Abstract
The implantation of bioprosthetic heart valves (BHVs) is increasingly becoming the treatment of choice in patients requiring heart valve replacement surgery. Unlike mechanical heart valves, BHVs are less thrombogenic and exhibit superior hemodynamic properties. However, BHVs are prone to structural valve degeneration (SVD), an unavoidable condition limiting graft durability. Mechanisms underlying SVD are incompletely understood, and early concepts suggesting the purely degenerative nature of this process are now considered oversimplified. Recent studies implicate the host immune response as a major modality of SVD pathogenesis, manifested by a combination of processes phenocopying the long‐term transplant rejection, atherosclerosis, and calcification of native aortic valves. In this review, we summarize and critically analyze relevant studies on (1) SVD triggers and pathogenesis, (2) current approaches to protect BHVs from calcification, (3) obtaining low immunogenic BHV tissue from genetically modified animals, and (4) potential strategies for SVD prevention in the clinical setting.
Keywords: bioprosthesis, calcification, genetically modified animals, immune rejection, inflammation, structural valve degeneration, valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- BHV
bioprosthetic heart valve
- CaP
calcium phosphate
- ECM
extracellular matrix
- MHV
mechanical heart valve
- MMP
matrix metalloproteinase
- NeuGc
N‐glycolylneuraminic acid
- SBVT
subclinical bioprosthetic valve thrombosis
- SVD
structural valve degeneration
- TA
tannic acid
- TGF‐β
transforming growth factor β
- THV
transcatheter heart valve
- VIC
valve interstitial cell
- α‐Gal
galactose‐α‐1,3‐galactose
Valve replacement surgery is the first‐line therapy for patients with valvular heart disease. 1 , 2 In such treatment modality, a dysfunctional native valve is replaced by an artificial one, which can be a mechanical heart valve (MHV) or a bioprosthetic heart valve (BHV). MHVs are made of pyrolytic carbon, various polymers, and metal alloys, whereas BHVs represent valve substitutes that can be of 3 types: (1) chemically stabilized tissues of animal origin (xenografts), (2) valves obtained from cadavers or live donors during heart transplantation (homografts), or (3) patients' own valves (autografts) transplanted from one position to another. Both MHVs and BHVs have their advantages and disadvantages. 1 , 2 , 3 MHVs are durable yet highly thrombogenic, which necessitates life‐long use of anticoagulants. In contrast, BHVs do not require anticoagulant therapy and demonstrate excellent hemodynamic properties similar to those of native valves; nonetheless, their durability is limited because of inevitable structural valve degeneration (SVD), a dangerous condition eventually requiring redo valve replacement, a major surgical intervention. 4 , 5
SVD is an irreversible process manifested by gradual degenerative changes in the prosthesis, such as pannus growth, leaflet fibrosis and calcification, delamination of the connective tissue, and emergence of ruptures and perforations. 4 , 5 Ultimately, this results in a BHV failure, a critical drop in hemodynamic efficacy of the valve attributable to stenosis and regurgitation. 6 Mechanisms underlying SVD are still incompletely understood, and the earlier vision of SVD development as a passive physicochemical phenomenon is now considered oversimplified. Recent studies provided evidence that multiple active processes are involved in SVD pathogenesis, including long‐term immune rejection and atherosclerosis‐like tissue remodeling. 6
More than 200 000 heart valve replacement surgeries are performed annually worldwide, with a predicted increment to 850 000 per year by 2050. 7 For subjects needing heart valve replacement, BHV implantation is increasingly becoming the treatment of choice. 7 Because of the high prevalence of BHVs in surgical practice, there is an increasing need to predict SVD development and develop novel treatment modalities for SVD management. In this article, we will summarize and critically review: (1) in‐depth pathophysiological features of SVD, focusing on both passive degeneration and cell‐mediated valve destruction; (2) current approaches to protect BHVs from calcification; (3) generation of low‐immunogenic biomaterial for BHVs in genetically modified animals; and (4) experimental therapy regimens for SVD prevention.
Types of BHVs
Xenografts
Because biomaterials of animal origin are widely available, xenografts account for most of BHVs. The main sources of BHV xenografts are bovine or porcine pericardia and porcine aortic valves pretreated with fixatives and detergents. 8 Such pretreatment stabilizes xenografts and improves their durability, as well as abates immunogenicity, which would otherwise lead to graft‐versus‐host disease. Most commercially available BHVs are pretreated with glutaraldehyde. 9
Xenografts can be of 2 subtypes: surgically implantable BHVs and transcatheter heart valves (THVs). The former may have several design options, including stented, stentless, and sutureless models. 8 THV implantation is a relatively new approach that emerged in 2002 and, initially recommended exclusively for high‐risk patients, is now also indicated to middle‐ and low‐risk patients. 1 , 2 , 10 THVs consist of a balloon‐expandable or self‐expanding metal stent with leaflets inside. 8 During minimally invasive surgery, such THV is delivered to the heart via a catheter inserted through the femoral or radial artery, or through a small incision between the ribs (transapically), and then unfolds inside the affected native valve or failed BHV. 8
Homografts
Homografts are rarely used in clinical practice; they represent the aortic root and pulmonary trunk excised from cadavers or obtained during heart transplantation. 11 Unlike xenograft BHVs, homografts do not undergo a pretreatment with fixatives; instead, they are subjected to antibiotic disinfection and optional decellularization, followed by freezing or short‐term storage at 4°C until implantation. 11 Akin to xenografts, homografts are prone to SVD over time. 11 Also, homograft implantation is more difficult than that of MHVs or most of xenografts, and their use is limited by the availability of allogeneic material. 11
Autografts
Autografts are patient’s own tissues explanted from one position and implanted to another. Through the Ross procedure, the patient’s pulmonary valve is moved to the aortic position, while homografts or xenografts are then used as the patient’s pulmonary valve. 12 Autograft BHVs are especially beneficial in children with congenital heart disease because of immune compatibility, inherent adaptation to somatic growth, and low risk of thrombosis. 12 In addition, autografts display the best hemodynamic parameters among all BHVs. 12 Despite multiple advantages, the Ross procedure is a difficult surgery with multiple perioperative risks; thus, such interventions are rare and mostly limited to pediatric patients. 12 Nevertheless, autografts represent the only living bioprosthetic valve that is currently available (although tissue‐engineered valves are expected to undergo clinical testing in the next few years), whereas xenografts and homografts do not have a significant amount of living cells and are not capable of regeneration.
Durability of Xenografts and Homografts
The onset of SVD generally occurs 7 to 8 years after BHV implantation, with freedom from SVD rates substantially decreasing 10 to 15 years after surgery. 6 , 13 The durability of xenograft BHVs implanted in the aortic position has been well investigated. 14 On the contrary, THV durability is less well studied because of relative newness of this approach as well as poor health conditions of candidates for such minimally invasive intervention. 8 Although clinical studies report similar durability between THVs and classical BHVs for the aortic position 5 to 10 years after intervention, 15 , 16 , 17 , 18 there is currently a lack of data on the durability of THVs for a longer follow‐up period. 19 , 20
Similar to xenograft BHVs, homografts are also prone to SVD development, thus having a limited lifespan. 11 Several clinical studies compared the durability of xenografts and homografts, demonstrating conflicting results. 21 , 22 , 23 , 24 Although the actuarial freedom from evolving aortic valve dysfunction was 86% for patients implanted with a stentless Medtronic Freestyle xenograft BHV versus 37% for patients with a homograft 8 years postimplantation, 21 no differences were found in the durability between the Perimount stented bovine pericardial BHVs and homografts over a 12‐year period. 22 Xenografts and homografts implanted in the pulmonary position of young (<20 years old) patients displayed similar freedom from SVD rates 5 and 10 years after surgery 23 ; however, xenograft implantation posed a substantially higher risk of SVD development 15 years postoperation. In another study, younger patients (10–20 years old) subjected to homograft pulmonary valve replacement showed 92% freedom from SVD after 5 years, whereas those implanted with xenografts displayed only 53% freedom from SVD. 24
The durability of both xenografts and homografts also depends on host factors. For example, young age of graft recipient is one of the most significant risk factors determining early SVD onset, whereas patients >60 years of age often do not outlive the durability of BHVs because of relatively low life expectancy after valve surgery. 6 Unfortunately, there is a lack of studies comparing age‐related reintervention rates in patients who received the same BHV model and underwent surgery in the similar clinical setting. Other risk factors include arterial hypertension, hyperparathyroidism, diabetes mellitus, end‐stage renal disease, and prosthesis‐patient mismatch. 6
Pathophysiological Features of SVD
Native heart valves are a complex multicomponent system enabling self‐regulation because of valve interstitial cells (VICs) that produce and remodel the extracellular matrix (ECM). 25 They provide a compensatory adaptive response to changing hydrodynamic and biochemical parameters of the body. 25 In the absence of VICs, the lifespan of BHV directly depends on the durability of the chemically cross‐linked ECM. This chapter critically reviews the mechanisms that underlie SVD development.
Calcification
Prosthesis‐Related Dystrophic Calcification
Dystrophic calcification is designated as a purely passive process not regulated by recipient’s cells and determined by precipitation of calcium phosphates (CaPs) on cell debris and fibrous components of BHV. 26 Treatment of porcine aortic VICs by glutaraldehyde resulted in their gradual calcification accompanied by a depletion of calcium ions (Ca2+) from the culture medium. 27 Electron microscopy of treated cells revealed the presence of CaP crystals, mainly associated with the inner surface of the plasma membrane and apoptotic bodies; as calcification progressed, multiple organelles also underwent mineralization. 27 Under physiological conditions, live cells maintain low Ca2+ concentrations because of the calcium‐dependent ATPase that pumps Ca2+ out of the cell through the plasma membrane. As a result of cell death caused by glutaraldehyde, ion pumps cease to function, triggering the influx of Ca2+ into the cell. In porcine VICs treated by glutaraldehyde, intracellular Ca2+ concentrations exceed those in control cells by a million times. 27
Because cell membranes and organelles are rich in organic phosphate, accumulated cellular Ca2+ can concentrate on their surface, binding to acidic phospholipids and calcium‐binding proteins. 26 Cells treated with glutaraldehyde also display high concentrations of inorganic phosphate, possibly deriving as a result of protein degradation, cessation of ATP synthesis in mitochondria, and residual activity of alkaline phosphatase. 27 Ultimately, the influx of Ca2+ into the inorganic phosphate–rich cytosol creates a microenvironment favoring nucleation of CaP crystals in dead cells. Although physiological concentrations of Ca2+ and phosphate in the blood are insufficient for spontaneous precipitation of hydroxyapatite, they are enough to support the growth of newly formed crystal cores. Thus, the contact of a glutaraldehyde‐fixed BHV with the blood leads to the gradual calcification of the graft.
In addition to cell debris, fibrous components of the BHV can also undergo mineralization. Type I collagen is the predominant collagen type in heart valves and pericardia of the human and swine, also playing a role in bone formation. Precipitation and subsequent growth of CaP crystals on collagen fibers begins in spaces in its 3‐dimensional structure, called hole zones. 26 It is suggested that proteoglycans residing near hole zones shield collagen fibers from calcification 26 ; however, proteoglycans cannot be cross‐linked by glutaraldehyde and therefore undergo degradation. 28 Thus, destruction and gradual loss of proteoglycans and glycosaminoglycans, as well as damage to collagen fibers, result in unmasking of calcification‐prone areas that contributes to the mineralization of BHV collagens. 26 Loss of proteoglycans and damage to collagen fibers may be promoted by mechanical stress, oxidative stress, or enzymatic activity in BHV.
Unlike collagen, glutaraldehyde does not stabilize elastin, which does not have sufficiently active amino groups; therefore, BHV elastin is vulnerable to degradation caused by mechanical stress, proteolysis, and subsequent calcification. 26 Inhibiting elastin degradation through administration of matrix metalloproteinase (MMP) inhibitors significantly reduced the calcification of subcutaneous elastin implants in rats. 29 Also in a rat model of subcutaneous implantation of porcine aortic fragments, chemically mediated fragmentation of elastin resulted in a more pronounced tissue calcification compared with untreated samples or those depleted of cells or collagen. 30
Recipient‐Related Dystrophic Calcification
Another BHV calcification modality is recipient‐related calcification, where mineral ions and bioactive factors of the patient provoke or aggravate calcium deposition. For instance, BHVs adsorb Ca2+‐binding proteins from the serum, 31 and calcified regions of explanted BHVs contain a Ca2+‐binding protein osteopontin, whereas it is absent in unaffected prosthesis areas, therefore implicating osteopontin in promoting mineralization. 32 Epidemiological studies indicated that higher CaP product (Ca×inorganic phosphate) in the serum directly correlates with BHV calcification. 33 Furthermore, patients taking calcium supplements or subjects with end‐stage renal disease or hyperparathyroidism are also at risk of early BHV calcification. 6 Presumably, a faster calcium metabolism accompanying a rapid growth partially accounts for the BHV calcification in pediatric patients.
In some patients, BHV dystrophic calcification may be associated with inherited deficiency in proteins inhibiting CaP precipitation, such as fetuin‐A. 34 Fetuin‐A–deficient mice fed with diet rich in calcium, phosphates, and vitamin D3 (calcitriol) displayed severe calcification of blood vessels and heart valves. 34 Lower fetuin‐A serum levels were found in patients with calcification of native valves and also positively correlated with the progression of valvular calcification. 35 In comparison with healthy subjects, patients on dialysis have substantially lower fetuin‐A serum levels and the ability of their serum to inhibit CaP precipitation is significantly impaired. 36
Recipient’s own cells can also contribute to BHV calcification without acquiring an osteoblast‐like phenotype. BHV‐infiltrating macrophages can form calcium deposits by undergoing mineralization through apoptosis. 37 Another hypothesis suggests that dystrophic calcification can be caused by the demise of red blood cells (RBCs) diffusing into BHV leaflets under the influence of blood pressure. In particular, our group repeatedly observed blood‐filled capillary‐like cavities in explanted BHVs, as well as accumulations of RBCs in areas of tissue loosening and delamination (Figure 1). Intraleaflet hemorrhage is a sign of the native valve degeneration and associates with a loss of endothelial integrity and neovascularization. 38 In support of the mentioned hypothesis, hemorrhage areas in native aortic valves are colocalized with the sites of ectopic calcification, and intraleaflet hemorrhage correlates with the progression of calcification. 39 Possibly, accumulation of iron from dying RBCs provokes oxidative stress in the affected valves and thus promotes transition of VICs to the osteoblast phenotype. 38 Such putative mechanism would not work for BHVs, which are devoid of VICs. Nevertheless, destruction of RBCs trapped in the BHV can lead to oxidation‐driven SVD. 40 , 41 In this scenario, oxidized ECM and fragments of RBCs can serve as nucleation sites for calcification.
Figure 1. The importance of red blood cells for structural valve degeneration development.
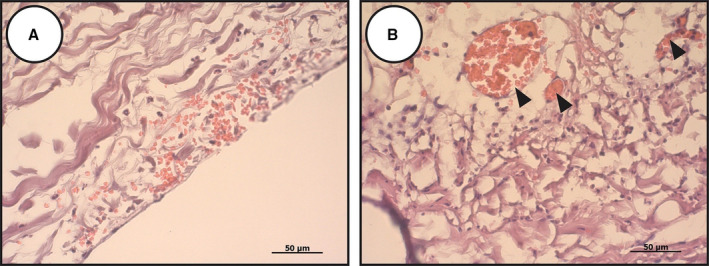
Red blood cells may penetrate the bioprosthetic heart valves through the extracellular matrix disintegration (A) or capillary‐like tubes (B) formed as a result of mechanical stress or chronic inflammation. Red blood cell demise causes iron deposition and further oxidation‐driven degradation of the prosthetic tissue.
To summarize, the proposed mechanisms responsible for dystrophic calcification of the BHV are presented in Figure 2.
Figure 2. Mechanisms driving dystrophic calcification responsible for structural valve degeneration.
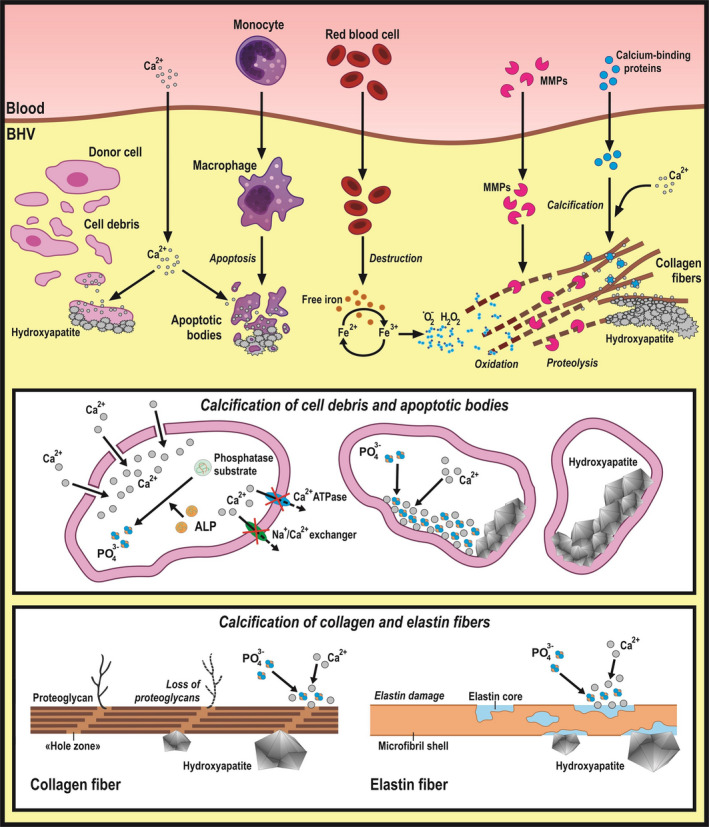
Both implant‐related (residual donor cells and their debris, loss of glycosaminoglycans, and damage of collagen/elastin fibers during chemical treatment and storage) and recipient‐related (immune cell and red blood cell penetration, serum proteolytic enzymes, and calcium‐binding proteins) factors promote dystrophic calcification of bioprosthetic heart valves (BHVs). ALP indicates alkaline phosphatase; and MMP, matrix metalloproteinase.
Mechanical Degeneration
Structural Differences Between Native and Artificial Heart Valves
Heart valves operate in complex hydrodynamic conditions, experiencing significant shear stress, bending deformations, and leaflet tension. However, BHVs are more prone to mechanical degeneration than native valves because of the altered structure of the chemically treated ECM, which is unable to self‐repair.
Native aortic valves and BHVs have major structural differences. Leaflets of the native aortic valve consist of 3 ECM layers: fibrosa, spongiosa, and ventricularis, all having different mechanical properties that enable load damping, have high elasticity, and provide the nonlinear response to stress. 42 Xenopericardial BHVs lack such distinctive layer structure, and, despite some anisotropy, their mechanical characteristics are different from those of the native valve. In addition, xenopericardial BHVs gradually lose glycosaminoglycans and elastin during preservation, chemical treatment, and functioning that adversely affects their mechanical properties. Collagen cross‐linking resulting from glutaraldehyde treatment makes xenografts rigid, thus restricting fiber rearrangements during the cardiac cycle. Strips of glutaraldehyde‐fixed bovine pericardium displayed more pronounced destruction of collagen and elastin fibers during cyclic deformation compared with untreated samples. 43 Finally, xenografts have a reduced ability to absorb strain energy that further increases a mechanical load, accelerating delamination and destruction of collagen fibers.
Biomechanics of tissues surrounding the native valve also plays a major role in its function and load distribution. In stented BHVs, the mechanical interaction between the leaflets and valvular annulus is impaired (Figure 3). Cumulatively, different mechanical properties attributable to a harsh chemical treatment and anatomical differences between the native heart valves and BHVs lead to a stress concentration in leaflets, especially their bending areas, eventually resulting in a mechanical destruction of the graft.
Figure 3. Mechanical stress and structural valve degeneration.
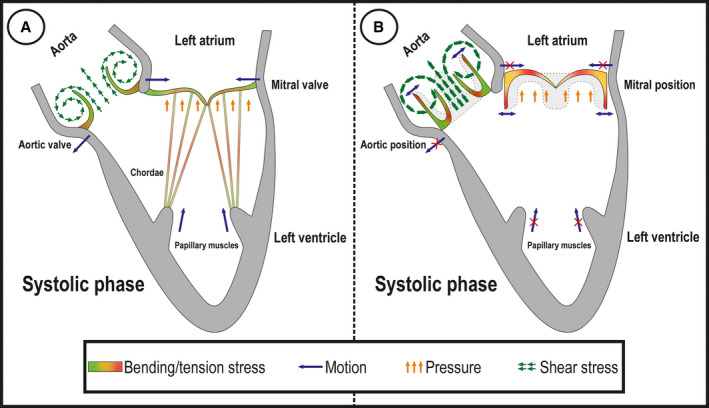
Mechanical load and stress distribution in systolic phase in native mitral and aortic valves (A) and in the case of heart valve implantation into mitral and aortic positions (B).
Relationship Between Mechanical Stress and Calcification
Fatigue failure of BHVs occurs independently of their calcification. 44 Yet, these processes are interrelated because BHV calcification mainly develops in areas of high mechanical stress. This association can be explained by more rapid delamination of fibrous components in calcified areas. Accelerated stress‐driven deterioration of the ECM integrity accompanied by proteolysis can potentially promote the deposition of Ca2+ on damaged collagen and elastin fibers. In turn, leaflet calcification and BHV stenosis affect valve hemodynamics, further promoting mechanical stress.
Newly implanted BHVs enable a physiological blood flow; however, calcification and SVD development lead to several‐fold increase in transvalvular gradient and jet velocity, phenocopying calcific aortic valve disease. This leads to a vicious cycle: an increased rigidity of the leaflets limits their mobility and changes the hydrodynamic characteristics of the prosthetic valve, inflicting additional damage to BHV and further promoting its calcification. The relationship between hemodynamic conditions and calcification of porcine xenografts was well observed during accelerated wear testing in a rapid calcification solution. 45
Inflow valves of HeartMate XVE left ventricular assist device that are exposed to the higher stress values are more frequently and heavily calcified in comparison with outflow valves. 46 Computer‐aided design of the BHV calcification confirmed that calcification is initiated in leaflet margins, where the mechanical stress is maximum across the valve and further extends to the center. 47 To conclude, there is currently a consensus that high mechanical stress applied to the BHVs in the human organism and potentially affected by prosthesis‐patient mismatch promotes their calcification.
Cyclic Loading as a Major Determinant of BHV Mechanical Degeneration
In heart valves, hemodynamic load is of cyclic nature. On average, native valves undergo ≈600 million cycles of opening and closing during 15 years of operation and ≈3 billion cycles during a lifetime. 43 Cyclic stress, derived from the combination of stretching, flexure, and shear, may inflict delamination of the leaflets, leading to calcification and eventually resulting in valve failure.
In glutaraldehyde‐treated BHVs, damage of collagen molecules occurs as early as after 20 million cycles, which is equivalent to 6 months of in vivo valve operation. 48 Because BHVs are not capable of ECM regeneration and remodeling, any changes in the collagen meshwork (eg, delamination, structural rearrangements, and destruction) resulting from cyclic loading are irreversible. In a model of porcine BHV degeneration under prolonged cyclic loading during accelerated wear testing, a marked decrease in radial extensibility was observed because of the stiffening of the effective collagen fiber network. 49 Alternatively, a decrease in xenograft BHV stiffness and its extension under cyclic loading can lead to an excess length of the leaflets and asymmetry, which are characteristic of dysfunctional BHVs and further aggravate mechanical stress and SVD progression. Because collagen is a basic component of BHVs, its fiber structure determines both the strength and the fatigue limit of the biomaterial; however, current manufacturers of commercial BHVs generally do not take into account the fiber geometry in pericardium sheets.
The durability of modern BHVs exceeds current standards for both stented and stentless models; for example, the Carpentier‐Edwards PERIMOUNT Magna Ease prosthesis retains its original hemodynamics even after 1 billion cycles, corresponding to 25 years of operation. 50 Yet, most BHVs show failure signs significantly earlier, in particular in young patients. Thus, SVD development cannot be attributed solely to biomaterial fatigue and mechanical degeneration.
Mechanical Degeneration of THVs
The first THV implantations were performed in 2002 and were initially conducted mainly in high‐risk patients, which limits our understanding of their long‐term dysfunction (albeit transcatheter aortic valve replacement is currently indicated for low‐risk patients as well). In comparison with classic BHVs, THVs display significantly higher stress and fatigue damage on identical loading conditions. 51 Computer simulation indicated that durability of THVs is substantially reduced compared with BHVs to ≈7.8 years. 51 More important, the stress‐strain state of THV leaflets depends on their postimplantation diameter. On complete stent expansion, high stress‐strain areas are limited to commissures in diastole. 52 However, its incomplete expansion by ≥2 mm (≥9.1%) induces the relocation of high stress regions 52 and significantly affects THV durability despite the fact that incomplete stent expansion by 10% to 15% is commonly considered acceptable in clinical practice.
During stent deployment, stress distribution in surrounding tissues and the final shape of THV substantially depend on topography, shape, and maturity of calcium deposits, whereas THV geometry after implantation defines the mechanical durability and affects calcification rate. 53 Despite the fact that experimental data on both factors and mechanisms of SVD in THVs are lacking, thin pericardium from which these prostheses are manufactured and unavoidable need in stent deployment might promote SVD. Dynamic numerical analysis indicated a correlation between reduced THV leaflet thickness and increased stress: the peak stress experienced by the leaflets augmented by 178% in systole and 507% in diastole after reducing their thickness from 0.5 to 0.18 mm. 54 Crimping and balloon expanding of THVs during the implantation might lead to the structural rearrangements in collagen fibers and impair mechanical properties of the prosthetic tissue. 55
However, clinical results of THV implantation are optimistic, showing similar mortality and significantly lower SVD rate (4.8%) compared with surgical aortic valve replacement (24.0%) after 6 years of functioning. 56 Prevalence of severe SVD at 5 years postoperation was 1% to 3% in THV recipients. 57 , 58 , 59 , 60 In other studies, THVs showed similar freedom from SVD after 8 years of follow‐up, yet patient survival was only 27% to 30%. 61 , 62 More important, high‐risk patients receiving THVs exhibit acceptable freedom of severe SVD (97.5%) and moderate to severe SVD (87%) 5 years postoperation. 63 Even redo transcatheter aortic valve replacement surgery is a relatively safe option comparable in this regard to redo surgical aortic valve replacement. 64 , 65 A recent study reported 80% to 90% survival in patients who underwent transcatheter replacement of THVs after 1 year of follow‐up. 66 Among the THVs, self‐expanding and balloon‐expandable prostheses demonstrated similar rates of all‐cause or cardiovascular death, stroke, and repeated hospitalization in their recipients. 67 Yet, moderate to severe SVD was more frequent in those who received balloon‐expandable THVs, presumably because of their worse hemodynamic properties. 67
Because the maximum duration of follow‐up in patients who received THVs is currently limited to 8 to 9 years, further studies are needed to make a clear conclusion on actual SVD rate after a THV implantation. However, it seems to be that in silico and in vitro predictions of higher degeneration rate in THVs have not been proved in clinical studies; vice versa, THVs demonstrate even better results than conventional BHVs, possibly because of the minimally invasive surgery benefits. Increasing rates of THV implantation, which was initially recommended for high‐risk patients, to middle‐ and low‐risk individuals might be useful in determining the actual rate of SVD on THV replacement.
Immune Response to BHV Implantation
Foreign Body Reaction and Pannus Growth
The foreign body reaction is a nonspecific reaction of the innate immunity in response to implantation of a medical device. 68 It is initiated by tissue damage inflicted during the implantation and further enhanced by adsorption of serum proteins on the implant's surface. 68 This triggers the contact activation system as well as fibrinolysis and complement cascades, resulting in adhesion of platelets and activated leukocytes on the implant's surface and ultimately leading to inflammation and thrombosis in the peri‐implant area. 68 Platelets and immune cells can further release various bioactive factors to induce fibroblast‐mediated encapsulation of the foreign body. 68
The implantation of BHVs resembles foreign body reaction in certain aspects. Immune infiltrates, emerging as a result of implantation, lead to fibrovascular tissue outgrowths in areas where recipient’s tissues and the prosthesis contact each other. Moderate tissue outgrowths serve as a nonthrombogenic surface along the seam and improve BHV attachment. However, excessive outgrowth of the ECM, known as pannus, may negatively affect leaflets by limiting their movement, thereby contributing to stenosis and valve dysfunction.
In terms of cellular composition, pannus is populated by endothelial cells, myofibroblasts, and various immune cells, including macrophages, neutrophils, lymphocytes, and foreign‐body giant cells. 69 Detailed mechanisms of pannus formation and the contribution of each cell population to this process have not been well studied; nonetheless, it was reported that pannus‐derived endothelial and immune cells display high expression levels of transforming growth factor β (TGF‐β) and its type 1 receptor. 69 In addition, high expression of TGF‐β type 1 receptor but not TGF‐β was observed in pannus myofibroblasts, suggestive of a TGF‐β–driven intercellular cross talk within the pathologically growing tissue. 69 In agreement, patients with BHV pannus have elevated plasma TGF‐β levels compared with those without pannus growth. 70 TGF‐β pathway is recognized to be in control of tissue regeneration and fibrosis, thus probably playing a key role in the formation of pannus.
Immune Infiltration in BHVs
The role of the immune system in SVD has long been considered with skepticism, because glutaraldehyde fixation was believed to eliminate the immunogenicity of xenografts. Nonetheless, current research suggests that glutaraldehyde treatment is insufficient to completely negate the host immune response. Multiple groups observed leukocyte infiltrates in explanted dysfunctional xenografts and homografts. 37 , 71 , 72 Immune cell populations found in BHVs mostly consist of macrophages, whereas foreign‐body giant cells, foam cells, T‐lymphocytes and B‐lymphocytes, neutrophils, and eosinophils are less frequent. 37 , 71 , 72
In BHVs, immune infiltrates are commonly detected in areas of ECM deterioration with evidence of phagocytosis of matrix fibers and large amounts of macrophage‐derived proteolytic enzymes, such as MMP‐9 and plasminogen. 37 , 72 In combination, the MMP‐dependent proteolysis and the fibrinolytic system can cleave most ECM proteins. Noncalcified BHVs, explanted because of a leaflet rupture, exhibited higher MMP‐9 levels compared with calcified implants or native bovine pericardium. 73 This suggests that MMP‐9 appears in BHVs on the implantation; probable sources include immune cells and plasma. 73 In addition, plasmin is a potent proinflammatory mediator, stimulating the activation and migration of macrophages, as well as enforcing them to produce multiple cytokines and chemokines. 74 Macrophages are known to express almost the entire family of MMPs and several cathepsins (in particular, B, K, L, S, and V), yet there are no studies profiling their expression in BHVs. In addition, macrophages can produce calcium‐binding proteins, such as osteopontin and osteonectin, as well as secrete extracellular vesicles capable of inducing mineralization. 75 Consistently, the expression of osteopontin, osteocalcin, and osteonectin was colocalized with macrophage infiltrates and calcification sites in explanted BHVs. 76 In a clinical setting, steroid therapy of concomitant aortitis in patients undergoing aortic valve replacement correlated with lower degree of BHV degeneration. 77 These data suggest that immune cells and their secretome are involved in SVD, yet detailed mechanisms of their action in this scenario are unknown.
BHV‐infiltrating macrophages and neutrophils can also promote SVD through a generation of reactive oxygen species released by these cells during phagocytosis and inducing oxidative destruction of the prosthesis. In support of this hypothesis, explanted BHVs demonstrated high concentrations of tyrosine oxidation products. 40 , 41 Furthermore, incubation of glutaraldehyde‐treated bovine pericardium under oxidizing conditions led to collagen destruction, loss of glutaraldehyde cross‐links, and increased susceptibility to collagenase degradation. 40
SVD rates are substantially higher in young subjects and especially infants, cohorts of patients characterized by an overactive immune system. In keeping with these observations, homograft BHVs, characterized by poor immunogenicity, exhibit a good performance in subjects <20 years old compared with xenografts. 23 , 24 On the contrary, patients >70 years old most commonly undergo redo valve replacement because of a prosthetic valve endocarditis rather than SVD, a trend perfectly explained by the host immunity factor. 78 Thus, age‐related changes in the immune function can potentially affect SVD development.
To elucidate the role of BHV‐induced immune rejection in the development of SVD, Manji et al transplanted untreated or glutaraldehyde‐fixed ascending aortas/valves from guinea pigs (xenogeneic model) or rats (syngeneic model) into the infrarenal aortas of young rats. 79 In addition, a xenogeneic group was treated with steroids until the graft harvest. 79 Expectedly, rat‐to‐rat transplant demonstrated a weak inflammatory response, albeit it was more pronounced in animals bearing glutaraldehyde‐treated samples. 79 Guinea pig‐to‐rat transplant showed a pronounced inflammatory response, increased serum IgG levels, and significant destruction of transplanted tissues caused by immune cell infiltration. 79 Steroid treatment substantially reduced the inflammatory response, although it was still more severe than in rat‐to‐rat transplant groups. 79 Notably, this study also observed a direct correlation between the intensity of inflammation and the degree of implant calcification. 79
In conclusion, the host immune response may promote SVD onset via multiple mechanisms; however, current evidence mostly comes from observational studies, and the dynamics of immune cell infiltration into BHVs as well as in‐detail mechanisms of their action remain completely unstudied. It is not known whether BHV‐infiltrating immune cells directly degrade the ECM and inflict calcification, or they emerge after these pathological changes have occurred. More research is needed to elucidate the impact of immune response on SVD development.
Triggers of the Immune Response Against Xenografts
Essential components of BHV ECM‐specific glycans, galactose‐α‐1,3‐galactose (α‐Gal) and N‐glycolylneuraminic acid (NeuGc), are highly abundant in most mammals but not humans, who lost their corresponding genes during the evolution. 80 Expectedly, antibodies against these carbohydrates are associated with a rapid xenograft rejection. 80 Together, α‐Gal and NeuGc are the main obstacles for live tissue xenotransplantation to humans. 80
Both α‐Gal and NeuGc are expressed in native porcine heart valves and porcine/bovine pericardia as well as in commercially available BHVs. 81 Glutaraldehyde cannot efficiently cross‐link carbohydrates because of the absence of amino groups and cannot mask α‐Gal and NeuGc, which therefore can trigger immune response against BHVs. Indeed, explanted BHVs demonstrated high levels of IgM, IgG, and C4d complement fragment, suggestive of immune reactivity. 71 Furthermore, the implantation of xenografts markedly elevated anti‐Gal antibody titer in the blood of patients undergoing valve replacement. 82 However, no elevation of anti‐Gal antibody titer was observed in subjects implanted with decellularized and glutaraldehyde‐fixed BHVs. 82 Interestingly, several reports attributed BHV failure to the α‐Gal syndrome, 83 a rare allergy to α‐Gal–containing substances, generally caused by tick bites or consumption of red meat. In addition, human monocytes can recognize α‐Gal–containing epitopes via galectin‐3, suggesting that xenogeneic glycans found in BHVs may also promote the innate immune response. 84
Besides carbohydrates, proteins within BHV can also be immunogenic. Serum from patients implanted with glutaraldehyde‐fixed BHVs exhibited reactivity toward 19 graft proteins, some of which are homologous to human proteins responsible for autoantibody formation in various human diseases. 85 In a different study, patients implanted with BHVs displayed high titers of serum antibodies against porcine albumin and type IV collagen, indicating an immune reaction against the graft. 86
Finally, inflammatory response in BHVs can be triggered by subclinical bioprosthetic valve thrombosis (SBVT). It can potentially provoke pannus formation, inflammatory cell infiltration, and subsequent calcification of BHVs. Deposition of a key thrombogenic molecule fibrinogen on graft leaflets has been well documented 37 ; thus, it may potentially contribute to recurrent SBVT, which, in turn, can trigger chronic inflammation and attract leukocytes to xenograft. In addition, fibrinogen and its degradation products act as proinflammatory factors per se, promoting the migration, adhesion, and activation of immune cells. Thus, SBVT may uphold an immune response in BHVs, potentially contributing to their dysfunction.
Native Valve Calcification, Atherosclerosis, and SVD: Are There Common Mechanisms?
SVD shares some risk factors with atherosclerosis and calcific aortic stenosis, including the metabolic syndrome, diabetes mellitus, smoking, and hyperlipidemia. 6 Therefore, common mechanisms are conceivable for these diseases. Both calcific aortic stenosis and atherosclerosis are characterized by endothelial dysfunction, lipid deposition in the subendothelial layer, and intense lipid‐driven inflammatory reaction, all leading to the activation of resident cells (eg, VICs or smooth muscle cells), and their fibroproliferative response with ultimate tissue mineralization. Dysfunctional BHVs display a considerable lipid deposition and contain foam cells, an atherosclerosis‐specific cell type. 73 More important, these lipid deposits primarily consist of oxidized low‐density lipoprotein (LDL), another characteristic marker of atherosclerosis. 72 Oxidized LDL is recognized for the stimulation of macrophages and foam cells to secrete MMPs. In agreement, immunohistochemistry studies of explanted BHVs revealed that macrophages and foam cells produce MMP‐9, whereas samples without lipid deposition showed no evidence of MMP‐9 expression. 72 LDL can be a source of multiple enzymes, such as lipoprotein‐associated phospholipase A2 and autotaxin, which promote inflammation in calcified native aortic valves 25 and thus may possibly contribute to SVD. Furthermore, oxidized LDL enhances the release of proinflammatory cytokines by immune cells that can be relevant for immunity‐driven SVD.
Clinical studies documented the relationship between SVD and impaired lipid metabolism. High risk of SVD was associated with an increased ratio of LDL/high‐density lipoprotein, which reflects the balance between proatherogenic and antiatherogenic lipoproteins. 87 Along similar lines, dysregulated hemodynamic parameters of BHVs correlated with insulin resistance, increased lipoprotein‐associated phospholipase A2 activity, and higher levels of subtilisin‐kexin type 9 proprotein convertase. 88
The mechanism behind LDL deposition and oxidation in BHVs is poorly understood. It is possible that LDL may be trapped by glycosaminoglycans, 72 thus phenocopying dysfunctional native aortic valves and atherosclerotic lesions. Oxidation of LDL is likely to be mediated by infiltrating neutrophils and macrophages, which contribute to release of reactive oxygen species into the BHV microenvironment.
The putative inflammatory and atherogenic phenocopies contributing to SVD onset are presented in Figure 4.
Figure 4. The molecular basis of chronic inflammation in relation to structural valve degeneration.
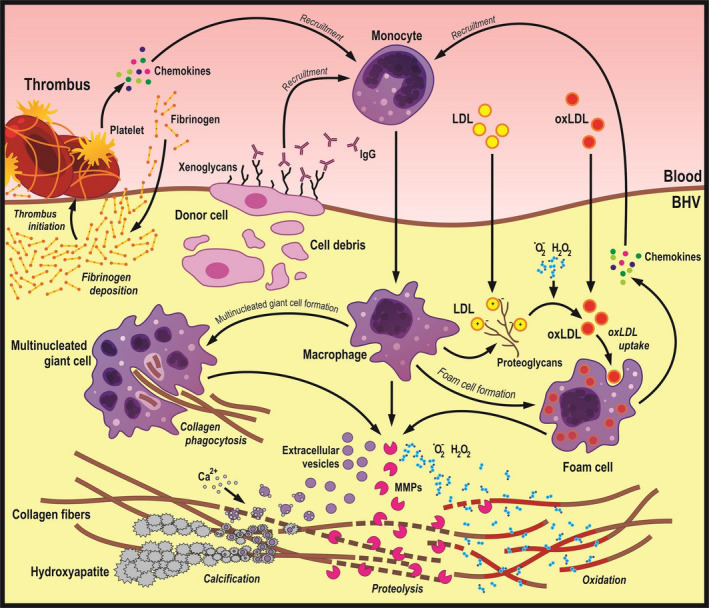
Xenogeneic glycans and thrombi adhered to bioprosthetic heart valves (BHVs) recruit monocytes, which can penetrate the tissue with the subsequent differentiation into macrophages and multinucleated giant cells. Immune cells internalize disintegrated fragments of collagen fibers and release reactive oxygen species, proteolytic enzymes, and calcium‐binding extracellular vesicles, altogether promoting degradation and calcification of the extracellular matrix. In addition, immune cells produce proteoglycans, which bind low‐density lipoprotein (LDL) from the plasma. Macrophages engulfing LDL are then transformed into the foam cells reminiscent of the pathophysiological scenarios observed in atherosclerotic plaques and calcific aortic valve disease. MMP indicates matrix metalloproteinase; and oxLDL, oxidized LDL.
Strategies for SVD Prevention
Anticalcification Treatment of Glutaraldehyde‐Treated Xenografts
As described above, the major disadvantage of glutaraldehyde treatment is gradual xenograft calcification. Its exact mechanism is unknown, yet possible explanations include the toxic effect of unstable glutaraldehyde polymers persisting in the interstices of cross‐linked tissues, negative surface charge of glutaraldehyde‐treated grafts attracting positively charged Ca2+ ions, and binding of host plasma Ca2+ to glutaraldehyde aldehyde groups.
Multiple studies attempted to diminish graft calcification by modifying the protocol of glutaraldehyde fixation. In a circulatory sheep model, glutaraldehyde detoxification by urazole combined with diamine extension of glutaraldehyde cross‐links led to a mitigation of leaflet calcification. 89 Masking of free aldehyde groups through pretreatment of the Perimount mitral valve with a proprietary compound showed a significantly reduced Ca2+ content in comparison with the control group in a juvenile sheep model of orthotopic valve implantation. 90
Treatment by 2‐amino oleic acid reduced the diffusion of Ca2+ by 16.5‐fold in glutaraldehyde‐fixed porcine BHVs implanted into sheep. 91 In rabbits intramuscularly implanted with porcine or bovine BHVs, 2‐amino oleic acid pretreatment resulted in 2‐fold decrease in tissue mineralization. 92 Incubation of glutaraldehyde‐fixed porcine valve leaflets in ethanol showed a significant decrease in calcification on subcutaneous implantation into rats and sheep that can be explained by depletion of calcification‐promoting phospholipids. 93
Alternative Fixation Regimens
The use of glutaraldehyde as a fixative has undeniable advantages, such as high chemical reaction rate, water solubility, superior cross‐linking properties, and high cost‐effectiveness. Yet, several alternative fixation regimens demonstrated substantially slower rates of graft calcification, posing as candidates for commercial use (Figure 5).
Figure 5. Chemical modifications of collagen induced by various fixatives.
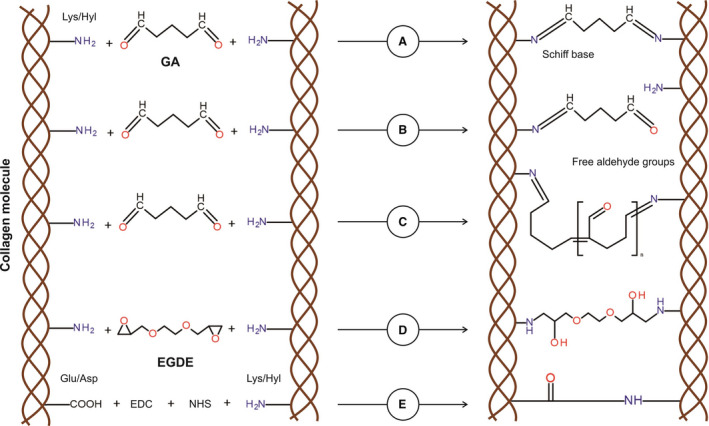
A, Aldehyde groups of glutaraldehyde (GA) interact with amino group of lysine (Lys) or hydroxylysine (Hyl) residues within the collagen, thereby forming a stable chemical bond (Schiff base) for a stable cross‐linking. B, One of GA aldehyde groups interacts with a collagen amino group, resulting in a cross‐linking, whereas the second aldehyde group remains free to other chemical interactions, including calcium binding. C, Polymerization of GA is performed through aldol condensation. Despite collagen molecules that are cross‐linked, free aldehyde groups still remain. D, All epoxy groups of ethylene glycol diglycidyl ether (EGDE) interact with amino group of Lys or Hyl residues within the collagen, forming a stable covalent bond for a stable cross‐linking. E, Collagen fixation with 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide (EDC) and N‐hydroxysuccinimide (NHS) is conducted via the activation of carboxyl groups of aspartic acid/glutamic acid residues in the peptide chain and through the formation of intermediate compound, which is able to interact with free amino groups of lysine or hydroxylysine.
Epoxy compounds were proposed as an alternative to glutaraldehyde several decades ago. Among them are ethylene glycol diglycidyl ether, having chain length and conformation similar to glutaraldehyde, 1,4‐butanediol diglycidyl ether, polyepoxy compounds, and triglycidylamine. Structurally, diepoxides have ≥2 functional groups capable of forming stable covalent bonds with amino groups of collagen. The benefits of epoxy fixatives include low toxicity, high solubility, and their ability to additionally bind hydroxyl and carboxyl groups of collagen. Subcutaneous implantation of polyepoxy‐treated bovine pericardia into rabbits resulted in a 1.5‐fold reduced calcification compared with glutaraldehyde‐fixed samples, 94 whereas no significant differences have been revealed about the calcification of porcine aortic valves treated with glutaraldehyde or 1,4‐butanediol diglycidyl ether. 95 Currently, xenografts fixed by ethylene glycol diglycidyl ether are commercially available in Russia.
Decellularization of the porcine pericardium followed by incubation in methacrylic anhydride is able to introduce methacryloyl groups that are subsequently cross‐linked by radical polymerization. 96 When implanted subcutaneously into rats, such grafts demonstrated a 10‐fold decrease in Ca2+ content compared with glutaraldehyde‐fixed samples. 96 However, this method is limited by its complexity and the use of inorganic catalysts requiring thorough sample washing because of a high risk of sample contamination by reaction by‐products.
Genipin is a natural heterocyclic compound binding the free amino groups of lysine, hydroxylysine, and arginine, and further radically polymerizing to form intermolecular and intramolecular cross‐links. Intramuscular implantation in rabbits showed that genipin‐fixed bovine pericardia had 30% less CaP amount than those fixed by glutaraldehyde. 97 Also, subcutaneously implanted genipin‐fixed pericardia exhibited a weaker inflammatory reaction than glutaraldehyde‐treated samples. 98 However, genipin is difficult and expensive to source, thus limiting its applicability in the clinic.
A polyphenol tannic acid (TA) is another compound capable of binding elastin. Glutaraldehyde‐fixed bovine pericardia preincubated in TA displayed a markedly diminished calcification in vivo. 99 Samples treated with both glutaraldehyde and TA demonstrated fewer infiltrating macrophages and reduced levels of MMP‐9 compared with the samples fixed with glutaraldehyde alone. 99 In addition, TA‐treated porcine pericardia displayed resistance to enzymatic degradation in vitro. 100 Last, combined treatment of porcine pericardium with glutaraldehyde, TA, and ferric chloride reduced the amount of calcium by 4‐fold compared with glutaraldehyde fixation alone. 101
Another experimental tissue fixative is 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide, a compound stabilizing the ECM without linkers through forming an adduct O‐acylisourea with carboxyl groups of glutamic and aspartic acids, followed by a nucleophilic substitution of amino groups of lysine or hydroxylysine. To increase the number of cross‐links, 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide can be additionally supplemented with the affinity reagent N‐hydroxysuccinimide. 1‐Ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide–fixed tissues display low toxicity and retain native softness. A combined treatment of bovine pericardium by 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide, N‐hydroxysuccinimide, neomycin trisulfate, and pentagalloyl glucose resulted in superior mechanical characteristics of the graft as well as resistance to enzymatic cleavage in vitro and calcification in vivo. 9 In this regimen, an essential TA component, pentagalloyl glucose, acts as elastin cross‐linker, whereas neomycin binds to glycosaminoglycans, thus inhibiting their enzymatic degradation. 102
Decellularization of Xenografts and Homografts
Decellularization of BHVs before implantation can be a viable method for SVD prevention, because degeneration of xenografts is accelerated by residual donor cells and cell debris (see Prosthesis‐related dystrophic calcification and Triggers of the immune response against xenografts). Tissue decellularization can be achieved through physical or chemical treatments, whereby cell and organelle membranes are mechanically destroyed or lysed. 103 For efficient decellularization, it is imperative to ensure the preservation of ECM 3‐dimensional architecture, which ultimately determines graft biomechanical properties and its resistance to biodegradation.
Physical methods of decellularization include heat, ultrasound, and high pressure, being especially effective when coupled with chemical treatment. 103 The most commonly used decellularization chemicals are detergents, which lyse the cell membrane and separate DNA from proteins. Ionic detergents, such as SDS, enable better decellularization than nonionic counterparts (eg, Triton X‐100), yet SDS treatment is more aggressive toward the ECM. 103 In a rat subcutaneous model, glutaraldehyde‐fixed bovine pericardial samples preincubated with SDS showed a substantial decrease in Ca2+ up to 90 days compared with samples treated with glutaraldehyde only. 104 In the experimental setting, tissue engineering techniques can be used to repopulate decellularized matrices with host cells to improve their function. 105
Decellularization can serve as a tool to eliminate xenogeneic antigens. Heuschkel et al used SDS to remove α‐Gal from the bovine pericardium while preserving native ECM structure and its mechanical properties. 106 The combined treatment of bovine pericardium with freeze‐thaw cycles, Triton X‐100, and sodium deoxycholate (an alternative to SDS) completely depleted α‐Gal and resulted in a reduced immune response and calcification on its subcutaneous implantation in rats. 107 Yet, the observed differences in the immune response cannot be attributable exclusively to α‐Gal removal because it is widely expressed in rat tissues. Another group used a model of subcutaneous implantation into α‐Gal–deficient mice to demonstrate that glutaraldehyde‐fixed pericardia that were either decellularized or decellularized and α‐Gal treated are less prone to calcification than those treated with glutaraldehyde alone. 108
Despite the advantages of decellularization, most commercial xenograft manufacturing protocols omit this technique, probably because of the lack of consensus on standardized procedures to preserve the intact ECM. Unlike xenografts, decellularization is frequently used for homograft implantation. In patients with right ventricular outflow tract reconstruction, freedom from conduit dysfunction at 10 years postimplantation was 83% for those implanted with decellularized pulmonary homografts, yet only 58% for those whose homografts were cryopreserved. 109 In addition, decellularization using SDS in the presence of protease inhibitors was shown to reduce the immune response toward homografts. As such, implantation of cryopreserved homografts during the Ross procedure led to elevated anti–human leukocyte antigen class I and II antibody titers that were not observed in subjects implanted with decellularized homografts. 110
Genetically Modified Animals as a Source of Low Immunogenic Material for BHVs
Another SVD management strategy is to manufacture xenografts from pericardia of genetically modified animals lacking certain immunogenic molecules (eg, strains of pigs and cows with a knockout of α‐Gal [GGTA1 −/−] and NeuGc [CMAH −/−] genes and overexpression of several human proteins inhibiting both innate and adaptive immune response). 80 The depletion of xenogeneic carbohydrates may significantly improve the immune tolerance toward grafts manufactured from such animal tissues, yet overexpression of human immunosuppressive ligands is to a large extent negated by glutaraldehyde cross‐linking of grafts.
Heart transplantation from wild‐type pigs to baboons results in short‐term rejection within several hours. However, hearts transplanted from GGTA1 −/− pigs to baboons displayed normal organ functioning for up to several days and even months when administered with immunosuppressive drugs. 111 Accordingly, xenografts produced from GGTA1 −/− pigs demonstrated significantly weaker immunogenicity compared with those manufactured from wild‐type pigs in a nonhuman primate model. 112
Regardless of glutaraldehyde fixation, heart valves and pericardia from wild‐type pigs as well as commercially available porcine BHVs actively bound IgM/IgG after incubation in human serum in vitro. 81 However, biomaterials derived from GGTA1 −/− CMAH −/− hCD46 +/+ pigs had almost no affinity to human serum antibodies comparable to that of the human heart valves. 81 In agreement, human serum IgM/IgG minimally binds to the pericardium of GGTA1 −/− CMAH −/− B4GALNT −/− pigs additionally deficient of β‐1,4‐N‐acetylgalactosaminyl transferase, 113 to which many people develop antibodies.
More important, pericardia of GGTA1 −/− and wild‐type pigs have identical collagen content, morphological features, and tensile strength. 114 Recently, preliminary in vitro tests of the first experimental BHVs produced from GGTA1 −/− pericardium demonstrated excellent hemodynamics and durability after 200 million cycles. 115 Thus, α‐Gal– and NeuGc‐deficient animals may become a valuable source of xenograft biomaterial in the future if their clinical benefit would be proved. However, such xenografts are unlikely to be widely available because of the high costs of genetic engineering in large animals.
Experimental Therapy for SVD Prevention
Currently, there is no clinically approved treatment to manage SVD. Some authors suggested that statins could be beneficial for patients with SVD because of commonality of certain risk factors between this condition and atherosclerosis. Consistent with this hypothesis, a few retrospective studies demonstrated that statin‐treated patients had lower rates of SVD progression compared with the control group. 116 Similarly, statin administration reduces CRP (C‐reactive protein) concentrations in valve tissues and serum of patients with BHVs and calcific aortic stenosis, indicating anti‐inflammatory effect of this therapeutic regimen. 117 However, the results of the by far largest observational study, including data on 1193 subjects, could not find any benefit of lipid‐lowering therapy in slowing the degeneration of BHVs implanted in the aortic position 1, 5, and 10 years after surgery. 118 On the basis of the existing evidence, it is impossible to draw a conclusion about the effect of statins on SVD progression because of a limited number of studies. Also, statins generally have not demonstrated efficacy in the treatment of calcific aortic valve disease. 25
Because SBVT is implicated as a culprit of inflammatory response and subsequent calcification of BHVs, another possible way to control SVD could be an anticoagulant therapy. Current American Heart Association/American College of Cardiology and European Society of Cardiology guidelines do not recommend long‐term (>3 months) anticoagulant intake for subjects implanted with BHVs unless otherwise indicated. 1 , 2 In addition, even if the link between SBVT and SVD is proved, the administration of anticoagulants will deprive BHVs of their main advantage over MHVs; thus, this strategy can hardly be considered justified.
Several in vivo experiments 81 and limited clinical studies 77 indicate the potential usefulness of immunosuppressive therapy to improve BHV durability. However, because of multiple adverse effects, this strategy is unacceptable for most patients with BHVs, and its effectiveness in inhibiting the development of SVD requires clinical examination. Given the association between SVD with hypertension, hyperparathyroidism, and diabetes mellitus, therapies aimed at improving these conditions may also help in reducing SVD rates.
Finally, MMP inhibitors could diminish BHV deterioration by host enzymes, thus potentially retaining the xenograft integrity. However, currently there are no effective highly specific MMP inhibitors that would not have serious adverse effects and could be administered orally or by injection. Possibly, in the future, MMP inhibitors can be sewn into the BHVs to inhibit their proteolytic degradation.
Conclusions
SVD is a complex multifactorial process implemented through several interrelated mechanisms, both passive and active. Passive deterioration of xenografts and homografts is inevitable because of the lack of live resident cells capable of maintaining the valvular homeostasis and repairing damaged ECM within the graft. The major driving force of such passive deterioration is graft fatigue, resulting from persistent damage to its ECM under the influence of cyclic loads and in some cases accelerated by a prosthesis‐patient mismatch, a modifiable yet underestimated risk factor. 119 , 120 , 121 Another passive mechanism is dystrophic calcification emerging from CaP precipitation on a graft surface, which is caused by abundance of potential nucleation foci, such as fragmented fibers or cell debris.
Tackling the fatigue‐driven passive graft deterioration is no easy task. Compared with first‐generation models, modern commercially available BHVs have substantially longer service life because of decades of optimization of their design and anticalcification treatment regimens deactivating free aldehyde groups emerging as a result of glutaraldehyde fixation. Nonetheless, the possibilities of these approaches are limited and by now have been almost exhausted. Novel alternative approaches for xenograft fixation are either poorly studied or overly complicated and/or expensive for application in the clinical setting. Therefore, further research should focus on targeting host‐mediated mechanisms of SVD development.
Active mechanisms of BHV deterioration are mediated by recipient cells. As evidenced by multiple studies, both xenografts and homografts are capable of provoking both humoral and cellular immune responses in most patients. BHVs are actively infiltrated by immune cells producing proteolytic enzymes, calcium‐binding proteins, and reactive oxygen species. More important, chemical cross‐linking of BHVs does not provide a complete resistance to proteolytic cleavage and oxidative degradation. Under certain conditions, the inflammatory reaction within the BHV may acquire atherosclerosis‐like phenotype, whereby deposition and subsequent oxidation of LDLs may subsequently result in graft dysfunction. In addition, BHV tissues can induce the production of human antibodies against α‐Gal and NeuGc, which may further enhance immune cell recruitment and associated calcification.
Apparently, both active and passive mechanisms of SVD occur simultaneously and may reinforce each other in a feed‐forward manner. For example, mechanical stress induces the proteolytic cleavage and delamination of graft ECM, thereby facilitating the infiltration of immune cells, which further deteriorate the ECM and create areas susceptible to mechanical destruction. It is unclear, however, to which extent the active immune response toward BHVs contributes to SVD progression. Perhaps, it can vary greatly from one patient to another. Some case reports documented early BHV dysfunction caused by aggressive immune cell infiltration into the graft and accompanying ECM degradation, indicating that in certain subjects the active host‐mediated processes can be the dominant cause of SVD. This especially applies to children and young patients whose immune system is hyperactive. In some subjects, an extremely rapid SVD development was associated with an allergic response to BHV components. It is clear that in such individuals controlling the immune response can significantly alleviate SVD. Reducing the immunogenicity of homografts is another important problem; to this end, decellularization of homografts before implantation demonstrated excellent long‐term results in comparison with their cryopreserved counterparts.
Despite solid evidence on the role of the immune system in SVD development, it has brought little therapeutic benefit to patients with dysfunctional BHVs. In this regard, decellularization of graft materials and developing the new strains of genetically modified animals seem to be promising directions for the production of low immunogenic material for BHV manufacture. Nevertheless, both methods have limitations. The major caveat of decellularization techniques is potential emergence of structural rearrangements within the graft ECM, leading to reduction of its tensile strength, whereas the cost of BHVs made from genetically modified animal tissue is extremely high. In addition, BHVs made from low immunogenic biomaterial have not yet been used in surgical practice, and therefore their real clinical benefit is unknown. Potentially, such novel immunologically inert BHVs could be more durable, especially in children and young subjects. This hypothesis is indirectly confirmed by the fact that these cohorts of patients exhibit longer functioning of less immunogenic homografts compared with relatively more immunogenic xenografts.
As of today, there is no Food and Drug Administration–approved therapy to control SVD. Conflicting evidence on the effectiveness of statins to treat SVD may be explained by multifactorial nature of this condition. Potentially, lipid‐lowering therapy may only be effective in patients whose SVD is mainly driven by inflammatory mechanisms. Other approaches, such as systemic immunosuppression or anticoagulant therapy, are not feasible options because of adverse effects and annulling the main advantage of BHVs over MHVs.
Another point that may be important in evaluating the treatment outcomes is that SVD is a process rather than an event and ideally should be measured repeatedly over time, albeit in certain cases even serial echocardiography fails to provide a reliable snapshot of SVD. Hence, temporal patterns of SVD development, including the rate of its progression, might be taken into account when assessing the clinical efficacy of the respective therapy. In addition, consideration of the redo surgery as the only clinical definition of SVD results in a bias because some patients with SVD are not eligible for the reintervention. Therefore, a need to perform a repeated heart valve replacement surgery (which may also depend on the comorbid conditions of the patient), but not reintervention itself, should be more frequently used as an SVD definition in clinical studies.
Given the rising demand for BHV implantation worldwide, an increase in the service life of valve prostheses by an average of 3 to 5 years will have a tremendous clinical impact. SVD prevention strategies discussed in this review may be well used in the foreseeable future to improve the durability of BHVs (Figure 6). However, it is unlikely that they will eliminate SVD completely. For the breakthrough in SVD management, conceptually new approaches, such as the use of tissue‐engineered valves repopulated in vitro with host cells, are required.
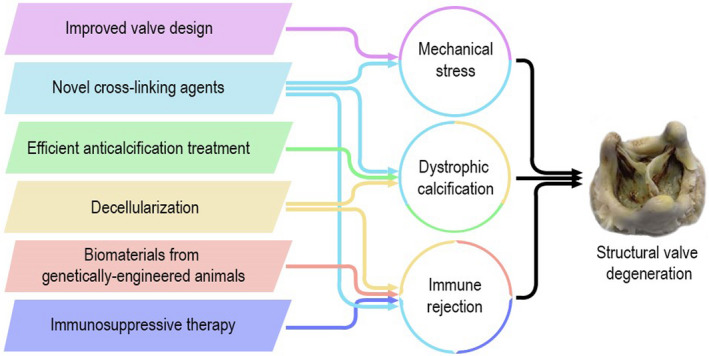
Figure 6. Key factors of structural valve degeneration (SVD) development and strategies to retard SVD.
Sources of Funding
This study was supported by the Complex Program of Basic Research under the Siberian Branch of the Russian Academy of Sciences within the Basic Research Topic of Research Institute for Complex Issues of Cardiovascular Diseases No. 0546‐2019‐0002 “Pathogenetic basis for the development of cardiovascular implants from biocompatible materials using patient‐oriented approach, mathematical modeling, tissue engineering, and genomic predictors.”
Disclosures
None.
(J Am Heart Assoc. 2020;9:e018506 DOI: 10.1161/JAHA.120.018506.)
For Sources of Funding and Disclosures, see page 16.
References
- 1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 3. Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183–2191. [DOI] [PubMed] [Google Scholar]
- 4. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, et al. Standardized definitions of structural deterioration and valve failure in assessing long‐term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017;38:3382–3390. [DOI] [PubMed] [Google Scholar]
- 5. Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388–399. [DOI] [PubMed] [Google Scholar]
- 6. Cote N, Pibarot P, Clavel MA. Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr Opin Cardiol. 2017;32:123–129. [DOI] [PubMed] [Google Scholar]
- 7. Bax JJ, Delgado V. Bioprosthetic heart valves, thrombosis, anticoagulation, and imaging surveillance. JACC Cardiovasc Interv. 2017;10:388–390. [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez‐Gabella T, Voisine P, Puri R, Pibarot P, Rodés‐Cabau J. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol. 2017;70:1013–1028. [DOI] [PubMed] [Google Scholar]
- 9. Tam H, Zhang W, Infante D, Parchment N, Sacks M, Vyavahare N. Fixation of bovine pericardium‐based tissue biomaterial with irreversible chemistry improves biochemical and biomechanical properties. J Cardiovasc Transl Res. 2017;10:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolte D, Vlahakes GJ, Palacios IF, Sakhuja R, Passeri JJ, Inglessis I, Elmariah S. Transcatheter versus surgical aortic valve replacement in low‐risk patients. J Am Coll Cardiol. 2019;74:1532–1540. [DOI] [PubMed] [Google Scholar]
- 11. Lisy M, Kalender G, Schenke‐Layland K, Brockbank KG, Biermann A, Stock UA. Allograft heart valves: current aspects and future applications. Biopreserv Biobank. 2017;15:148–157. [DOI] [PubMed] [Google Scholar]
- 12. Mazine A, El‐Hamamsy I, Verma S, Peterson MD, Bonow RO, Yacoub MH, David TE, Bhatt DL. Ross procedure in adults for cardiologists and cardiac surgeons: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:2761–2777. [DOI] [PubMed] [Google Scholar]
- 13. Bourguignon T, Espitalier F, Pantaleon C, Vermes E, El‐Arid JM, Loardi C, Karam E, Candolfi P, Ivanes F, Aupart M. Bioprosthetic mitral valve replacement in patients aged 65 years or younger: long‐term outcomes with the Carpentier‐Edwards PERIMOUNT pericardial valve. Eur J Cardiothorac Surg. 2018;54:302–309. [DOI] [PubMed] [Google Scholar]
- 14. Fatima B, Mohananey D, Khan FW, Jobanputra Y, Tummala R, Banerjee K, Krishnaswamy A, Mick S, Tuzcu EM, Blackstone E, et al. Durability data for bioprosthetic surgical aortic valve: a systematic review. JAMA Cardiol. 2019;4:71–80. [DOI] [PubMed] [Google Scholar]
- 15. Daubert MA, Weissman NJ, Hahn RT, Pibarot P, Parvataneni R, Mack MJ, Svensson LG, Gopal D, Kapadia S, Siegel RJ, et al. Long‐term valve performance of TAVR and SAVR: a report from the PARTNER I Trial. JACC Cardiovasc Imaging. 2017;10:15–25. [DOI] [PubMed] [Google Scholar]
- 16. Douglas PS, Leon MB, Mack MJ, Svensson LG, Webb JG, Hahn RT, Pibarot P, Weissman NJ, Miller DC, Kapadia S, et al. Longitudinal hemodynamics of transcatheter and surgical aortic valves in the PARTNER Trial. JAMA Cardiol. 2017;2:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackman DJ, Saraf S, MacCarthy PA, Myat A, Anderson SG, Malkin CJ, Cunnington MS, Somers K, Brennan P, Manoharan G, et al. Long‐term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. 2019;73:537–545. [DOI] [PubMed] [Google Scholar]
- 18. Durand E, Sokoloff A, Urena‐Alcazar M, Chevalier B, Chassaing S, Didier R, Tron C, Litzler PY, Bouleti C, Himbert D, et al. Assessment of long‐term structural deterioration of transcatheter aortic bioprosthetic valves using the new European definition. Circ Cardiovasc Interv. 2019;12:e007597. [DOI] [PubMed] [Google Scholar]
- 19. Foroutan F, Guyatt GH, Otto CM, Siemieniuk RA, Schandelmaier S, Agoritsas T, Vandvik PO, Bhagra S, Bagur R. Structural valve deterioration after transcatheter aortic valve implantation. Heart. 2017;103:1899–1905. [DOI] [PubMed] [Google Scholar]
- 20. Sawaya F, Jørgensen TH, Søndergaard L, De Backer O. Transcatheter bioprosthetic aortic valve dysfunction: what we know so far. Front Cardiovasc Med. 2019;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El‐Hamamsy I, Clark L, Stevens LM, Sarang Z, Melina G, Takkenberg JJ, Yacoub MH. Late outcomes following freestyle versus homograft aortic root replacement: results from a prospective randomized trial. J Am Coll Cardiol. 2010;55:368–376. [DOI] [PubMed] [Google Scholar]
- 22. Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg. 2006;131:558–564. [DOI] [PubMed] [Google Scholar]
- 23. Marathe SP, Bell D, Betts K, Sayed S, Dunne B, Ward C, Whight C, Jalali H, Venugopal P, Andrews D, et al. Homografts versus stentless bioprosthetic valves in the pulmonary position: a multicentre propensity‐matched comparison in patients younger than 20 years. Eur J Cardiothorac Surg. 2019;56:377–384. [DOI] [PubMed] [Google Scholar]
- 24. Bell D, Prabhu S, Betts KS, Chen Y, Radford D, Whight C, Ward C, Jalali H, Venugopal P, Alphonso N. Long‐term performance of homografts versus stented bioprosthetic valves in the pulmonary position in patients aged 10–20 years. Eur J Cardiothorac Surg. 2018;54:946–952. [DOI] [PubMed] [Google Scholar]
- 25. Kostyunin AE, Yuzhalin AE, Ovcharenko EA, Kutikhin AG. Development of calcific aortic valve disease: do we know enough for new clinical trials? J Mol Cell Cardiol. 2019;132:189–209. [DOI] [PubMed] [Google Scholar]
- 26. Simionescu DT. Prevention of calcification in bioprosthetic heart valves: challenges and perspectives. Expert Opin Biol Ther. 2004;4:1971–1985. [DOI] [PubMed] [Google Scholar]
- 27. Kim KM, Herrera GA, Battarbee HD. Role of glutaraldehyde in calcification of porcine aortic valve fibroblasts. Am J Pathol. 1999;154:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovekamp JJ, Simionescu DT, Mercuri JJ, Zubiate B, Sacks MS, Vyavahare NR. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27:1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vyavahare N, Jones PL, Tallapragada S, Levy RJ. Inhibition of matrix metalloproteinase activity attenuates tenascin‐C production and calcification of implanted purified elastin in rats. Am J Pathol. 2000;157:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bailey MT, Pillarisetti S, Xiao H, Vyavahare NR. Role of elastin in pathologic calcification of xenograft heart valves. J Biomed Mater Res A. 2003;66:93–102. [DOI] [PubMed] [Google Scholar]
- 31. Shen M, Carpentier SM, Berrebi AJ, Chen L, Martinet B, Carpentier A. Protein adsorption of calcified and noncalcified valvular bioprostheses after human implantation. Ann Thorac Surg. 2001;71:S406–S407. [DOI] [PubMed] [Google Scholar]
- 32. Shen M, Marie P, Farge D, Carpentier S, De Pollak C, Hott M, Chen L, Martinet B, Carpentier A. Osteopontin is associated with bioprosthetic heart valve calcification in humans. C R Acad Sci III. 1997;320:49–57. [DOI] [PubMed] [Google Scholar]
- 33. Mahjoub H, Mathieu P, Larose E, Dahou A, Sénéchal M, Dumesnil JG, Després JP, Pibarot P. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart. 2015;101:472–477. [DOI] [PubMed] [Google Scholar]
- 34. Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller‐Esterl W, Schinke T, Jahnen‐Dechent W. The serum protein alpha 2‐Heremans‐Schmid glycoprotein/fetuin‐A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koos R, Brandenburg V, Mahnken AH, Mühlenbruch G, Stanzel S, Günther RW, Floege J, Jahnen‐Dechent W, Kelm M, Kühl HP. Association of fetuin‐A levels with the progression of aortic valve calcification in non‐dialyzed patients. Eur Heart J. 2009;30:2054–2061. [DOI] [PubMed] [Google Scholar]
- 36. Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R, Metzger T, Wanner C, Jahnen‐Dechent W, Floege J. Association of low fetuin‐A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross‐sectional study. Lancet. 2003;361:827–833. [DOI] [PubMed] [Google Scholar]
- 37. Sakaue T, Nakaoka H, Shikata F, Aono J, Kurata M, Uetani T, Hamaguchi M, Kojima A, Uchita S, Yasugi T, et al. Biochemical and histological evidence of deteriorated bioprosthetic valve leaflets: the accumulation of fibrinogen and plasminogen. Biol Open. 2018;7:bio034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morvan M, Arangalage D, Franck G, Perez F, Cattan‐Levy L, Codogno I, Jacob‐Lenet MP, Deschildre C, Choqueux C, Even G, et al. Relationship of iron deposition to calcium deposition in human aortic valve leaflets. J Am Coll Cardiol. 2019;73:1043–1054. [DOI] [PubMed] [Google Scholar]
- 39. Akahori H, Tsujino T, Naito Y, Matsumoto M, Lee‐Kawabata M, Ohyanagi M, Mitsuno M, Miyamoto Y, Daimon T, Hao H, et al. Intraleaflet haemorrhage is associated with rapid progression of degenerative aortic valve stenosis. Eur Heart J. 2011;32:888–896. [DOI] [PubMed] [Google Scholar]
- 40. Christian AJ, Lin H, Alferiev IS, Connolly JM, Ferrari G, Hazen SL, Ischiropoulos H, Levy RJ. The susceptibility of bioprosthetic heart valve leaflets to oxidation. Biomaterials. 2014;35:2097–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S, Levy RJ, Christian AJ, Hazen SL, Frick NE, Lai EK, Grau JB, Bavaria JE, Ferrari G. Calcification and oxidative modifications are associated with progressive bioprosthetic heart valve dysfunction. J Am Heart Assoc. 2017;6:e005648 DOI: 10.1161/JAHA.117.005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arjunon S, Rathan S, Jo H, Yoganathan AP. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng. 2013;41:1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dalgliesh AJ, Parvizi M, Noble C, Griffiths LG. Effect of cyclic deformation on xenogeneic heart valve biomaterials. PLoS One. 2019;14:e0214656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vesely I, Barber JE, Ratliff NB. Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J Heart Valve Dis. 2001;10:471–477. [PubMed] [Google Scholar]
- 45. Barannyk O, Fraser R, Oshkai P. A correlation between long‐term in vitro dynamic calcification and abnormal flow patterns past bioprosthetic heart valves. J Biol Phys. 2017;43:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao KK, Li X, John R, Amatya DM, Joyce LD, Park SJ, Bianco R, Bolman RM III. Mechanical stress: an independent determinant of early bioprosthetic calcification in humans. Ann Thorac Surg. 2008;86:491–495. [DOI] [PubMed] [Google Scholar]
- 47. Arzani A, Mofrad MRK. A strain‐based finite element model for calcification progression in aortic valves. J Biomech. 2017;65:216–220. [DOI] [PubMed] [Google Scholar]
- 48. Sellaro TL, Hildebrand D, Lu Q, Vyavahare N, Scott M, Sacks MS. Effects of collagen fiber orientation on the response of biologically derived soft tissue biomaterials to cyclic loading. J Biomed Mater Res A. 2007;80:194–205. [DOI] [PubMed] [Google Scholar]
- 49. Sacks MS. A review on the biomechanical effects of fatigue on the porcine bioprosthetic heart valve. J Long Term Eff Med Implants. 2017;27:181–197. [DOI] [PubMed] [Google Scholar]
- 50. Raghav V, Okafor I, Quach M, Dang L, Marquez S, Yoganathan AP. Long‐term durability of Carpentier‐Edwards Magna Ease valve: a one billion cycle in vitro study. Ann Thorac Surg. 2016;101:1759–1765. [DOI] [PubMed] [Google Scholar]
- 51. Martin C, Sun W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech. 2015;48:3026–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abbasi M, Azadani AN. Leaflet stress and strain distributions following incomplete transcatheter aortic valve expansion. J Biomech. 2015;48:3663–3671. [DOI] [PubMed] [Google Scholar]
- 53. Sturla F, Ronzoni M, Vitali M, Dimasi A, Vismara R, Preston‐Maher G, Burriesci G, Votta E, Redaelli A. Impact of different aortic valve calcification patterns on the outcome of transcatheter aortic valve implantation: a finite element study. J Biomech. 2016;49:2520–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abbasi M, Azadani AN. Stress analysis of transcatheter aortic valve leaflets under dynamic loading: effect of reduced tissue thickness. J Heart Valve Dis. 2017;26:386–396. [PubMed] [Google Scholar]
- 55. Bourget JM, Zegdi R, Lin J, Wawryko P, Merhi Y, Convelbo C, Mao J, Fu Y, Xu T, Merkel NO, et al. Correlation between structural changes and acute thrombogenicity in transcatheter pericardium valves after crimping and balloon deployment. Morphologie. 2017;101:19–32. [DOI] [PubMed] [Google Scholar]
- 56. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJ, Chang Y, Steinbrüchel DA, Olsen PS, Petronio AS, et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol. 2019;73:546–553. [DOI] [PubMed] [Google Scholar]
- 57. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB Jr, Heiser J, et al. 5‐Year outcomes of self‐expanding transcatheter versus surgical aortic valve replacement in high‐risk patients. J Am Coll Cardiol. 2018;72:2687–2696. [DOI] [PubMed] [Google Scholar]
- 58. Gerckens U, Tamburino C, Bleiziffer S, Bosmans J, Wenaweser P, Brecker S, Guo J, Linke A. Final 5‐year clinical and echocardiographic results for treatment of severe aortic stenosis with a self‐expanding bioprosthesis from the ADVANCE Study. Eur Heart J. 2017;38:2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barbanti M, Petronio AS, Ettori F, Latib A, Bedogni F, De Marco F, Poli A, Boschetti C, De Carlo M, Fiorina C, et al. 5‐Year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Interv. 2015;8:1084–1091. [DOI] [PubMed] [Google Scholar]
- 60. Toggweiler S, Humphries KH, Lee M, Binder RK, Moss RR, Freeman M, Ye J, Cheung A, Wood DA, Webb JG. 5‐Year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:413–419. [DOI] [PubMed] [Google Scholar]
- 61. Holy EW, Kebernik J, Abdelghani M, Stämpfli SF, Hellermann J, Allali A, El‐Mawardy M, Sachse S, Lüscher TF, Tanner FC, et al. Long‐term durability and haemodynamic performance of a self‐expanding transcatheter heart valve beyond five years after implantation: a prospective observational study applying the standardised definitions of structural deterioration and valve failure. EuroIntervention. 2018;14:e390–e396. [DOI] [PubMed] [Google Scholar]
- 62. Barbanti M, Costa G, Zappulla P, Todaro D, Picci A, Rapisarda G, Di Simone E, Sicuso R, Buccheri S, Gulino S, et al. Incidence of long‐term structural valve dysfunction and bioprosthetic valve failure after transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7:e008440 DOI: 10.1161/JAHA.117.008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Didier R, Eltchaninoff H, Donzeau‐Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lièvre M, Prat A, Teiger E, et al. Five‐year clinical outcome and valve durability after transcatheter aortic valve replacement in high‐risk patients. Circulation. 2018;138:2597–2607. [DOI] [PubMed] [Google Scholar]
- 64. Tam DY, Vo TX, Wijeysundera HC, Dvir D, Friedrich JO, Fremes SE. Transcatheter valve‐in‐valve versus redo surgical aortic valve replacement for the treatment of degenerated bioprosthetic aortic valve: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2018;92:1404–1411. [DOI] [PubMed] [Google Scholar]
- 65. Tam DY, Dharma C, Rocha RV, Ouzounian M, Wijeysundera HC, Austin PC, Chikwe J, Gaudino M, Fremes SE. Transcatheter ViV versus redo surgical AVR for the management of failed biological prosthesis: early and late outcomes in a propensity‐matched cohort. JACC Cardiovasc Interv. 2020;13:765–774. [DOI] [PubMed] [Google Scholar]
- 66. Landes U, Webb JG, De Backer O, Sondergaard L, Abdel‐Wahab M, Crusius L, Kim WK, Hamm C, Buzzatti N, Montorfano M, et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75:1882–1893. [DOI] [PubMed] [Google Scholar]
- 67. Abdel‐Wahab M, Landt M, Neumann FJ, Massberg S, Frerker C, Kurz T, Kaur J, Toelg R, Sachse S, Jochheim D, et al. 5‐Year outcomes after TAVR with balloon‐expandable versus self‐expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. 2020;13:1071–1082. [DOI] [PubMed] [Google Scholar]
- 68. Morris AH, Stamer DK, Kyriakides TR. The host response to naturally‐derived extracellular matrix biomaterials. Semin Immunol. 2017;29:72–91. [DOI] [PubMed] [Google Scholar]
- 69. Teshima H, Hayashida N, Yano H, Nishimi M, Tayama E, Fukunaga S, Akashi H, Kawara T, Aoyagi S. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg. 2003;126:401–407. [DOI] [PubMed] [Google Scholar]
- 70. Teshima H, Fukunaga S, Takaseya T, Tomoeda H, Akashi H, Aoyagi S. Obstruction of St. Jude Medical valves in the aortic position: plasma transforming growth factor type beta 1 in patients with pannus overgrowth. Artif Organs. 2010;34:210–215. [DOI] [PubMed] [Google Scholar]
- 71. Nair V, Law KB, Li AY, Phillips KR, David TE, Butany J. Characterizing the inflammatory reaction in explanted Medtronic Freestyle stentless porcine aortic bioprosthesis over a 6‐year period. Cardiovasc Pathol. 2012;21:158–168. [DOI] [PubMed] [Google Scholar]
- 72. Shetty R, Pibarot P, Audet A, Janvier R, Dagenais F, Perron J, Couture C, Voisine P, Després JP, Mathieu P. Lipid‐mediated inflammation and degeneration of bioprosthetic heart valves. Eur J Clin Invest. 2009;39:471–480. [DOI] [PubMed] [Google Scholar]
- 73. Simionescu A, Simionescu DT, Deac RF. Matrix metalloproteinases in the pathology of natural and bioprosthetic cardiac valves. Cardiovasc Pathol. 1996;5:323–332. [DOI] [PubMed] [Google Scholar]
- 74. Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–519. [DOI] [PubMed] [Google Scholar]
- 75. New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage‐derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Srivatsa SS, Harrity PJ, Maercklein PB, Kleppe L, Veinot J, Edwards WD, Johnson CM, Fitzpatrick LA. Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J Clin Invest. 1997;99:996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Eishi K, Ishibashi‐Ueda H, Nakano K, Kosakai Y, Sasako Y, Kobayashi J, Yutani C. Calcific degeneration of bioprosthetic aortic valves in patients receiving steroid therapy. J Heart Valve Dis. 1996;5:668–672. [PubMed] [Google Scholar]
- 78. Forcillo J, Pellerin M, Perrault LP, Cartier R, Bouchard D, Demers P, Carrier M. Carpentier‐Edwards pericardial valve in the aortic position: 25‐years experience. Ann Thorac Surg. 2013;96:486–493. [DOI] [PubMed] [Google Scholar]
- 79. Manji RA, Zhu LF, Nijjar NK, Rayner DC, Korbutt GS, Churchill TA, Rajotte RV, Koshal A, Ross DB. Glutaraldehyde‐fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–327. [DOI] [PubMed] [Google Scholar]
- 80. Perota A, Lagutina I, Duchi R, Zanfrini E, Lazzari G, Judor JP, Conchon S, Bach JM, Bottio T, Gerosa G, et al. Generation of cattle knockout for galactose‐α1,3‐galactose and N‐glycolylneuraminic acid antigens. Xenotransplantation. 2019;26:e12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee W, Long C, Ramsoondar J, Ayares D, Cooper DK, Manji RA, Hara H. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration? Xenotransplantation. 2016;23:370–380. [DOI] [PubMed] [Google Scholar]
- 82. Bloch O, Golde P, Dohmen PM, Posner S, Konertz W, Erdbrügger W. Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves. Tissue Eng Part A. 2011;17:2399–2405. [DOI] [PubMed] [Google Scholar]
- 83. Hawkins RB, Frischtak HL, Kron IL, Ghanta RK. Premature bioprosthetic aortic valve degeneration associated with allergy to galactose‐alpha‐1,3‐galactose. J Card Surg. 2016;31:446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha‐GAL. J Immunol. 2006;177:1289–1295. [DOI] [PubMed] [Google Scholar]
- 85. Gates KV, Xing Q, Griffiths LG. Immunoproteomic identification of noncarbohydrate antigens eliciting graft‐specific adaptive immune responses in patients with bovine pericardial bioprosthetic heart valves. Proteomics Clin Appl. 2019;13:e1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Böer U, Buettner FFR, Schridde A, Klingenberg M, Sarikouch S, Haverich A, Wilhelmi M. Antibody formation towards porcine tissue in patients implanted with crosslinked heart valves is directed to antigenic tissue proteins and αGal epitopes and is reduced in healthy vegetarian subjects. Xenotransplantation. 2017;24:e12288. [DOI] [PubMed] [Google Scholar]
- 87. Nsaibia MJ, Mahmut A, Mahjoub H, Dahou A, Bouchareb R, Boulanger MC, Després JP, Bossé Y, Arsenault BJ, Larose E, et al. Association between plasma lipoprotein levels and bioprosthetic valve structural degeneration. Heart. 2016;102:1915–1921. [DOI] [PubMed] [Google Scholar]
- 88. Salaun E, Mahjoub H, Dahou A, Mathieu P, Larose É, Després JP, Rodés‐Cabau J, Arsenault BJ, Puri R, Clavel MA, et al. Hemodynamic deterioration of surgically implanted bioprosthetic aortic valves. J Am Coll Cardiol. 2018;72:241–251. [DOI] [PubMed] [Google Scholar]
- 89. Trantina‐Yates AE, Human P, Zilla P. Detoxification on top of enhanced, diamine‐extended glutaraldehyde fixation significantly reduces bioprosthetic root calcification in the sheep model. J Heart Valve Dis. 2003;12:93–101. [PubMed] [Google Scholar]
- 90. Flameng W, Hermans H, Verbeken E, Meuris B. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149:340–345. [DOI] [PubMed] [Google Scholar]
- 91. Chen W, Schoen FJ, Levy RJ. Mechanism of efficacy of 2‐amino oleic acid for inhibition of calcification of glutaraldehyde‐pretreated porcine bioprosthetic heart valves. Circulation. 1994;90:323–329. [DOI] [PubMed] [Google Scholar]
- 92. Shang H, Claessens SM, Tian B, Wright GA. Aldehyde reduction in a novel pericardial tissue reduces calcification using rabbit intramuscular model. J Mater Sci Mater Med. 2017;28:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, Kruth HS, Zand R, Levy RJ. Prevention of bioprosthetic heart valve calcification by ethanol preincubation: efficacy and mechanisms. Circulation. 1997;95:479–488. [DOI] [PubMed] [Google Scholar]
- 94. Xi T, Ma J, Tian W, Lei X, Long S, Xi B. Prevention of tissue calcification on bioprosthetic heart valve by using epoxy compounds: a study of calcification tests in vitro and in vivo. J Biomed Mater Res. 1992;26:1241–1251. [DOI] [PubMed] [Google Scholar]
- 95. van Wachem PB, Brouwer LA, Zeeman R, Dijkstra PJ, Feijen J, Hendriks M, Cahalan PT, van Luyn MJ. In vivo behavior of epoxy‐crosslinked porcine heart valve cusps and walls. J Biomed Mater Res. 2000;53:18–27. [DOI] [PubMed] [Google Scholar]
- 96. Guo G, Jin L, Jin W, Chen L, Lei Y, Wang Y. Radical polymerization‐crosslinking method for improving extracellular matrix stability in bioprosthetic heart valves with reduced potential for calcification and inflammatory response. Acta Biomater. 2018;82:44–55. [DOI] [PubMed] [Google Scholar]
- 97. Lim HG, Kim SH, Choi SY, Kim YJ. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. Eur J Cardiothorac Surg. 2012;41:383–390. [DOI] [PubMed] [Google Scholar]
- 98. Chang Y, Tsai CC, Liang HC, Sung HW. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials. 2002;23:2447–2457. [DOI] [PubMed] [Google Scholar]
- 99. Wang D, Jiang H, Li J, Zhou JY, Hu SS. Mitigated calcification of glutaraldehyde‐fixed bovine pericardium by tannic acid in rats. Chin Med J (Engl). 2008;121:1675–1679. [PubMed] [Google Scholar]
- 100. Cwalina B, Turek A, Nozynski J, Jastrzebska M, Nawrat Z. Structural changes in pericardium tissue modified with tannic acid. Int J Artif Organs. 2005;28:648–653. [DOI] [PubMed] [Google Scholar]
- 101. Jin W, Guo G, Chen L, Lei Y, Wang Y. Elastin stabilization through polyphenol and ferric chloride combined treatment for the enhancement of bioprosthetic heart valve anticalcification. Artif Organs. 2018;42:1062–1069. [DOI] [PubMed] [Google Scholar]
- 102. Lei Y, Ning Q, Tang Y, Wang Y. Exogenous hyaluronic acid and chondroitin sulfate crosslinking treatment for increasing the amount and stability of glycosaminoglycans in bioprosthetic heart valves. J Mater Sci Mater Med. 2019;30:38. [DOI] [PubMed] [Google Scholar]
- 103. Boccafoschi F, Botta M, Fusaro L, Copes F, Ramella M, Cannas M. Decellularized biological matrices: an interesting approach for cardiovascular tissue repair and regeneration. J Tissue Eng Regen Med. 2017;11:1648–1657. [DOI] [PubMed] [Google Scholar]
- 104. Collatusso C, Roderjan JG, Vieira ED, Costa FD, Noronha LD, Fornazari DdeF. Effect of SDS‐based decelullarization in the prevention of calcification in glutaraldehyde‐preserved bovine pericardium: study in rats. Rev Bras Cir Cardiovasc. 2012;27:88–96. [DOI] [PubMed] [Google Scholar]
- 105. VeDepo MC, Detamore MS, Hopkins RA, Converse GL. Recellularization of decellularized heart valves: progress toward the tissue‐engineered heart valve. J Tissue Eng. 2017;8:2041731417726327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Heuschkel MA, Leitolis A, Roderjan JG, Suss PH, Luzia CAO, da Costa FDA, Correa A, Stimamiglio MA. In vitro evaluation of bovine pericardium after a soft decellularization approach for use in tissue engineering. Xenotransplantation. 2019;26:e12464. [DOI] [PubMed] [Google Scholar]
- 107. Li N, Li Y, Gong D, Xia C, Liu X, Xu Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact Cardiovasc Thorac Surg. 2018;26:768–776. [DOI] [PubMed] [Google Scholar]
- 108. Kim MS, Lim HG, Kim YJ. Calcification of decellularized and alpha‐galactosidase‐treated bovine pericardial tissue in an alpha‐Gal knock‐out mouse implantation model: comparison with primate pericardial tissue. Eur J Cardiothorac Surg. 2016;49:894–900. [DOI] [PubMed] [Google Scholar]
- 109. Bibevski S, Ruzmetov M, Fortuna RS, Turrentine MW, Brown JW, Ohye RG. Performance of SynerGraft decellularized pulmonary allografts compared with standard cryopreserved allografts: results from multiinstitutional data. Ann Thorac Surg. 2017;103:869–874. [DOI] [PubMed] [Google Scholar]
- 110. da Costa FD, Dohmen PM, Duarte D, von Glenn C, Lopes SV, Filho HH, da Costa MB, Konertz W. Immunological and echocardiographic evaluation of decellularized versus cryopreserved allografts during the Ross operation. Eur J Cardiothorac Surg. 2005;27:572–578. [DOI] [PubMed] [Google Scholar]
- 111. Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, et al. Heart transplantation in baboons using alpha1,3‐galactosyltransferase gene‐knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. [DOI] [PubMed] [Google Scholar]
- 112. McGregor CG, Kogelberg H, Vlasin M, Byrne GW. Gal‐knockout bioprostheses exhibit less immune stimulation compared to standard biological heart valves. J Heart Valve Dis. 2013;22:383–390. [PubMed] [Google Scholar]
- 113. Zhang R, Wang Y, Chen L, Wang R, Li C, Li X, Fang B, Ren X, Ruan M, Liu J, et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically‐deleting three major glycan antigens, GGTA1/β4GalNT2/CMAH. Acta Biomater. 2018;72:196–205. [DOI] [PubMed] [Google Scholar]
- 114. McGregor C, Byrne G, Rahmani B, Chisari E, Kyriakopoulou K, Burriesci G. Physical equivalency of wild type and galactose α 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta Biomater. 2016;41:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rahmani B, McGregor C, Byrne G, Burriesci G. A durable porcine pericardial surgical bioprosthetic heart valve: a proof of concept. J Cardiovasc Transl Res. 2019;12:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Antonini‐Canterin F, Zuppiroli A, Popescu BA, Granata G, Cervesato E, Piazza R, Pavan D, Nicolosi GL. Effect of statins on the progression of bioprosthetic aortic valve degeneration. Am J Cardiol. 2003;92:1479–1482. [DOI] [PubMed] [Google Scholar]
- 117. Skowasch D, Schrempf S, Preusse CJ, Likungu JA, Welz A, Lüderitz B, Bauriedel G. Tissue resident C reactive protein in degenerative aortic valves: correlation with serum C reactive protein concentrations and modification by statins. Heart. 2006;92:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kulik A, Masters RG, Bédard P, Hendry PJ, Lam BK, Rubens FD, Mesana TG, Ruel M. Postoperative lipid‐lowering therapy and bioprosthesis structural valve deterioration: justification for a randomised trial? Eur J Cardiothorac Surg. 2010;37:139–144. [DOI] [PubMed] [Google Scholar]
- 119. Johnston DR, Soltesz EG, Vakil N, Rajeswaran J, Roselli EE, Sabik JF III, Smedira NG, Svensson LG, Lytle BW, Blackstone EH. Long‐term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Une D, Ruel M, David TE. Twenty‐year durability of the aortic Hancock II bioprosthesis in young patients: is it durable enough? Eur J Cardiothorac Surg. 2014;46:825–830. [DOI] [PubMed] [Google Scholar]
- 121. Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis‐patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–2129. [DOI] [PubMed] [Google Scholar]


