Abstract
Background
The improved life expectancy of patients with congenital heart disease is often accompanied by the development of atrial tachyarrhythmias. Similarly, the number of patients requiring redo operations is expected to continue to rise as these patients are aging. Consequently, the role of arrhythmia surgery in the treatment of atrial arrhythmias is likely to become more important in this population. Although atrial arrhythmia surgery is a well‐established part of Fontan conversion procedures, evidence‐based recommendations for arrhythmia surgery for macroreentrant atrial tachycardia and atrial fibrillation in other patients with congenital heart disease are still lacking.
Methods and Results
Twenty‐eight studies were included in this systematic review. The median reported arrhythmia recurrence was 13% (interquartile range, 4%–26%) during follow‐up ranging from 3 months to 15.2 years. A large variation in surgical techniques was observed. Based on the acquired data, biatrial lesions are more effective in the treatment of atrial fibrillation than exclusive right‐sided lesions. Right‐sided lesions may be more appropriate in the treatment of macroreentrant atrial tachycardia; evidence for the superiority of additional left‐sided lesions is lacking. There are not enough data to support the use of exclusive left‐sided lesions. Theoretically, prophylactic atrial arrhythmia surgery may be beneficial in this population, but evidence is currently limited.
Conclusions
To be able to provide recommendations for arrhythmia surgery in patients with congenital heart disease, future studies should report outcomes according to the type of preoperative arrhythmia, underlying congenital heart disease, lesion set, and energy source. This is essential for determining which surgical techniques should ideally be applied under which circumstances.
Keywords: arrhythmia surgery, atrial fibrillation, atrial tachycardia, congenital heart disease, systematic review
Subject Categories: Arrhythmias, Atrial Fibrillation, Congenital Heart Disease, Cardiovascular Surgery, Treatment
Nonstandard Abbreviations and Acronyms
- AAD
anti‐arrhythmic drugs
- ATA
atrial tachyarrhythmias
- MRAT
macroreentrant atrial tachycardia
- SND
sinus node dysfunction
Clinical Perspective
What Is New?
Concrete, evidence‐based recommendations for arrhythmia surgery for atrial fibrillation or macroreentrant atrial tachycardia in patients with congenital heart disease—other than those undergoing Fontan conversion—are currently lacking.
This systematic review, including 28 studies published over a time span of 25 years, provides an overview of the striking variation in surgical techniques applied over the past decades.
Outcomes of arrhythmia recurrence and adverse events, such as new‐onset atrial tachyarrhythmias and permanent pacemaker implantation, are summarized.
What Are the Clinical Implications?
Based on the acquired data, biatrial lesions are preferred in the treatment of atrial fibrillation, whereas exclusive right‐sided lesions are likely more appropriate in the treatment of macroreentrant atrial tachycardias.
Evidence supporting prophylactic atrial arrhythmia surgery is currently limited, and findings from this review emphasize the need for uniformity of surgical techniques in this unique population.
To be able to determine which surgical techniques should ideally be applied under which circumstances, detailed documentation of methodology (indication, underlying congenital heart defect, lesion set, and energy source) in future studies is essential.
Congenital heart disease (CHD) is the most common cause of congenital anomalies, with an estimated prevalence of 9 per 1000 live births and 4 per 1000 adults. 1 , 2 Although surgical correction or palliation is often performed in childhood, a considerable number of patients (20%) require primary or redo surgery in adulthood. 3 , 4 As a result of improved life expectancy in these patients, the number of redo operations is expected to continue to rise. Patients may not only require redo operations for their primary defect, but also for acquired coronary or valvular heart disease. 5 , 6 Moreover, the improved life expectancy in CHD patients is often accompanied by the development of atrial tachyarrhythmia (ATA), including macroreentrant atrial tachycardia (MRAT) and atrial fibrillation (AF). 7 , 8 ATAs in this population occur at a relatively young age and show rapid progression, resulting in impaired quality‐of‐life, morbidity, and mortality. 8 , 9 , 10
Therefore, the role of arrhythmia surgery in the treatment of atrial arrhythmias may become more important in this specific population. For patients undergoing Fontan conversion, class I recommendations were provided by the 2014 Pediatric and Congenital Electrophysiology Society (PACES)/Heart Rhythm Society (HRS) guidelines in favor of performing concomitant atrial arrhythmia surgery, which is supported by a large body of evidence. 11 However, on atrial arrhythmia surgery in other patients with CHD, recommendations provided by multiple guidelines are either largely extrapolated from studies on patients without CHD, 11 patients undergoing Fontan conversion, 12 or they are based on only a small number of published studies in this population. 12 , 13 In addition, the 2017 Society of Thoracic Surgeons guidelines for surgical treatment of AF do not yet provide specific recommendations for patients with CHD at all. 14
Therefore, this systematic review aimed to evaluate and summarize outcomes of atrial arrhythmia surgery for MRAT and AF in patients with CHD undergoing cardiac surgery other than Fontan conversion.
Methods
The systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines. 15 The data that support the findings of this study are available from the corresponding author upon reasonable request.
Search Strategy and Selection Criteria
We searched Embase, MEDLINE, Web‐of‐Science Core Collection, Cochrane Library, and Google Scholar for relevant articles using terms associated with congenital heart disease and atrial arrhythmia surgery up to November 20, 2019, with no start date restriction. The complete search strategy is provided in Data S1. Additionally, we manually searched reference lists of identified articles and relevant reviews.
Eligibility assessment of identified articles was performed independently by 2 reviewers (C. H., E. M.); disagreements were resolved by consensus. Studies were first screened based on title and abstract. If potentially relevant, the full text was assessed. Studies were included if they reported outcomes of arrhythmia surgery for AF or MRAT in patients with CHD undergoing surgery other than Fontan conversion. Studies were excluded if they only reported outcomes of arrhythmia surgery for focal atrial tachycardia, accessory pathways, or atrioventricular nodal reentry tachycardia; if duration of follow‐up was <3 months; or if >25% of the study population consisted of patients undergoing Fontan conversion surgery. We excluded review articles, book chapters, conference abstracts, editorials, case reports, and studies written in languages other than English. If double reporting of the same patient populations was suspected, the most recent publication was included. Both publications were included if it was possible to exclude duplicate data from 1 of the publications, or if both publications also included a substantial amount of unique data.
Data Extraction and Data Appraisal
Data extraction was performed by 1 reviewer (C. H.) into a predetermined template and the extracted data were subsequently checked for accuracy by the second reviewer (E. M.). Disagreements were resolved by discussion and where necessary, a third reviewer with expertise in the field (A. B.) was consulted.
Available data on study characteristics (study period, study design), patient characteristics (age, sex, CHD type, preoperative arrhythmias), procedural characteristics (location of lesions, energy source) and follow‐up (duration, arrhythmia recurrence, new‐onset ATA, permanent pacemaker implantation) were collected. The number of arrhythmia recurrences was derived from Kaplan‐Meier curves, where possible, if it was not explicitly described. If a distinction was made between early (generally <3 months) and late recurrences, the number of late recurrences was selected.
Quality assessment of the included articles was performed using the Newcastle‐Ottawa Scale (nonrandomized studies) or the Cochrane Risk of Bias 2 tool (randomized controlled trials). 16 , 17 The Newcastle Ottawa Scale assesses risk of bias and ranges from 0 points (high risk) to 9 points (low risk). The following items were assessed: (1) representativeness of the exposed cohort (1 point), (2) selection of the non‐exposed cohort (1 point), (3) ascertainment of exposure (ie, arrhythmia surgery) (1 point), (4) demonstration that the outcome of interest was not present at the start of the study (1 point), (5) comparability of cohorts on the basis of the design or analysis (2 points), (6) assessment of outcome (1 point), (7) follow‐up being long enough (ie, mean/median >6 months) for outcomes to occur (1 point), and (8) adequacy of follow‐up of cohorts (1 point). The Risk of Bias 2 tool assesses risk of bias in 5 domains: randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result.
Data Analysis
Outcomes of arrhythmia surgery were presented for relevant subcategories (type of arrhythmia surgery or CHD). When calculating the proportion of patients with a recurrence, the preferred denominator was the number of patients who had long‐term follow‐up available (excluding early deaths and those lost to follow‐up); otherwise, the number of patients at the start of the study was used. The 95% CI was calculated using the normal approximation method; when conditions were not appropriate for approximation of the binomial distribution by the normal distribution, a Clopper‐Pearson interval was calculated. 18 Pronounced heterogeneity within and between studies (eg, large variation in follow‐up duration) precluded meaningful meta‐analyses, even after dividing the studies into relevant subcategories. 19 To provide an overall indication of the outcomes of the studies anyhow, the median (interquartile range [IQR]) was provided for the following parameters: duration of inclusion period, quality score, and the number of arrhythmia recurrences or permanent pacemaker implantations.
Results
As illustrated in Figure 1, our initial search identified 2175 records after removal of duplicates and addition of 1 article identified by searching reference lists. After exclusion of records based on screening of title and abstract, 132 full‐text articles were assessed for eligibility, resulting in 28 studies included in this review. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47
Figure 1. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flowchart of the study selection.
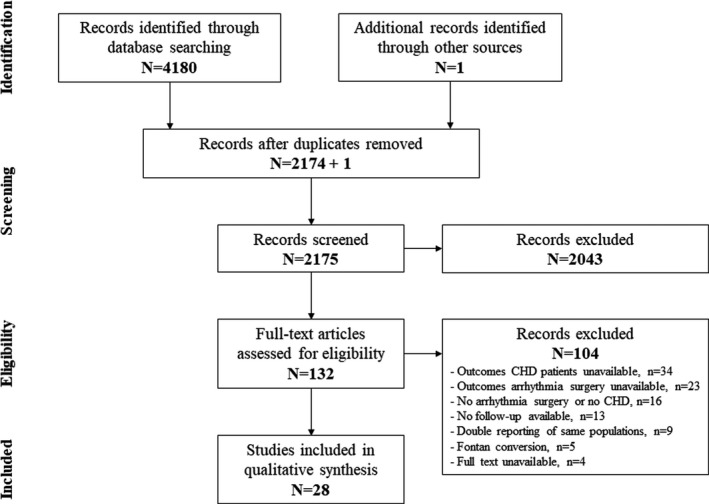
CHD indicates congenital heart disease.
A summary of the included studies is provided in Table 1. Of the included studies, 27 were cohort studies and 1 was a randomized controlled trial. First of all, quality of the included studies as assessed by the Newcastle‐Ottawa Scale was relatively good. As we only included data from patients (cases) and not from controls (where applicable), items (2) and (5) of the scale were not assessed, resulting in a maximum score of 6 points. The median score was 5 (IQR 5–6). Most scores <6 were because of the lack of information with regard to follow‐up duration or loss to follow‐up if the study population of interest was a subset of a larger group of patients. In some cases, follow‐up was short, 35 loss to follow‐up was relatively high, 23 or the authors did not provide specific information about patient acquisition 34 or the objective assessment of rhythm outcomes. 25 , 27 , 46 The randomized controlled trial was judged to be at low risk for bias in all 5 domains.
Table 1.
Summary of Included Studies
| Author, y | Sample Size | Study Period | NOS | CHD Types | Age (y) | Male sex | Preoperative Arrhythmia | Location of Lesions | Follow‐Up (y) | Arrhythmia Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Sakamoto et al, 2019 40 | 29 | 1993–2014 | 6 | ASD | 54.6±10.2 | 66% | AF, 29 | Biatrial, 29 | 7 (1.7–21.9) | 12/29 (41%)* |
| Gonzalez Corcia et al, 2019 23 | 166 | 1998–2016 | 5 | Various† |
24.8 (23.6–51.4)‡ |
54% |
AF, 25 MRAT, 69 AF+MRAT, 28 Unspecified, 15 None, 29 |
RA, 105 LA, 6 Biatrial, 55 |
1.9 (0.4–5.7)‡ | 33% at 5 y* |
|
Ramdjan and Mouws 2018 39 |
66 | 2001–2017 | 6 | Various† | 56±14 | 47% |
AF, 46 MRAT, 6 AF+MRAT, 14 |
RA, 6 LA, 39 Biatrial, 21 |
2 (1–4)‡ |
AF: 27/60 (45%) MRAT: 6/20 (30%) |
| Engelsgaard et al, 2018 21 | 41 | 2006–2010 | 5 | N/A | 69.2±8.8|| | 72%|| | AF, 41 | Biatrial, 41 | 7.4 (2.7)‡|| | 32/41 (78%)* |
|
Lim et al, 2017 33 |
27 | 1997–2003 | 6 | Various | 3.4±3.7 | 56% | None, 27 | RA, 27 | 15.2±2.9 | 1/27 (4%) |
| Giamberti et al, 2017 22 | 80 | 2002–2013 | 6 | Various† | 39 (18–72) | 60% |
AF, 38 MRAT, 42 |
RA, 47 Biatrial, 33 |
6 (1–12.9) | 15/75 (20%)* |
|
Stulak et al, 2015 44 |
86 | 1995–2012 | 6 | Ebstein | 40 (0.8–72) | 57% |
AF, 61 MRAT, 21 AF+MRAT, 4 |
RA, 62 Biatrial, 24 |
4.5 (0.3–17.1) | 9%*,¶ |
|
Wi et al, 2013 47 |
15 | 2001–2010 | 5 | ASD | 57.8±12.6|| | 55%|| | AF, 15 |
RA, 1 Biatrial, 14 |
3.8±2.3|| | 3/15 (20%) |
|
Shim et al, 2013 41 |
42 | 2000–2011 | 6 | ASD | 52.5±9.5 | 60% | AF, 42 | Biatrial, 42 | 3.2±2.5 | 9/42 (21%) |
|
Nitta et al, 2013 37 |
10 | N/A | 6 | ASD | 54±11 | 70% | AF, 10 |
LA, 2 Biatrial, 8 |
10.8±3.8 | 2/10 (20%) |
|
Im et al, 2013 26 |
56 | 1998–2011 | 6 | ASD | 59 (34–79) | 50% | AF, 56 |
RA, 23 Biatrial, 33 |
4.1 (0.4–12.4) | 10/53 (19%) |
|
Gutierrez 2013 24 |
24 | 2004–2010 | 6 | Various† | 40.9 (14–66) * | 46% |
AF, 5 MRAT, 19 |
RA, 14 LA, 1 Biatrial, 9 |
2.8 (0.1–5.7) * | 5/19 (26%) |
|
Stulak et al, 2012 42 |
187 | 1994–2009 | 5 | Various† | 45 (1–75)|| | 45%|| | AF, 187 |
RA, 146 LA, 10 Biatrial, 31 |
4.1 (0.3–17.2)|| | 11% |
|
Atallah et al, 2012 20 |
29 | 1999–2001 | …# | Various |
2.4 (0.5)‡ 2.7 (1.9)‡ |
53% 43% |
None, 15 None, 14 |
RA, 15 None, 14 |
9.0 (1.2)‡ 9.3 (1.1)‡ |
0/15 (0%) 0/14 (0%) |
|
Mavroudis et al, 2008 36 |
55 | 1987–2007 | 5 | Various | 15.9±12.5|| | N/A |
AF, 11 MRAT, 44 |
RA, 44 Biatrial, 11 |
5±N/A|| | 2/55 (4%) |
|
Lai et al, 2008 32 |
7 | 2003–2007 | 6 | Mostly ASD | 47.1 (19–60) * | 14% | AF, 7 | Biatrial, 7 | 2 (0.3–4) * | 0/7 (0%)* |
|
Lukac et al, 2007 35 |
17 | N/A | 5 | Mostly ASD | 48 (32–58) | 35% |
AF, 5 MRAT, 1 AF+MRAT, 1 None, 10 |
RA, 17 | 0.4 | 2/17 (12%) |
|
Stulak et al, 2006 43 |
99 | 1993–2003 | 6 | Various† | 43 (9–72) | 47% |
AF, 77 MRAT, 22 |
RA, 99 | 2 (N/A–8) | 6/87 (7%)* |
|
Karamlou et al, 2006 28 |
34 | 1969–2005 | 5 | TOF | 37.7 (11.1–62.3)|| | 65%|| | AF/MRAT, 34 | RA, 34 | 5.4 (N/A–31)|| | 3/34 (9%)* |
|
Ohtsuka et al, 2005 38 |
2 | 2002–2005 | 5 | ASD | 56.5±19.8|| | 82%|| | AF, 2 | Biatrial, 2 | 1±0.7|| | 0/2 (0%) |
|
Khositseth et al, 2004 29 |
48 | 1990–2001 | 6 | Ebstein | 56.5±19.8|| | 43%|| | AF/MRAT, 48 | RA, 48 |
RSM: 3.3±2.1 Isthmus: 1.6±1.5 |
11/44 (24%)* |
|
Huang et al, 2000 25 |
3 | 1973–1997 | 4 | Ebstein | 23.9±14.0|| | 47%|| |
AF, 2 AF+MRAT, 1 |
RA, 3 | 13.2±7.1|| | 0/3 (0%)* |
|
Kobayashi et al, 1998 30 |
26 | 1992–1997 | 6 | ASD | 58.2±9.1 | 58% | AF, 26 |
RA, 3 Biatrial, 23 |
2.7±1.7 | 3/26 (12%) |
|
Kamata et al, 1997 27 |
8 | 1993–1995 | 5 | Mostly ASD | 59.8±9.8|| | 48%|| | AF, 8 | Biatrial, 8 | 1 | 2/8 (25%) |
|
Vigano et al, 1996 46 |
8 | 1989–1994 | 5 | ASD | N/A | N/A | AF, 8 | RA, 8 | 0.3 to 4.3 | 1/8 (13%) |
|
Lin et al, 1996 34 |
2 | N/A | 5 | ASD | 53, 64 | 50% | AF, 2 | RA, 2 | 1.3, 2.7 | 1/2 (50%) |
|
Kosakai et al, 1995 31 |
2 | 1992–1994 | 5 | VSD, Ebstein | 57.7±9.0|| | 43%|| | AF, 2 | Biatrial, 2 | 1.9±0.5|| | 0/2 (0%) |
|
Suwalski et al, 1994 45 |
3 | 1993–1994 | 4 | ASD | 43 (27–55)|| | 71%|| | AF, 3 | Biatrial, 3 | 0.4 (0.3–1.2)|| | 0/3 (0%) |
AF indicates atrial fibrillation; ASD, atrial septal defect; CHD, congenital heart disease; LA, left atrium; MRAT, macroreentrant atrial tachycardia; N/A, not available; NOS, Newcastle Ottawa Scale; RA, right atrium; RSM, right‐sided maze; TOF, tetralogy of Fallot; and VSD, ventricular septal defect.
* Recurrence of preoperative arrhythmia or other atrial tachyarrhythmias (not specified).
<25% Fontan conversions.
Age and follow‐up duration expressed as mean±SD, median (minimum–maximum) or minimum–maximum unless indicated otherwise: ‡Median (interquartile range), §mean (minimum–maximum).
Study population was part of a larger cohort; data were not specified. Data from the entire cohort or most appropriate subgroup are displayed.
Outcome measure: recurrence or on anti‐arrhythmic drugs.
Randomized controlled trial. Overall risk of bias: low.
Year of publication ranged from 1994 to 2019 (median 2010) and patients were included over a median span of 10 years (IQR, 4–17). Overall, the reported number of ATA recurrences during variable follow‐up periods ranged between 0% and 78% (median 13%, IQR, 4%–26%; Table 1). Potentially duplicate data were presented in 4 studies 29 , 42 , 43 , 44 ; the decision to include these studies was based on the presence of a significant amount of unique data in each study according to the inclusion period and inclusion criteria.
Types of Arrhythmia Surgery
Most studies provided a comprehensive description of their methods for arrhythmia surgery (n=18, 64%), which was accompanied by a detailed figure of lesion sets and/or references in 14 studies. Six studies described their method only by referring to a previously published study providing a comprehensive description, 24 , 28 , 36 , 42 , 43 , 45 and in 4 studies, the method applied was referred to only by name. 37 , 39 , 44 , 47 The studies demonstrated a large variation in methods used for arrhythmia surgery, including the locations of lesions within the atria and the use of different energy sources. Not only did these methods vary between studies, but also between patients within studies.
Biatrial Arrhythmia Surgery
Biatrial arrhythmia surgery, consisting of lesions in both the right and left atrium, was performed in 19 studies (68%) (Table 1), including 10 studies in which biatrial lesions were applied in >20 patients. Lesions were generally applied according to the Cox Maze III/IV lesion set, sometimes with modifications. 48 , 49 Four studies only performed isolation of the pulmonary veins instead of the full left atrial lesion set in a subset of patients. Specific outcomes for these variations were only provided in the study by Sakamoto et al, who showed similar outcomes for the full left atrial lesion set versus exclusive isolation of the pulmonary veins in the context of biatrial arrhythmia surgery (P=0.70). 37 , 39 , 40 , 44 In most studies (n=16), biatrial arrhythmia surgery was performed in patients with AF; the 3 other studies did not specify the type of preoperative arrhythmia. 24 , 39 , 44
Fourteen of the 19 studies provided separate outcomes of biatrial arrhythmia surgery, which are summarized in the upper panel of Figure 2. The number of arrhythmia recurrences reported in these studies varied between 0% and 78% (median, 13%; IQR, 0%–27%), during follow‐up ranging from 0.4 to 7.4 years. Even though sample sizes of studies published from 2013 onwards were larger than those of earlier studies, 95% CIs were still relatively wide, spanning a range of ≈20%. When only considering the 8 studies with sample size >20, the median reported amount of arrhythmia recurrences was 20% (IQR, 11%–39%) during follow‐up ranging from 1 to 7.4 years.
Figure 2. Outcomes of biatrial arrhythmia surgery (upper panel) and right‐sided arrhythmia surgery (lower panel).
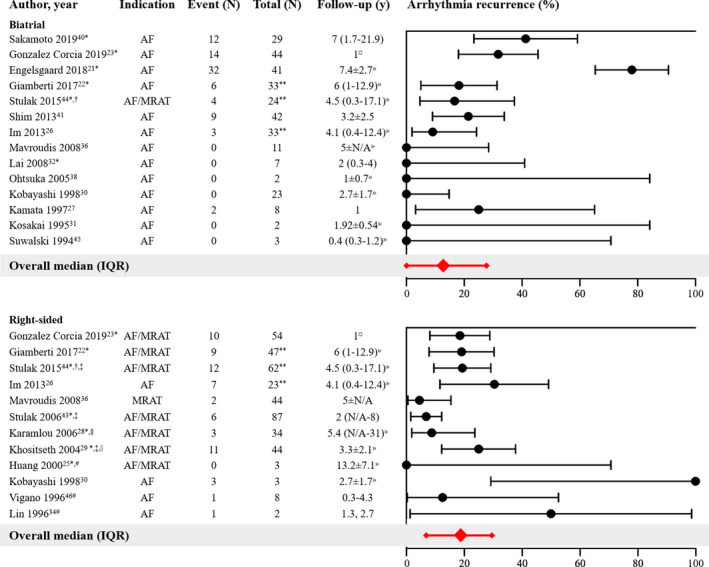
Forest plot of the proportions of patients with arrhythmia recurrence and corresponding 95% CIs. The overall median and interquartile range of proportions are displayed in red. AF/MRAT indicates that both arrhythmias were regarded as indication: outcomes of arrhythmia surgery were not further specified according to the type of preoperative arrhythmia. AR indicates atrial fibrillation; IQR, interquartile range; and MRAT, macroreentrant atrial tachycardia; *Outcome measure: recurrence of preoperative arrhythmia or other atrial tachyarrhythmias (not specified). †Outcome measure: recurrence or on anti‐arrhythmic drugs. ‡Partially duplicate data but studies also contain a significant amount of unique data. §Including n=22 patients with exclusive isthmus ablation; outcomes not specified. ||Including n=9 patients with exclusive isthmus ablation; outcomes specified in text. #Right atrial compartment or isolation techniques applied. **Number of patients at start of study. ¤Separate outcomes only available in sub‐analysis at 1 year follow‐up. »Follow‐up not specified for subgroup.
Interestingly, 1 study reported outcomes of biatrial arrhythmia surgery according to type of preoperative AF and found no difference in the number of recurrences between patients with paroxysmal AF and those with non‐paroxysmal AF (45% versus 44%; P values not provided). 40 Also, the presence of CHD did not appear to influence the results of biatrial arrhythmia surgery in the study of Engelsgaard et al. In their study, they reported outcomes of the Cox maze IV procedure for AF in 144 patients, including 41 patients with CHD, during a median follow‐up of 7.4 years (IQR, 2.7). 21 Despite their strict definition of recurrent arrhythmias (>3 months after the procedure, lasting ≥30 seconds, documented on ECG, Holter monitoring, or device interrogations), a relatively high number of recurrences was observed in both patients without CHD (79%) and patients with CHD (78%; P value not provided).
Right‐Sided Arrhythmia Surgery
Exclusive right‐sided arrhythmia surgery was performed in 19 studies (68%) (Table 1). In the majority of these studies (n=11), lesions were generally applied according to the right‐sided maze procedure, which is a modification of the traditional Cox maze III procedure. This modification was proposed and published in 1998 and was specifically intended for patients with CHD affecting the right side of the heart. 50 Several older studies used the right atrial compartment or isolation technique, which generally consisted of a single incision parallel to the sulcus terminalis, extending posteriorly and anteriorly towards the tricuspid valve annulus, including cryolesions between the incision and the tricuspid valve. 25 , 34 , 46 Solely cryoablation of the cavotricuspid isthmus was performed in 3 studies. 25 , 28 , 29 The indication for right‐sided arrhythmia surgery was AF (n=6 26 , 30 , 34 , 42 , 46 , 47 ), MRAT (n=1 36 ) or both (n=9 22 , 23 , 24 , 25 , 28 , 29 , 39 , 43 , 44 ). Three studies performed prophylactic right‐sided arrhythmia surgery. 20 , 33 , 35
Twelve of the 19 studies provided separate results of right‐sided arrhythmia surgery in patients with AF or MRAT (lower panel Figure 2). The proportion of arrhythmia recurrences was below 30% in most studies (median 19%, IQR 7–29%) during follow‐up ranging from 1 to 13.2 years. Studies performed before 2004 had smaller sample sizes resulting in more imprecise estimates of true arrhythmia recurrence. The 2 studies reporting the highest proportion of arrhythmia recurrence (50% and 100%) had only small study populations (respectively n=2 34 and n=3 30 ).
Separate outcomes of the right‐sided maze procedure versus exclusive cyroablation of the isthmus were provided in the study of Khositseth et al. In patients undergoing surgery for Ebstein anomaly, they found no significant difference in the recurrence rate between the 2 procedures (10/35 [29%] versus 1/9 [11%], P=0.50), although the type of preoperative arrhythmia (AF or MRAT) was not specified. 29 Outcomes of the right‐sided maze procedure according to the type of preoperative arrhythmia were reported in the study of Stulak et al: though not statistically significant, arrhythmias appeared to recur more often when the indication was AF (6/62 [10%]) rather than MRAT (0/21 [0%], P value not provided) and when the indication was non‐paroxysmal AF (3/11 [27%]) rather than paroxysmal AF (3/51 [6%], P=0.15). 43
Biatrial Versus Right‐Sided Arrhythmia Surgery
Three studies performed both biatrial and right‐sided arrhythmia surgery for similar indications in their study population and reported separate outcomes for each procedure. 26 , 30 , 44 When comparing biatrial and exclusive right‐sided lesions for the treatment of AF in patients with an atrial septal defect (ASD), 2 studies showed that exclusive right‐sided lesions appeared to be less effective than biatrial lesions (recurrence right versus biatrial: 7/23 [30%] versus 3/33 [9%] 26 and 3/3 [100%] versus 0/23 [0%]). 30 In addition, exclusive right‐sided lesions appeared to be less effective than biatrial lesions in the treatment of non‐paroxysmal AF/MRAT (recurrence right versus biatrial: 7/19 [37%] versus 3/29 [10%] 26 and 29% versus 14% [P=0.053]), 44 whereas the number of recurrences in patients with paroxysmal AF/MRAT was fairly similar for both lesion sets (recurrence right versus biatrial: 0/4 [0%] versus 0/4 [0%] 26 and 12% versus 23% [P=0.08]). 44 In line with these observations, authors of several studies explicitly state that their current policy—which is in contrast to that during the study period in some cases—is to perform biatrial arrhythmia surgery in patients with AF (regardless of duration) or longstanding ATA, also in patients with predominantly right‐sided CHD. 22 , 26 , 37 , 44
Left‐Sided Arrhythmia Surgery
Only 5 studies (18%) performed exclusive left‐sided arrhythmia surgery in a relatively small subset of their study populations (median, 5%; IQR, 4%–40%; Table 1). None of these studies provided solid indications for performing exclusively left‐sided rather than biatrial arrhythmia surgery and none provided separate results on the outcomes of exclusively left‐sided arrhythmia surgery. Two studies performed isolated pulmonary vein isolation in only a small subset of patients (2/10 37 and 5/66 39 ), but they did not report outcomes in these patients.
Energy Sources
The original Cox Maze III procedure consists of a set of atrial incisions (also called “the cut‐and‐sew technique”) which makes it a technically complex and long procedure, with a relatively high incidence of postoperative bleeding. 48 , 49 , 51 Over the years, various alternative energy sources have been used in an attempt to simplify the technique, which also applies to the studies included in this review. Most studies published up until 2007 (9 of 12) used a combination of incisions and cryolesions. The other 3 studies only used cryoablation. 29 , 35 , 38 The first study included in this review to report the use of radiofrequency energy is that of Lai et al. 32 Nine of the 15 studies published thereafter used radiofrequency energy. 21 , 22 , 37 , 39 , 40 , 41 , 42 , 44 , 47 None of the studies compared outcomes of different types of energy sources.
Prophylactic Arrhythmia Surgery
As shown in Table 2, 4 studies described outcomes of prophylactic arrhythmia surgery in patients with CHD undergoing surgery other than Fontan conversion. 20 , 23 , 33 , 35 One study only provided the general location of the lesions (right‐sided or biatrial), whereas the other 3 applied a standardized lesion or lesion set, which included a lesion between the right atriotomy and the right atrioventricular valve annulus in all. The randomized controlled trial of Atallah et al was not able to detect differences in terms of efficacy or safety between the intervention and control group, although sample size was small and follow‐up may not have been long enough to detect late occurrence of MRAT. Prophylactic arrhythmia surgery in the study of Lim et al included 2 additional lesions and modifications of suture lines; 4 cases of spontaneous (1) and inducible non‐sustained MRAT (3) were observed during long‐term follow‐up. 20 Interestingly, the 4 cases of atrial flutter (either spontaneous or induced) in the study of Lukac et al 35 occurred in the 4 patients in whom bidirectional block was not obtained because of incomplete cryolesions, thereby creating an isthmus between the atriotomy scar and the tricuspid annulus and facilitating the development of reentry tachycardias. This led the authors to conclude that, although effective when bidirectional block is achieved, this prophylactic lesion may be proarrhythmogenic in the absence of bidirectional block.
Table 2.
Prophylactic Arrhythmia Surgery
| Author, y | No. | CHD | Lesions | Outcome |
|---|---|---|---|---|
| Gonzalez Corcia et al, 2019 23 | 29 | Mainly Ebstein |
Right‐sided lesions*: 28 Biatrial lesions*: 1 |
Freedom from ATA at 1, 3, 5 y: 97%, 97%, 80% |
| Lim et al, 2017 33 | 27 | Initial LT Fontan |
|
Spontaneous MRAT: 1/27 (3.7%) at 12.6 y Inducible non‐sustained MRAT, 3/19 (11.1%) at 5.2 to 11.8 y |
| Atallah et al, 2012 20 | 15 | Initial LT Fontan | Atrial incision right atriotomy—RAVV |
Spontaneous MRAT, 0/15 (0%) at 9 y Inducible MRAT, 0/2 (0%) at 9 y |
| 14 | Initial LT Fontan | Control group (no prophylactic lesion) |
Spontaneous MRAT, 0/14 (0%) at 9.3 y Inducible MRAT, 0/5 (0%) at 9.3 y |
|
| Lukac et al, 2007 35 | 17 | Mainly ASD | Cryolesion right atriotomy—RAVV |
Spontaneous MRAT, 2/17 (12%) at 3 mo Inducible MRAT, 2/17 (12%) at 3 mo |
ASD indicates atrial septal defect; ATA, atrial tachyarrhythmias; CHD, congenital heart disease; CS, coronary sinus; LT, lateral tunnel; MRAT, macroreentrant atrial tachycardia; and RAVV, right atrioventricular valve.
Lesion locations and energy sources not further specified.
Anti‐Arrhythmic Drugs
Table 3 provides an overview of available data on perioperative use of anti‐arrhythmic drugs (AAD). Twelve studies (39%) provided information on their policy on postoperative AAD use. These policies were more or less in accordance with the 2017 guidelines for surgical treatment of AF, which advise the use of a class III AAD, eg, amiodarone, for at least 2 to 3 months after surgery until stable sinus rhythm is achieved. 14 However, several studies only prescribed AAD in case of early postoperative AF. Thirteen studies (46%) specified the number of patients using perioperative AAD, which varied considerably: preoperative AAD use ranged from 35% to 100% and postoperative AAD use from 0% to 83%. Two studies reported a substantial decrease in the number of patients using AAD after arrhythmia surgery, 22 , 43 whereas use of AAD remained stable or showed only a minimal decrease in 3 studies. 23 , 24 , 39 Data comparing the use of AAD in patients with or without arrhythmia recurrence were reported in 2 studies (54% versus 30%, P=0.04 24 and 81% versus 79%, P value not available 39 ).
Table 3.
Anti‐Arrhythmic Drugs
| Author, y |
Postoperative AAD Policy (Indication, Duration, Class) |
Preoperative AAD Use | Postoperative AAD Use |
|---|---|---|---|
| Sakamoto et al, 2019 40 | All patients, max. 3 mo, AAD class N/A | … | … |
| Gonzalez Corcia et al, 2019 23 | … |
Class I, III*: 24/77 without recurrence 11/24 with recurrence |
Class I, III*: 23/77 without recurrence 13/24 with recurrence |
| Ramdjan and Mouws 2018 39 | … |
Class I to IV, digoxin: MRAT, 5/6 AF, 58/60 |
Class I to IV, digoxin: MRAT, N/A AF, 50/60 27/33 without recurrence 23/27 with recurrence |
| Engelsgaard et al, 2018 21 | All patients, at least early postoperative, AAD class N/A | … | … |
| Lim et al, 2017 33 | … | … |
Class II, 2/27 |
| Giamberti et al, 2017 22 | All patients, at least 3 mo, AAD class III (amiodarone) |
AAD class N/A 51/80 |
AAD class N/A 12/75 |
| Stulak et al, 2015 44 | AF, 3 mo, AAD class III | … | … |
| Wi et al, 2013 47 | … | … |
Class I/III: PAF, : 0/3 PeAF, 5/12 without recurrence |
| Shim et al, 2013 41 |
AF, duration N/A AAD class III (amiodarone) |
… | … |
| Nitta et al, 2013 37 | … | … | … |
| Im et al,2013 26 | AF, AAD class I/III 3 mo, digoxin >3 mo | … | … |
| Gutierrez et al, 2013 24 | … |
Class I, III: 8/19 Class II, digoxin: 16/19 |
Class I, III: 4/19 Class II, digoxin: 13/19 |
| Stulak et al, 2012 42 | … | … | … |
| Atallah et al, 2012 20 | … | … | … |
| Mavroudis et al, 2008 36 | … | … | … |
| Lai et al, 2008 32 | All patients, max. 3 mo, AAD class III (amiodarone) | … | 0/7 |
| Lukac et al, 2007 35 | … | … |
Class I, III: 0/17 Class II, digoxin: 1/17 |
| Stulak et al, 2006 43 | AF, 3 mo, AAD class III |
Cardiac medications: 77/99 Class II: 22% Class III (amiodarone): 15% Digoxin: 42% |
Class I: 1/87 Class II: 12/87 Class III (amiodarone): 8/87 Class IV: 1/87 Digoxin: 24/87 |
| Karamlou et al, 2006 28 | … | … | … |
| Ohtsuka et al, 2005 38 | All patients, duration N/A, digoxin | … | … |
| Khositseth et al, 2004 29 | … | … |
RSM, 4/35 AAD class N/A CTI, 1/9 AAD class III (amiodarone) |
| Huang et al, 2000 25 | … | … | … |
| Kobayashi et al, 1998 30 | … | … | … |
| Kamata et al, 1997 27 | … | … | … |
| Vigano et al, 1996 46 | All patients, duration N/A, AAD class III (amiodarone) | … | Class III (amiodarone): 1/7 |
| Lin et al, 1996 34 | … | Class I, digoxin: 1/2 | 0/2 |
| Kosakai et al, 1995 31 | All patients, until stable SR, AAD class N/A | … | … |
| Suwalski et al, 1994 45 | All patients, 3 mo, AAD class II | Class I to IV, digoxin: 3/3 | … |
AAD indicates anti‐arrhythmic drugs; AF, atrial fibrillation; CTI, exclusive cavotricuspid isthmus ablation; MRAT, macroreentrant atrial tachycardia; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; RSM, right‐sided maze; and SR, sinus rhythm.
Outcomes from sub‐analysis at 1‐year follow‐up
Outcomes According to Type of Congenital Heart Disease
As summarized in Table 1, a considerable number of studies reported outcomes of arrhythmia surgery in a cohort of patients with a variety of CHD. CHD‐specific outcomes were provided in 12 studies for patients with an ASD, in 4 studies for patients with Ebstein anomaly, and in 1 study for patients with tetralogy of Fallot.
Atrial Septal Defect
Most studies (n=8) performed biatrial arrhythmia surgery in patients with an ASD, 4 studies performed right‐sided arrhythmia surgery and 2 did not specify outcomes according to the location of lesions (Figure 3). As described before, 2 studies compared outcomes after biatrial versus right‐sided arrhythmia surgery for AF in patients with ASD, and showed that biatrial lesions were more effective. 26 , 30 Overall, as illustrated in Figure 3, the reported proportion of arrhythmia recurrence after biatrial arrhythmia surgery (median, 5%; IQR, 0%–30%, follow‐up range, 0.4–7 years) appeared to be somewhat smaller than after right‐sided arrhythmia surgery (median, 40%; IQR, 17%–88%, follow‐up range, 2–4.1 years). However, this should be interpreted with great caution as 95% CIs of the proportions in a considerable number of studies were wide.
Figure 3. Outcomes of arrhythmia surgery in patients with an atrial septal defect.
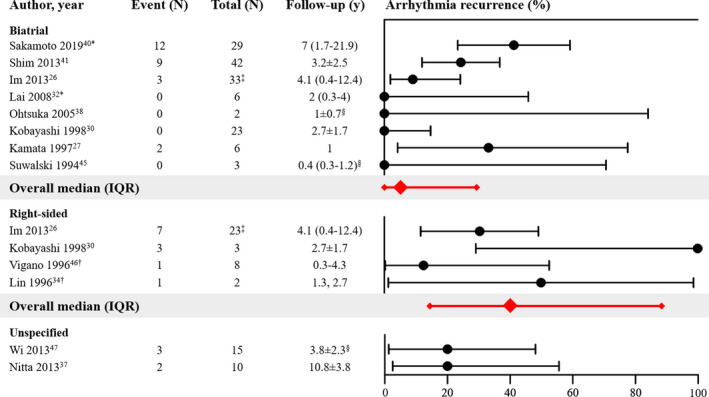
Forest plot of the proportions of patients with arrhythmia recurrence and corresponding 95% CIs. The overall median and interquartile range of proportions are displayed in red. IQR indicates interquartile range. *Outcome measure: recurrence of preoperative arrhythmia or other atrial tachyarrhythmias (not specified). †Right atrial compartment or isolation techniques applied. ‡Number of patients at start of study. § Follow‐up not specified for subgroup.
Two studies demonstrated the positive effect of concomitant arrhythmia surgery on the occurrence of postoperative AF when compared with ASD repair only. In the study of Kobayashi et al, 26 patients with a history of AF underwent ASD repair and concomitant arrhythmia surgery; AF persisted after surgery in 3 patients (12%). However, postoperative AF occurred less often in the 23 patients who regained sinus rhythm after arrhythmia surgery (0/23, 0%) than in patients without a history of AF who only underwent ASD repair (8/45, 18%). 30 In patients with preoperative non‐paroxysmal AF, Wi et al showed that the prevalence of postoperative AF was higher in those undergoing ASD repair only (14/17, 82%) than in those undergoing concomitant arrhythmia surgery (3/12, 25%, P=0.006). 47
Ebstein Anomaly
As displayed in Table 1, 4 studies provided separate outcomes of atrial arrhythmia surgery in patients with Ebstein anomaly, who are particularly at risk of developing ATA because of their often severely enlarged right atrium. 25 , 29 , 31 , 44 Of note, the 2 largest studies of these 4 were performed in the same center and their inclusion period showed an overlap of 6 years (1995–2001); hence, duplicate data may be present in these studies. 29 , 44 However, both studies also provide unique data for parts of their inclusion periods that lasted 5 years (1990–1995 29 ) and 11 years (2001–2012 44 ) respectively. In the initial study, 48 patients underwent right‐sided arrhythmia surgery (right‐sided maze procedure: 38, isthmus ablation: 10), resulting in an overall freedom from recurrent ATA of 74.6%±7.1% during a mean follow‐up of 2.8 years. In the more recent study, 86 patients underwent either right‐sided (n=62) or biatrial arrhythmia surgery (n=24), resulting in an overall freedom from recurrent ATA of 91% during a median follow‐up of 4.5 years. As described before, biatrial lesions were more effective than right‐sided lesions in the treatment of non‐paroxysmal ATA in these patients. In the 2 smaller studies, either right‐sided or biatrial arrhythmia surgery was performed. Both studies reported no recurrence of ATA in any of the patients (0/3 at mean follow‐up of 13.2 years 25 and 0/1 at mean follow‐up of 1.9 years 31 ).
Tetralogy of Fallot
Only 1 study documented the prevalence of arrhythmias in 249 patients with tetralogy of Fallot undergoing reoperation and evaluated the outcomes of arrhythmia surgery in a subset of these patients. 28 Their results showed great advantage of performing arrhythmia surgery in those with documented preoperative arrhythmias. ATA were present before surgery in 41/249 (16%) patients, and 34 of these patients underwent concomitant right‐sided arrhythmia surgery (isthmus ablation: 22, right‐sided maze procedure: 12). The 7.5‐year survival free of recurrent ATA was 75% in patients undergoing arrhythmia surgery, as opposed to 34% of the 7 patients without concomitant arrhythmia intervention (P<0.001).
Factors Associated With Atrial Arrhythmia Recurrence
As shown in Table 4, several studies analyzed the effect of clinical and surgical characteristics on the outcomes of atrial arrhythmia surgery. Independent predictors of arrhythmia recurrence included older age at the time of surgery 23 , 39 , 40 and preoperative atrial arrhythmia duration ≥3 years. 22 One study analyzed factors associated with time‐to‐event (event being the first episode of AF recurrence, new‐onset ATA, or permanent pacemaker implantation), which was decreased in patients undergoing right‐sided maze procedure (versus biatrial arrhythmia surgery) and those with significant preoperative tricuspid regurgitation. 26
Table 4.
Factors Associated With Arrhythmia Recurrence
| Author, y | Variable | Outcome | HR (95% CI) |
|---|---|---|---|
| Sakamoto et al, 2019 40 | Age at surgery | Recurrence |
1.067 (1.001–1.137) P=0.04 |
| Gonzalez Corcia et al, 2019 23 | Age at surgery | Recurrence |
N/A P=0.0018 |
| Ramdjan and Mouws, 2018 39 | Age at surgery | Recurrence |
1.05 (1.015–1.092)* P=0.0006 |
| Giamberti et al, 2017 22 | Duration ATA ≥3 y | Recurrence |
11.95 (2.6–52) P=0.001 |
| Im et al, 2013 26 |
|
Time‐to‐event‡ |
1. 5.11 (1.59–16.44) P=0.006 2. 4.67 (1.38–15.87) P=0.014 |
ATA indicates atrial tachyarrhythmia; HR, hazard ratio; N/A, not available; and TR, tricuspid regurgitation.
Odds ratio.
vs biatrial maze.
Event: recurrence, new‐onset atrial tachyarrhythmia, permanent pacemaker implantation.
New‐Onset Atrial Tachyarrhythmia After Arrhythmia Surgery
Three studies reported on the development of new‐onset regular ATA after arrhythmia surgery, which may arise as a result of incomplete lesions. Two studies reported a relatively high prevalence of new‐onset regular ATA of, respectively 20% and 24% after 3.8 and 2 years of follow‐up in patients who had AF before arrhythmia surgery. 39 , 47 One of these studies even demonstrated that the prevalence of new‐onset ATA was higher in patients with arrhythmia surgery (20%) than in those without (8%; P value not provided). 47 In addition, Lukac et al investigated the outcome of prophylactic cryolesions between the right atriotomy and the tricuspid annulus. In their study, new‐onset spontaneous or inducible atrial flutter was observed in patients without bidirectional block within 3 months after arrhythmia surgery. 35 Hence, this study supports the hypothesis of incomplete lesions as a potential cause of the development of new‐onset regular ATA after arrhythmia surgery.
Permanent Pacemaker Implantation
As displayed in Figure 4, 20 studies (71%) reported the number of patients requiring permanent pacemaker implantation, which varied between 0% and 42% (median, 9.6%; IQR, 0%–20%) during follow‐up ranging from 0.3 to 15.2 years. When only considering the 13 studies with sample size >20 patients, the median reported number of pacemaker implantations increased to 15% (IQR, 3%–28%) during follow‐up ranging from 1 to 15.2 years.
Figure 4. Permanent pacemaker implantation.
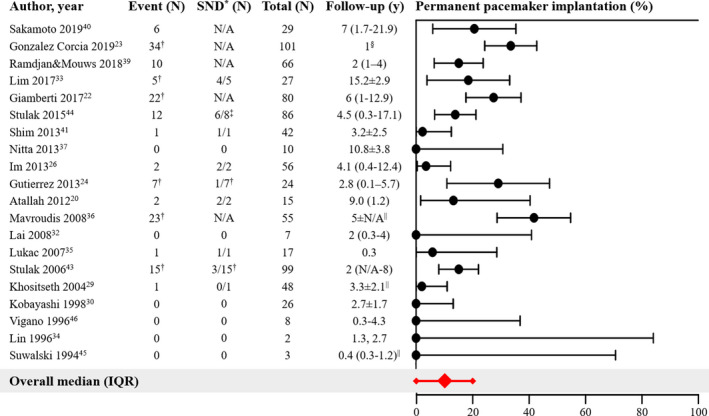
Forest plot of the proportions of patients with permanent pacemaker implantation and corresponding 95% CIs. The overall median and interquartile range of proportions are displayed in red. IQR indicates interquartile range; and SND, sinus node dysfunction. *Indication for permanent pacemaker implantation. †Including intraoperatively implanted pacemakers. ‡Indication only provided for the 8 early pacemaker implantations. §Separate outcomes only available in sub‐analysis at 1‐year follow‐up. ||Follow‐up not specified for subgroup.
Only 9 studies provided indications for pacemaker implantation, which was sinus node dysfunction (SND) in most cases. In 6 studies, the number of permanent pacemakers implanted included those implanted intraoperatively. Indications for intraoperative pacemaker implantation were atrioventricular conduction block or sinus node dysfunction, 24 , 33 , 43 or implantation as part of the Fontan conversion procedure (<25% of the population). 22 , 23 , 24 , 43 In 3 studies, the indications of some or all intraoperative pacemaker implantations were not provided (23/23, 36 2/22, 22 unknown proportion of 34 23 ).
Discussion
Summary of Evidence
In this systematic review, we aimed to evaluate the outcomes of arrhythmia surgery for MRAT and AF in patients with CHD undergoing cardiac surgery other than Fontan conversion. The variation in lesion sets and energy sources used was striking, an observation that was appropriately captured by Gonzalez Corcia et al: “Over time, ‘maze’ has become synonymous with just about any lesion that is applied to the atria as therapy for arrhythmias.” 23 Not only did surgical techniques vary between studies, but the indications for which specific procedures were performed also differed. Even though these significant variations precluded any meaningful meta‐analyses, the following conclusions can be drawn from the qualitative synthesis of the data.
Based on the available data included in this review, we can conclude that the creation of biatrial lesions (rather than exclusive right‐sided lesions) is the preferred strategy in the surgical treatment of paroxysmal or non‐paroxysmal AF in this population. More specifically, patients with an ASD and a history of AF (paroxysmal or non‐paroxysmal) appear to benefit from concomitant biatrial arrhythmia surgery during ASD repair. Although evidence is limited, the right‐sided maze procedure is likely the most appropriate treatment of MRAT without documented AF, also because there is no evidence for superiority of biatrial lesions in this situation. To date, there is not enough data to support the use of exclusive left‐sided lesions in patients with CHD. CHD‐specific outcomes were not only provided for ASD, but also for Ebstein anomaly and tetralogy of Fallot, although the amount of data was limited. It may however be reasonable to assume that the conclusions stated above also apply to these lesions, as they are associated with predominantly right‐sided disease and outcomes of arrhythmia surgery were not vastly different from those in patients with ASD. As none of the studies compared outcomes of different energy sources, we cannot provide specific recommendations on the type of energy sources to be used for creation of lesions in this population.
Arrhythmia Surgery Techniques
In 1998, the right‐sided maze procedure was proposed for patients with right‐sided CHD, after several reports had published their experience with exclusive left‐sided lesions. 50 It was assumed that the left atrium was relatively unaffected in patients with right‐sided CHD. Limiting the creation of lesions to the supposedly affected atrium resulted in a significant simplification and shortening of the original procedure, with a lower risk of complications. 43 , 50 However, also in 1998, Kobayashi et al disputed the efficacy of exclusive right‐sided lesions compared with biatrial lesions in the treatment of AF in patients with ASD. 30 Years later, Im et al confirmed in a larger cohort of patients with ASD who exclusive right‐sided lesions were less effective in the treatment of AF than biatrial lesions. 26 These results indicate that the left atrium may contribute at least in part to the substrate of AF in these patients, even if their CHD is predominantly right‐sided.
In line with these observations, most studies in this review performed biatrial arrhythmia surgery for paroxysmal or non‐paroxysmal AF. The median reported number of arrhythmia recurrences after biatrial arrhythmia surgery was 13% (IQR, 0%–27%) in all studies and 20% (IQR, 11%–39%) in studies with sample size >20. The type of preoperative AF did not appear to affect late success, although this was only reported by one study in this review. 40 Extensive variations in follow‐up durations and surgical techniques limit the ability to comment on the efficacy of this procedure in the CHD population relative to that in a general AF population without CHD (7% after 1 year, 22% after 5 years; no difference between types of AF). 52
Although the right‐sided maze is not as effective as the biatrial maze for treatment of AF, it is likely the most appropriate treatment strategy in patients with MRAT (without prior documented AF). From a mechanistic point of view, this can be explained by the fact that most MRATs are located in the right atrium, which is subject to longstanding pressure or volume overload and, often, surgical scarring. 53 One study included in this review reported no recurrences during a median follow‐up of 2 years after right‐sided arrhythmia surgery for MRAT. 43 Importantly, not 1 study provided evidence in favor of performing biatrial arrhythmia surgery for MRAT. There were no studies specifically investigating the effect of duration of preoperative MRAT (paroxysmal versus non‐paroxysmal) on the outcomes of arrhythmia surgery. Stulak et al indicate that they would favor biatrial lesions over right‐sided lesions in case of “longer‐standing arrhythmias”, although they did not differentiate between AF and MRAT in this recommendation nor in their results. 44
Since only few studies included in this review (5/28) performed exclusive left‐sided arrhythmia surgery in a small subset of patients without providing separate outcomes, we cannot form a solid conclusion on this matter. However, prior studies on a more general surgical population demonstrated the superiority of biatrial lesions over left atrial lesions only, particularly in case of persistent AF. 54 , 55 In turn, a complete left atrial lesion set—generally consisting of pulmonary vein isolation, connecting lesions to the mitral valve annulus and the left atrial appendage, and excision of the left atrial appendage—has also been shown to be more effective than pulmonary vein isolation alone. 56 , 57
Energy Sources
The complexity of arrhythmia surgery has decreased somewhat because of the emergence of alternative energy sources replacing the cut‐and‐sew lesions of the original Cox Maze III procedure. 26 However, in contrast to the cut‐and‐sew technique, continuity and transmurality of lesions created by alternative energy sources may be incomplete. 58 Various energy sources have been applied in the studies in this review, although none provided separate outcomes. Radiofrequency ablation was the most commonly applied method in the more recent studies (>2008). A large systematic review by Khargi et al including 48 studies and 3832 patients compared outcomes of surgical AF ablation using either the cut‐and‐sew technique or alternative energy sources. 58 There was no difference in freedom from AF between the 2 groups. As patients with CHD often have thickened and scarred myocardium, the risk of incomplete lesions when using alternative sources may still be relevant in this specific population. To minimize this risk, irrigated radiofrequency may be used. Cooling of the catheter tip allows for higher power levels and hence the ability to create larger and deeper lesions. 59 A recent study of Ad et al demonstrated the superiority of cryothermal energy over radiofrequency energy, particularly in patients with larger left atrial size and longer AF duration. 60 Of note, the successful use of cryothermal energy is dependent on tissue thickness, requiring multiple freezes to obtain complete lesions in thicker target tissue.
Prophylactic Arrhythmia Surgery
The 2014 PACES/HRS guidelines and a 2018 position paper by the European Heart Rhythm Association (EHRA)/Association for European Paediatric and Congenital Cardiology (AEPC)/European Society of Cardiology (ESC) recommend that prophylactic arrhythmia surgery may be considered in certain situations (patients with Ebstein anomaly or atrial dilatation undergoing surgery, patients with CHD undergoing re‐operation). 11 , 12 However, these recommendations were based on expert opinion or extrapolated from studies on therapeutic arrhythmia surgery in patients with CHD or prophylactic arrhythmia surgery in patients with non‐congenital mitral valve disease. Our extensive literature search only identified 4 studies describing outcomes of prophylactic arrhythmia surgery in only a small number of patients. The only randomized controlled trial included in this review was not able to draw conclusions on the efficacy of a prophylactic lesion during the lateral tunnel Fontan procedure, given the lack of occurrence of the primary end point in both the intervention and the control group. Another study applied prophylactic right‐sided lesions in most patients, which were not standardized and may have varied from patient to patient. 23 Postoperative occurrences of MRAT (either spontaneous or induced) were observed in 2 studies, in which a prophylactic lesion between the right atriotomy and the right atrioventricular valve annulus was applied using cryoenergy. 33 , 35 In 1 of these studies, the authors confirmed that these arrhythmias were caused by incomplete cryolesions. 35 Similar observations were described in a study reporting characteristics of new‐onset ATA after catheter ablation of AF in a mixed population. 61 In this study, nearly all ATA were related to gaps in prior ablation lines. As prophylactic arrhythmia surgery is performed without knowing if the patient will ever develop atrial arrhythmias, the development of arrhythmias attributable to incomplete lesions is an extremely undesirable outcome. Although prophylactic arrhythmia surgery may be beneficial for patients with CHD with specific anatomic substrates predisposing them to the development of ATA, it is yet unknown which lesion sets should be applied and which energy sources should be used. Based on the 4 studies that we identified in our literature search, we are unable to provide recommendations on specific surgical techniques for this matter. This indicates the need for studies investigating the outcomes of prophylactic arrhythmia in patients with CHD; ideally these are randomized studies with a sufficient sample size and follow‐up duration following a standardized approach. This was also acknowledged by Mavroudis et al, who suggested prophylactic lesion sets to be used in standardized experimental protocols. 62
Permanent Pacemaker Implantation
Twenty of the studies included in this review reported numbers of patients requiring permanent pacemaker implantation varying between 0% and 42%. Apart from those implanted in the context of Fontan conversion, the most common indication for pacemaker implantation was SND. It is widely recognized that permanent pacemaker implantation for SND is a potential adverse outcome of atrial arrhythmia surgery. 63 Underlying SND may be unmasked once the ATA is successfully abolished. 64 Furthermore, injury to the sinoatrial node or its arteries may cause postoperative SND, although this is less likely to occur because of technical improvements and increased experience over the years. 63 , 65
The wide range in reported numbers of pacemaker implantations may be because of the fact that policies differ between centers, for example on the indications for intraoperative pacemaker implantation. Furthermore, policies may have changed over the years as experience with arrhythmia surgery has evolved. Whereas in earlier years, early postoperative junctional rhythm may have been an indication for pacemaker implantation, experience has shown that stable sinus rhythm returns in many of these patients. 63 , 65 In addition, the number of pacemaker implantations in some studies included those implanted as a part of the Fontan conversion procedure. For these reasons, and given the large heterogeneity in follow‐up durations, study populations and arrhythmia surgery techniques, it is not possible to draw conclusions on whether pacemaker implantation in patients with CHD is more often required than in the general population (around 10%). 65
Strengths and Limitations at Study and Outcome Level
Except for some of the more recent studies, sample sizes were relatively small, as is often the case in studies involving patients with CHD. Similar to studies evaluating the outcomes of endovascular AF ablation in patients with CHD, 66 ASD was the predominant CHD type in most studies. Study designs were non‐randomized and mostly retrospective in nature. Despite limitations generally associated with these designs, overall quality of the studies was acceptable. In some studies, patients with CHD were a subset of a larger group of patients not included in this review. As a result, more detailed information beyond the number of patients with an arrhythmia recurrence was often not provided (eg, outcomes according to lesion set or CHD type, pacemaker implantation). Although most studies reported the number of arrhythmia recurrences, a considerable number of studies (n=10) did not differentiate between recurrence versus new‐onset ATA, and 1 study reported outcomes as recurrence or use of anti‐arrhythmic drugs. Data on the use of perioperative AAD were relatively scarce and heterogeneous, thereby limiting the ability to provide solid conclusions on the possible influence of AAD on outcomes of arrhythmia surgery in this population. Furthermore, indications for permanent pacemaker implantation were not always provided. The number of pacemaker implantations in some studies included those implanted intraoperatively, which may be attributable to a variety of indications other than those directly related to arrhythmia surgery.
The large variation in follow‐up durations among the included studies complicates the interpretation of outcomes, particularly since most studies did not report yearly event rates or the number of recurrences at fixed time points (eg 1 year, 5 years). It may be reasonable to expect that the duration of follow‐up is related to the number of arrhythmia recurrences. We chose not to calculate yearly event rates, because we did not have individual study data at our disposal. Also, since the distribution of follow‐up duration appeared skewed in many studies, extrapolation to rates merely based on presented number of recurrences and mean or median follow‐up duration may potentially have led to incorrect results. This limited our options for performing a meaningful meta‐analysis. In addition, the proportion of arrhythmia recurrence in a considerable number of studies was 0 or 1; small sample size contributed to this. For inclusion in a meta‐analysis, corrections that account for such proportions would have to be made, with arguable consequences for the results. Finally, even after dividing results into relevant subcategories, significant heterogeneity remained regarding study populations, definitions of outcome measures and variations in lesion sets and energy sources. For these reasons, we deemed a meta‐analysis unable to provide meaningful results here and chose to refrain from it.
Strengths and Limitations at Review Level
This review was conducted according to the PRISMA guidelines, thereby providing transparency of the methods and a systematic and uniform approach to answering our primary research question. 15 We included studies from many different countries and centers, which broadens the perspective on the one hand, but is accompanied by various levels of expertise, patients volumes, and center‐specific policies on the other hand, which should be considered when interpreting the results. We did not set a start date restriction for the literature search, as there was no concrete evidence on the basis of which a specific year or time period should have been selected. Furthermore, this approach resulted in a complete overview of the evolution of atrial arrhythmia surgery in patients with CHD. Inevitably, this decision in itself causes heterogeneity among studies, given the changes in surgical techniques over the years. Only studies written in the English language were included, which may have led to the exclusion of potentially relevant studies. We decided not to exclude studies including also patients undergoing Fontan conversion, as this would often have led to the exclusion of a substantial number of other patients relevant to the primary research question. Instead, by setting specific inclusion criteria (ie, <25% of patients undergoing Fontan conversion) we limited the influence of these patients on the outcomes.
Conclusions
This systematic review summarized outcomes of atrial arrhythmia surgery in patients with CHD published over a time span of 25 years. Regardless of the many variations in indications, surgical techniques, and follow‐up durations, this review reports a median arrhythmia recurrence of 13% (IQR, 4%–26%). More specifically, based on the acquired data, biatrial lesions are preferred in the treatment of AF, whereas exclusive right‐sided lesions may be more appropriate in the treatment of MRAT. To date, it is unclear whether the addition of left‐sided lesions would be beneficial to the treatment of MRAT. Theoretically, prophylactic atrial arrhythmia surgery may be beneficial in this population, but evidence is currently limited. To be able to provide more specific recommendations, future studies should specifically report outcomes according to the type of preoperative arrhythmia, underlying CHD, lesion set, and energy source, as this is essential for determining which surgical technique should ideally be applied under which circumstances. Additionally, differentiation between recurrence and new‐onset regular ATA should be made and indications for pacemaker implantation clearly described, to be able to assess potential adverse outcomes.
Sources of Funding
This work was supported by grants from CardioVasculair Onderzoek Nederland, 914728; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Vidi grant 91717339; Biosense Webster USA, ICD 783454; and Medical Delta to prof. dr. de Groot.
Disclosures
None.
Supporting information
Data S1
(J Am Heart Assoc. 2020;9:e016921 DOI: 10.1161/JAHA.120.016921.)
For Sources of Funding and Disclosures, see page 16.
Footnotes
References
- 1. Khairy P, Ionescu‐Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;1149–1157. [DOI] [PubMed] [Google Scholar]
- 2. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;2241–2247. [DOI] [PubMed] [Google Scholar]
- 3. Verheugt CL, Uiterwaal CS, Vaartjes I, van der Velde ET, Zomer AC, Meijboom FJ, Pieper PG, Post MC, Vliegen HW, Hazekamp MG, et al. Chance of surgery in adult congenital heart disease. Eur J Prev Cardiol. 2017;1319–1327. [DOI] [PubMed] [Google Scholar]
- 4. Zomer AC, Verheugt CL, Vaartjes I, Uiterwaal CS, Langemeijer MM, Koolbergen DR, Hazekamp MG, van Melle JP, Konings TC, Bellersen L, et al. Surgery in adults with congenital heart disease. Circulation. 2011;2195–2201. [DOI] [PubMed] [Google Scholar]
- 5. Srinathan SK, Bonser RS, Sethia B, Thorne SA, Brawn WJ, Barron DJ. Changing practice of cardiac surgery in adult patients with congenital heart disease. Heart. 2005;207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;50–60. [DOI] [PubMed] [Google Scholar]
- 7. Avila P, Oliver JM, Gallego P, Gonzalez‐Garcia A, Rodriguez‐Puras MJ, Cambronero E, Ruiz‐Cantador J, Campos A, Peinado R, Prieto R, et al. Natural history and clinical predictors of atrial tachycardia in adults with congenital heart disease. Circ Arrhythm Electrophysiol. 2017;9:e005396 10.1161/CIRCEP.117.005396. [DOI] [PubMed] [Google Scholar]
- 8. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;1679–1686. [DOI] [PubMed] [Google Scholar]
- 9. Ramdjan T, Mouws E, Teuwen CP, Sitorus GDS, Houck CA, Bogers A, de Groot NMS. Progression of late postoperative atrial fibrillation in patients with tetralogy of fallot. J Cardiovasc Electrophysiol. 2018;30–37. [DOI] [PubMed] [Google Scholar]
- 10. Teuwen CP, Ramdjan TT, Gotte M, Brundel BJ, Evertz R, Vriend JW, Molhoek SG, Dorman HG, van Opstal JM, Konings TC, et al. Time course of atrial fibrillation in patients with congenital heart defects. Circ Arrhythm Electrophysiol. 2015;1065–1072. [DOI] [PubMed] [Google Scholar]
- 11. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot N, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;e102–e165. [DOI] [PubMed] [Google Scholar]
- 12. Hernandez‐Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown‐up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;1719–1753. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;2893–2962. [DOI] [PubMed] [Google Scholar]
- 14. Badhwar V, Rankin JS, Damiano RJ Jr, Gillinov AM, Bakaeen FG, Edgerton JR, Philpott JM, McCarthy PM, Bolling SF, Roberts HG, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2017;329–341. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;9:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P.The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 7, 2020.
- 17. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898. [DOI] [PubMed] [Google Scholar]
- 18. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;404–413. [Google Scholar]
- 19. 9.1.4 when not to use meta‐analysis in a review. Available at: http://handbook-5-1.cochrane.org/chapter_9/9_1_4_when_not_to_use_meta_analysis_in_a_review.htm. Accessed January 29, 2020.
- 20. Atallah J, Collins KK, Jonas RA, Mayer JE Jr, Triedman JK. Follow‐up of a modified fontan randomized trial for intraatrial reentrant tachycardia prophylaxis. Congenit Heart Dis. 2012;219–225. [DOI] [PubMed] [Google Scholar]
- 21. Engelsgaard CS, Pedersen KB, Riber LP, Pallesen PA, Brandes A. The long‐term efficacy of concomitant maze iv surgery in patients with atrial fibrillation. Int J Cardiol Heart Vasc. 2018;20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giamberti A, Pluchinotta FR, Chessa M, Varrica A, Vitale R, Frigiola A, Pappone C, Ranucci M. Surgery for supraventricular tachycardia and congenital heart defects: long‐term efficacy of the combined approach in adult patients. Europace. 2017;1542–1548. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez Corcia MC, Walsh EP, Emani S. Long‐term results of atrial maze surgery in patients with congenital heart disease. Europace. 2019;1345–1352. [DOI] [PubMed] [Google Scholar]
- 24. Gutierrez SD, Earing MG, Singh AK, Tweddell JS, Bartz PJ. Atrial tachyarrhythmias and the Cox‐maze procedure in congenital heart disease. Congenit Heart Dis. 2013;434–439. [DOI] [PubMed] [Google Scholar]
- 25. Huang CJ, Chiu IS, Lin FY, Chen WJ, Lin JL, Lo HM, Wu MH, Chu SH. Role of electrophysiological studies and arrhythmia intervention in repairing Ebstein's anomaly. Thorac Cardiovasc Surg. 2000;347–350. [DOI] [PubMed] [Google Scholar]
- 26. Im YM, Kim JB, Yun SC, Lee JW, Chung CH, Park JJ, Yun TJ. Arrhythmia surgery for atrial fibrillation associated with atrial septal defect: right‐sided maze versus biatrial maze. J Thorac Cardiovasc Surg. 2013;648–655.e641. [DOI] [PubMed] [Google Scholar]
- 27. Kamata J, Kawazoe K, Izumoto H, Kitahara H, Shiina Y, Sato Y, Nakai K, Ohkubo T, Tsuji I, Hiramori K. Predictors of sinus rhythm restoration after cox maze procedure concomitant with other cardiac operations. Ann Thorac Surg. 1997;394–398. [DOI] [PubMed] [Google Scholar]
- 28. Karamlou T, Silber I, Lao R, McCrindle BW, Harris L, Downar E, Webb GD, Colman JM, Van Arsdell GS, Williams WG. Outcomes after late reoperation in patients with repaired tetralogy of Fallot: the impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg. 2006;1786–1793. [DOI] [PubMed] [Google Scholar]
- 29. Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg. 2004;826–833. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi J, Yamamoto F, Nakano K, Sasako Y, Kitamura S, Kosakai Y. Maze procedure for atrial fibrillation associated with atrial septal defect. Circulation. 1998;II399–II402. [PubMed] [Google Scholar]
- 31. Kosakai Y, Kawaguchi AT, Isobe F, Sasako Y, Nakano K, Eishi K, Kito Y, Kawashima Y. Modified maze procedure for patients with atrial fibrillation undergoing simultaneous open heart surgery. Circulation. 1995;II359–II364. [DOI] [PubMed] [Google Scholar]
- 32. Lai YQ, Li JH, Li JW, Xu SD, Luo Y, Zhang ZG. Concomitant irrigated monopolar radiofrequency ablation of atrial fibrillation in adults with congenital heart disease. Interact Cardiovasc Thorac Surg. 2008;80–82. [DOI] [PubMed] [Google Scholar]
- 33. Lim HG, Lee JR, Kim YJ. The effects of modification to lateral tunnel fontan procedure for prophylactic arrhythmia surgery. Ann Thorac Surg. 2017;197–204. [DOI] [PubMed] [Google Scholar]
- 34. Lin FY, Huang JH, Lin JL, Chen WJ, Lo HM, Chu SH. Atrial compartment surgery for chronic atrial fibrillation associated with congenital heart defects. J Thorac Cardiovasc Surg. 1996;231–237. [DOI] [PubMed] [Google Scholar]
- 35. Lukac P, Hjortdal VE, Pedersen AK, Mortensen PT, Jensen HK, Hansen PS. Prevention of atrial flutter with cryoablation may be proarrhythmogenic. Ann Thorac Surg. 2007;1717–1723. [DOI] [PubMed] [Google Scholar]
- 36. Mavroudis C, Deal BJ, Backer CL, Tsao S. Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg. 2008;857–868. [DOI] [PubMed] [Google Scholar]
- 37. Nitta T, Sakamoto SI, Miyagi Y, Fujii M, Ishii Y, Ochi M. Reentrant and focal activations during atrial fibrillation in patients with atrial septal defect. Ann Thorac Surg. 2013;1266–1272. [DOI] [PubMed] [Google Scholar]
- 38. Ohtsuka T, Ninomiya M, Maemura T. Cardioscopic trans‐septal cryoablation of left atrium in nonmitral cases. Innovations (Phila). 2005;48–50. [DOI] [PubMed] [Google Scholar]
- 39. Ramdjan TTTK, Mouws EMJP, Kik C, Roos‐Hesselink JW, Bogers AJJC, De Groot NMS. Concomitant arrhythmia surgery in patients with congenital heart disease. Interact Cardiovasc Thorac Surg. 2018;902–909. [DOI] [PubMed] [Google Scholar]
- 40. Sakamoto SI, Hiromoto A, Ishii Y, Sasaki T, Miyagi Y, Nitta T. Surgical outcomes of modified‐maze procedures in adults with atrial septal defect. Surg Today. 2019;124–129. [DOI] [PubMed] [Google Scholar]
- 41. Shim H, Yang JH, Park PW, Jeong DS, Jun TG. Efficacy of the maze procedure for atrial fibrillation associated with atrial septal defect. Korean J Thorac Cardiovasc Surg. 2013;98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stulak JM, Dearani JA, Burkhart HM, Park SJ, Suri RM, Schaff HV. The surgical treatment of concomitant atrial arrhythmias during redo cardiac operations. Ann Thorac Surg. 2012;1894–1900. [DOI] [PubMed] [Google Scholar]
- 43. Stulak JM, Dearani JA, Puga FJ, Zehr KJ, Schaff HV, Danielson GK. Right‐sided maze procedure for atrial tachyarrhythmias in congenital heart disease. Ann Thorac Surg. 2006;1780–1785. [DOI] [PubMed] [Google Scholar]
- 44. Stulak JM, Sharma V, Cannon BC, Ammash N, Schaff HV, Dearani JA. Optimal surgical ablation of atrial tachyarrhythmias during correction of ebstein anomaly. Ann Thorac Surg. 2015;1700–1705. [DOI] [PubMed] [Google Scholar]
- 45. Suwalski K, Pytkowski M, Zelazny P, Majstrak F, Kaszczynski T, Pasierski T, Rzaczynska M, Wojciechowski D. Surgery as an effective nonpharmacological mode of treatment of atrial fibrillation resistant to standard therapy. Pacing Clin Electrophysiol. 1994;2167–2171. [DOI] [PubMed] [Google Scholar]
- 46. Viganò M, Graffigna A, Ressia L, Minzioni G, Pagani F, Aiello M, Gazzoli F. Surgery for atrial fibrillation. Eur J Cardiothorac Surg. 1996;490–497. [DOI] [PubMed] [Google Scholar]
- 47. Wi J, Choi JY, Shim JM, Uhm JS, Hwang HJ, Kim JY, Pak HN, Joung B, Lee M. Fate of preoperative atrial fibrillation after correction of atrial septal defect. Circ J. 2013;109–115. [DOI] [PubMed] [Google Scholar]
- 48. Cox JL, Jaquiss RD, Schuessler RB, Boineau JP. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg. 1995;485–495. [DOI] [PubMed] [Google Scholar]
- 49. Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, Moon MR, Moazami N, Lawton JS, Damiano RJ Jr. The effect of ablation technology on surgical outcomes after the Cox‐maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;389–396. [DOI] [PubMed] [Google Scholar]
- 50. Theodoro DA, Danielson GK, Porter CBJ, Warnes CA. Right‐sided maze procedure for right atrial arrhythmias in congenital heart disease. Ann Thorac Surg. 1998;149–154. [DOI] [PubMed] [Google Scholar]
- 51. Giamberti A, Chessa M, Abella R, Butera G, Negura D, Foresti S, Carminati M, Cappato R, Frigiola A. Surgical treatment of arrhythmias in adults with congenital heart defects. Int J Cardiol. 2008;37–41. [DOI] [PubMed] [Google Scholar]
- 52. Henn MC, Lancaster TS, Miller JR, Sinn LA, Schuessler RB, Moon MR, Melby SJ, Maniar HS, Damiano RJ Jr. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2015;1168–1176, 1178.e1161–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;534–545. [DOI] [PubMed] [Google Scholar]
- 54. Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta‐analysis. J Thorac Cardiovasc Surg. 2006;1029–1035. [DOI] [PubMed] [Google Scholar]
- 55. Onorati F, Mariscalco G, Rubino AS, Serraino F, Santini F, Musazzi A, Klersy C, Sala A, Renzulli A. Impact of lesion sets on mid‐term results of surgical ablation procedure for atrial fibrillation. J Am Coll Cardiol. 2011;931–940. [DOI] [PubMed] [Google Scholar]
- 56. Gaita F, Riccardi R, Caponi D, Shah D, Garberoglio L, Vivalda L, Dulio A, Chiecchio A, Manasse E, Gallotti R. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long‐term clinical results. Circulation. 2005;136–142. [DOI] [PubMed] [Google Scholar]
- 57. Melby SJ, Zierer A, Bailey MS, Cox JL, Lawton JS, Munfakh N, Crabtree TD, Moazami N, Huddleston CB, Moon MR, et al. A new era in the surgical treatment of atrial fibrillation: the impact of ablation technology and lesion set on procedural efficacy. Ann Surg. 2006;583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khargi K, Hutten BA, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;258–265. [DOI] [PubMed] [Google Scholar]
- 59. Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R, Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline‐irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995;2264–2273. [DOI] [PubMed] [Google Scholar]
- 60. Ad N, Holmes SD, Rongione AJ, Massimiano PS, Fornaresio LM. Does surgical ablation energy source affect long‐term success of the concomitant Cox maze procedure? Ann Thorac Surg. 2017;29–35. [DOI] [PubMed] [Google Scholar]
- 61. Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford T, Wells D, Sarrazin JF, Chalfoun N, Kuhne M, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;1781–1787. [DOI] [PubMed] [Google Scholar]
- 62. Mavroudis C, Stulak JM, Ad N, Siegel A, Giamberti A, Harris L, Backer CL, Tsao S, Dearani JA, Weerasena N, et al. Prophylactic atrial arrhythmia surgical procedures with congenital heart operations: review and recommendations. Ann Thorac Surg. 2015;352–359. [DOI] [PubMed] [Google Scholar]
- 63. Cox JL, Ad N, Churyla A, Malaisrie SC, Pham DT, Kruse J, Kislitsina ON, McCarthy PM. The maze procedure and postoperative pacemakers. Ann Thorac Surg. 2018;1561–1569. [DOI] [PubMed] [Google Scholar]
- 64. Cox JL. Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol. 2004;250–262. [DOI] [PubMed] [Google Scholar]
- 65. Stulak JM, Sundt TM III, Dearani JA, Daly RC, Orsulak TA, Schaff HV. Ten‐year experience with the Cox‐maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg. 2007;1319–1324. [DOI] [PubMed] [Google Scholar]
- 66. Pranata R, Tondas AE, Yonas E, Chintya V, Yamin M. Efficacy and safety of catheter ablation for atrial fibrillation in congenital heart disease—a systematic review and meta‐analysis. Indian Pacing Electrophysiol J. 2019;216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


