasound imaging results. Therefore, if these patients with HF already had disorders of liver stiffness or fibrosis, it would not be related to only cardiac load. The relationships between SWE and other fibrosis evaluations, such as liver biopsy, which is not generally performed in patients with HF, or imaging (eg, computed tomography, magnetic resonance imaging) should be assessed in further studies. In addition, novel imaging technique such as shear wave dispersion may be useful for evaluating LS. 29 , 45 Third, we conducted the present study using only variables regarding hospitalization, without taking into consideration changes in medical parameters or treatments. Fourth, since the attending physician decided to perform RHC, there might be potential selection bias. Therefore, the present results should be viewed as preliminary, and further studies with a larger population are needed.
Conclusions
Intrahepatic congestion and hypoperfusion determined by abdominal ultrasonography (liver SWE and celiac PSV) are associated with LFT abnormality, cardiac function, and adverse prognosis in patients with HF.
Sources of Funding
This work was supported in part by a grant‐in‐aid for Scientific Research (Grant Number 20K07828) from the Japan Society for the Promotion of Science, Tokyo, Japan.
Disclosures
Drs Yoshihisa and Misaka belong to the Department of Advanced Cardiac Therapeutics, which is supported by Fukuda‐Denshi Co, Ltd. This company is not associated with the contents of the current study. Drs Yokokawa and Sugimoto belong to the Department of Pulmonary Hypertension, which is supported by ACTELION PHARMA Co, Ltd. This company is also not associated with the contents of the current study. The remaining authors have no disclosures to report.
Acknowledgments
The authors thank Tomiko Miura, Kumiko Watanabe, and Hitomi Kobayashi for their outstanding technical assistance. The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
(J Am Heart Assoc. 2020;9:e016689 DOI: 10.1161/JAHA.120.016689.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al.; Authors/Task Force M and Document R . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804. [DOI] [PubMed] [Google Scholar]
- 3. Ikeda Y, Ishii S, Yazaki M, Fujita T, Iida Y, Kaida T, Nabeta T, Nakatani E, Maekawa E, Yanagisawa T, et al. Portal congestion and intestinal edema in hospitalized patients with heart failure. Heart Vessels. 2018;33:740–751. [DOI] [PubMed] [Google Scholar]
- 4. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485–495. [DOI] [PubMed] [Google Scholar]
- 6. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–2405. [DOI] [PubMed] [Google Scholar]
- 7. Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804–2811. [DOI] [PubMed] [Google Scholar]
- 8. Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, Paugam‐Burtz C, Cai D, Pohjanjousi P, Laterre PF, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013;34:742–749. [DOI] [PubMed] [Google Scholar]
- 9. Poelzl G, Auer J. Cardiohepatic syndrome. Curr Heart Fail Rep. 2015;12:68–78. [DOI] [PubMed] [Google Scholar]
- 10. Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Misaka T, Sato T, Suzuki S, Oikawa M, et al. Liver stiffness assessed by Fibrosis‐4 index predicts mortality in patients with heart failure. Open Heart. 2017;4:e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abe S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Sato T, et al. Liver dysfunction assessed by model for end‐stage liver disease excluding INR (MELD‐XI) scoring system predicts adverse prognosis in heart failure. PLoS One. 2014;9:e100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takiguchi M, Yoshihisa A, Miura S, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, et al. Impact of body mass index on mortality in heart failure patients. Eur J Clin Invest. 2014;44:1197–1205. [DOI] [PubMed] [Google Scholar]
- 14. Taniguchi T, Ohtani T, Kioka H, Tsukamoto Y, Onishi T, Nakamoto K, Katsimichas T, Sengoku K, Chimura M, Hashimoto H, et al. Liver stiffness reflecting right‐sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging. 2019;12:955–964. [DOI] [PubMed] [Google Scholar]
- 15. Soloveva A, Kobalava Z, Fudim M, Ambrosy AP, Villevalde S, Bayarsaikhan M, Garmash I, Naumenko M. Relationship of liver stiffness with congestion in patients presenting with acute decompensated heart failure. J Card Fail. 2019;25:176–187. [DOI] [PubMed] [Google Scholar]
- 16. Taniguchi T, Sakata Y, Ohtani T, Mizote I, Takeda Y, Asano Y, Masuda M, Minamiguchi H, Kanzaki M, Ichibori Y, et al. Usefulness of transient elastography for noninvasive and reliable estimation of right‐sided filling pressure in heart failure. Am J Cardiol. 2014;113:552–558. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto K, Kinugasa Y, Sugihara S, Mukai‐Yatagai N, Kato M. Ultrasonographic assessment of organs other than the heart in patients with heart failure. J Med Ultrason (2001). 2019;46:389–397. [DOI] [PubMed] [Google Scholar]
- 18. Colli A, Pozzoni P, Berzuini A, Gerosa A, Canovi C, Molteni EE, Barbarini M, Bonino F, Prati D. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257:872–878. [DOI] [PubMed] [Google Scholar]
- 19. Hakui H, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ishimi M, et al. Usefulness of cardiac metaiodobenzylguanidine imaging to improve prognostic power of the model for end‐stage liver disease scoring system in patients with mild‐to‐moderate chronic heart failure. Am J Cardiol. 2016;117:1947–1952. [DOI] [PubMed] [Google Scholar]
- 20. Jalal Z, Iriart X, De Ledinghen V, Barnetche T, Hiriart JB, Vergniol J, Foucher J, Thambo JB. Liver stiffness measurements for evaluation of central venous pressure in congenital heart diseases. Heart. 2015;101:1499–1504. [DOI] [PubMed] [Google Scholar]
- 21. Hopper I, Kemp W, Porapakkham P, Sata Y, Condon E, Skiba M, Farber L, Porapakkham P, Williams TJ, Menahem S, et al. Impact of heart failure and changes to volume status on liver stiffness: non‐invasive assessment using transient elastography. Eur J Heart Fail. 2012;14:621–627. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 23. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 24. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; Initiative S . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 27. Yoo J, Lee JM, Joo I, Lee DH, Yoon JH, Kang HJ, Ahn SJ. Prospective validation of repeatability of shear wave dispersion imaging for evaluation of non‐alcoholic fatty liver disease. Ultrasound Med Biol. 2019;45:2688–2696. [DOI] [PubMed] [Google Scholar]
- 28. Iijima H, Tada T, Kumada T, Kobayashi N, Yoshida M, Aoki T, Nishimura T, Nakano C, Ishii A, Takashima T, et al. Comparison of liver stiffness assessment by transient elastography and shear wave elastography using six ultrasound devices. Hepatol Res. 2019;49:676–686. [DOI] [PubMed] [Google Scholar]
- 29. Lee DH, Lee JY, Bae JS, Yi NJ, Lee KW, Suh KS, Kim H, Lee KB, Han JK. Shear‐wave dispersion slope from US shear‐wave elastography: detection of allograft damage after liver transplantation. Radiology. 2019;293:327–333. [DOI] [PubMed] [Google Scholar]
- 30. Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, Sato T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64:256–264. [DOI] [PubMed] [Google Scholar]
- 31. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 32. Yoshihisa A, Kimishima Y, Kiko T, Sato Y, Watanabe S, Kanno Y, Abe S, Miyata‐Tatsumi M, Sato T, Suzuki S, et al. Liver fibrosis marker, 7S domain of collagen type IV, in patients with pre‐capillary pulmonary hypertension. Int J Cardiol. 2018;258:269–274. [DOI] [PubMed] [Google Scholar]
- 33. Leeming DJ, Nielsen MJ, Dai Y, Veidal SS, Vassiliadis E, Zhang C, He Y, Vainer B, Zheng Q, Karsdal MA. Enzyme‐linked immunosorbent serum assay specific for the 7S domain of collagen type IV (P4NP 7S): a marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res. 2012;42:482–493. [DOI] [PubMed] [Google Scholar]
- 34. Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002;39:1901–1908. [DOI] [PubMed] [Google Scholar]
- 35. Yoshihisa A, Abe S, Sato Y, Watanabe S, Yokokawa T, Miura S, Misaka T, Sato T, Suzuki S, Oikawa M, et al. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2018;7:330–338. [DOI] [PubMed] [Google Scholar]
- 36. Mohamed BA, Schnelle M, Khadjeh S, Lbik D, Herwig M, Linke WA, Hasenfuss G, Toischer K. Molecular and structural transition mechanisms in long‐term volume overload. Eur J Heart Fail. 2016;18:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Poschl G, Buchler MW, Seitz HK, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206–210. [DOI] [PubMed] [Google Scholar]
- 40. Yoshitani T, Asakawa N, Sakakibara M, Noguchi K, Tokuda Y, Kamiya K, Iwano H, Yamada S, Kudou Y, Nishida M, et al. Value of virtual touch quantification elastography for assessing liver congestion in patients with heart failure. Circ J. 2016;80:1187–1195. [DOI] [PubMed] [Google Scholar]
- 41. Cogger VC, Fraser R, Le Couteur DG. Liver dysfunction and heart failure. Am J Cardiol. 2003;91:1399. [DOI] [PubMed] [Google Scholar]
- 42. Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070. [DOI] [PubMed] [Google Scholar]
- 43. Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 1985;5:367–375. [DOI] [PubMed] [Google Scholar]
- 44. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. 2018;5:e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Yoshimasu Y, Kasai Y, Itoi T. Clinical utilization of shear wave dispersion imaging in diffuse liver disease. Ultrasonography. 2020;39:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


