Abstract
Background
Ruxolitinib is a novel oral Janus kinase inhibitor that is used for treatment of myeloproliferative diseases. It exhibits potent anti-inflammatory and immunosuppressive effects, and may increase the risk of opportunistic infections. Here, we report a rare case of Cryptococcus neoformans and Mycobacterium haemophilum coinfection in a myelofibrosis patient who was receiving ruxolitinib.
Case presentation
A 70-year-old Thai man who was diagnosed with JAK2V617F-mutation-positive primary myelofibrosis had been treated with ruxolitinib for 4 years. He presented with cellulitis at his left leg for 1 week. Physical examination revealed fever, dyspnea, desaturation, and sign of inflammation on the left leg and ulcers on the right foot. Blood cultures showed positive for C. neoformans. He was prescribed intravenous amphotericin B deoxycholate with a subsequent switch to liposomal amphotericin B due to the development of acute kidney injury. He developed new onset of fever after 1 month of antifungal treatment, and the lesion on his left leg had worsened. Biopsy of that skin lesion was sent for mycobacterial culture, and the result showed M. haemophilum. He was treated with levofloxacin, ethambutol, and rifampicin; however, the patient eventually developed septic shock and expired.
Conclusions
This is the first case of C. neoformans and M. haemophilum coinfection in a patient receiving ruxolitinib treatment. Although uncommon, clinicians should be aware of the potential for multiple opportunistic infections that may be caused by atypical pathogens in patients receiving ruxolitinib.
Keywords: Ruxolitinib, Myelofibrosis, Cryptococcosis, Cryptococcus, Mycobacterium haemophilum
Background
Ruxolitinib is a novel biologic agent used for the treatment of myeloproliferative diseases, that inhibits Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2). Although it is effective and had a survival benefit in such patients, this immunosuppressive agent may increase the risk of opportunistic infections. However, reports related to its infectious complications is limited. Previous studies reported bacteria, Mycobacterium tuberculosis, Cryptococcus neoformans, Pneumocystis jirovecii, herpes simplex, and varicella zoster virus to be etiologic agents that were isolated from patients receiving ruxolitinib [1]. Furthermore, no previous case has been reported of Mycobacterium haemophilum infection or concurrent infection by two pathogens in a patient receiving ruxolitinib treatment. Here, we report a ruxolitinib-treated patient with rare infectious complication of C. neoformans fungemia who was co-infected with M. haemophilum cellulitis at his left leg.
Case presentation
A 70-year-old Thai man with a 5-year history of primary myelofibrosis, hypertension, pulmonary hypertension due to left-sided heart disease, asthma, and osteoporosis presented with an erythematous swollen left leg for 7 days (Fig. 1). He also complained of fever, dyspnea on exertion, and orthopnea for 6 days. He had multiple ulcers on his right foot for 10 days, and was treated with ceftriaxone for 3 days prior to hospitalization. He received ruxolitinib for treatment of primary myelofibrosis for 4 years. His other medications included warfarin, omeprazole, furosemide, diltiazem, spironolactone, bisoprolol, and seretide evohaler for his pulmonary and heart diseases.
Fig. 1.
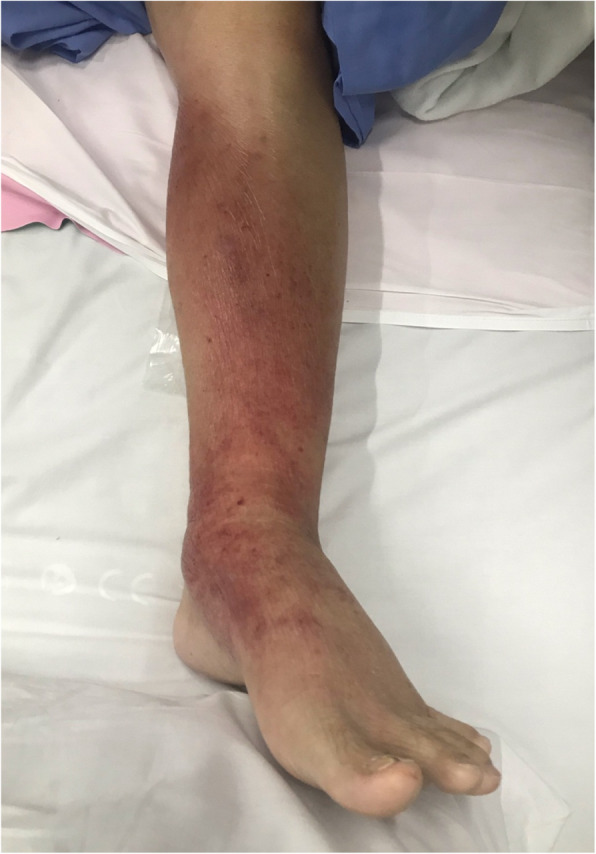
Patient’s left leg showed erythematous lesion with swelling and tenderness
Ruxolitinib was initiated in November 2015 after the patient developed pancytopenia following hydroxyurea and allopurinol treatment. Total duration of ruxolitinib therapy was 48 months, and the most recent dose was 20 mg/day. He denied smoking and alcohol drinking. He reported no exposure to herbal drug use or animals, and he had no known tuberculosis contact.
Physical examination revealed a low-grade fever [temperature (T) 38.3 °C], blood pressure (BP) 117/64 mmHg, pulse 72/min, respiratory rate 24/min, oxygen saturation 85% at room air, and 98% with oxygen cannula 3 l per minute (LPM). He was alert, had mild pallor, and was tachypneic. Cardiovascular examination showed jugular vein distension with apical and parasternal heaving, loud P2, and irregular pulse. Fine crepitations were detected at both lower lung fields. Abdominal examination revealed mild splenomegaly. Ill-defined erythematous swelling of the left leg with mild tenderness, and multiple ulcers at his right foot with minimal pus discharge were noted. Neurological examination and other systems were unremarkable.
Complete blood count (CBC) revealed a white blood cell count (WBC) of 21,130 cells/μL (81% neutrophil, 9% band form, 5% metamyelocyte, and 2% promyelocyte), hemoglobin (Hb) level of 7.2 g/dL, platelet (PLT) count of 536,000 cells/μL, blood urea nitrogen (BUN) of 23.9 mg/dL, and serum creatinine (SCr) of 1.21 mg/dL. Liver function tests showed direct bilirubin (DBIL) of 0.66 mg/dL, total bilirubin (TBIL) of 0.45 mg/ dL, aspartate transaminase (AST) of 16 U/L, alanine transaminase (ALT) of 10 U/L, alkaline phosphatase (ALP) of 84 U/L, albumin (ALB) of 2.8 g/dL, and total protein of 5.4 g/dL. The urinalysis was unremarkable. Chest radiograph (CXR) showed bilateral interstitial infiltration with blunt costophrenic angles and increased cardiothoracic ratio. One of his 2 blood cultures grew round budding yeasts on the fourth day after blood collection, which were identified as C. neoformans (Fig. 2). Serum cryptococcal antigen was positive at a titer of 1:2. Lumbar puncture was attempted; however, no cerebrospinal fluid was collected.
Fig. 2.
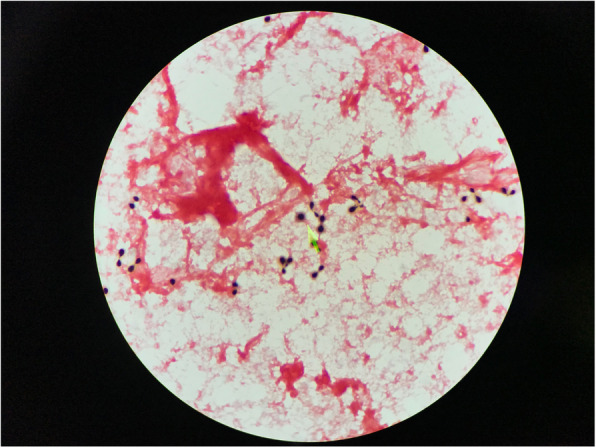
Gram stain from blood culture showed encapsulated budding yeasts
On the admission date, the patient was empirically treated with intravenous ceftazidime and azithromycin. Following the blood culture result, intravenous amphotericin B deoxycholate 50 mg/day (0.87 mg/kg/day) and oral fluconazole 800 mg/day were added. The patient was improved. Subsequently, the patient developed acute kidney injury (AKI) and the antifungal agent was changed to liposomal amphotericin B 180 mg (3 mg/kg/day). The patient received induction therapy with 28 days of intravenous amphotericin B plus fluconazole 800 mg/day, followed by oral fluconazole at the dose of 400 mg/day thereafter. However, ruxolitinib was still continued.
After 1 month of hospitalization and antifungal treatment, the patient developed new onset of fever and worsening of the lesion on his left leg. Intravenous vancomycin was initiated for empirical treatment of suspected nosocomial skin and soft tissue infection. Skin biopsy at the left leg lesion was performed. The tissue pathology showed suppurative granuloma involving dermis and subcutis that suggested mycobacterial skin and soft tissue infection, and Ziehl-Neelsen staining demonstrated numerous acid-fast bacilli (Fig. 3). Tissue mycobacterial culture revealed positive organisms from acid-fast stain, and isolate identification by INNO-LiPA assay technique from liquid medium was compatible with M. haemophilum. The mycobacterium colony grew on chocolate plate agar after 42 days of incubation, but failed to grow on solid (Lowenstein-Jensen) medium.
Fig. 3.
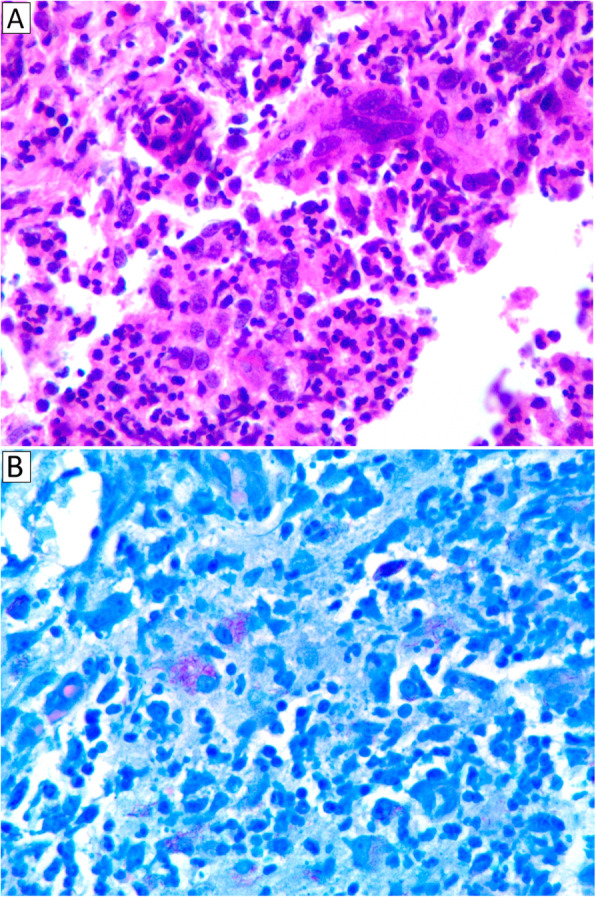
Histopathological findings of skin biopsy from the patient’s left leg revealed suppurative granuloma involving dermis and subcutis (a) with numerous acid-fast bacilli visualized by Ziehl-Neelsen staining method (b)
The patient was treated with levofloxacin 750 mg/day, rifampicin 300 mg/day, and ethambutol 800 mg/day; however, the patient was not improved after 7 days of antimicrobial treatment. He then developed septic shock and expired on the 48th day of hospitalization.
Discussion and conclusions
Abnormal regulation of the Janus kinase (JAK) - signal transducer and activator of transcription (STAT) signaling pathway is the major pathophysiology of myelofibrosis. In addition to chemotherapy and hematopoietic stem cell transplantation, many immunomodulatory agents have recently been approved for treatment of this condition [1]. On the other hand, ruxolitinib adversely affects host immune response [2]. The involved immune response components include both innate and adaptive immunity, such as natural killer cells, dendritic cells, T helper cells, regulatory T cells and several cytokines. Therefore, either intracellular or extracellular pathogens can proliferate in the absence of an effective immune response [3].
We searched the term “ruxolitinib AND infection” and “ruxolitinib AND infectious” in the PUBMED database, and there were 59 results of clinical trials, case reports, and literature reviews. From those results, the most common opportunistic pathogens that benefit from these immune defects include bacteria, mycobacteria, viruses, and fungi, similar to those seen in human immunodeficiency virus (HIV)-related infections. The causative pathogens in previously reported cases of ruxolitinib-associated infections are summarized in Table 1 [1–17]. Consistent with the foregoing, it appears that Cryptococcus and nontuberculous mycobacteria were able to simultaneously infect our patient due to the downregulation of multiple cytokines.
Table 1.
Types of organisms and infections in previously reported cases of ruxolitinib-associated infection [1–17]
| Organisms | Infections | Number of Patients |
|---|---|---|
| Bacteria | Cellulitis/necrotizing fasciitis | 1 |
| Klebsiella pneumoniae liver abscess | 1 | |
| Bronchitis/pneumonia | NA | |
| Urinary tract infection | NA | |
| Osteomyelitis | NA | |
| Sepsis | NA | |
| Mycobacteria | Tuberculosis | 91 |
| NTM | 23 | |
| Virus | HSV/VZV infection | 4 |
| HBV reactivation | 3 | |
| EBV-related disease (CNS lymphoma, gastric ulcer) | 2 | |
| CMV retinitis | 1 | |
| Disseminated molluscum contagiosum | 1 | |
| JC virus (meningitis, granule cell neuronopathy, PML) | 3 | |
| Fungus | Coccidioidomycosis | 8 |
| Cryptococcosis | 6 | |
| Pneumocystis jiroveci pneumonia | 3 | |
| Invasive aspergillosis | 2 | |
| Disseminated Talaromyces marneffei infection | 1 | |
| Rhino-orbital mucormycosis | 1 | |
| Parasite | Toxoplasma retinitis | 1 |
Abbreviations: CMV Cytomegalovirus, CNS Central nervous system, EBV Epstein-Barr virus, HBV Hepatitis B virus, HSV Herpes simplex virus, NA Not available, NTM Nontuberculous mycobacterium, PML Progressive multifocal leukoencephalopathy, VZV Varicella zoster virus
From previous case reports of cryptococcal infection, which are summarized in Table 2 [4–6, 9–11], 6 patients with a history of ruxolitinib treatment developed cryptococcal infection. Wysham NG, et al. [4] and Hirano A, et al. [5] reported 2 cases of pulmonary cryptococcosis, but the other four cases presented as fungemia, pericardial involvement, and meningitis or meningoencephalitis. The duration of ruxolitinib treatment was 5–46 months. One patient had a concurrent infection with Histoplasma capsulatum [6]. Three of six patients received intravenous amphotericin B deoxycholate for induction therapy, and the other antifungal drugs that were used were fluconazole, voriconazole, flucytosine, and isavuconazole. The outcome was favorable in 5 of 6 patients, and one patient deteriorated during treatment and expired. These observations suggest a benefit of discontinuing ruxolitinib to achieve a good outcome in management of ruxolitinib-associated cryptococcal diseases.
Table 2.
Literature review for reported cases of cryptococcal infection in patients receiving ruxolitinib
| Author [Year] | Patient | Gender/Age | Clinical diagnosis | Underlying disease(s) | Ruxolitinib duration | Symptoms | Laboratory findings | Imaging | Organism/site | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wysham N, et al. [2013] [4] | 1 | Male/66 | Pneumonia | Polycythemia vera and myelofibrosis | 18 months | Dyspnea, cough, and intermittent fever |
- Mild leukocytosis - Serum cryptococcal Ag 1:4 |
Multifocal consolidations with surrounding ground glass opacities | Cryptococcus neoformans (BAL) |
- Oral fluconazole (5 months) - Discontinue ruxolitinib |
Survived |
| Chen C, et al. [2016] [10] | 2 | Female/69 | Meningoencephalitis | Myelofibrosis | 46 months | Fever, alteration of consciousness |
- Blood chemistry: NA - CSF cryptococcal Ag 1:512 |
Normal brain imaging |
- Cryptococcus neoformans (Fungal CSF culture) |
- Amphotericin B and high-dose fluconazole | Survived |
| Hirano A, et al. [2017] [5] | 3 | Male/79 | Pulmonary nodules | Primary myelofibrosis | 6 months | Asymptomatic |
- Normal CBC - Serum cryptococcal Ag 1:8 |
Multiple pulmonary nodules | Cryptococcus spp. (TBBx) |
- Oral voriconazole (5 months) - Discontinue ruxolitinib |
Survived |
| Liu J, et al. [2018] [9] | 4 | Male/71 | Pericardial effusion | Chronic myelomonocytic leukemia | NA | Cardiac tamponade |
- Marked leukocytosis - AKI, metabolic acidosis, liver dysfunction |
Bilateral pulmonary infiltrations (CXR) |
Cryptococcus neoformans (Blood and pericardial culture) |
- IV micafungin - IV fluconazole |
Died |
| Prakash K and Richman D [2019] [6] | 5 | Male/51 | Meningitis | Polycythemia vera | 18 months | Fever, lethargy, headache, weight loss, oral ulcer |
- AKI, hyponatremia, elevated ALP - CSF pleocytosis - Serum cryptococcal Ag 1:128 |
- Innumerable rim enhancing lesions at the gray-white junction - Retrocardiac mass |
- Cryptococcus neoformans (Fungal CSF culture) - Histoplasma capsulatum |
- Amphotericin B and flucytosine - Isavuconazole - Discontinue ruxolitinib |
Survived |
| Tsukui D, et al. [2020] [11] | 6 | Male/76 | Meningitis | Myelofibrosis | 5 months | Fever, alteration of consciousness |
- Anemia with normal WBC and platelet - CSF pleocytosis - Serum cryptococcal Ag positive (qualitative) |
Normal brain imaging |
- Cryptococcus neoformans (CSF- quantitative assay titers positive 1:16) |
- Amphotericin B 37 days, then oral fluconazole - Discontinue ruxolitinib |
Survived |
Abbreviations: Ag Antigen, AKI Acute kidney injury, ALP Alkaline phosphatase, BAL Bronchoalveolar lavage, CBC Complete blood count, CSF Cerebrospinal fluid, CXR Chest X-ray, IV Intravenous, NA Not available, TBBx Transbronchial biopsy, WBC White blood cell
Ruxolitinib treatment is also associated with mycobacterial reactivation [1, 2, 7]. Case reports of ruxolitinib-associated mycobacterial infections during 2011–2018 are shown in Table 3 [1, 8, 18, 19]. Anand K, et al. reported 91 cases of M. tuberculosis, and 23 cases of atypical mycobacterial infections [8]. In previous reports, the site of M. tuberculosis infection was identified in 17 cases, with the most common form being disseminated disease, followed by lymphadenitis, pulmonary diseases, and peritonitis [1, 18, 19]. For infections caused by nontuberculous mycobacteria, there were 11 (47.8%) cases of Mycobacterium avium complex, 9 (39.1%) unspecified atypical mycobacterium infections, 2 (8.7%) Mycobacterium kansasii, and 1 (4.3%) Mycobacterium abscessus [8]. Salvator H, et al. reported 1 case of M. abscessus lung infection in a 66-year-old female diagnosed with a myelodysplastic syndrome with blastic transformation who underwent allogeneic hematopoietic stem cell transplantation. The patient received ruxolitinib treatment for severe chronic cutaneous graft-versus-host disease, and she developed dyspnea 8 months later. Mycobacterium abscessus was identified from lung biopsies. She was treated with imipenem, amikacin, and azithromycin, and ruxolitinib was discontinued. Her symptoms were rapidly improved [20]. To our knowledge, there is no previous case report of M. haemophilum infection in a patient treated with ruxolitinib. Here, we report a rare case of primary myelofibrosis treated with ruxolitinib for 4 years who had bloodstream infection with C. neoformans, and concurrent cellulitis at his left leg due to M. haemophilum.
Table 3.
Causative organism in reported cases of mycobacterial infection in patients receiving ruxolitinib [1, 8, 18, 19]
| Organism | Patients |
|---|---|
| Mycobacterium tuberculosis | 91 |
| • Unknown site of infection | 74 (81.3%) |
| • Disseminated tuberculosis | 8 (8.8%) |
| • Tuberculous lymphadenitis | 5 (5.5%) |
| • Pulmonary tuberculosis | 3 (3.3%) |
| • Tuberculous peritonitis | 1 (1.1%) |
| Atypical mycobacterial infections | 23 |
| • Mycobacterium avium complex | 11 (47.8%) |
| • Unspecified atypical mycobacterium infection | 9 (39.1%) |
| • Mycobacterium kansasii | 2 (8.7%) |
| • Mycobacterium abscessus | 1 (4.3%) |
M. haemophilum causes skin infections as well as disseminated infection in immunocompromised individuals. It is classified as a nonchromogen by Runyon group classification. Routine microbiological laboratory may report “no growth”, despite the presence of acid fast bacilli in the stain. This is because the bacterium requires heme or iron supplementation in culture media. There is no standard guideline for management of M. haemophilum infection, but treatment with a combination of macrolides, fluoroquinolones, and rifampicin has been suggested, with the details of treatment determined on a case-by-case basis [21, 22].
In conclusion, this is the first case of dual infection with C. neoformans and M. haemophilum in a patient diagnosed with primary myelofibrosis that was being treated with ruxolitinib. This case highlights the possibility of infection caused by multiple atypical pathogens in immunosuppressed patients, especially those receiving ruxolitinib.
Acknowledgements
The authors gratefully acknowledge the family of the patient profiled in this report for granting us permission to describe his case. We would like to thank Ms. Amiroh Waesamaae for her assistance with manuscript formatting and preparation.
Abbreviations
- ALT
Alanine transaminase
- ALP
Alkaline phosphatase
- AST
Aspartate transaminase
- BP
Blood pressure
- FDA
Food and Drug Administration
- HIV
Human immunodeficiency virus
- IL
Interleukin
- LPM
Liters per minute
- PMF
Primary myelofibrosis
- STAT
Signal transducer and activator of transcription
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- US
United States
Authors’ contributions
NS wrote the draft, and critically revised the manuscript; MC critically revised the manuscript and served as the guarantor; and, PC provided laboratory data and approved the manuscript. All authors read and approved the final manuscript.
Funding
There was no funding source for this article. Dr. Methee Chayakulkeeree and Dr. Piriyaporn Chongtrakool were both supported by a Chalermphrakiat Grant from the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
This article was exempted from institutional review board (IRB) approval because our center does not require IRB approval for single-case reports.
Consent for publication
Written informed consent was obtained from the family of the patient for publication of this case report and any accompanying images. A copy of the signed consent form is available for review by the editor of BMC Infectious Diseases upon request.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dioverti MV, Abu Saleh OM, Tande AJ. Infectious complications in patients on treatment with ruxolitinib: case report and review of the literature. Infect Dis (Lond) 2018;50(5):381–387. doi: 10.1080/23744235.2017.1390248. [DOI] [PubMed] [Google Scholar]
- 2.Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339–347. doi: 10.1002/ajh.24976. [DOI] [PubMed] [Google Scholar]
- 3.Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol. 2019;9:1186. doi: 10.3389/fonc.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest. 2013;143(5):1478–1479. doi: 10.1378/chest.12-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano A, Yamasaki M, Saito N, Iwato K, Daido W, Funaishi K, Ishiyama S, Deguchi N, Taniwaki M, Ohashi N. Pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis. Respir Med Case Rep. 2017;22:87–90. doi: 10.1016/j.rmcr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakash K, Richman D. A case report of disseminated histoplasmosis and concurrent cryptococcal meningitis in a patient treated with ruxolitinib. BMC Infect Dis. 2019;19(1):287. doi: 10.1186/s12879-019-3922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lescuyer S, Ledoux MP, Gravier S, Natarajan-Amé S, Duval C, Maloisel F, Mauvieux L, Toussaint E, Fornecker LM, Herbrecht R. Tuberculosis and atypical mycobacterial infections in ruxolitinib-treated patients with primary or secondary myelofibrosis or polycythemia vera. Int J Infect Dis. 2019;80:134–136. doi: 10.1016/j.ijid.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Anand K, Burns EA, Ensor J, Rice L, Pingali SR. Mycobacterial infections with ruxolitinib: a retrospective pharmacovigilance review. Clin Lymphoma Myeloma Leuk. 2020;20(1):18–23. doi: 10.1016/j.clml.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Mouhayar E, Tarrand JJ, Kontoyiannis DP. Fulminant Cryptococcus neoformans infection with fatal pericardial tamponade in a patient with chronic myelomonocytic leukaemia who was treated with ruxolitinib: case report and review of fungal pericarditis. Mycoses. 2018;61(4):245–255. doi: 10.1111/myc.12735. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Chen YY, Huang CE. Cryptococcal meningoencephalitis associated with the long-term use of ruxolitinib. Ann Hematol. 2016;95(2):361–362. doi: 10.1007/s00277-015-2532-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsukui D, Fujita H, Suzuki K, Hirata K. A case report of cryptococcal meningitis associated with ruxolitinib. Medicine (Baltimore) 2020;99(13):e19587. doi: 10.1097/MD.0000000000019587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadjadian P, Wille K, Griesshammer M. Ruxolitinib-associated infections in polycythemia vera: review of literature, clinical significance, and recommendations. Cancers (Basel) 2020;12(11):e3132. doi: 10.3390/cancers12113132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusne Y, Kimes KE, Feller FF, Patron R, Banacloche JG, Blair JE, Vikram HR, Ampel NM. Coccidioidomycosis in patients treated with ruxolitinib. Open Forum Infect Dis. 2020;7(6):ofaa167. doi: 10.1093/ofid/ofaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moruno-Rodríguez A, Sánchez-Vicente JL, Rueda-Rueda T, Lechón-Caballero B, Muñoz-Morales A, López-Herrero F. Invasive aspergillosis manifesting as retinal necrosis in a patient treated with ruxolitinib. Arch Soc Esp Oftalmol. 2019;94(5):237–241. doi: 10.1016/j.oftal.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Sylvine P, Thomas S, Pirayeh E. Infections associated with ruxolitinib: study in French pharmacovigilance database. Ann Hematol. 2018;97(5):913–914. doi: 10.1007/s00277-018-3242-8. [DOI] [PubMed] [Google Scholar]
- 16.Ballesta B, González H, Martín V, Ballesta JJ. Fatal ruxolitinib-related JC virus meningitis. J Neuro-Oncol. 2017;23(5):783–785. doi: 10.1007/s13365-017-0558-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K, Nakamura M, Konishi A, Kaneko S, Nakamichi K, Saijo M, Yakushiji Y, Kusaka H. JC virus granule cell neuronopathy associated with ruxolitinib: A case report and review of the literature. eNeurologicalSci. 2020;21:100269. doi: 10.1016/j.ensci.2020.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakiyama E, Chinen Y, Tsukamoto T, Takimoto-Shimomura T, Kuwahara-Ota S, Matsumura-Kimoto Y, Shimura Y, Kobayashi T, Horiike S, Kuroda J. Tuberculosis peritonitis during treatment of polycythemia vera with ruxolitinib. Infect Drug Resist. 2020;13:1017–1021. doi: 10.2147/IDR.S249030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepeler MS, Özkurt ZN, Güzel ÖT, Akyürek N. Tuberculosis reactivation related with ruxolitinib in a patient with primary myelofibrosis. J Infect Dev Ctries. 2018;12(10):926–928. doi: 10.3855/jidc.9993. [DOI] [PubMed] [Google Scholar]
- 20.Salvator H, Berti E, Catherinot E, Rivaud E, Chabrol A, Nguyen S, Zemoura L, Cardot E, Tcherakian C, Couderc LJ. Foch Hospital Lung Immune Deficiencies Study Group. Pulmonary alveolar proteinosis and Mycobacterium abscessus lung infection related to ruxolitinib after allogeneic stem cell transplantation. Eur Respir J. 2018;51(5):1701960. doi: 10.1183/13993003.01960-2017. [DOI] [PubMed] [Google Scholar]
- 21.Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, Prins JM, Kuijper EJ. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24(4):701–717. doi: 10.1128/CMR.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nookeu P, Angkasekwinai N, Foongladda S, Phoompoung P. Clinical characteristics and treatment outcomes for patients infected with Mycobacterium haemophilum. Emerg Infect Dis. 2019;25(9):1648–1652. doi: 10.3201/eid2509.190430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


