Abstract
Background
Cardiac rehabilitation is an established performance measure for adults with ischemic heart disease, but patient participation is remarkably low. Home‐based cardiac rehabilitation (HBCR) may be more practical and feasible, but evidence regarding its efficacy is limited. We sought to compare the effects of HBCR versus facility‐based cardiac rehabilitation (FBCR) on functional status in patients with ischemic heart disease.
Methods and Results
This was a pragmatic trial of 237 selected patients with a recent ischemic heart disease event, who enrolled in HBCR or FBCR between August 2015 and September 2017. The primary outcome was 3‐month change in distance completed on a 6‐minute walk test. Secondary outcomes included rehospitalization as well as patient‐reported physical activity, quality of life, and self‐efficacy. Characteristics of the 116 patients enrolled in FBCR and 121 enrolled in HBCR were similar, except the mean time from index event to enrollment was shorter for HBCR (25 versus 77 days; P<0.001). As compared with patients undergoing FBCR, those in HBCR achieved greater 3‐month gains in 6‐minute walk test distance (+95 versus +41 m; P<0.001). After adjusting for demographics, comorbid conditions, and indication, the mean change in 6‐minute walk test distance remained significantly greater for patients enrolled in HBCR (+101 versus +40 m; P<0.001). HBCR participants reported greater improvements in quality of life and physical activity but less improvement in exercise self‐efficacy. There were no deaths or cardiovascular hospitalizations.
Conclusions
Patients enrolled in HBCR achieved greater 3‐month functional gains than those enrolled in FBCR. Our data suggest that HBCR may safely derive equivalent benefits in exercise capacity and overall program efficacy in selected patients.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02105246.
Keywords: cardiac rehabilitation, exercise, outcomes, telemedicine
Subject Categories: Rehabilitation, Exercise, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- 6MWT
6‐minute walk test
- CABG
coronary artery bypass graft
- CR
cardiac rehabilitation
- FBCR
facility‐based cardiac rehabilitation
- HBCR
home‐based cardiac rehabilitation
- IHD
ischemic heart disease
- PCI
percutaneous coronary intervention
- VA
Veterans Administration
Clinical Perspective
What Is New?
A pragmatic trial of home‐based cardiac rehabilitation compared with traditional facility‐based cardiac rehabilitation was similarly efficacious in terms of functional status improvement and multiple patient‐reported measures.
Home‐based cardiac rehabilitation could be efficiently and safely provided to patients with a wide range of clinical indications and comorbidities.
What Are the Clinical Implications?
Home‐based cardiac rehabilitation may be an effective alternative for selected patients who are unable or unwilling to participate in facility‐based cardiac rehabilitation and can help reduce delays to enrollment compared with facility‐based programs.
Cardiac rehabilitation (CR) constitutes a multidisciplinary intervention that is recommended for most patients after hospitalization for multiple conditions including myocardial infarction, percutaneous coronary intervention (PCI), or coronary artery bypass graft surgery (CABG). 1 , 2 , 3 , 4 In a meta‐analysis of randomized trials, facility‐based CR (FBCR) was associated with fewer hospital readmissions and a 26% reduction in cardiovascular mortality. 5 On the basis of overwhelming evidence that participation in such programs diminishes morbidity and mortality and reduces rehospitalizations, referral of patients to FBCR has become a performance measure for adults with ischemic heart disease (IHD). 2 , 6 Yet, despite such strong recommendations, FBCR remains vastly underused after IHD events. Less than 20% of eligible patients participate in the United States. 7 , 8
A significant barrier to enrollment in FBCR is the requirement by Medicare and other health insurance providers that outpatient FBCR be delivered in a physician's office or hospital‐based facility setting. Patients are expected to travel to an FBCR location for supervised exercise sessions 2 to 3 times per week for 12 to 36 sessions. However, many patients live too far from an FBCR program for this to be practical, and even when nearby programs are available, many patients do not have the time, transportation, social support, or financial resources to attend a facility‐based program. 9 For patients in rural areas, participation is especially unlikely. Additional barriers to participation have been well‐described and are numerous. 10 , 11 , 12 , 13 In 2011, an American Heart Association Presidential Advisory called for a reengineering of CR programs to better accommodate patient needs 7 and for trials that demonstrate their value.
Home‐based CR (HBCR) is an alternative model of delivery that has been suggested as one important opportunity to improve participation. 14 It is logistically more realistic and potentially less costly. A Cochrane meta‐analysis of 17 randomized trials found that HBCR and FBCR had similar effects on exercise capacity, mortality, cardiovascular risk factors, and health‐related quality of life. 15 Moreover, the 2011 American Heart Association/American College of Cardiology Foundation guidelines explicitly stated that HBCR can be substituted for FBCR in low‐risk patients 1 and released a joint scientific statement in 2019 in support of HBCR. 14 Despite these endorsements, there is no reimbursement model for HBCR in the United States. A key issue is lack of adequate high‐quality data to justify coverage. 7 , 14 In addition, skepticism about the benefits of HBCR may stem from lack of standardization across programs. While HBCR and FBCR have similar core components (exercise training, behavioral modification, and psychosocial support), 14 , 16 , 17 trials of HBCR have entailed highly variable interventions including differences in staffing, communication, education, and transitions. In contrast, most FBCR programs undergo a standardized certification process by the American Association of Cardiovascular and Pulmonary Rehabilitation that leads to general consistency in format, content, and process.
In this study, we sought to compare the effects of HBCR and FBCR in patients with IHD ( myocardial infarction, PCI, or CABG). Since functional gain is often used as an objective marker of program efficacy, we evaluated 3‐month change in 6‐minute walk test (6MWT) distance as the primary outcome measure. We also evaluated several patient‐reported outcomes, including physical activity, quality of life, and self‐efficacy.
Methods
The data that support the findings of this study may be made available from the corresponding author upon review of reasonable request.
Design and Setting
This was a pragmatic, nonrandomized trial with parallel interventions to evaluate 3‐month change in 6MWT distances for cardiac patients after participation in HBCR versus FBCR (ie, standard of care) at 3 Veterans Administration (VA) medical centers. Clinicians at all 3 sites were contacted and educated about the recommendations to refer patients hospitalized for IHD to CR. After discharge, patients at the San Francisco VA who enrolled in the study received a 12‐week HBCR program, patients at the VA Pittsburgh received a 12‐week FBCR program, and patients enrolled at the VA Ann Arbor received a 6‐week FBCR program. Study assessments were conducted at baseline and 3 months after enrolling in CR.
Study Participants
All patients with recent hospitalization for myocardial infarction, PCI, or CABG who were enrolling in HBCR (at the San Francisco VA) or FBCR (at the VA Pittsburgh or VA Ann Arbor facilities) were invited to participate in the study. Participants at the HBCR site were referred using a semiautomated system in which a check box for CR evaluation was included in the post‐CABG and post‐PCI order sets, and a daily list of patients with elevated troponin levels was generated by the clinical laboratory. 18 For HBCR, patients were evaluated for interest and eligibility during their index hospitalization. At FBCR sites, patients were referred at or after hospital discharge and contacted via telephone to assess their interest. Those interested in CR were invited to return to the hospital for subsequent evaluation. Most patients were contacted within 2 days of receipt of consultation to determine interest in CR and begin the evaluation process. VA Pittsburgh enrolled patients in a rolling fashion while VA Ann Arbor's standard process was prespecified start dates for groups of participants.
Exclusion criteria included age <18 years; a planned cardiovascular procedure (pacemaker, defibrillator, staged PCI, or vascular procedure); pregnancy; significant cognitive or behavioral limitation; being blind, deaf, or mute; uncontrolled atrial arrhythmia; high‐grade atrioventricular block without a pacemaker; or discharge to a skilled nursing facility. HBCR participants had additional exclusion criteria including no telephone or clinical indication for an implantable cardioverter defibrillator but without an implantable cardioverter defibrillator implanted at time of study enrollment. Each site's institutional review board approved the study (University of California, San Francisco and San Francisco VA Institutional Review Board #14‐14‐075; VA Ann Arbor Institutional Review Board #2015‐050323; University of Pittsburgh Institutional Review Board #15100383; VA Pittsburgh Institutional Review Board #00001453). Informed consent was obtained from all study participants.
Baseline Evaluation
All study participants underwent comprehensive baseline assessments at the time of enrollment including submaximal exercise testing by a standardized 6MWT protocol, 19 blood pressure, lipid and glucose levels, as well as self‐reported measures of physical function. Participants also completed questionnaires regarding their quality of life and physical activity, including a single‐item assessment of physical activity, 20 , 21 , 22 Godin physical activity scale, 23 , 24 and the Duke Activity Status Index. 25 The Godin physical activity scale categorizes subjects as “active” for ≥24 points, “moderately active” 14 to 23 points, or “insufficiently active/sedentary” for <14 points. The Duke Activity Status Index provides an estimate of functional capacity based on subjects' reported ability to perform various activities and estimates the maximum metabolic equivalent a subject could achieve. Patients' belief in their ability to manage the challenges of coronary artery disease (ie, cardiac self‐efficacy) was measured, with ≥15 points used to define good cardiac disease self‐efficacy. 26 Patients' belief and confidence in their ability to exercise (ie, exercise self‐efficacy) was also measured, with ≥24 points used to define good exercise self‐efficacy. 27 Cognition was measured using the Montreal Cognitive Assessment. 28 Patients enrolled in HBCR received and were taught how to use a pedometer as well as equipment for monitoring heart rate (Fitbit Charge 2), blood pressure, body weight, and (if indicated) blood sugar to enable self‐monitoring and data tracking. Patients were classified as rural or urban based on the location of their primary residence using the VA's method of Rural‐Urban Commuting Areas based on the US Census Bureau criteria. 29
Intervention
Both FBCR programs are certified by the American Association of Cardiovascular and Pulmonary Rehabilitation and followed standard American Heart Association/American College of Cardiology Foundation protocols 30 with 2 to 3 sessions per week of patient education and monitored exercise. The HBCR intervention was based on these same standards as well as the American Heart Association's Active Partnership for the Health of Your Heart program 18 , 31 , 32 , 33 (Table 1). The type of staff and percent effort providing clinical care at each site was similar (Table S1). As previously described, 18 HBCR patients underwent 6 weekly followed by 3 biweekly telephone calls from clinical staff (nurse care manager, dietitian, or exercise physiologist) to provide individualized coaching and cardiac disease education. Home physical activity was encouraged in all patients but not directly supervised or monitored. HBCR participants received exercise bands, pedalers, and a Fitbit Charge 2 device allowing for continuous heart rate self‐monitoring during exercise and recorded the duration and intensity of exercise in a logbook. Access to Fitbit data was not authorized. At each weekly call, the prior week's exercise was reviewed, and goals for the following week were adjusted with an updated exercise prescription based on the patient's goals and symptoms.
Table 1.
Comparison of Home‐Based Versus Facility‐Based Cardiac Rehabilitation Programs
| Program Details | HBCR | FBCR |
|---|---|---|
| Patient education | X | X |
| Risk assessment* | X | X |
| Exercise training | X | X |
| Dietary counseling | X | X |
| Psychosocial support | X | X |
| Medication adherence | X | X |
| Smoking cessation | X | X |
| Home exercise equipment | X | |
| Referral | Before discharge | At or after discharge |
| Sessions | 9–12 sessions | 12–36 sessions |
| Duration | 12 wk | 6–12 wk |
FBCR indicates facility‐based cardiac rehabilitation; and HBCR, home‐based cardiac rehabilitation.
Measurement of baseline exercise capacity, body mass index, blood pressure, heart rate, lipid levels, creatinine, and glycohemoglobin.
In both HBCR and FBCR programs, patients were encouraged to complete all CR sessions and to increase their physical activity as tolerated. For HBCR, participation was assessed by the number of weekly phone calls, if they lasted at least 15 minutes and included components of education and physical activity. For FBCR, participation was assessed by the number of CR sessions attended. HBCR participants were considered lost to follow‐up after 3 unanswered phone calls or unreturned messages. FBCR participants were considered lost to follow‐up after 3 sessions in which they failed to show.
Outcome Assessment
Follow‐up assessments were conducted upon CR program completion or 3 months after enrollment. We assessed a number of outcomes, including 6MWT distance using a standardized protocol, 19 time to enrollment in CR, quality of life, cardiac and exercise self‐efficacy, and physical function. Use of distance as the primary outcome was chosen because it would be easier to obtain in a pragmatic, remotely conducted study in which patients may not have easy access to formal exercise testing on a treadmill but is proven to have prognostic value related to fitness in this population. 34 Twenty‐eight patients (33%) who participated in HBCR had follow‐up 6MWT performed by a visiting nurse near their home; all other study participants had follow‐up 6MWT performed by study personnel at the San Francisco VA, VA Ann Arbor, or VA Pittsburgh. Adverse events considered included death, cardiac‐related hospitalization, and cardiac procedures, which were identified by patient report and review of electronic health records.
Statistical Analysis
We compared baseline characteristics of the 2 groups using unpaired t‐tests for continuous variables and chi‐square tests for dichotomous or categorical variables. Percentages and means with 95% CIs were reported for categorical and continuous variables respectively. Imbalanced baseline measures were identified using a P value of <0.05 and included as covariates in outcome analyses. Our primary outcome of change in 6MWT distance was analyzed using a multivariate linear regression model using an intent‐to‐treat principle so that all subjects with follow‐up data were analyzed regardless of their adherence to CR, and a P value of <0.05 for statistical significance was used. We tested for interactions with type of CR to see whether outcomes varied by age, sex, race, indication for CR, or rurality. Our secondary outcomes from questionnaires were compared with ANOVA for continuous variables and chi‐square for categorical variables. Analyses were performed using SAS Enterprise (version 7; SAS Institute Inc, Cary, NC) and Stata (version 15.1; StataCorp, College Station, TX).
Results
A total of 237 subjects were enrolled in the study. Those in HBCR were similar to those in FBCR in terms of age, sex, total cholesterol, left ventricular ejection fraction, body mass index, waist circumference, glycated hemoglobin, blood pressure, and renal function (Table 2). HBCR patients were more likely to be Latino/Hispanic and have hyperlipidemia, prior myocardial infarction prior PCI, and posttraumatic stress disorder, but less likely to have recent CABG or chronic obstructive pulmonary disease. FBCR patients reported being more physically active by single‐item self‐report (35% versus 14%; P<0.001), but Godin physical activity scale and Duke Activity Status Index scores suggested that both groups were similarly active at baseline (Table 3). Mean 6MWT distances at baseline were 346 m (95% CI, 326–366) in the 121 patients in HBCR versus 349 m (95% CI, 329–369) in the 116 patients in FBCR (P=0.82) (Table 3). 35
Table 2.
Characteristics of Home‐Based Versus Facility‐Based CR Participants
| Characteristics | HBCR (n=121) | FBCR (n=116) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y (mean±SD) | 65±8 | 65±8 | 0.64 |
| Male, n (%) | 119 (98.3) | 115 (99.1) | 0.59 |
| Race, n (%) | |||
| White | 91 (77) | 89 (77) | 0.02 |
| Black | 13 (11) | 22 (19) | |
| Other | 14 (12) | 4 (4) | |
| Latino/Hispanic | 11 (9) | 2 (2) | 0.02 |
| Rural residence | 36 (31) | 17 (16) | 0.009 |
| Indication, n (%) | 0.02 | ||
| Myocardial infarction | 26 (22) | 12 (10) | |
| Percutaneous coronary intervention | 63 (52) | 57 (49) | |
| Coronary artery bypass graft surgery | 32 (26) | 47 (41) | |
| Comorbid conditions, n (%) | |||
| Hypertension | 112 (93) | 102 (88) | 0.23 |
| Hyperlipidemia | 113 (93) | 85 (78) | <0.001 |
| Prior myocardial infarction | 55 (46) | 30 (28) | 0.002 |
| Prior percutaneous coronary intervention | 63 (55) | 36 (34) | 0.004 |
| Heart failure | 25 (21) | 21 (19) | 0.56 |
| Atrial fibrillation | 20 (17) | 22 (19) | 0.60 |
| Diabetes mellitus | 48 (40) | 54 (47) | 0.28 |
| Stroke | 17 (14) | 18 (16) | 0.73 |
| Peripheral arterial disease | 11 (10) | 8 (8) | 0.09 |
| Chronic kidney disease | 19 (17) | 16 (15) | 0.35 |
| Chronic obstructive pulmonary disease | 11 (9) | 22 (19) | 0.03 |
| Depression | 39 (32) | 16 (26) | 0.41 |
| Posttraumatic stress disorder | 41 (34) | 10 (9) | <0.001 |
| Current or past smoker | 84 (70) | 87 (75.0) | 0.34 |
| Measurements, n (%) | |||
| Total cholesterol, mg/dL | 161±48 | 170±48 | 0.18 |
| LDL cholesterol, mg/dL | 90±44 | 100±42 | 0.005 |
| HDL cholesterol, mg/dL | 41±14 | 41±10 | 0.64 |
| Triglycerides, mg/dL | 159±91 | 160±112 | 0.51 |
| Left ventricular ejection fraction <50%, n (%) | 24 (22) | 28 (25) | 0.65 |
| Body mass index | 30±5 | 31±6 | 0.16 |
| Waist circumference, cm | 108±14 | 110±15 | 0.56 |
| Glycated hemoglobin, % | 6.4±1.5 | 6.4±1.7 | 0.26 |
| Systolic blood pressure, mm Hg | 127±15 | 127±18 | 0.94 |
| Diastolic blood pressure, mm Hg | 73±8 | 75±11 | 0.09 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 72±23 | 75±26 | 0.76 |
CR indicates cardiac rehabilitation; FBCR, facility‐based cardiac rehabilitation; HBCR, home‐based cardiac rehabilitation; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
Table 3.
Cardiac Rehabilitation Baseline Measures in Home‐Based vs Facility‐Based CR Participants
| Characteristics | HBCR | FBCR | P Value |
|---|---|---|---|
| Clinical measures of functional status | |||
| 6MWT distance, m, mean (95% CI) | 346 (326–366) | 349 (329–369) | 0.82 |
| Estimated metabolic equivalents,* mean (95% CI) | 2.6 (2.6–2.7) | 2.7 (2.6–2.8) | 0.82 |
| Patient‐reported measures of functional status | |||
| Quality of life: excellent or very good, n (%) | 25 (34) | 37 (37) | 0.68 |
| Physical activity self‐report excellent or very good, n (%) | 13 (14 | 37 (35) | <0.001 |
| Godin physical activity scale, mean±SD | 22±25 | 28±28 | 0.11 |
| Duke Activity Status Index, mean±SD | 25±4 | 25±4 | 0.56 |
| Cardiac self‐efficacy ≥15, n (%) | 77 (83) | 88 (79) | 0.55 |
| Exercise self‐efficacy ≥24, n (%) | 18 (20) | 19 (19) | 0.81 |
| Montreal Cognitive Assessment, mean±SD | 25±3 | 25±4 | 0.94 |
| Days from index event to | |||
| Referral, mean (95% CI) | 6 (2–10) | 16 (12–21) | 0.003 |
| Clinical evaluation, mean (95% CI) | 9 (5–14) | 37 (31–43) | <0.001 |
| Enrollment, first session, mean (95% CI) | 25 (19–31) | 77 (65–89) | <0.001 |
6MWT indicates 6‐minute walk test; CR, cardiac rehabilitation; FBCR, facility‐based cardiac rehabilitation; and HBCR, home‐based cardiac rehabilitation.
Estimated metabolic equivalents=3.5+([6MWT×0.1]/6)/3.5. 35
Structure and Feasibility
Among 175 patients who initially agreed to participate in HBCR, 152 (87%) enrolled. Among 237 eligible patients who initially agreed to participate in FBCR, 192 (81%) enrolled (Figure 1). HBCR program patients experienced shorter times from index event to outpatient CR referral (6 versus 16 days; P=0.003), to clinical evaluation for CR appropriateness (9 versus 37 days; P<0.001), and to enrollment in the first CR session (25 versus 77 days; P<0.001). HBCR participants were more likely to complete all 9 recommended sessions over 3 months compared with FBCR participants, who were expected to complete 12 to 36 sessions over 3 months (54% versus 26%; P<0.001).
Figure 1. Overview of participant flow in study.
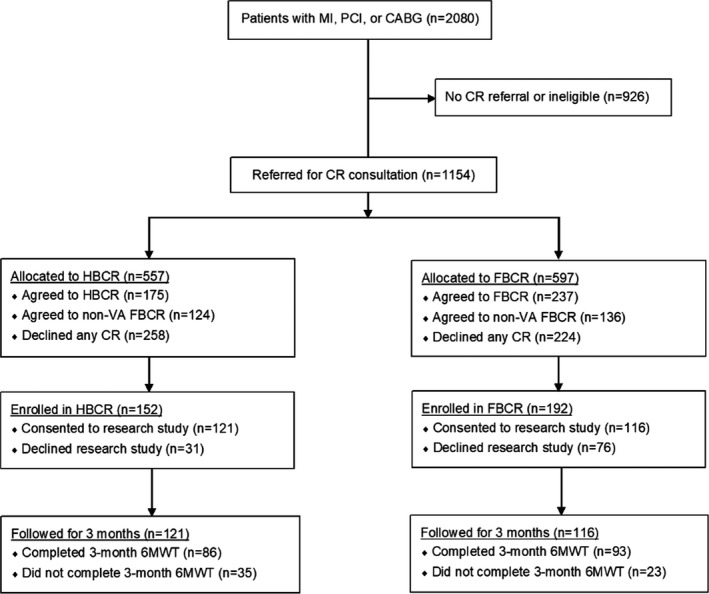
6MWT indicates 6‐minute walk test; CABG, coronary artery bypass graft; CR, cardiac rehabilitation; FBCR, facility‐based cardiac rehabilitation; HBCR, home‐based cardiac rehabilitation; MI, myocardial infarction; PCI, percutaneous coronary intervention; and VA, Veteran's Administration.
Efficacy
A total of 86 (71%) HBCR participants and 93 (80%) FBCR participants completed the 3‐month 6MWT. Compared with FBCR, participants in HBCR had a greater increase in mean 6MWT distance at 3 months (95 versus 41 m; P<0.001) (Figure 2). After adjusting for demographics, clinical indication, and comorbid conditions, the mean change in 6MWT at 3 months remained significantly greater for HBCR participants (101 versus 40 m; P<0.001) (Table 4 and Table S2). Further adjustment for time to enrollment and percent of sessions completed did not change the results. There were no significant differences in the outcomes of change in 6MWT distance within specific subpopulations including age, sex, race, ethnicity, clinical indication, or rural residence (Table 5).
Figure 2. 6MWT distance at baseline and 3 months after enrollment.
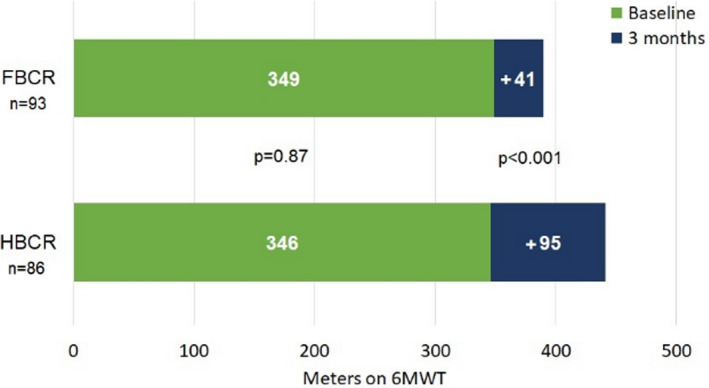
6MWT indicates 6‐minute walk test; FBCR, facility‐based cardiac rehabilitation; and HBCR, home‐based cardiac rehabilitation.
Table 4.
Change in Outcomes in Home‐Based Versus Facility‐Based CR Participants at 3 Months
| Outcomes | HBCR | FBCR | P Value |
|---|---|---|---|
| (n=Participants With Complete Data) | |||
| Mean increase in 6MWT distance | n=86 | n=93 | |
| Unadjusted | 95 (77–113) | 41 (23–58) | <0.001 |
| Adjusted | 101 (81–121) | 40 (18–61) | <0.001 |
| Completion of recommended CR sessions | n=121 | n=116 | |
| >50% sessions, n (%) | 88 (73 | 70 (60) | 0.03 |
| >85% sessions, n (%) | 73 (60) | 38 (33) | <0.001 |
| Quality of life | n=52 | n=52 | |
| Increase, n (%) | 23 (44) | 6 (12) | <0.001 |
| Physical activity | n=55 | n=85 | |
| Increase, n (%) | 44 (80) | 50 (59) | 0.03 |
| Godin physical activity scale | n=52 | n=81 | |
| Increase, n (%) | 37 (71) | 51 (64) | 0.45 |
| Duke Activity Status Index | n=50 | n=86 | |
| Increase | 38 (76%) | 51 (59%) | 0.13 |
| Cardiac self‐efficacy | n=57 | n=91 | |
| Increase, n (%) | 43 (75) | 53 (58) | 0.06 |
| Exercise self‐efficacy | n=54 | n=80 | |
| Increase, n (%) | 21 (39) | 49 (61) | 0.03 |
6MWT indicates 6‐minute walk test; CR, cardiac rehabilitation; FBCR, facility‐based cardiac rehabilitation; and HBCR, home‐based cardiac rehabilitation.
Table 5.
Mean Increase in 6MWT Distance Stratified by Subgroups
| Stratum | Total Patients | Mean Increase in 6MWT (m) | P Value | |
|---|---|---|---|---|
| HBCR | FBCR | |||
| All participants | 179 | +95 | +41 | <0.001 |
| Age ≤65 | 69 | +83 | +55 | 0.16 |
| Age >65 | 110 | +102 | +32 | <0.001 |
| White | 139 | +94 | +41 | <0.001 |
| Non‐White | 40 | +98 | +40 | 0.13 |
| Compliant (participated in ≥85% sessions) | 109 | +96 | +37 | 0.003 |
| Noncompliant (<85% sessions) | 70 | +93 | +43 | 0.02 |
| Urban | 130 | +104 | +45 | <0.001 |
| Rural | 45 | +73 | +37 | 0.17 |
6MWT indicates 6‐minute walk test; FBCR, facility‐based cardiac rehabilitation; and HBCR, home‐based cardiac rehabilitation.
Since one‐third of HBCR participants had the follow‐up 6MWT performed by non‐VA staff, we conducted a sensitivity analysis to compare 3‐month change in 6MWT distances between those who had the test performed by VA staff versus non‐VA staff. There was no statistical difference between the 2 groups in terms of mean 6MWT change at 3 months (76±104 m versus 91±108 m; P=0.52).
Participants who started CR within 30 days showed greater improvement in their 6MWT distances at 3 months compared with those who started more than 30 days after their IHD event (101 versus 45 m; P<0.001). Results were consistent with the primary findings even after stratification by type of CR or time to enrollment. There was no difference in change in 6MWT distance stratified by adherence to at least 85% of CR sessions attended.
After participation in CR at 3 months of follow‐up, most participants in either HBCR or FBCR showed favorable changes in self‐reported measures of quality of life, functional status, and self‐efficacy. A greater proportion of participants in the HBCR program reported improvement in self‐reported quality of life (44% versus 12%, P<0.001) and physical activity (80% versus 59%; P=0.03) when compared with facility‐based participants (Table 4). However, functional status measured by the Godin physical activity scale and Duke Activity Status Index showed similar improvements in both groups. More FBCR participants reported improvement in exercise self‐efficacy (61% versus 39%; P=0.03) when compared with home‐based participants.
Adverse Events
Adverse events were assessed for 100% of patients. During the 3‐month follow‐up period after enrollment in either CR program, there were no deaths, cardiovascular hospitalizations, or elective hospitalizations for a cardiac procedure among either the HBCR or FBCR participants. In the HBCR group, 1 participant, who subsequently withdrew from the study, was hospitalized for a broken foot after a fall that occurred in his bathroom while at home. In the FBCR group, 3 patients were hospitalized and withdrew from the study. The reasons for hospitalization were recurrent lower‐extremity cellulitis before starting FBCR, a new diagnosis of esophageal cancer requiring urgent surgery, and sepsis caused by a urinary tract infection.
Discussion
Our study responds to a critical need to provide evidence of the value of HBCR in comparison with traditional FBCR in a wider range of patients than previously studied. We compared similar patients enrolled in home‐based and facility‐based models to compare efficacy and safety. Participants enrolled in HBCR achieved relatively greater improvement in 6MWT distance, self‐reported physical activity, and quality of life than those in FBCR but less improvement in exercise self‐efficacy. We focused on 6MWT distance as a primary outcome metric of physical function because it was easier to obtain in this pragmatic, remotely conducted study than stress testing and provides related prognostic value related to cardiorespiratory fitness and other facets of physiological reserve. 34 Patients in HBCR were more likely to complete >85% of sessions than those in FBCR. Our sample was not powered to detect significant differences in rehospitalization or mortality, but we observed no signals of adverse events in either group. While we recognize that this was not a randomized trial with completely comparable intervention and control groups, our results suggest that HBCR has at least equivalent benefits to FBCR and no evidence of harm among selected patients with IHD who are willing to participate in HBCR.
Several possible explanations could account for the greater improvement in 6MWT distance in the HBCR cohort. Foremost, the earlier initiation of CR facilitated by HBCR may have contributed to earlier functional gain. The mean time between index event and enrollment in CR was over 7 weeks shorter in the HBCR group compared with the FBCR group. Some of this difference could have been attributed to the automated enrollment provided at the VA facility providing HBCR or the staggered enrollment at VA Ann Arbor, but this also reflects the fact that HBCR is particularly conducive to such speed and efficiency as it is not limited by space and staffing limitations. After adjustment for time between index event and enrollment, 3‐month change in 6MWT distance remained higher in HBCR versus FBCR participants. Nonetheless, it remains possible that shorter initiation time could have enabled patients to more quickly increase physical activity and reduce sedentary habits.
HBCR resulted in similar improvements despite being delivered only once per week and via telephone rather than up to 3 times per week under direct supervision. This is an important finding because it supports the belief that patients may benefit from similar improvements in functional capacity without as frequent direct provider contact and site‐based participation. Furthermore, while number of sessions has been proposed as a CR quality measure, 36 gains in physical function may be less dependent on the number of provider encounters than on the tailoring of CR programs to each patient's individual needs.
Objective 6MWT distance as well as subjective self‐reported measures of physical function, physical activity, quality of life, and self‐efficacy improved in both groups of participants. Longer‐term sustainability has been postulated because patients can more easily integrate the recommendations and activity from HBCR into their lifestyle and requires additional investigation. Measures of quality of life also improved more frequently among HBCR participants, suggesting that functional gains were conducive to greater satisfaction with care. However, an increase in exercise self‐efficacy was more common among FBCR participants, which may indicate that direct monitoring contributes to patients' confidence in exercising and physical activity.
HBCR programs can be quite variable in terms of systematic referrals, timing of evaluation, degree of follow‐up, complexity of the intervention, and whether it can be tailored to needs of individual patients. No single HBCR protocol has been identified as the optimal model. The HBCR program in our study was modeled on current standards for FBCR in respect to education and exercise guidance to best compare these 2 models of care. However, there are also burgeoning opportunities to integrate wearable devices and mobile technologies to enrich enhance clinician surveillance and communication with patients remotely. 37 These may ultimately help advance the efficacy of HBCR with respect to supervision and even patient‐to‐patient camaraderie to levels that were previously unattainable. Safety may also be enhanced, potentially expanding application of HBCR to those who are relatively more frail or high‐risk by enabling tracking of activity, body position, hemodynamics, ECGs, and other safety and physiological parameters in almost real time.
More investigation of which subpopulations might benefit more from HBCR is needed. Some patients might prefer HBCR for logistical reasons, with respect to both distance and time. Others might prefer HBCR because they can schedule their participation at times when FBCR services are not available. 38 Others may prefer the context of home and family, as it may make CR more personally relevant and sustainable. Still others may be less comfortable in a group setting. These are some of the logistical barriers that are reduced or removed by an HBCR program and integrating exercise with a patient's regular home routine where they live, reducing enrollment delays, enabling individually tailored coaching, allowing for different types of exercise (swimming, bicycling, etc), providing flexible and convenient scheduling, minimizing need for travel or transportation, and providing greater privacy. However, proponents of FBCR cite the availability of direct supervision and feedback, camaraderie of other patients and staff, access to high‐quality exercise equipment, and the immediate availability of medical care as critical for established successes of CR to date. Some patients are currently believed to be too high risk for unmonitored physical activity but may benefit from a staged approach that facilitates from site‐based to home‐based activity. There is much excitement regarding “hybrid” approaches that link FBCR to HBCR in ways that mitigate patient anxieties and ensure smoother transitions to independence despite medical complexities (ClinicalTrials.gov: NCT03922529). For now, this remains an active area of investigation.
Our study has a number of limitations. The most significant limitation is that it was a pragmatic, nonrandomized trial of 2 different forms of CR delivery in real‐world clinical practice. Had we limited study participation to patients who were willing and able to participate in either FBCR or HBCR, we would have excluded our most important target group, namely, those who do not participate in FBCR. Since randomization was impractical, we selected sites that offered different methods of CR delivery (HBCR versus FBCR). While we used rigorous methods to adjust for differences in patients who completed HBCR versus FBCR, it remains possible that residual confounding still differentiated patients who enrolled in HBCR versus FBCR. FBCR requires a patient to commit more time, but it can also accommodate limitations that would be unsafe exercising at home alone such as frailty or underlying apprehension regarding physical declines. Similarly, FBCR may have been more appealing to patients with relatively greater fall risks, cognitive limitations, and other parameters that would make them more reluctant to enroll in HBCR. Thus, even while we adjusted for a long list of relevant clinical comorbidities, it is improbable that the populations were identical.
Other notable limitations include:
HBCR was associated with a greater loss to follow‐up at 3 months, which might contribute to selection bias. However, we compared the characteristics of patients with and without follow‐up and there were no differences in baseline demographics, comorbidities, left ventricular ejection fraction, body mass index, or 6MWT distances.
Differences in quantity and quality of exercise performed as part of HBCR and FBCR may have differed. We did not objectively monitor the quantity or intensity of exercise and therefore could be different between the 2 groups.
Most participants were men and therefore it is unknown how our results will translate to women with IHD.
Reliability of follow‐up 6MWT by nonstudy personnel in HBCR participants is uncertain, 34 although our sensitivity analysis including only those with follow‐up 6MWT with study personnel did not changes our results.
Although no 2 CR programs are identical, both Pittsburgh and Ann Arbor offer professionally certified programs that achieve the same benchmarks and standards of care for CR.
Our results are at 3 months of follow‐up, and sustainability of these changes over a longer follow‐up are unknown.
Finally, our study only included VA‐based CR programs and may not generalize to non‐VA settings.
Conclusions
We found that patients enrolled in HBCR achieved functional gains and improvements in quality of life that were as good as and even better than those of similar patients enrolled in FBCR. Our results do not detract from the value of FBCR but provide evidence for the benefits of HBCR, especially for patients who might not otherwise participate in CR. Expansion of HBCR programs could increase short‐term functional status in many eligible patients.
Sources of Funding
This work was funded by a contract with the Patient‐Centered Outcomes Research Institute (IH‐1304‐6787), Washington, DC, and by the Veterans Affairs Health Services Research & Development Quality Enhancement Research Initiative (QUE 15‐283), Washington, DC. Dr Forman currently receives funding from National Institute of Aging, Grant numbers: R01 AG060499, R01 AG058883, and P30 AG024827.
Disclosures
None.
Supporting information
Tables S1‐S2
(J Am Heart Assoc. 2020;9:e016456 DOI: 10.1161/JAHA.120.016456.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016456
For Sources of Funding and Disclosures, see page 9.
References
- 1. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;2458–2473. [DOI] [PubMed] [Google Scholar]
- 2. Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circ Cardiovasc Qual Outcomes. 2018;9:e000037 DOI: 10.1161/HCQ.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 3. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;e344–e426. [DOI] [PubMed] [Google Scholar]
- 4. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;e362–e425. [DOI] [PubMed] [Google Scholar]
- 5. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;1–12. [DOI] [PubMed] [Google Scholar]
- 6. Drozda J Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, Bonow RO, Burkiewicz JS, Crouch M, Goff DC Jr, et al. ACCF/AHA/AMA‐PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association‐Physician Consortium for Performance Improvement. Circulation. 2011;248–270. [DOI] [PubMed] [Google Scholar]
- 7. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;2951–2960. [DOI] [PubMed] [Google Scholar]
- 8. Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and Veterans Affairs populations: opportunity for improvement. Circulation. 2018;1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schopfer DW, Priano S, Allsup K, Helfrich CD, Ho PM, Rumsfeld JS, Forman DE, Whooley MA. Factors associated with utilization of cardiac rehabilitation among patients with ischemic heart disease in the Veterans Health Administration: a qualitative study. J Cardiopulm Rehabil Prev. 2016;167–173. [DOI] [PubMed] [Google Scholar]
- 10. Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, Roger VL. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grace SL, Shanmugasegaram S, Gravely‐Witte S, Brual J, Suskin N, Stewart DE. Barriers to cardiac rehabilitation: does age make a difference? J Cardiopulm Rehabil Prev. 2009;183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parashar S, Spertus JA, Tang F, Bishop KL, Vaccarino V, Jackson CF, Boyden TF, Sperling L. Predictors of early and late enrollment in cardiac rehabilitation, among those referred, after acute myocardial infarction. Circulation. 2012;1587–1595. [DOI] [PubMed] [Google Scholar]
- 13. Evenson KR, Fleury J. Barriers to outpatient cardiac rehabilitation participation and adherence. J Cardiopulm Rehabil. 2000;241–246. [DOI] [PubMed] [Google Scholar]
- 14. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home‐based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;e69–e89. [DOI] [PubMed] [Google Scholar]
- 15. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;2675–2682. [DOI] [PubMed] [Google Scholar]
- 17. Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (writing committee to develop clinical performance measures for cardiac rehabilitation). Circulation. 2010;1342–1350. [DOI] [PubMed] [Google Scholar]
- 18. Rohrbach G, Schopfer DW, Krishnamurthi N, Pabst M, Bettencourt M, Loomis J, Whooley MA. The design and implementation of a home‐based cardiac rehabilitation program. Fed Pract. 2017;30–39. [PMC free article] [PubMed] [Google Scholar]
- 19. American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;111–117. [DOI] [PubMed] [Google Scholar]
- 20. Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self‐reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;422–428. [DOI] [PubMed] [Google Scholar]
- 21. Bowles HR, FitzGerald SJ, Morrow JR Jr, Jackson AW, Blair SN. Construct validity of self‐reported historical physical activity. Am J Epidemiol. 2004;279–286. [DOI] [PubMed] [Google Scholar]
- 22. Gill DP, Jones GR, Zou G, Speechley M. Using a single question to assess physical activity in older adults: a reliability and validity study. BMC Med Res Methodol. 2012;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;141–146. [PubMed] [Google Scholar]
- 24. Amireault S, Godin G. The Godin‐Shephard Leisure‐Time Physical Activity Questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;604–622. [DOI] [PubMed] [Google Scholar]
- 25. Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self‐administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;651–654. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan MD, LaCroix AZ, Russo J, Katon WJ. Self‐efficacy and self‐reported functional status in coronary heart disease: a six‐month prospective study. Psychosom Med. 1998;473–478. [DOI] [PubMed] [Google Scholar]
- 27. Marcus BH, Selby VC, Niaura RS, Rossi JS. Self‐efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;60–66. [DOI] [PubMed] [Google Scholar]
- 28. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;695–699. [DOI] [PubMed] [Google Scholar]
- 29. Rural‐urban commuting area codes. 2016.
- 30. Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise‐based rehabilitation for patients with coronary heart disease: systematic review and meta‐analysis of randomized controlled trials. Am J Med. 2004;682–692. [DOI] [PubMed] [Google Scholar]
- 31. DeBusk RF, Haskell WL, Miller NH, Berra K, Taylor CB, Berger WE III, Lew H. Medically directed at‐home rehabilitation soon after clinically uncomplicated acute myocardial infarction: a new model for patient care. Am J Cardiol. 1985;251–257. [DOI] [PubMed] [Google Scholar]
- 32. American Heart Association . An Active Partnership for the Health of Your Heart. San Bruno, CA: Krames; 2008. [Google Scholar]
- 33. Krishnamurthi N, Schopfer DW, Ahi T, Bettencourt M, Piros K, Ringer R, Shen H, Kehler JP, Whooley MA. Predictors of patient participation and completion of home‐based cardiac rehabilitation in the Veterans Health Administration for patients with coronary heart disease. Am J Cardiol. 2019;19–24. [DOI] [PubMed] [Google Scholar]
- 34. Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, Clare RM, Ellis SJ, Dunlap ME, Bittner V. 6‐min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bittner V. Role of the 6‐minute walk test in cardiac rehabilitation In: Kraus WE, Keteyian SJ, eds. Contemporary Cardiology: Cardiac Rehabilitation. Totowa, NJ: Humana Press Inc.; 2007:131–139. [Google Scholar]
- 36. Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;9:e000568 DOI: 10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home‐based versus hospital‐based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;24–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2


