Abstract
Background
Chronic kidney disease (CKD) confers increased cardiovascular risk, not fully explained by traditional factors. Proteins regulate biological processes and inform the risk of diseases. Thus, in 938 patients with stable coronary heart disease from the Heart and Soul cohort, we quantified 1054 plasma proteins using modified aptamers (SOMAscan) to: (1) discern how reduced glomerular filtration influences the circulating proteome, (2) learn of the importance of kidney function to the prognostic information contained in recently identified protein cardiovascular risk biomarkers, and (3) identify novel and even unique cardiovascular risk biomarkers among individuals with CKD.
Methods and Results
Plasma protein levels were correlated to estimated glomerular filtration rate (eGFR) using Spearman‐rank correlation coefficients. Cox proportional hazard models were used to estimate the association between individual protein levels and the risk of the cardiovascular outcome (first among myocardial infarction, stroke, heart failure hospitalization, or mortality). Seven hundred and nine (67.3%) plasma proteins correlated with eGFR at P<0.05 (ρ 0.06–0.74); 218 (20.7%) proteins correlated with eGFR moderately or strongly (ρ 0.2–0.74). Among the previously identified 196 protein cardiovascular biomarkers, just 87 remained prognostic after correction for eGFR. Among patients with CKD (eGFR <60 mL/min per 1.73 m2), we identified 21 protein cardiovascular risk biomarkers of which 8 are unique to CKD.
Conclusions
CKD broadly alters the composition of the circulating proteome. We describe protein biomarkers capable of predicting cardiovascular risk independently of glomerular filtration, and those that are prognostic of cardiovascular risk specifically in patients with CKD and even unique to patients with CKD.
Keywords: cardiovascular disease, chronic kidney disease, proteomics
Subject Categories:
Non‐Standard Abbreviation and Acronym
- IPA
Ingenuity Pathway Analysis
Clinical Perspective
What Is New?
We used modified aptamers for high throughput proteomic analyses to identify the importance of kidney function on the circulating proteome.
We determined the effect of kidney function on the prognostic information of previously identified cardiovascular risk biomarkers and newly identified novel and even unique cardiovascular biomarkers among the population of patients with chronic kidney disease.
What Are the Clinical Implications?
Chronic kidney disease is known to confer significantly increased cardiovascular risk that is not fully explained by traditional risk factors such as hypertension and diabetes mellitus.
Herein, we describe protein biomarkers capable of predicting cardiovascular risk that is independent of glomerular filtration.
We also identify proteins that are prognostic of cardiovascular risk specifically in patients with chronic kidney disease and even unique to patients with impaired renal function.
Chronic kidney disease (CKD) confers a substantial increase in cardiovascular risk.1, 2 While traditional risk factors such as diabetes mellitus and hypertension predict adverse cardiovascular outcomes in patients with CKD, they do not fully explain the excess cardiovascular risk in this population.3 CKD is associated with changes in the concentrations of some circulating proteins, partly because of their reduced renal clearance, but also the result of biochemical alterations associated with the “uremic milieu”.4, 5, 6 Such changes in the circulating proteome may give rise to biomarkers that are strongly prognostic of cardiovascular risk in CKD and even unique to CKD.7 Current approaches designed to elucidate the excess cardiovascular risk among CKD patients have targeted a relatively small number of candidate biomarkers to explain what is likely a complex mechanism. Large‐scale proteomic scanning removes the limitations of educated guesses and has the potential to reveal a much larger set of cardiovascular risk biomarkers in CKD than has otherwise been possible.
The field of proteomics has matured over the past 2 decades.8 Technologies have been developed that can currently measure the levels of hundreds and even thousands of proteins from a small sample of blood.9, 10, 11 We recently used one such technology, modified aptamers, to successfully measure the levels of 1054 proteins in 938 participants with stable coronary heart disease from the Heart and Soul observational study.9 We discovered 196 proteins that are prognostic of cardiovascular risk in this cohort. Notably, despite its relatively recent ascendance as a leading technology, aptamer‐based proteomics has been well‐validated. The assay's specificity has been confirmed by orthogonal approaches including mass spectrometry12 and by linking the effects of cis‐genetic variants to measurements of protein expression.12, 13, 14
Kidney function in the patients from the Heart and Soul cohort ranges from normal to moderately impaired and this cohort has been well‐suited for investigations of kidney‐related outcomes.15, 16, 17, 18, 19 Specifically, the presence of CKD in Heart and Soul has been associated with increased cardiovascular risk.16, 17 Our proteomic analysis of the Heart and Soul cohort had 3 objectives: (1) To discern how reduced glomerular filtration rates impact the composition of the circulating proteome, by surveying the plasma concentrations of 1054 distinct proteins, (2) To learn of the importance of kidney function in the prognostic information contained in the previously identified 196 cardiovascular risk biomarkers9 by correcting their hazard ratios for glomerular filtration rate; this correction also generates a set of biomarker proteins that are capable of predicting cardiovascular risk independently of kidney function and (3) To identify cardiovascular risk biomarkers specifically in patients with CKD by conducting a de novo biomarker discovery among the previously measured 1054 plasma proteins. Of great interest was discovery of any cardiovascular risk biomarkers that are unique to patients with CKD and to help identify the effect of variations in glomerular filtration rates on disease‐related pathways involved in processes such as atherosclerosis, inflammation, and angiogenesis.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study population
The cohort and study protocol was approved by the appropriate institutional review boards: the University of California San Francisco Committee on Human Research, the Research and Development Committee at the San Francisco Veterans Affairs Medical Center, the Medical Human Subjects Committee at Stanford University, the Human Subjects Committee at the Veterans Affairs Palo Alto Health Care System, and the Data Governance Board of the Community Health Network of San Francisco. All participants provided written informed consent. The Heart and Soul is a prospective cohort of patients with stable coronary artery disease from 12 clinics in the San Francisco Bay Area. Participants were enrolled from September 2000 to December 2002, with last follow‐up in November 2011.9 They were recruited based on ≥1 of the following eligibility criteria: history of myocardial infarction (MI), angiographic evidence of at least 50% stenosis in ≥1 coronary vessels, prior evidence of inducible ischemia by stress testing, or history of coronary revascularization. Exclusion criteria included MI within the previous 6 months, those unable to walk a block, or those planning to relocate within 2 years. Race was self‐identified in a questionnaire with categories of white, black, Asian, Latino, or other. The Heart and Soul study was approved by the appropriate institutional review board and all participants provided written informed consent.9 Events were defined as the first among: myocardial infarction, stroke, heart failure hospitalization, or mortality.
Assessment of Kidney Function and Definition of CKD
The design of the present study is summarized in a flowchart in Figure 1. Consistent with prior publications from Heart and Soul, we defined CKD as estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2.15, 18, 19, 20 An important objective of this study was to evaluate the association of plasma proteins with cardiovascular risk that is independent of kidney function. For this purpose, we adjusted the cardiovascular risk hazard ratios of prognostic proteins for eGFR, using the creatinine based CKD‐Epi equation.21, 22, 23
Figure 1. Study flowchart.9 .
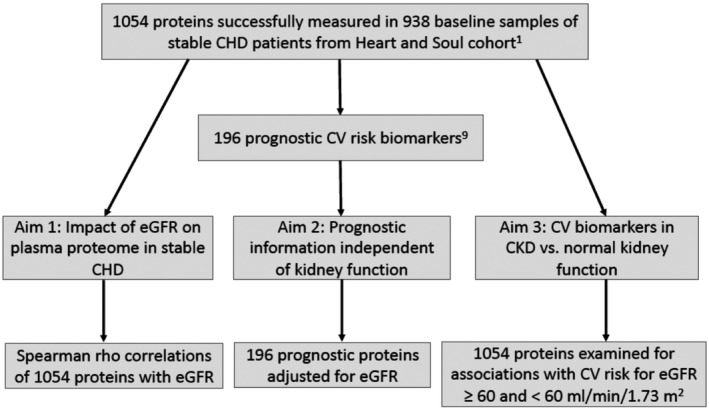
CHD indicates coronary heart disease, CKD, chronic kidney disease; and eGFR, estimated glomerular filtration rate.
Quantification of Proteins in Human Plasma by Modified Aptamers
The proteomic analysis consisted of the 938 baseline plasma samples from the Heart and Soul study.9 Sample collection was standardized with fasted samples collected at the same time of day and centrifuged and frozen within 1 hour of sample collection. The method of quantification of plasma proteins using modified aptamers has been described previously.9, 24, 25 In brief, each of the 1130 individual proteins measured has its binding reagent made of chemically modified DNA, referred to as a modified aptamer. Each sample of plasma was incubated with the mixture of modified aptamers to generate modified aptamer‐protein complexes under equilibrium conditions. Unbound modified aptamers and unbound or non‐specifically bound proteins were eliminated by 2 bead‐based immobilization steps. After eluting the modified aptamers from the target protein, the fluorescently labeled modified aptamers were directly quantified on a hybridization array (Agilent Technologies, Santa Clara, CA). Calibrators were included so that the degree of fluorescence was a consistent reflection of protein concentration. In a prior study we reported the repeatability of the modified aptamer assay by establishing the coefficients of variation for 1129 proteins (one fewer protein than in the current study).25 The distribution of coefficients of variation across these 1129 proteins is provided in Table S1. The median coefficient of variation was 3.9% and 95% of proteins had a coefficient of variation ≤10.5%. The 1054 proteins that passed all the quality control metrics9 are the focus of the analyses in the present study. We have reported that 200 proteins were prognostic of cardiovascular risk from the analyses of 2 cohorts, Heart and Soul and HUNT3 (Helseundersøkelsen i Nord‐Trøndelag).9 As the present analysis included only data from the Heart and Soul cohort, the applicable number of prognostic biomarkers proteins is 196, rather than 200. The Ingenuity Pathway Analysis (IPA) tool,9, 25 was used to evaluate common functional groups related to cardiovascular and renal disease. IPA recognized 1045 of the 1054 proteins measured, and 217 of the 218 proteins whose plasma level correlated with estimated glomerular filtration rates at ρ≥0.2.
Statistical Analysis
For population characteristics, we divided the cohort according to eGFR ≥60 mL/min per 1.73 m2 (non‐CKD group) and eGFR<60 mL/min per 1.73 m2 (CKD group). Differences among continuous variables were tested using the Wilcoxon Rank Sum Test. Comparisons of categorial measures were made using Chi‐squared analysis. Levels of plasma proteins were correlated with eGFR by Spearman rank correlation coefficients. Associations that were statistically significant at P<0.05 were defined as weak for absolute (ie, positive or negative) value of ρ<0.2, moderate for absolute ρ 0.2 to <0.5 and strong for absolute ρ≥0.5. Absolute values of rho are referred to as “rho”. The primary outcome (referred to as the cardiovascular outcome) in this study was defined as the first event among MI, stroke/transient ischemic attack (referred to as stroke), heart failure hospitalization, or all‐cause death.9 Cox proportional hazard models were used to estimate the association between individual protein levels and risk of the primary outcome. In single‐variable analysis of the associations between individual proteins and the primary outcome, Bonferroni‐corrected significance levels were reported (padj) adjusting for all 1054 proteins measured. This resulted in a nominal significance level of P adj=4.74×10−5. To find proteins prognostic of the primary outcome separately in patients without or with CKD, we divided the cohort according to the non‐CKD group and CKD group, respectively.21, 22 Percentages reported are of the total 1054 proteins unless otherwise stated.
Results
Population Characteristics
The baseline characteristics of the study participants, divided according to non‐CKD (n=679) or CKD (n=258) populations are summarized in Table 1. One patient was excluded because of incomplete data. Median (interquartile range) eGFR was 80.9 (71.1–93.1) mL/min per 1.73 m2 for non‐CKD participants and 50.0 (40.1–55.4) mL/min per 1.73 m2 for CKD participants (P=2.0×10−16). As expected, participants with CKD were older, more frequently diabetic, and had higher systolic blood pressures. They also had lower rates of current smoking. Also expected, time to events were shorter and the cardiovascular event rates (MI, stroke, heart failure, and death) were nearly twice as high in participants with CKD.
Table 1.
Baseline Characteristics of the Study Participants
| Non‐CKD | CKD | P value | |
|---|---|---|---|
| No. | 679 | 258 | |
| Follow‐up, y | 8.9 (6–10) | 7.6 (4–9) | <0.00001 |
| Age, y | 65 (57–72) | 74 (66–79) | <0.00001 |
| Men | 555 (82) | 217 (84) | 0.39 |
| Ethnicity | |||
| White | 401 (59) | 164 (64) | 0.6 |
| Black | 116 (17) | 35 (14) | |
| Asian | 80 (12) | 27 (11) | |
| Latino | 60 (9) | 22 (9) | |
| Diabetes mellitus | 166 (24) | 80 (31) | 0.04 |
| Current smoker | 152 (22) | 32 (12) | 0.003 |
| Event rate, %/y | 5.4 | 10.6 | |
| Events during follow‐up, No. | 284 (42) | 180 (70) | <0.00001 |
| Time to event, y | 8.9 (4.3–9) | 5 (1.9–8.9) | <0.00001 |
| BMI, kg/m2 | 27.7 (24.8–31.4) | 27.4 (24.9–30.5) | 0.37 |
| HDL‐C, mg/dL | 43 (36–54) | 42 (34–52) | 0.1 |
| LDL‐C, mg/dL | 99 (82–124) | 99 (82–119) | 0.32 |
| Total cholesterol, mg/dL | 173 (150–199) | 168 (146–195) | 0.12 |
| Triglycerides, mg/dL | 108 (71–166) | 119 (81–173) | 0.12 |
| Creatinine, mg/dL | 0.8 (0.9–1.1) | 1.4 (1.2–1.6) | <0.00001 |
| eGFR, mL/min per 1.73 m2 | 80.9 (71.1–93.1) | 50.0 (40.1–55.4) | <0.00001 |
| CRP, mg/L | 2.1 (0.8–4.7) | 2.7 (1.1–6.3) | 0.004 |
| Systolic blood pressure, mm Hg | 130 (118–143) | 135 (120–148) | 0.001 |
| Diastolic blood pressure, mm Hg | 74 (68–80) | 72 (68–80) | 0.41 |
Continuous measures are presented as median (interquartile range); categorical measures are presented as n (%) of the column total. BMI indicates body mass index; CKD, chronic kidney disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; and LDL, low‐density lipoprotein cholesterol.
Impact of eGFR on the Circulating Plasma Proteome
Table S2 displays the Spearman rho correlations between eGFR and plasma levels for all 1054 proteins. Plasma levels of 709 proteins (67.3%) correlated with eGFR significantly (P<0.05). Of these, 24 proteins (2.3%), correlated with eGFR strongly (ρ≥0.5), 194 proteins (18.4%) moderately (ρ 0.2 to <0.5) and 491 proteins (46.6%) weakly (ρ 0.06 to <0.2). The histogram in Figure 2 depicts the distribution of these correlations. Notably, for the 24 proteins that correlated with eGFR strongly, all the rho correlations were negative (ie, in each case, lower eGFR was associated with higher plasma protein levels). For the 194 proteins that correlated eGFR moderately, levels of 131 (12.4%) proteins correlated with eGFR negatively and 63 (6.0%) positively.
Figure 2. Distribution of Spearman rho correlations between the 1054 proteins measured and estimated glomerular filtration rate.
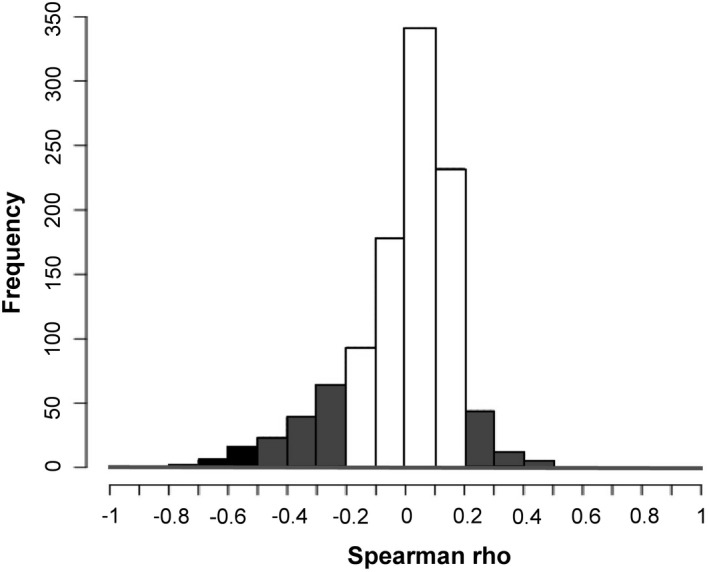
Strong correlation (black bars), moderate correlation (gray bars), weak correlation (white bars). The Spearman rho correlations for each of the 1054 proteins are shown in Table S2.
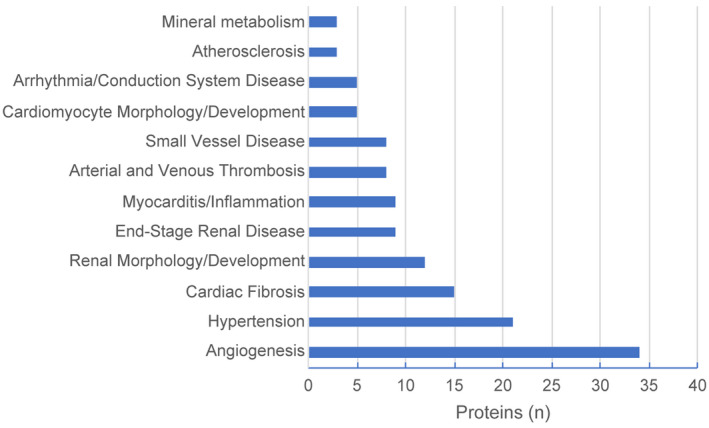
Figure 3 displays the number of proteins within 12 cardiovascular and renal disease‐related biological functions whose plasma level is impacted by eGFR at ρ≥0.2. The top 3 functional groups based on the largest number of proteins affected by eGFR relate to angiogenesis (n=34 proteins), hypertension (n=21 proteins), and cardiac fibrosis (n=15 proteins).
Figure 3. The number of plasma proteins whose level is impacted by estimated glomerular filtration rate at ρ≥0.2 for 12 cardiovascular and renal disease‐related biological functions, derived from Ingenuity Pathway Analysis.
Contribution of Kidney Function to Prognostic Information Contained Within 196 Cardiovascular Risk Biomarkers and Biomarkers Independent of Kidney Function
From the Heart and Soul cohort, we previously identified 196 proteins that are prognostic of cardiovascular risk.9 By adjusting the hazard ratios of these biomarkers for eGFR, we discerned how much of the prognostic information they carry is attributable to kidney function. Only 87 of 196 proteins (44%) survived this adjustment (at a Bonferroni corrected P value<0.05), as shown in Table S3 with the hazard ratios and levels of statistical significance for all 196 proteins, before and after the eGFR correction.
Cardiovascular Risk Biomarkers in Chronic Kidney Disease Versus Normal Kidney Function
To discover biomarkers that are prognostic of cardiovascular risk in patients with CKD as compared with those with normal kidney function, we divided the cohort according to eGFR (Table 1). There were 679 non‐CKD participants who had 285 cardiovascular events (MI, stroke, heart failure events, and death) and 258 participants with CKD who had 180 events.
At a Bonferroni corrected P value<P adj, there were 84 proteins prognostic of cardiovascular risk in non‐CKD patients (Table S3) and 21 proteins that were prognostic of cardiovascular risk in CKD patients (Table 2). Among these 21 proteins, 8 proteins were unique to CKD (highlighted in grey color in Table 2). The plasma levels of these 8 proteins correlated significantly with eGFR, for one protein positively: dual 3’,5’‐cyclic‐AMP and ‐GMP phosphodiesterase 11A (ie, lower eGFR was associated with lower concentration of this protein) and for 7 proteins negatively (Table 2). The molecular weights and key biological functions derived from the literature and IPA annotations for these 21 protein cardiovascular risk biomarkers, including those 8 that are unique to CKD, are provided in Table 2.
Table 2.
Twenty‐One Proteins Prognostic of Cardiovascular Risk in Patients With CKD
| Protein | UniProt ID | Molecular Weight (kDa) | HR | P value | Spearman ρ | P value ρ | Biological Function |
|---|---|---|---|---|---|---|---|
| Tumor necrosis factor receptor superfamily member 1A | P19438 | 50.5 | 1.31 | 1.63×10−05 | −0.65 | 8.84×10−112 |
Soluble form of TNF‐alpha receptor. Antagonizes activity of TNF‐alpha |
| Beta‐2‐microglobulin | P61769 | 14 | 1.34 | 1.10×10−05 | −0.63 | 2.33×10−100 |
Part of major histocompatibility complex. Involved in inflammatory response |
| Tumor necrosis factor receptor superfamily member 27 | Q9HAV5 | 32.8 | 1.32 | 4.44×10−06 | −0.53 | 9.33×10−66 | Involved in ectodermal and epidermal development |
| Metalloproteinase inhibitor 1 | P01033 | 23 | 1.51 | 7.23×10−08 | −0.43 | 1.32×10−42 | Extracellular matrix remodeling and turnover. |
| Hepatitis A virus cellular receptor 2 | Q8TDQ0 | 33.4 | 1.39 | 4.05×10−05 | −0.41 | 3.04×10−37 | Co‐inhibitory molecule that regulates T‐cell activation or tolerance |
| Platelet‐activating factor acetylhydrolase IB subunit beta | P68402 | 30 | 1.36 | 7.98×10−07 | −0.38 | 1.91×10−32 |
Degrades platelet‐activating factor. Mediator of inflammation |
| Tumor necrosis factor receptor superfamily member 9 | Q07011 | 27.9 | 1.35 | 1.47×10−05 | −0.33 | 1.57×10−23 | Co‐stimulator of T‐cells |
| Phosphatidylethanolamine‐binding protein 1 | P30086 | 21.1 | 1.33 | 4.55×10−05 | −0.28 | 7.64×10−17 | Tumor suppressor gene |
| Fibroblast growth factor 7 | P21781 | 36 | 1.39 | 1.84×10−06 | −0.25 | 4.18×10−14 |
Tissue repair. Tumor growth and survival |
| Troponin I, cardiac muscle | P19429 | 24.0 | 1.31 | 1.67×10−05 | −0.23 | 1.08×10−11 | Marker of myocardial damage |
| Tyrosine‐protein kinase, yes | P07947 | 60.8 | 1.39 | 1.25×10−05 | −0.21 | 8.46×10−10 |
Src family of oncogenes. Regulates cell growth and survival |
| Angiopoietin‐2 | O15123 | 56.9 | 1.66 | 5.62×10−12 | −0.14 | 2.85×10−05 | Modulates vascular development and remodeling during angiogenesis and inflammation |
| PSA:alpha‐1‐antichymotrypsin complex | P07288, P01011 | 76.4 | 1.42 | 5.12×10−06 | −0.13 | 0.00011 | Acute phase reactant protein. Expressed in several malignancies |
| Complement component C7 | P10643 | 110 | 1.57 | 1.79×10−09 | −0.12 | 0.00065 | Involved in innate immunity. |
| Ubiquitin+1, truncated mutation for UbB | P62979 | 18 | 1.32 | 2.92×10−05 | −0.12 | 0.00048 |
Frameshift mutation of Ubiquitin. Associated with neurodegenerative disorders |
| Interleukin‐8 | P10145 | 11.1 | 1.3 | 2.12×10−05 | 0.03 | 0.36 | Chemoattractant that promotes activation of monocytes and neutrophils |
| Cadherin‐3 | P22223 | 91.4 | 0.71 | 3.37×10−06 | 0.26 | 1.60×10−14 |
Calcium‐dependent cell adhesion molecule. Tumor suppressor role |
| Growth hormone receptor | P10912 | 71.5 | 0.69 | 6.45×10−07 | 0.26 | 3.70×10−15 | Activates JAK‐STAT signaling pathways that alter calcium signaling in contractile and cytoskeletal proteins. |
| Proto‐oncogene tyrosine‐protein kinase receptor Ret | P07949 | 124.3 | 0.7 | 2.65×10−05 | 0.31 | 3.04×10−21 | Gain of function mutation results in cancer syndromes |
| Dual 3’,5’‐cyclic‐AMP and ‐GMP phosphodiesterase 11A | Q9HCR9 | 104.8 | 0.71 | 2.98×10−05 | 0.37 | 3.29×10−30 |
Binds cAMP and cGMP. Similar to PDE‐5 |
| Epidermal growth factor receptor | P00533 | 134.3 | 0.71 | 1.41×10−05 | 0.44 | 5.89×10−45 | Regulates epithelial tissue development and homeostasis |
Proteins unique to patients with chronic kidney disease are shown in gray color. CKD indicates chronic kidney disease; HR, hazard ratio; and TNF, tumor necrosis factor.
Dividing the Heart and Soul cohort based on eGFR created 2 smaller groups, each with fewer outcome events. This likely reduced the statistical power to detect prognostic proteins within each smaller group by widening the CIs around each hazard ratio point estimate. To shed light on this issue, Figure S1 shows the point estimates and CIs of hazard ratios for each of the 196 proteins that were prognostic of cardiovascular risk in the full cohort and for the smaller groups of non‐CKD and CKD participants.
Discussion
Among patients with CKD, increased cardiovascular risk is a well‐recognized cause of mortality and morbidity.2, 3 As this risk is not fully accounted for by traditional risk factors,3 identification of new cardiovascular risk biomarkers has been a high priority in CKD research. Prior studies have suggested that reduced GFR and the biochemical changes associated with “uremic milieu” can impact the concentration of some circulating proteins4, 5, 6 but the extent to which this occurs in patients with coronary heart disease, many of whom also have CKD,16, 17 has not been previously defined. The present study reached a new milestone by measuring a far greater number of plasma proteins than has otherwise been possible with targeted approaches. We used the Heart and Soul cohort to successfully accomplish 3 aims: (1) We conducted the largest survey to date of the impact of glomerular filtration rates on the composition of the plasma proteome in patients with coronary heart disease, making a total of nearly 1 million individual protein measurements. We found that reductions in eGFR broadly impact the circulating proteome and affect biological functions involved in cardiovascular and renal diseases. (2) We determined how much of the information among previously identified 196 cardiovascular biomarkers,9 relates specifically to kidney function by adjusting their hazard ratios for eGFR. We found that kidney function contributes prominently to the prognostic information carried by cardiovascular protein biomarkers—fewer than half of these biomarkers survived the adjustment for eGFR. In this process, we identified 87 protein biomarkers that remained prognostic of cardiovascular risk, and that can contribute to cardiovascular risk modeling independently of kidney function. (3) We conducted a de novo proteomic discovery in the Heart and Soul cohort that informed 21 biomarkers of cardiovascular risk specifically in patients with CKD. From this analysis, we identified 8 cardiovascular biomarkers that are unique to patients with CKD.
Notably, levels of approximately of the 1054 proteins measured (67.3%) were significantly correlated with eGFR demonstrating that a large portion of the circulating proteome is impacted to some degree by a decline in kidney function (rho coefficients ranged from 0.06–0.74, Table S2). For one fifth of the circulating proteome (20.7%) levels of proteins were moderately or strongly correlated with eGFR (ρ≥0.2). As many of the proteins we measured are cytokines, chemokines, adipokines, growth factors and hormones that orchestrate biological functions,12 any changes in their circulating concentrations have implications for the risk of cardiovascular and other diseases. Concretely, Figure 3 displays cardiovascular and renal disease‐related pathways derived from IPA for the 217 proteins recognized by the IPA, whose circulating levels are associated with eGFR at ρ≥0.2. The observed shift in the proteome as eGFR declines involves critical functions in cardiovascular and renal diseases, such as angiogenesis, control of blood pressure (hypertension) and cardiac fibrosis (Figure 3), providing a plausible biological underpinning to the host of cardiovascular and non‐cardiovascular co‐morbidities reported in patients with CKD. Interestingly, in addition to the cardiovascular and renal disease pathways shown in Figure 3, there was a substantial number of proteins involved in pathways leading to the development of gastrointestinal tract tumors (not shown). Notably, increased risk of gastrointestinal, urinary tract, and endocrine tumors has been reported in patients with end‐stage renal disease.26
Our findings of the impact of reduced eGFR on the circulating proteome is supported by a recent report from Lund, Sweden.5 In their study, 389 participants whose kidney function ranged from stage I to stage V CKD had their glomerular filtration assessed directly with iohexol and a total of 2893 circulating proteins were measured with modified aptamers. Despite major differences between their and our studies (eg. the Lund cohort consisted of patients referred for evaluation of suspected kidney disease, regardless of their cardiovascular status which was not reported;5 their samples consisted of LiHeparin plasma whereas ours were EDTA plasma; 2 different versions of the modified aptamer SOMAscan platform were used; the Lund cohort had greater representation of patients with advanced CKD), our findings and those from Lund5 are remarkably consistent, providing important external validation to our findings. This is illustrated in Table S4 which compares the rho coefficients for the top 100 proteins from our Heart and Soul cohort to the same proteins assessed in the Lund cohort (98 proteins in common to the 2 cohorts are shown). The scatterplot of these 98 rho coefficients shows generally good agreement between findings in our Heart and Soul and the Lund cohorts (Figure S2).
The observed changes in the plasma proteome across eGFR can be explained through several potential mechanisms. (1) Concentration of specific proteins may vary by the function of the kidney which filters, potentially reabsorbs, and catabolizes many low molecular weight proteins. As filtration by glomeruli occurs freely for proteins <15 kDa and relatively rapidly for proteins 15 to 45 kDa,6 any plasma accumulation of proteins of that size can be at least partly attributable to their reduced glomerular filtration. To inform this issue, Table S2 also lists the molecular weights for all 1054 proteins that we measured along with their rho correlations with eGFR. (2) Additionally, healthy kidneys serve as a rich source of circulating proteins.27, 28 Accordingly, the loss of renal parenchyma in CKD can reduce the plasma levels of some proteins, with circulating erythropoietin in CKD as a well‐known clinical example.29 (3) Lastly, kidney function is susceptible to damage from many of the same risk factors that lead to atherosclerotic cardiovascular disease, with diabetes mellitus or hypertension as familiar examples.7 Changes in the circulating proteome in patients with CKD can thus reflect the effect of shared cardiovascular and renal risk factors.7
Determining disease‐based risk through identification of novel biomarkers, as well as development of individualized risk prediction models are the key objectives of the field of precision (personalized) medicine.30 We previously constructed a proteomic cardiovascular risk model based on a discovery program for new biomarkers using a large‐scale, agnostic proteomics approach.9 Likewise, the current study relied on the same large‐scale methodology to identify protein biomarkers that are prognostic of cardiovascular risk in patients with CKD. We first focused on the 196 cardiovascular risk biomarkers that we had identified9 that did not previously account for kidney function in their discovery. After correcting for eGFR, the number of prognostic proteins fell from 196 to just 87, signifying that kidney function contributes prominently to cardiovascular risk information contained in many circulating proteins. The hazard ratios for all 196 protein cardiovascular biomarkers before and after correction for eGFR are listed in Table S3. Conversely, the 87 proteins that survived the eGFR correction (shown in Table S3) are able to serve as cardiovascular risk biomarkers independently of eGFR.9
In our second approach to elucidating cardiovascular risk protein biomarkers in CKD, we conducted de novo discovery for such biomarkers, separately for individuals with CKD and those with normal kidney function. At a Bonferroni corrected P value<P adj, among the 1054 proteins measured, we identified 84 proteins that were prognostic of cardiovascular risk in participants with normal kidney function (eGFR≥60 mL/min per 1.73 m2, shown in Table S5) and 21 proteins that were prognostic of cardiovascular risk specifically in participants with CKD (Table 2). As summarized in Table 2, based on literature review and IPA annotations, these 21 proteins are involved in a number of pathobiological processes including angiogenesis, inflammation, cell signaling with extracellular matrix interactions, tissue repair, and malignant transformation of cells. These cardiovascular risk biomarkers in patients with CKD should be investigated in future studies as potential causal mediators in this setting. Of particular interest, among these 21 proteins are 8 proteins that are prognostic of cardiovascular risk only among individuals with CKD but not among those with normal kidney function (highlighted in grey color in Table 2). The molecular weights of these 21 protein biomarkers in CKD and their rho correlations with eGFR are described in Table 2. Notably, only 2 of these 21 prognostic proteins were strongly associated with eGFR (ρ≥0.5) while, conversely, 22 of 24 proteins strongly associated with eGFR (Table S2) were not prognostic of cardiovascular risk in the setting of CKD (Table S2). These results suggest that these 21 proteins carry prognostic information that extends beyond glomerular filtration.
The high fidelity of the SOMAscan proteomic assay has been previously established in several ways. The effect of cis‐genetic variants on protein expression measured by the modified aptamer (SOMAscan) assay has been published for 55212 and 104613 variants and confirmed that the assay was measuring the intended protein target. Furthermore, orthogonal validation of the target protein by mass spectrometry has been performed for ~1000 aptamer reagents.13 Overall, these data have suggested that the large majority of modified aptamers are highly specific for their cognate protein targets.12 Notably, the specificity of the modified aptamers for their target proteins has been verified for 19 of the 21 protein cardiovascular risk biomarkers in CKD that we have discovered in the present analysis (tumor necrosis factor receptor superfamily member 9 and phosphatidylethanol‐amine‐binding protein 1 were not evaluated), as part of a larger study to confirm the specificity of 920 aptamers by mass spectometry.12 In addition, for 16 of these 19 aptamers tested, there was no cross‐reactivity with proteins closely related to the target proteins (defined as proteins with >40% sequence identity with the target protein or members of the same protein family), while for 3 proteins (angiopoeitin‐2, tyrosine kinase YES, and epidermal growth factor receptor), weak cross‐reactivity with closely related proteins was noted.12 Other methods for orthogonal correlations such as ELISA, are not readily available for a wide range of proteins that we measured and have their own challenges with respect to target specificity and cross‐reactivity.
Among the 8 prognostic proteins unique to CKD, shown in Table 2, platelet‐activating factor acetylhydrolase IB subunit beta (also known as lipoprotein‐associated phospholipase A2) degrades platelet‐activating factor and regulates inflammation.31 FGF‐7 (fibroblast growth factor) is an epithelial specific growth factor involved in tissue repair as well as tumor growth and survival.32, 33 Tyrosine kinase YES is a member of the Src family of oncogenes and regulates cell growth and survival.34, 35 Tumor necrosis factor receptor superfamily member 9 is a member of the TNF‐receptor family and functions a co‐stimulator of T‐cells.36 Tumor necrosis factor receptor superfamily member 1A is a soluble form of the TNF‐alpha receptor and antagonizes its activity.37, 38, 39 Tumor necrosis factor receptor superfamily member 1A has been established as a predictor of cardiovascular outcomes in advanced CKD and may play a role in heart failure.37, 38, 39, 40 Ubiquitin+1 is a frameshift mutation of Ubiquitin, affecting the proteasome degradation system.41, 42, 43, 44 Ubiqutin+1 has been associated with neurodegenerative disorders. Dual 3’,5’‐cyclic‐AMP and ‐GMP phosphodiesterase‐11 binds both cAMP and cGMP and bears similarity to PDE‐5.45, 46 Phosphatidylethanolamine‐binding protein 1 acts as a tumor suppressor gene and also activates the beta‐adrenergic receptor, playing an adaptive role in heart failure.47, 48, 49 From the cardiovascular risk standpoint, among these 8 proteins, platelet‐activating factor acetylhydrolase IB subunit beta (lipoprotein‐associated phospholipase A2) has been suspected of being involved in the pathogenesis of atherosclerosis. Although clinical trials of lipoprotein‐associated phospholipase A2 inhibition with darapladib did not demonstrate a clinical benefit,50, 51 given our findings of a strong association of this biomarker with cardiovascular risk in patients with CKD, one might plausibly ask if the result may have been different in a trial that specifically targeted the CKD population. Admittedly, we do not yet know if our lipoprotein‐associated phospholipase A2 aptamer assay measures protein abundance (as expected of a binding assay) or whether it also informs its enzymatic activity. In the subgroup analysis of cardiovascular biomarkers in CKD or no CKD, the total number of participants and cardiovascular outcome events in each group was smaller than in the total cohort, likely leading to fewer prognostic biomarkers in each group. Notably, among the 196 prognostic proteins, there were additional proteins that trended towards statistical significance threshold within the CKD or no CKD smaller groups (shown in Figure S1).
In conclusion, by scanning the plasma proteome for 1054 distinct proteins, we have shown in patients with coronary heart disease that kidney function has a broad impact on the circulating proteome and specifically on biological functions relevant to cardiovascular and renal diseases. We have described protein biomarkers that predict cardiovascular risk in CKD independently of glomerular filtration and biomarkers that were newly discovered specifically to predict cardiovascular risk in CKD. The impact of eGFR on the plasma levels of 1054 individual proteins shown in Table S2, the hazard ratios for the associations of proteins with cardiovascular risk in the 1054 proteins before and after eGFR correction also shown here in Table S2 as well as the discovery of 21 proteins that specifically predict cardiovascular risk in CKD shown in Table 2, will provide a wealth of new leads for future academic investigations and drug discovery in the field of CKD. Future studies, for example using Mendelian randomization, will determine which of the cardiovascular risk biomarkers in CKD that we have described are also causal cardiovascular disease mediators and thus desirable new therapeutic targets to reduce this risk in patients with CKD.
Our study had many strengths but also some limitations. The present study from the Heart and Soul cohort focuses on patients with kidney function ranging from normal to moderately reduced. Future studies with greater representation of patients with CKD stages 4 and 5 are needed. Furthermore, in the present study, our rigorous statistical approach that adjusted for multiplicity of testing during biomarker discovery led to a statistical significance threshold of P adj=4.74×10−5. This stringent threshold excluded some familiar cardiovascular risk biomarkers in CKD, notably fibroblast growth factor 23 (which had hazard ratio, 1.18; P=0.0039), whereas the 21 aforementioned protein biomarkers had stronger associations with cardiovascular risk (Table 2). Lastly, we present convincing external validation for the impact of eGFR on the composition of the circulating proteome (ie, the Lund cohort) but further validation in external cohorts will be needed for the 21 cardiovascular risk biomarkers that we report in patients with CKD.
Sources of Funding
The proteomic analysis was supported by SomaLogic, Inc. Dr Ganz's proteomic research is supported by National Institutes of Health grants 1RO1HL129856, 1UO1DK108809, and 1R01AG052964. The Heart and Soul cohort was supported by the Department of Veterans Affairs; the National Heart, Lung, and Blood Institute (R01 HL079235); the American Federation for Aging Research; the Robert Wood Johnson Foundation; and the Ischemia Research and Education Foundation.
Disclosures
Dr. Ganz serves on a medical advisory board to SomaLogic, Inc., for which he accepts no salary, honoraria, or any other financial incentives. Drs. Brody, Mehler, Weiss, DeLisle, Ostroff, and Williams are employees of SomaLogic, Inc. SomaLogic had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the article. Dr Ganz had the ultimate responsibility for all aspects of this study. SomaLogic, Inc., had no veto rights concerning the decision to submit the article for publication. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5 Figures S1–S2
(J Am Heart Assoc. 2020;9:e016463 DOI: 10.1161/JAHA.120.016463.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Saran R, Robinson B, Abbott KC, Agodoa LYC, Albertus P, Ayanian J, Balkrishnan R, Bragg‐Gresham J, Cao J, Chen JLT, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the united states. Am J Kidney Dis. 2017;69:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 3. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. [DOI] [PubMed] [Google Scholar]
- 4. deFilippi CR, Herzog CA. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin Chem. 2017;63:59–65. [DOI] [PubMed] [Google Scholar]
- 5. Christensson A, Ash JA, DeLisle RK, Gaspar FW, Ostroff R, Grubb A, Lindström V, Bruun L, Williams SA. The impact of the glomerular filtration rate on the human plasma proteome. Proteomics Clin Appl. 2018;12:e1700067. [DOI] [PubMed] [Google Scholar]
- 6. Jia L, Zhang L, Shao C, Song E, Sun W, Li M, Gao Y. An attempt to understand kidney's protein handling function by comparing plasma and urine proteomes. PLoS ONE. 2009;4:e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Husain‐Syed F, McCullough PA, Birk H‐W, Renker M, Brocca A, Seeger W, Ronco C. Cardio‐pulmonary‐renal interactions: a multidisciplinary approach. J Am Coll Cardiol. 2015;65:2433–2448. [DOI] [PubMed] [Google Scholar]
- 8. Lindsey ML, Mayr M, Gomes AV, Delles C, Arrell DK, Murphy AM, Lange RA, Costello CE, Jin Y‐F, Laskowitz DT, et al. Transformative impact of proteomics on cardiovascular health and disease: a scientific statement from the american heart association. Circulation. 2015;132:852–872. [DOI] [PubMed] [Google Scholar]
- 9. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and validation of a protein‐based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. 2016;315:2532–2541. [DOI] [PubMed] [Google Scholar]
- 10. Sabatine MS. Using aptamer‐based technology to probe the plasma proteome for cardiovascular disease prediction. JAMA. 2016;315:2525–2526. [DOI] [PubMed] [Google Scholar]
- 11. Ngo D, Sinha S, Shen D, Kuhn EW, Keyes MJ, Shi X, Benson MD, O'Sullivan JF, Keshishian H, Farrell LA, et al. Aptamer‐based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation. 2016;134:270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, Hoover H, Gudmundsdottir V, Horman SR, Aspelund T, et al. Co‐regulatory networks of human serum proteins link genetics to disease. Science. 2018;361:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benson MD, Yang Q, Ngo D, Zhu Y, Shen D, Farrell LA, Sinha S, Keyes MJ, Vasan RS, Larson MG, et al. Genetic architecture of the cardiovascular risk proteome. Circulation. 2018;137:1158–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the heart and soul study. J Am Soc Nephrol. 2004;15:2908–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bansal N, Katz R, De Boer IH, Peralta CA, Fried LF, Siscovick DS, Rifkin DE, Hirsch C, Cummings SR, Harris TB, et al. Development and validation of a model to predict 5‐year risk of death without ESRD among older adults with CKD. Clin J Am Soc Nephrol. 2015;10:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park M, Hsu C, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park M, Vittinghoff E, Shlipak MG, Mishra R, Whooley M, Bansal N. Associations of N‐terminal pro‐B‐type natriuretic peptide with kidney function decline in persons without clinical heart failure in the Heart and Soul Study. Am Heart J. 2014;168(931–9):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park M, Vittinghoff E, Ganz P, Peralta CA, Whooley M, Shlipak MG. Role of soluble endothelial cell‐selective adhesion molecule biomarker in albuminuria and kidney function changes in patients with coronary artery disease: the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2014;34:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the heart and soul study. J Am Soc Nephrol. 2003;14:3233–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD‐EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD epidemiology collaboration (CKD‐EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, et al. Aptamer‐based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5:e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams SA, Murthy AC, DeLisle RK, Hyde C, Malarstig A, Ostroff R, Weiss SJ, Segal MR, Ganz P. Improving assessment of drug safety through proteomics: early detection and mechanistic characterization of the unforeseen harmful effects of torcetrapib. Circulation. 2017;137:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong G, Staplin N, Emberson J, Baigent C, Turner R, Chalmers J, Zoungas S, Pollock C, Cooper B, Harris D, et al. Chronic kidney disease and the risk of cancer: an individual patient data meta‐analysis of 32,057 participants from six prospective studies. BMC Cancer. 2016;16:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue‐based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 28. Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, et al. Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Mol Cell Proteomics. 2014;13:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erslev AJ, Besarab A. Erythropoietin in the pathogenesis and treatment of the anemia of chronic renal failure. Kidney Int. 1997;51:622–630. [DOI] [PubMed] [Google Scholar]
- 30. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez‐Jimenez F. Association between lipoprotein‐associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159–165. [DOI] [PubMed] [Google Scholar]
- 32. Mei C, Mao Z, Shen X, Wang W, Dai B, Tang B, Wu Y, Cao Y, Zhang S, Zhao H, et al. Role of keratinocyte growth factor in the pathogenesis of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2005;20:2368–2375. [DOI] [PubMed] [Google Scholar]
- 33. Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang X‐F, Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci USA. 2003;100:6098–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. [DOI] [PubMed] [Google Scholar]
- 35. Sato A, Sekine M, Virgona N, Ota M, Yano T. Yes is a central mediator of cell growth in malignant mesothelioma cells. Oncol Rep. 2012;28:1889–1893. [DOI] [PubMed] [Google Scholar]
- 36. Jeon HJ, Choi J‐H, Jung I‐H, Park J‐G, Lee M‐R, Lee M‐N, Kim B, Yoo J‐Y, Jeong S‐J, Kim D‐Y, et al. CD137 (4‐1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. [DOI] [PubMed] [Google Scholar]
- 37. Bae E, Cha R‐H, Kim YC, An JN, Kim DK, Yoo KD, Lee SM, Kim M‐H, Park JT, Kang S‐W, et al. Circulating TNF receptors predict cardiovascular disease in patients with chronic kidney disease. Medicine. 2017;96:e6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carlsson AC, Östgren CJ, Nystrom FH, Länne T, Jennersjö P, Larsson A, Ärnlöv J. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Cardiovasc Diabetol. 2016;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neirynck N, Glorieux G, Schepers E, Verbeke F, Vanholder R. Soluble tumor necrosis factor receptor 1 and 2 predict outcomes in advanced chronic kidney disease: a prospective cohort study. PLoS ONE. 2015;10:e0122073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Safranow K, Dziedziejko V, Rzeuski R, Czyzycka E, Wojtarowicz A, Bińczak‐Kuleta A, Jakubowska K, Olszewska M, Ciechanowicz A, Kornacewicz‐Jach Z, et al. Plasma concentrations of TNF‐alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009;74:386–392. [DOI] [PubMed] [Google Scholar]
- 41. Dennissen FJA, Kholod N, Hermes DJHP, Kemmerling N, Steinbusch HWM, Dantuma NP, van Leeuwen FW. Mutant ubiquitin (UBB+1) associated with neurodegenerative disorders is hydrolyzed by ubiquitin C‐terminal hydrolase L3 (UCH‐L3). FEBS Lett. 2011;585:2568–2574. [DOI] [PubMed] [Google Scholar]
- 42. Herrmann J, Soares SM, Lerman LO, Lerman A. Potential role of the ubiquitin‐proteasome system in atherosclerosis: aspects of a protein quality disease. J Am Coll Cardiol. 2008;51:2003–2010. [DOI] [PubMed] [Google Scholar]
- 43. Herrmann J, Lerman LO, Lerman A. Ubiquitin and ubiquitin‐like proteins in protein regulation. Circ Res. 2007;100:1276–1291. [DOI] [PubMed] [Google Scholar]
- 44. van Leeuwen FW, de Kleijn DP, van den Hurk HH, Neubauer A, Sonnemans MA, Sluijs JA, Köycü S, Ramdjielal RD, Salehi A, Martens GJ, et al. Science. 1998;279:242–247. [DOI] [PubMed] [Google Scholar]
- 45. Ceyhan O, Birsoy K, Hoffman CS. Identification of biologically active PDE11‐selective inhibitors using a yeast‐based high‐throughput screen. Chem Biol. 2012;19:155–163. [DOI] [PubMed] [Google Scholar]
- 46. Makhlouf A, Kshirsagar A, Niederberger C. Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res. 2006;18:501–509. [DOI] [PubMed] [Google Scholar]
- 47. Lorenz K, Rosner MR, Brand T, Schmitt JP. Raf kinase inhibitor protein: lessons of a better way for β‐adrenergic receptor activation in the heart. J Physiol (Lond). 2017;595:4073–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmid E, Neef S, Berlin C, Tomasovic A, Kahlert K, Nordbeck P, Deiss K, Denzinger S, Herrmann S, Wettwer E, et al. Cardiac RKIP induces a beneficial β‐adrenoceptor‐dependent positive inotropy. Nat Med. 2015;21:1298–1306. [DOI] [PubMed] [Google Scholar]
- 49. Shi T, Moravec CS, Perez DM. Novel proteins associated with human dilated cardiomyopathy: selective reduction in α(1A)‐adrenergic receptors and increased desensitization proteins. J Recept Signal Transduct Res. 2013;33:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID‐TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 51. STABILITY Investigators , White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5 Figures S1–S2


