Abstract
Background
Angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (ARBs) may worsen the prognosis of coronavirus disease 2019, but any association could be confounded by the cardiometabolic conditions indicating ACE‐I/ARB use. We therefore examined the impact of ACE‐Is/ARBs on respiratory tract infection outcomes.
Methods and Results
This cohort study included all adult patients hospitalized with influenza or pneumonia from 2005 to 2018 in Denmark using population‐based medical databases. Thirty‐day mortality and risk of admission to the intensive care unit in ACE‐Is/ARBs users was compared with nonusers and with users of calcium channel blockers. We used propensity scores to handle confounding and computed propensity score‐weighted risks, risk differences (RDs), and risk ratios (RRs). Of 568 019 patients hospitalized with influenza or pneumonia, 100 278 were ACE‐I/ARB users and 37 961 were users of calcium channel blockers. In propensity score‐weighted analyses, ACE‐I/ARB users had marginally lower 30‐day mortality than users of calcium channel blockers (13.9% versus 14.5%; RD, −0.6%; 95% CI, −1.0 to −0.1; RR, 0.96; 95% CI, 0.93–0.99), and a lower risk of admission to the intensive care unit (8.0% versus 9.6%; RD, −1.6%; 95% CI, −2.0 to −1.2; RR, 0.83; 95% CI, 0.80–0.87). Compared with nonusers, current ACE‐I/ARB users had lower mortality (RD, −2.4%; 95% CI, −2.8 to −2.0; RR, 0.85; 95% CI, 0.83–0.87), but similar risk of admission to the intensive care unit (RD, 0.4%; 95% CI, 0.0–0.7; RR, 1.04; 95% CI, 1.00–1.09).
Conclusions
Among patients with influenza or pneumonia, ACE‐I/ARB users had no increased risk of admission to the intensive care unit and slightly reduced mortality after controlling for confounding.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitor, cohort study, infectious disease, intensive care unit
Subject Categories: Epidemiology, Hypertension, Mortality/Survival, Complications, Treatment
Nonstandard Abbreviations and Acronyms
- COVID‐19
coronavirus disease 2019
- PS
propensity score
- RD
risk difference
Clinical Perspective
What Is New?
In this large cohort study of more than 500 000 patients hospitalized with influenza or pneumonia, users of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers had no increased risk of intensive care unit admission and a slightly reduced mortality after controlling for confounding.
What Are the Clinical Implications?
Our data support the current recommendations to avoid discontinuation of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers during the current coronavirus disease 2019 pandemic, unless future evidence contradicts our findings.
Use of angiotensin‐converting enzyme inhibitors (ACE‐Is) and angiotensin receptor blockers (ARBs) may increase the risk of developing severe or fatal coronavirus disease 2019 (COVID‐19) by upregulating expression of the ACE2 enzyme, which is known to facilitate severe acute respiratory syndrome‐coronavirus 2 entry into cells. 1 , 2 , 3 , 4 , 5 Case series of hospitalized patients with severe and fatal COVID‐19 from China, 6 , 7 , 8 Italy, 9 and the United States 10 , 11 have reported a high prevalence (≈30%–40%) of hypertension, cardiovascular conditions, and diabetes mellitus—conditions often treated with ACE‐Is/ARBs. 12 Data are currently lacking to clarify whether treatment of these coexisting conditions, including ACE‐I/ARB use, contributed to the observed adverse health outcomes, or if patients had worse outcomes simply because they were older and suffered from cardiometabolic conditions (ie, confounding by indication). 2 , 12 , 13 , 14 , 15 A few studies have suggested an association between ACE‐I and ARB use and decreased mortality from bacterial pneumonia. 16 , 17 , 18 Although preadmission ACE‐I use has been associated with increased mortality in patients hospitalized for viral diseases, 19 two small studies of patients with COVID‐19 do not indicate any increased mortality in inpatient users of ACE‐Is/ARBs. 20 , 21 Most of the existing studies had limitations: They did not have ACE‐I/ARB use as their main exposure, had mixed pneumonia risk and outcome, were biased by studying in‐hospital medication after study inclusion, or were small with imprecise estimates.
Major institutions and societies have called for further research and issued warnings against ACE‐I/ARB discontinuation in patients with COVID‐19, 21 as drug discontinuation may worsen underlying cardiometabolic conditions. 22 Thus, there is an urgent need to clarify the impact of ACE‐Is/ARBs on outcomes of lower respiratory tract infections. We report here on a large population‐based cohort study investigating the impact of ACE‐I/ARB use on intensive care unit (ICU) admission and death following hospitalization for influenza or pneumonia, while controlling for potential confounding by indication by using active antihypertensive drug comparators.
METHODS
The study was approved by the Danish Data Protection Agency (record number 2014‐54‐0922) through registration at Aarhus University (record number KEA‐2017‐36/812). Ethics approval and informed consent are not required for registry‐based observational studies in Denmark.
Study Design and Setting
This nationwide cohort study included all patients diagnosed with influenza or pneumonia in Danish hospitals from January 1, 2005 through September 30, 2018, with follow‐up to October 31, 2018. The study design is summarized in Figure S1. Denmark has a tax‐supported healthcare system providing equal access to all acute care, including hospital care for pneumonia. 23 All Danish residents receive a personal identity number at birth or upon immigration, which allows individual‐level linkage among medical databases and registries. The Danish Civil Registration System is a population registry that contains data updated daily on the vital status (deceased or alive) and residence of all Danish residents. 24 (Because of the sensitive nature of the data collected for this study, requests to access the databases used in this study from researchers at authorized institutions may be sent to the Danish Health Data Authority by e‐mail to forskerservice@sundhedsdata.dk.)
Influenza and Pneumonia
The study examined all hospitalizations by adult members (aged ≥18 years) of the study cohort who had either a primary or secondary diagnosis of influenza or pneumonia using the Danish National Patient Registry data (Figure 1). 25 Among other variables, the patient registry includes data on the dates of hospital contact/admission and discharge, primary and secondary diagnoses, and procedure codes. Influenza or pneumonia contacts were not included if preceded by an influenza or pneumonia diagnosis within the prior 3 months, to avoid inclusion of readmissions relating to the same disease episode. Finally, we predefined subgroups diagnosed with influenza or diagnosed with pneumonia with bacterial or unspecified pathogen (codes provided in Table S1).
Figure 1. Patient flow diagram.
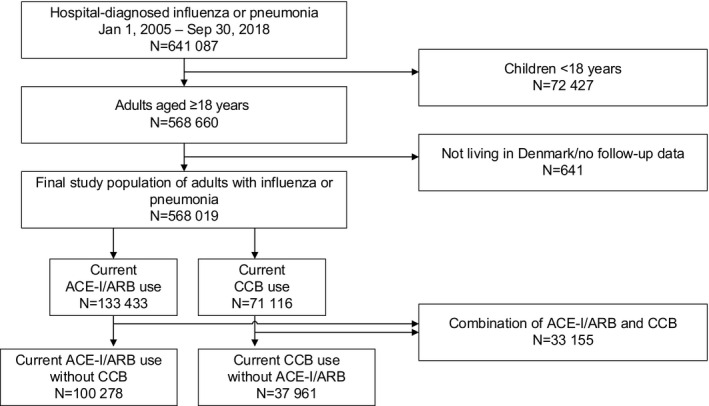
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; and CCB, calcium channel blocker.
Outcomes
The primary study outcomes were death within 30 days after hospital admission and ICU admissions during the index hospitalization, including transferals between departments and hospitals after the diagnosis of influenza or pneumonia. Secondary outcomes included organ‐supportive treatment during ICU admission with mechanical ventilation, noninvasive ventilation, and inotropes/vasopressors. Finally, dialysis‐treated acute kidney injury was defined as treatment with acute renal replacement therapy in patients with no history of previous dialysis for chronic kidney disease. Outcomes were ascertained using population registry data for all‐cause death, and diagnosis and procedure data from the patient registry for all other outcomes. 24 , 25 , 26
Exposures
Data on all filled prescriptions for cardiovascular medications were obtained from the Danish National Prescription Registry. 27 Our main exposure of interest was current use of either ACE‐Is or ARBs. We defined current use as a filled prescription within 90 days before the hospital contact for influenza or pneumonia; most prescriptions for ACE‐Is/ARBs filled in Danish pharmacies are for a 3‐month supply.
In our main analyses, current ACE‐I/ARB users were compared with current calcium channel blocker (CCB) use and with nonusers of ACE‐Is/ARBs (no prescription 365 days before index date). We chose CCBs as the active comparator drug because CCBs are also a first‐line treatment for hypertension, and have no known pharmacological effects on the renin–angiotensin–aldosterone system, in contrast to β blockers and thiazides. Current users of both ACE‐Is/ARBs and CCBs were excluded from the head‐to‐head comparison of these drugs.
In our second analysis, we examined the outcomes of former ACE‐I/ARB users (prescription filled 91 to 365 days before index date) versus nonusers to address potential uncontrolled confounding by indication.
Potential Confounders
We included a range of variables potentially associated both with cardiovascular drug use and with the outcomes of interest. Age and sex were obtained from the population registry. 24 We included information on coexisting conditions requiring inpatient or outpatient hospital contact within 10 years before the index admission from the patient registry (see Table 1 and Table S1 for included codes). 25 Prescriptions for important concurrent medications filled within 90 days before admission were also included. 27 Finally, as socioeconomic markers, we included data on marital status and on urban versus rural residence.
Table 1.
Characteristics of Current Users of ACE‐Is or ARBs and CCBs Admitted With Influenza or Pneumonia, Overall and After PS‐Weighting
| Overall Cohort | PS‐Weighted Cohort | |||||
|---|---|---|---|---|---|---|
|
Current ACE‐I/ARB Use, N (%) |
Current CCB Use, N (%) |
SD |
Current ACE‐I/ARB Use, N (%) |
Current CCB Use*, N (%) |
SD | |
| No. of patients | 100 278 (72.5) | 37 961 (27.5) | 100 278 (50.2) | 99 625 (49.8) | ||
| Age, median (Q1–Q3), y | 76.9 (68.5–83.9) | 78.8 (70.2–85.8) | 0.21 | 76.9 (68.5–83.9) | 77.5 (69.1–84.3) | 0.07 |
| Male | 53 833 (53.7) | 18 500 (48.7) | 0.14 | 53 833 (53.7) | 51 740 (51.9) | 0.05 |
| Coexisting conditions (within prior 10 y) | ||||||
| Hospital‐diagnosed hypertension | 51 716 (51.6) | 21 587 (56.9) | 0.15 | 51 716 (51.6) | 54 522 (54.7) | 0.09 |
| Previous myocardial infarction | 13 449 (13.4) | 3335 (8.8) | 0.21 | 13 449 (13.4) | 13 416 (13.5) | 0.00 |
| Diagnosis of stable angina pectoris | 18 861 (18.8) | 6440 (17.0) | 0.07 | 18 861 (18.8) | 18 714 (18.8) | 0.00 |
| Heart failure | 26 518 (26.4) | 4330 (11.4) | 0.55 | 26 518 (26.4) | 26 546 (26.6) | 0.01 |
| Stroke | 14 843 (14.8) | 6323 (16.7) | 0.07 | 14 843 (14.8) | 15 759 (15.8) | 0.04 |
| Atrial fibrillation/flutter | 25 816 (25.7) | 6925 (18.2) | 0.26 | 25 816 (25.7) | 26 164 (26.3) | 0.02 |
| Heart valve disease | 9678 (9.7) | 3329 (8.8) | 0.04 | 9678 (9.7) | 9991 (10.0) | 0.02 |
| Venous thromboembolism | 5381 (5.4) | 1965 (5.2) | 0.01 | 5381 (5.4) | 5710 (5.7) | 0.02 |
| Diabetes mellitus | 29 098 (29.0) | 8036 (21.2) | 0.26 | 29 098 (29.0) | 30 230 (30.3) | 0.04 |
| Chronic pulmonary disease | 30 900 (30.8) | 11 396 (30.0) | 0.02 | 30 900 (30.8) | 31 262 (31.4) | 0.02 |
| Renal disease | 9404 (9.4) | 5708 (15.0) | 0.25 | 9404 (9.4) | 10 275 (10.3) | 0.04 |
| End‐stage renal disease | 1417 (1.4) | 1610 (4.2) | 0.24 | 1417 (1.4) | 1574 (1.6) | 0.02 |
| Liver disease | 1681 (1.7) | 759 (2.0) | 0.03 | 1681 (1.7) | 1696 (1.7) | 0.00 |
| Dementia | 5735 (5.7) | 2716 (7.2) | 0.08 | 5735 (5.7) | 5901 (5.9) | 0.01 |
| Cancer | 18 573 (18.5) | 7784 (20.5) | 0.07 | 18 573 (18.5) | 18 288 (18.4) | 0.01 |
| Metastatic cancer | 2834 (2.8) | 1312 (3.5) | 0.05 | 2834 (2.8) | 2726 (2.7) | 0.01 |
| Peptic ulcer disease | 6523 (6.5) | 2824 (7.4) | 0.05 | 6523 (6.5) | 6777 (6.8) | 0.02 |
| Rheumatoid arthritis or connective tissue disease | 6562 (6.5) | 2697 (7.1) | 0.03 | 6562 (6.5) | 6595 (6.6) | 0.00 |
| Comedication (prescription within 90 d) | ||||||
| Total number of antihypertensive drugs† | ||||||
| 1 | 41 998 (41.9) | 21 008 (55.3) | 0.38 | 41 998 (41.9) | 42 571 (42.7) | 0.02 |
| 2 | 48 637 (48.5) | 14 472 (38.1) | 0.30 | 48 637 (48.5) | 47 098 (47.3) | 0.03 |
| 3 | 9352 (9.3) | 2421 (6.4) | 0.16 | 9352 (9.3) | 9618 (9.7) | 0.02 |
| 4 | 291 (0.3) | 60 (0.2) | 0.04 | 291 (0.3) | 339 (0.3) | 0.01 |
| Thiazides | 30 954 (30.9) | 6788 (17.9) | 0.43 | 30 954 (30.9) | 32 095 (32.2) | 0.04 |
| β Blockers | 35 525 (35.4) | 11 735 (30.9) | 0.14 | 35 525 (35.4) | 33 406 (33.5) | 0.06 |
| Other antihypertensive drugs | 1735 (1.7) | 971 (2.6) | 0.08 | 1735 (1.7) | 1849 (1.9) | 0.01 |
| Statins | 37 363 (37.3) | 11 547 (30.4) | 0.21 | 37 363 (37.3) | 37 279 (37.4) | 0.00 |
| Aspirin | 34 140 (34.0) | 12 295 (32.4) | 0.05 | 34 140 (34.0) | 34 304 (34.4) | 0.01 |
| Loop diuretics | 34 667 (34.6) | 11 574 (30.5) | 0.12 | 34 667 (34.6) | 35 103 (35.2) | 0.02 |
| Immunosuppressants | 1359 (1.4) | 521 (1.4) | 0.00 | 1359 (1.4) | 1279 (1.3) | 0.01 |
| Glucocorticoids | 15 050 (15.0) | 6081 (16.0) | 0.04 | 15 050 (15.0) | 15 310 (15.4) | 0.01 |
| NSAIDs | 12 334 (12.3) | 4653 (12.3) | 0.00 | 12 334 (12.3) | 12 231 (12.3) | 0.00 |
| Opioids | 27 563 (27.5) | 11 423 (30.1) | 0.08 | 27 563 (27.5) | 28 122 (28.2) | 0.02 |
| Vitamin K antagonists | 12 164 (12.1) | 2863 (7.5) | 0.22 | 12 164 (12.1) | 11 842 (11.9) | 0.01 |
| Proton pump inhibitors | 30 277 (30.2) | 12 954 (34.1) | 0.12 | 30 277 (30.2) | 30 906 (31.0) | 0.03 |
| Antidepressants | 22 736 (22.7) | 9793 (25.8) | 0.10 | 22 736 (22.7) | 23 337 (23.4) | 0.03 |
| Hypnotics/sedatives | 15 720 (15.7) | 6517 (17.2) | 0.06 | 15 720 (15.7) | 15 976 (16.0) | 0.01 |
| Antipsychotics | 5508 (5.5) | 2551 (6.7) | 0.07 | 5508 (5.5) | 5433 (5.5) | 0.00 |
| Antibiotics (prescription within 10 d) | 26 448 (26.4) | 9973 (26.3) | 0.00 | 26 448 (26.4) | 26 235 (26.3) | 0.00 |
| Antivirals (prescription within 10 d) | 226 (0.2) | 84 (0.2) | 0.00 | 226 (0.2) | 194 (0.2) | 0.01 |
| Lifestyle and social factors | ||||||
| Markers of smoking | 52 541 (52.4) | 19 033 (50.1) | 0.06 | 52 541 (52.4) | 52 447 (52.6) | 0.01 |
| Obesity | 10 262 (10.2) | 2719 (7.2) | 0.15 | 10 262 (10.2) | 10 785 (10.8) | 0.03 |
| Alcoholism | 6107 (6.1) | 2381 (6.3) | 0.01 | 6107 (6.1) | 5925 (5.9) | 0.01 |
| Marital status | ||||||
| Widow | 31 850 (31.8) | 13 837 (36.5) | 0.14 | 31 850 (31.8) | 33 295 (33.4) | 0.05 |
| Divorced | 14 243 (14.2) | 5368 (14.1) | 0.00 | 14 243 (14.2) | 14 118 (14.2) | 0.00 |
| Married | 46 171 (46.0) | 15 841 (41.7) | 0.12 | 46 171 (46.0) | 44 607 (44.8) | 0.04 |
| Unmarried | 8014 (8.0) | 2915 (7.7) | 0.02 | 8014 (8.0) | 7604 (7.6) | 0.02 |
| Urban residence | 34 592 (34.5) | 12 629 (33.3) | 0.04 | 34 592 (34.5) | 34 848 (35.0) | 0.01 |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; PS, propensity score; and SD, standardized difference.
Pseudopopulation of current CCB users weighted to the PS distribution of current ACE‐I/ARB users.
Number of antihypertensive drugs including the exposure of interest (ACE‐Is/ARBs or CCBs, thiazides, β blockers, and other (range from 1 to 4 because patients using both ACE‐I/ARBs and CCBs are not included).
Statistical Analysis
Patient characteristics were tabulated according to exposure groups before and after propensity score‐ (PS‐) weighting. The PS, which measures the probability of being exposed, was estimated using a logistic regression model, including calendar time and all covariates listed in Table 1. We used PS‐weighting to generate a pseudopopulation of relevant comparisons (eg, CCB users or ACE‐I/ARB nonusers) that resembled the number and covariate distribution of ACE‐I/ARB users. As we were interested in the average treatment effect in the treated, we applied PS‐weighting using average treatment effect in the treated weights to make the number and covariate distribution in the comparison groups resemble that of the ACE‐I/ARB users. Overlap in PS distributions was checked before weighting and found sufficient. Covariate balance was assessed by standardized differences, empirical cumulative distribution functions for continuous variables (age and index), and by PS‐weight percentiles. Overall, covariate balance was deemed acceptable, although there was some imbalance in small subgroups as reflected by high weights and standardized differences.
Follow‐up started on the date of hospital contact with influenza/pneumonia and continued until the specific outcome of interest, emigration, or up to 30 days, whichever occurred first. The 30‐day risk of death and ICU admission was computed and plotted, both crude and PS‐weighted. Risk differences (RDs) were computed for all outcomes by subtracting PS‐weighted risks. Risk ratios (RRs) were estimated as the ratio of PS‐weighted risk estimates. All estimates were presented with 95% CIs obtained through bootstrapping with 200 bootstrap samples.
Additional analyses were performed to examine the robustness of our findings.
First, we changed the exposure definition to make a head‐to‐head comparison between ACE‐I/ARB and β blockers and thiazides, respectively. Second, we compared monotherapy with ACE‐I/ARB with CCB monotherapy. Third, analyses were repeated using only the primary (first‐listed) diagnoses of influenza or pneumonia. Fourth, analyses were stratified by age at the index date (18–65 years and >65 years). Fifth, analyses were repeated according to potential underlying indications for treatment including patients with renal disease, with congestive heart failure, with ischemic heart disease (myocardial infarction or stable angina pectoris), with diabetes mellitus, and without any of these comorbidities, and in patients without any of these comorbidities who had a hospital diagnosis of hypertension. Sixth, we repeated the analyses restricted to patients currently using more than 1 antihypertensive medication (polytherapy). Finally, we stratified by hospital versus community‐acquired pneumonia defined as no hospital contract within 3 to 30 days before study inclusion.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
The final study cohort included 568 019 adult Danish residents hospitalized for influenza or pneumonia (Figure 1): 100 278 (17.7%) were current ACE‐I/ARB users, 37 961 (6.7%) were current CCB users, and 33 155 (5.8%) were current users of both. There were 9413 admissions for influenza and 518 296 for pneumonia with bacterial or unspecified pathogen. Of the overall cohort of influenza/pneumonia, 454 303 (79.8%) were community‐acquired and 114 716 (20.2%) were hospital‐acquired.
A total of 42 827 hospitalizations included admission to the ICU; for most of these patients, ICU transferal happened early (median of 1 day, 25th–75th percentiles: 0–5 days) after first hospital admission. A total of 76 762 died within 30 days. Patient characteristics of the final study cohort are provided in Table S2 overall, and by death within 30 days and ICU admission.
Descriptive Characteristics of ACE‐I/ARB and CCB Users
Median age was almost similar in ACE‐I/ARB and CCB users (76.9 versus 78.8 years). There were slightly more men among ACE‐I/ARB users than among CCB users (53.7% versus 48.7%). ACE‐I/ARB users had a higher prevalence of most cardiovascular diseases, including heart failure, myocardial infarction, and diabetes mellitus. In contrast, hypertension and renal disease were less frequent in ACE‐I/ARB users than in CCB users (Table 1). Comedication with most cardiovascular medications, including other antihypertensive drugs, was more frequent in ACE‐I/ARB users. In both groups, 26% of patients had received antibiotics within 10 days prior to hospital admission. Markers of smoking and alcohol‐related disorders were equally prevalent in both groups; obesity was more common in ACE‐I/ARB users.
After PS‐weighting of CCB users, treatment groups were well‐balanced on all measured covariates, with absolute standardized differences for all covariates decreasing from 0.00 to 0.55 before PS‐balancing to <0.10 (Table 1).
Outcomes in ACE‐I/ARB Versus CCB Users
Table 2 shows the number of outcome events, risks, RDs, and RRs after PS‐weighting of CCB users. The crude estimates are provided in Table 3. The cumulative incidence (risk) plots before (crude‐) and after PS‐weighting (adjusted) are given in Figure 2A and 2B.
Table 2.
Outcomes in Current Users of ACE‐Is or ARBs Compared With CCBs, Adjusted by PS‐Weighting and Stratified by Influenza and Pneumonia With Bacterial or Unspecified Pathogen
| Population | Event | ACE‐Is/ARBs | CCBs |
Risk Difference* % (95% CI) vs CCBs |
Risk Ratio* (95% CI) vs CCBs |
||
|---|---|---|---|---|---|---|---|
| Events/at Risk | Risk % | Events/at Risk* | Risk* % | ||||
| Any influenza or pneumonia | 30‐d mortality | 13 940/100 278 | 13.9% | 14 412/99 625 | 14.5% | −0.6 (−1.0 to −0.1) | 0.96 (0.93–0.99) |
| ICU admission | 7993/100 278 | 8.0% | 9558/99 625 | 9.6% | −1.6 (−2.0 to −1.2) | 0.83 (0.80–0.87) | |
| ICU+MV | 4218/100 278 | 4.2% | 5196/99 625 | 5.2% | −1.0 (−1.3 to −0.7) | 0.81 (0.76–0.86) | |
| ICU+NIV | 3039/100 278 | 3.0% | 4132/99 625 | 4.1% | −1.1 (−1.4 to −0.8) | 0.73 (0.68–0.79) | |
| ICU+inotropes/vasopressors | 3825/100 278 | 3.8% | 3877/99 625 | 3.9% | −0.1 (−0.3 to 0.2) | 0.98 (0.92–1.05) | |
| D‐AKI | 965/98 861 | 1.0% | 1296/98 534 | 1.3% | −0.3 (−0.5 to −0.1) | 0.74 (0.64–0.86) | |
| Influenza | 30‐d mortality | 82/1565 | 5.2% | 60/1516 | 3.9% | 1.3 (−1.2 to 3.8) | 1.33 (0.69–2.55) |
| ICU admission | 129/1565 | 8.2% | 146/1516 | 9.6% | −1.4 (−5.7 to 3.0) | 0.86 (0.53–1.39) | |
| ICU+MV | 70/1565 | 4.5% | 70/1516 | 4.6% | −0.2 (−2.9 to 2.6) | 0.96 (0.52–1.80) | |
| ICU+NIV | 63/1565 | 4.0% | 55/1516 | 3.6% | 0.4 (−1.6 to 2.3) | 1.11 (0.65–1.88) | |
| ICU+inotropes/vasopressors | 61/1565 | 3.9% | 55/1516 | 3.6% | 0.3 (−2.4 to 2.9) | 1.07 (0.51–2.25) | |
| D‐AKI | 15/1514 | 1.0% | <5/1504 | 0.3% | 0.7 (0.1–1.3) | 3.40 (0.96–12.02) | |
| Pneumonia with bacterial or unspecified pathogen† | 30‐d mortality | 13 140/93 193 | 14.1% | 13 572/92 542 | 14.7% | −0.6 (−1.1 to −0.0) | 0.96 (0.93–1.00) |
| ICU admission | 7456/93 193 | 8.0% | 8912/92 542 | 9.6% | −1.6 (−2.1 to −1.2) | 0.83 (0.79–0.87) | |
| ICU+MV | 3918/93 193 | 4.2% | 4788/92 542 | 5.2% | −1.0 (−1.3 to −0.7) | 0.81 (0.76–0.87) | |
| ICU+NIV | 2839/93 193 | 3.0% | 3890/92 542 | 4.2% | −1.2 (−1.5 to −0.9) | 0.72 (0.67–0.78) | |
| ICU+inotropes/vasopressors | 3567/93 193 | 3.8% | 3587/92 542 | 3.9% | −0.0 (−0.3 to 0.2) | 0.99 (0.92–1.07) | |
| D‐AKI | 886/91 899 | 1.0% | 1185/91 538 | 1.3% | −0.3 (−0.5 to −0.2) | 0.74 (0.65–0.86) | |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; D‐AKI, dialysis‐treated acute kidney injury; ICU, intensive care unit; MV, mechanical ventilation; NIV, noninvasive ventilation; and PS, propensity score.
Pseudopopulation of current CCB users weighted to the PS distribution of current ACE‐I/ARB users.
Not including viral pneumonia and influenza without proven influenza virus.
Table 3.
Crude Outcomes in Current Users of ACE‐Is or ARBs Compared With CCBs, Overall and Stratified by Influenza and Pneumonia With Bacterial or Unspecified Pathogen
| Population | Event | ACE‐Is/ARBs | CCBs |
Risk Difference* % (95% CI) vs CCBs |
Risk Ratio* (95% CI) vs CCBs |
||
|---|---|---|---|---|---|---|---|
| Events/At Risk | Risk % | Events/At Risk* | Risk* % | ||||
| Any influenza or pneumonia | 30‐d mortality | 13 940/100 278 | 13.9 | 6016/37 961 | 15.8 | −1.9 (−2.4 to −1.5) | 0.88 (0.85–0.90) |
| ICU admission | 7993/100 278 | 8.0 | 3321/37 961 | 8.7 | −0.8 (−1.1 to −0.5) | 0.91 (0.88–0.94) | |
| ICU+MV | 4218/100 278 | 4.2 | 1753/37 961 | 4.6 | −0.4 (−0.6 to −0.2) | 0.91 (0.87–0.95) | |
| ICU+NIV | 3039/100 278 | 3.0 | 1468/37 961 | 3.9 | −0.8 (−1.0 to −0.6) | 0.78 (0.74–0.83) | |
| ICU+inotropes/vasopressors | 3825/100 278 | 3.8 | 1322/37 961 | 3.5 | 0.3 (0.1–0.5) | 1.10 (1.04–1.16) | |
| D‐AKI | 965/98 861 | 1.0 | 438/36 351 | 1.2 | −0.2 (−0.4 to −0.1) | 0.81 (0.72–0.92) | |
| Influenza | 30‐d mortality | 82/1565 | 5.2 | 25/526 | 4.8 | 0.5 (−1.6 to 2.5) | 1.10 (0.71–1.71) |
| ICU admission | 129/1565 | 8.2 | 44/526 | 8.4 | −0.1 (−2.8 to 2.6) | 0.99 (0.70–1.38) | |
| ICU+MV | 70/1565 | 4.5 | 24/526 | 4.6 | −0.1 (−2.1 to 1.9) | 0.98 (0.62–1.56) | |
| ICU+NIV | 63/1565 | 4.0 | 28/526 | 5.3 | −1.3 (−3.5 to 0.9) | 0.76 (0.47–1.21) | |
| ICU+inotropes/vasopressors | 61/1565 | 3.9 | 20/526 | 3.8 | 0.1 (−1.8 to 2.0) | 1.03 (0.62–1.69) | |
| D‐AKI | 15/1514 | 1.0 | <5/449 | 0.7 | 0.3 (−0.5 to 1.2) | 1.48 (0.45–4.84) | |
| Pneumonia with bacterial or unspecified pathogen | 30‐d mortality | 13 140/93 193 | 14.1 | 5683/35 426 | 16.0 | −1.9 (−2.4 to −1.5) | 0.88 (0.85–0.91) |
| ICU admission | 7456/93 193 | 8.0 | 3123/35 426 | 8.8 | −0.8 (−1.2 to −0.5) | 0.91 (0.87–0.95) | |
| ICU+MV | 3918/93 193 | 4.2 | 1640/35 426 | 4.6 | −0.4 (−0.7 to −0.2) | 0.91 (0.86–0.96) | |
| ICU+NIV | 2839/93 193 | 3.0 | 1389/35 426 | 3.9 | −0.9 (−1.1 to −0.7) | 0.78 (0.73–0.83) | |
| ICU+inotropes/vasopressors | 3567/93 193 | 3.8 | 1240/35 426 | 3.5 | 0.3 (0.1–0.6) | 1.09 (1.02–1.17) | |
| D‐AKI | 886/91 899 | 1.0 | 407/33 983 | 1.2 | −0.2 (−0.3 to −0.1) | 0.80 (0.73–0.89) | |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; D‐AKI, dialysis‐treated acute kidney injury; ICU, intensive care unit; MV, mechanical ventilation; NIV, noninvasive ventilation; and PS, propensity score.
Pseudopopulation of current CCB users weighted to the PS distribution of current ACE‐I/ARB users.
Figure 2. Crude (A) and propensity score‐weighted (B) 30‐day cumulative incidence (risk) and relative risk (RR) of death and intensive care unit (ICU) admission in patients with influenza or pneumonia, with only influenza, and with pneumonia with bacterial or unspecified pathogen.
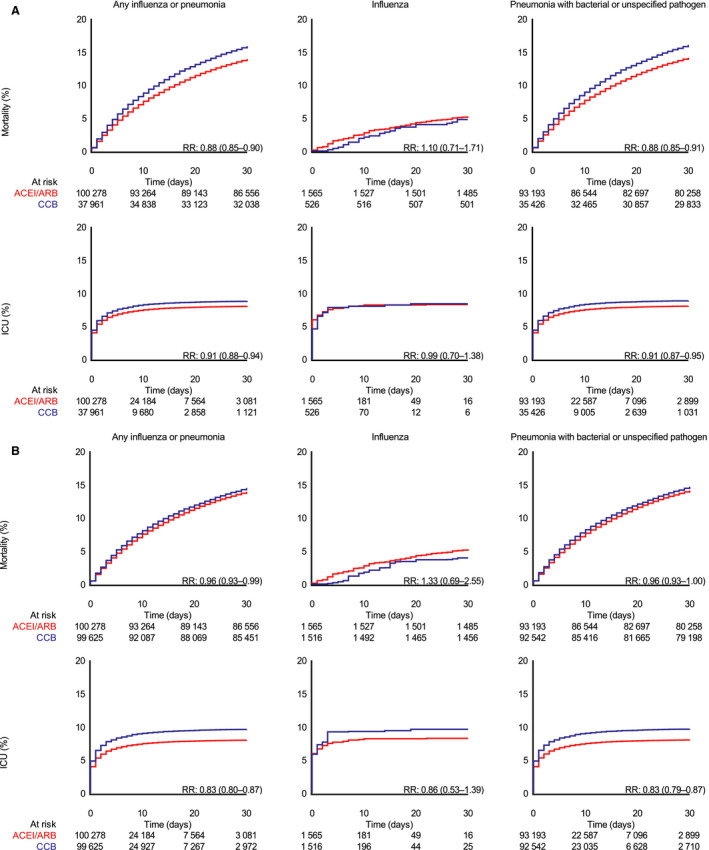
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Among all patients with influenza or pneumonia, 30‐day mortality was 13.9% in ACE‐I/ARB Users and 14.5% in CCB users, with a corresponding RD of −0.6% (95% CI, −1.0 to −0.1) and a RR of 0.96 (95% CI, 0.93–0.99). The risk of ICU admission was 8.0% in ACE‐I/ARB Users and 9.6% in CCB users, corresponding to an RD of −1.6% (95% CI, −2.0 to −1.2) and an RR of 0.83 (95% CI, 0.80–0.87) for ACE‐I/ARB users compared with CCB users. The estimates were similar for ICU admissions with need for mechanical ventilation, noninvasive ventilation, and dialysis‐treated acute kidney injury. There was no difference in risk of ICU admission including treatment with inotropes/vasopressors (RD, −0.1%; 95% CI, −0.3 to 0.2).
In the subgroup of patients diagnosed with influenza, 30‐day mortality was 5.2% in ACE‐I/ARB users and 3.9% in CCB users (RD, 1.3%; 95% CI, −1.2 to 3.8; RR, 1.33, 95% CI, 0.69–2.55). The risk of ICU admission was 8.2% in ACE‐I/ARB users and 9.6% in CCB users (RD, −1.4%; 95% CI, −5.7 to 3.0; RR, 0.86; 95%, CI, 0.53–1.39). Risk of ICU admission with need for mechanical ventilation was similar in ACE‐I/ARB and CCB users (4.5% versus 4.6%).
Characteristics and Outcomes in Current Users of ACE‐Is/ARBs Compared With Nonusers
The patient characteristics of all current ACE‐I/ARB users and nonusers were reasonably balanced after PS‐weighting, with only a few standardized differences above 0.10 (Table 4).
Table 4.
Characteristics of Current and Nonusers of ACE‐Is or ARBs, Overall and After PS‐Weighting
| Overall Cohort | PS‐Weighted Cohort | |||||
|---|---|---|---|---|---|---|
|
Current Use, N (%) |
Nonuse, N (%) |
SD |
Current Use, N (%) |
Nonuse*, N (%) |
SD | |
| No. of patients | 133 433 (25.6) | 388 790 (74.4) | 133 433 (46.6) | 153 032 (53.4) | ||
| Age, median (Q1–Q3), y | 76.8 (68.4–83.9) | 71.0 (55.5–82.2) | 0.72 | 76.8 (68.4–83.9) | 77.9 (70.0–84.5) | 0.14 |
| Male | 71 441 (53.5) | 193 862 (49.9) | 0.10 | 71 441 (53.5) | 80 274 (52.5) | 0.03 |
| Coexisting conditions (within prior 10 y) | ||||||
| Hospital‐diagnosed hypertension | 73 445 (55.0) | 69 624 (17.9) | 1.18 | 73 445 (55.0) | 96 194 (62.9) | 0.23 |
| Previous myocardial infarction | 16 679 (12.5) | 17 563 (4.5) | 0.41 | 16 679 (12.5) | 22 901 (15.0) | 0.10 |
| Diagnosis of stable angina pectoris | 24 532 (18.4) | 35 356 (9.1) | 0.39 | 24 532 (18.4) | 31 467 (20.6) | 0.08 |
| Heart failure | 31 094 (23.3) | 29 366 (7.6) | 0.63 | 31 094 (23.3) | 44 098 (28.8) | 0.18 |
| Stroke | 20 229 (15.2) | 38 078 (9.8) | 0.23 | 20 229 (15.2) | 25 042 (16.4) | 0.05 |
| Atrial fibrillation/flutter | 32 077 (24.0) | 51 014 (13.1) | 0.40 | 32 077 (24.0) | 40 708 (26.6) | 0.08 |
| Heart valve disease | 12 482 (9.4) | 17 585 (4.5) | 0.27 | 12 482 (9.4) | 17 586 (11.5) | 0.10 |
| Venous thromboembolism | 6780 (5.1) | 19 016 (4.9) | 0.01 | 6780 (5.1) | 8142 (5.3) | 0.02 |
| Diabetes mellitus | 40 513 (30.4) | 41 969 (10.8) | 0.71 | 40 513 (30.4) | 51 830 (33.9) | 0.11 |
| Chronic pulmonary disease | 39 469 (29.6) | 104 818 (27.0) | 0.08 | 39 469 (29.6) | 47 524 (31.1) | 0.05 |
| Renal disease | 14 090 (10.6) | 19 586 (5.0) | 0.29 | 14 090 (10.6) | 20 715 (13.5) | 0.13 |
| End‐stage renal disease | 2558 (1.9) | 4865 (1.3) | 0.08 | 2558 (1.9) | 3756 (2.5) | 0.05 |
| Liver disease | 2144 (1.6) | 12 069 (3.1) | 0.14 | 2144 (1.6) | 2327 (1.5) | 0.01 |
| Dementia | 7375 (5.5) | 25 108 (6.5) | 0.06 | 7375 (5.5) | 8408 (5.5) | 0.00 |
| Cancer | 24 429 (18.3) | 71 800 (18.5) | 0.01 | 24 429 (18.3) | 27 422 (17.9) | 0.01 |
| Metastatic cancer | 3823 (2.9) | 12 863 (3.3) | 0.04 | 3823 (2.9) | 4070 (2.7) | 0.02 |
| Peptic ulcer disease | 8495 (6.4) | 20 797 (5.3) | 0.06 | 8495 (6.4) | 10 630 (6.9) | 0.03 |
| Rheumatoid arthritis or connective tissue disease | 8593 (6.4) | 20 944 (5.4) | 0.06 | 8593 (6.4) | 10 141 (6.6) | 0.01 |
| Comedication (prescription within 90 d) | ||||||
| CCBs | 33 155 (24.8) | 30 720 (7.9) | 0.67 | 33 155 (24.8) | 47 023 (30.7) | 0.19 |
| Thiazides | 44 645 (33.5) | 31 089 (8.0) | 0.94 | 44 645 (33.5) | 57 991 (37.9) | 0.13 |
| β Blockers | 48 363 (36.2) | 50 612 (13.0) | 0.79 | 48 363 (36.2) | 64 996 (42.5) | 0.18 |
| Other antihypertensive drugs | 3304 (2.5) | 2165 (0.6) | 0.22 | 3304 (2.5) | 5179 (3.4) | 0.08 |
| Statins | 51 633 (38.7) | 49 818 (12.8) | 0.88 | 51 633 (38.7) | 67 673 (44.2) | 0.16 |
| Aspirin | 46 492 (34.8) | 62 983 (16.2) | 0.62 | 46 492 (34.8) | 60 158 (39.3) | 0.13 |
| Loop diuretics | 44 817 (33.6) | 68 607 (17.6) | 0.53 | 44 817 (33.6) | 58 864 (38.5) | 0.14 |
| Immunosuppressants | 1754 (1.3) | 4468 (1.1) | 0.02 | 1754 (1.3) | 1979 (1.3) | 0.00 |
| Glucocorticoids | 19 404 (14.5) | 52 371 (13.5) | 0.04 | 19 404 (14.5) | 23 187 (15.2) | 0.02 |
| NSAIDs | 16 746 (12.6) | 46 738 (12.0) | 0.02 | 16 746 (12.6) | 19 235 (12.6) | 0.00 |
| Opioids | 36 636 (27.5) | 94 717 (24.4) | 0.10 | 36 636 (27.5) | 43 555 (28.5) | 0.03 |
| Vitamin K antagonists | 15 165 (11.4) | 18 620 (4.8) | 0.34 | 15 165 (11.4) | 19 818 (13.0) | 0.07 |
| Proton pump inhibitors | 40 862 (30.6) | 92 464 (23.8) | 0.22 | 40 862 (30.6) | 50 863 (33.2) | 0.08 |
| Antidepressants | 30 223 (22.7) | 81 315 (20.9) | 0.06 | 30 223 (22.7) | 36 335 (23.7) | 0.04 |
| Hypnotics/sedatives | 20 601 (15.4) | 51 308 (13.2) | 0.09 | 20 601 (15.4) | 24 605 (16.1) | 0.02 |
| Antipsychotics | 7155 (5.4) | 29 802 (7.7) | 0.13 | 7155 (5.4) | 7880 (5.1) | 0.01 |
| Antibiotics (prescription within 10 d) | 35 169 (26.4) | 113 795 (29.3) | 0.09 | 35 169 (26.4) | 39 545 (25.8) | 0.02 |
| Antivirals (prescription within 10 d) | 289 (0.2) | 1160 (0.3) | 0.02 | 289 (0.2) | 326 (0.2) | 0.00 |
| Lifestyle and social factors | ||||||
| Markers of smoking | 68 378 (51.2) | 187 502 (48.2) | 0.09 | 68 378 (51.2) | 80 872 (52.8) | 0.05 |
| Obesity | 13 666 (10.2) | 25 742 (6.6) | 0.18 | 13 666 (10.2) | 17 001 (11.1) | 0.04 |
| Alcoholism | 7966 (6.0) | 34 773 (8.9) | 0.16 | 7966 (6.0) | 8522 (5.6) | 0.02 |
| Marital status | ||||||
| Widow | 42 492 (31.8) | 96 406 (24.8) | 0.22 | 42 492 (31.8) | 52 400 (34.2) | 0.07 |
| Divorced | 18 773 (14.1) | 60 204 (15.5) | 0.06 | 18 773 (14.1) | 20 810 (13.6) | 0.02 |
| Married | 61 321 (46.0) | 163 218 (42.0) | 0.11 | 61 321 (46.0) | 68 524 (44.8) | 0.03 |
| Unmarried | 10 847 (8.1) | 68 962 (17.7) | 0.41 | 10 847 (8.1) | 11 297 (7.4) | 0.04 |
| Urban residence | 45 019 (33.7) | 146 919 (37.8) | 0.12 | 45 019 (33.7) | 51 920 (33.9) | 0.01 |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; PS, propensity score; and SD, standardized difference.
Pseudopopulation of nonusers weighted to the PS distribution of current ACE‐I/ARB users.
ACE‐I/ARB users had lower 30‐day mortality than nonusers (13.5% versus 15.9%; Table 5). The corresponding RD was −2.4% (95% CI, −2.8 to −2.0) and the RR was 0.85 (95% CI, 0.83–0.87). The risk of ICU admission was similar among users and nonusers (8.4% versus 8.1%; RD, 0.4%; 95% CI, 0.0–0.7; RR, 1.04; 95% CI, 1.00–1.09). When compared with nonusers, former ACE‐I/ARB users did not have increased mortality, but they did have a decreased risk of ICU admission (Figure 3). Crude estimates are provided in Table 6.
Table 5.
Outcomes in Current Users of ACE‐Is or ARBs Compared With Nonusers, Adjusted by PS‐Weighting and Stratified by Influenza and Pneumonia With Bacterial or Unspecified Pathogen
| Population | Event | Current ACE‐I/ARB Use | ACE‐I/ARB nonuse |
Risk Difference % (95% CI) vs Nonuse* |
Risk Ratio (95% CI) vs Nonuse* |
||
|---|---|---|---|---|---|---|---|
| Events/At Risk | Risk % | Events/At Risk* | Risk* % | ||||
| Any influenza or pneumonia | 30‐d mortality | 18 017/133 433 | 13.5% | 24 273/153 032 | 15.9% | −2.4 (−2.8 to −2.0) | 0.85 (0.83–0.87) |
| ICU admission | 11 225/133 433 | 8.4% | 12 332/153 032 | 8.1% | 0.4 (0.0–0.7) | 1.04 (1.00–1.09) | |
| ICU+MV | 5965/133 433 | 4.5% | 6600/153 032 | 4.3% | 0.2 (−0.1 to 0.4) | 1.04 (0.98–1.10) | |
| ICU+NIV | 4424/133 433 | 3.3% | 5219/153 032 | 3.4% | −0.1 (−0.3 to 0.1) | 0.97 (0.91–1.04) | |
| ICU+inotropes/vasopressors | 5352/133 433 | 4.0% | 5294/153 032 | 3.5% | 0.6 (0.3–0.8) | 1.16 (1.08–1.24) | |
| D‐AKI | 1455/130 875 | 1.1% | 1617/150 516 | 1.1% | 0.0 (−0.1 to 0.2) | 1.03 (0.89–1.20) | |
| Influenza | 30‐d mortality | 110/2110 | 5.2% | 182/2447 | 7.4% | −2.2 (−5.0 to 0.5) | 0.70 (0.47–1.05) |
| ICU admission | 191/2110 | 9.1% | 212/2447 | 8.7% | 0.4 (−1.9 to 2.7) | 1.04 (0.80–1.37) | |
| ICU+MV | 118/2110 | 5.6% | 109/2447 | 4.4% | 1.1 (−0.5 to 2.8) | 1.26 (0.89–1.78) | |
| ICU+NIV | 94/2110 | 4.5% | 99/2447 | 4.1% | 0.4 (−1.3 to 2.1) | 1.10 (0.74–1.63) | |
| ICU+inotropes/vasopressors | 103/2110 | 4.9% | 93/2447 | 3.8% | 1.1 (−0.3 to 2.5) | 1.29 (0.91–1.82) | |
| D‐AKI | 22/2014 | 1.1% | 30/2363 | 1.3% | −0.2 (−1.3 to 0.9) | 0.86 (0.36–2.07) | |
| Pneumonia with bacterial or unspecified pathogen† | 30‐d mortality | 16 979/124 054 | 13.7% | 22 644/141 687 | 16.0% | −2.3 (−2.7 to −1.9) | 0.86 (0.83–0.88) |
| ICU admission | 10 482/124 054 | 8.4% | 11 361/141 687 | 8.0% | 0.4 (0.1–0.8) | 1.05 (1.01–1.10) | |
| ICU+MV | 5529/124 054 | 4.5% | 5990/141 687 | 4.2% | 0.2 (−0.0 to 0.5) | 1.05 (0.99–1.12) | |
| ICU+NIV | 4151/124 054 | 3.3% | 4853/141 687 | 3.4% | −0.1 (−0.3 to 0.2) | 0.98 (0.91–1.05) | |
| ICU+inotropes/vasopressors | 4973/124 054 | 4.0% | 4817 | 3.4% | 0.6 (0.4–0.9) | 1.18 (1.10–1.26) | |
| D‐AKI | 1343/121 749 | 1.1% | 1422 | 1.0% | 0.1 (−0.0 to 0.2) | 1.08 (0.95–1.23) | |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; D‐AKI, dialysis‐treated acute kidney injury; ICU, intensive care unit; MV, mechanical ventilation; NIV, noninvasive ventilation; and PS, propensity score.
Pseudopopulation of nonusers weighted to the PS distribution of current ACE‐I/ARB users.
Not including viral pneumonia and influenza without proven influenza virus.
Figure 3. Thirty‐day mortality and risk of intensive care unit (ICU) admission by different exposure definitions and subgroups.
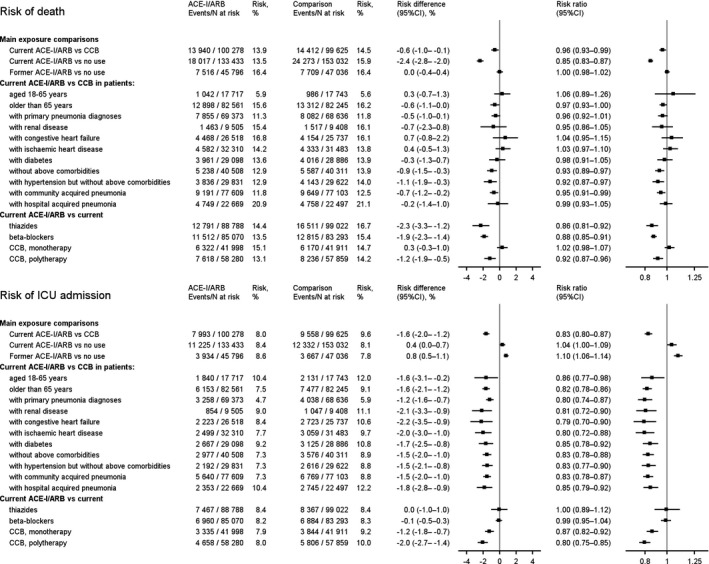
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; and CCB, calcium channel blocker.
Table 6.
Crude Outcomes in Current Versus Nonusers of ACE‐Is or ARBs, Overall and Stratified by Influenza and Pneumonia With Bacterial or Unspecified Pathogen
| Population | Event | ACE‐I/ARB use | Nonuse | Risk Difference % (95% CI) vs Nonuse | Risk Ratio (95% CI) vs Nonuse | ||
|---|---|---|---|---|---|---|---|
| Events/At Risk | Risk % | Events/At risk† | Risk† % | ||||
| Any influenza or pneumonia | 30‐d mortality | 18 017/133 433 | 13.5 | 51 229/388 790 | 13.2 | 0.3 (0.1–0.5) | 1.02 (1.01–1.04) |
| ICU admission | 11 225/133 433 | 8.4 | 27 668/388 790 | 7.1 | 1.3 (1.1–1.5) | 1.18 (1.16–1.21) | |
| ICU+MV | 5965/133 433 | 4.5 | 15 300/388 790 | 3.9 | 0.5 (0.4–0.7) | 1.14 (1.11–1.17) | |
| ICU+NIV | 4424/133 433 | 3.3 | 10 224/388 790 | 2.6 | 0.7 (0.6–0.8) | 1.26 (1.22–1.31) | |
| ICU+inotropes/vasopressors | 5352/133 433 | 4.0 | 11 547/388 790 | 3.0 | 1.0 (0.9–1.2) | 1.35 (1.31–1.40) | |
| D‐AKI | 1455/130 875 | 1.1 | 2561/383 925 | 0.7 | 0.4 (0.4–0.5) | 1.67 (1.55–1.79) | |
| Influenza | 30‐d mortality | 110/2110 | 5.2 | 296/6581 | 4.5 | 0.7 (−0.5 to 1.9) | 1.16 (0.92–1.45) |
| ICU admission | 191/2110 | 9.1 | 497/6581 | 7.6 | 1.5 (0.0–3.0) | 1.20 (1.01–1.42) | |
| ICU+MV | 118/2110 | 5.6 | 336/6581 | 5.1 | 0.5 (−0.7 to 1.7) | 1.10 (0.88–1.36) | |
| ICU+NIV | 94/2110 | 4.5 | 228/6581 | 3.5 | 1.0 (−0.0 to 2.0) | 1.29 (1.01–1.64) | |
| ICU+inotropes/vasopressors | 103/2110 | 4.9 | 282/6581 | 4.3 | 0.6 (−0.5 to 1.6) | 1.14 (0.91–1.43) | |
| D‐AKI | 22/2014 | 1.1 | 78/6386 | 1.2 | −0.1 (−0.6 to 0.4) | 0.89 (0.58–1.38) | |
| Pneumonia with bacterial or unspecified pathogen | 30‐d mortality | 16 979/124 054 | 13.7 | 48 206/351 722 | 13.7 | −0.0 (−0.2 to 0.2) | 1.00 (0.98–1.02) |
| ICU admission | 10 482/124 054 | 8.4 | 25 667/351 722 | 7.3 | 1.2 (1.0–1.3) | 1.16 (1.13–1.18) | |
| ICU+MV | 5529/124 054 | 4.5 | 14 056/351 722 | 4.0 | 0.5 (0.3–0.6) | 1.12 (1.08–1.15) | |
| ICU+NIV | 4151/124 054 | 3.3 | 9518/351 722 | 2.7 | 0.6 (0.5–0.8) | 1.24 (1.19–1.28) | |
| ICU+inotropes/vasopressors | 4973/124 054 | 4.0 | 10 632/351 722 | 3.0 | 1.0 (0.9–1.1) | 1.33 (1.28–1.37) | |
| D‐AKI | 1343/121 749 | 1.1 | 2320/347 374 | 0.7 | 0.4 (0.4–0.5) | 1.65 (1.54–1.77) | |
ACE‐Is indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; D‐AKI, dialysis‐treated acute kidney injury; ICU, intensive care unit; MV, mechanical ventilation; and NIV, noninvasive ventilation.
Additional Analyses
The head‐to‐head comparisons of ACE‐Is/ARBs with β blockers and thiazides, respectively, showed that 30‐day mortality was also lowered in ACE‐I/ARB users when compared with these other drugs, whereas there was no difference in risk of ICU admission (Figure 3).
ACE‐I/ARB monotherapy users did not have increased mortality, but decreased absolute and relative risk of ICU admission when compared with CCB monotherapy (Figure 3).
Restriction to patients with a first‐listed diagnosis of influenza or pneumonia reduced the number of current ACE‐I/ARB users to 69 373 and current CCB users to 26 006. PS‐weighted 30‐day mortality was 11.3% in ACE‐/ARB users and 11.8% in CCB users. The corresponding RR was 0.96 (95% CI, 0.92–1.01). Relative risk of ICU admission was also similar to that obtained in the main analyses (RR, 0.80; 95% CI, 0.74–0.87; Figure 3). The subgroup analyses by age and coexisting conditions were overall robust and compatible with our main findings. In patients without any other conditions than hypertension, current ACE‐I/ARB users had lower absolute and relative risks of both death and ICU admission (Figure 3). Our findings were robust also when restricting to patients on more than one antihypertensive medication (polytherapy), and when stratifying by hospital‐ and community‐acquired pneumonia (Figure 3).
DISCUSSION
In this cohort study of patients with influenza or pneumonia, we found that ACE‐I/ARB users had no increased risk of ICU admissions and a slightly reduced mortality, after controlling for coexisting conditions and comedications. The findings were robust across various different exposure definitions, subgroups, and also when compared with other antihypertensive drugs. We observed reduced risks of both ICU admission and mortality in some subgroups of ACE‐I/ARB users, including patients with hypertension and no other cardiometabolic conditions.
Our study extends findings from previous smaller studies of pneumonia mortality. Our observed associations were weaker than reported in previous studies. 16 , 17 , 18 A previous meta‐analysis of 3 trials and 4 observational studies with data on pneumonia‐related mortality reported decreased odds for mortality in ACE‐I users compared with nonusers (OR, 0.73; 95% CI, 0.58–0.92), but most of the studies examined were not designed to address this topic, as ACE‐I was not their main exposure, and several studies mixed pneumonia risk and outcomes. 16 In a later US study including 8652 pneumonia patients aged ≥65 years, ACE‐I use prior to hospitalization also was associated with reduced 30‐day mortality (adjusted OR, 0.80; 95% CI, 0.68–0.89). 17 Another US study that examined the association between cardiovascular medication in 21 985 elderly patients hospitalized with pneumonia reported an adjusted OR for 90‐day mortality of 0.82 (95% CI, 0.74–0.91) for ACE‐I users and 0.58 (95% CI, 0.44–0.77) for ARB users. 18 In contrast, a US study of patients hospitalized with mixed viral disease found that preadmission ACE‐I use was associated with increased mortality (OR, 3.02; 95% CI, 1.30–7.01) and increased intubation rates among the 539 patients admitted with pneumonia. 19 In that study, in‐hospital ACE‐I use was associated with substantially lowered mortality, which may be biased as the drug may only be continued during hospitalization in patients without severe illness. 19 Such bias may also explain the lower mortality (OR, 0.37; 95% CI, 0.15–0.89) among in‐hospital users of ACE‐Is/ARBs in a recent Chinese study of 1128 patients with COVID‐19. 20 Another recent Chinese study of 1178 patients found no association between in‐hospital use of ACE‐Is/ARBs and the severity and mortality of patients with COVID‐19. 21
The mechanism behind our findings is not clear. ACE2 expression, which is probably increased in ACE‐I/ARB‐treated patients, may protect against the development of severe acute lung injury, 13 , 28 , 29 In contrast, it has been suggested that the ACE‐I/ARB‐induced increase in cell‐surface ACE2 expression may increase the risk and severity of severe acute respiratory syndrome‐coronavirus and severe acute respiratory syndrome‐coronavirus 2 specifically. 13 The contradictions are addressed in planned trials on the impact of losartan on organ dysfunction and mortality in patients hospitalized with COVID‐19 (ClinicalTrials.gov Identifier: NCT04312009 and NCT04328012).
Although the current study was conducted in Denmark's population‐based hospital setting using validated registries with complete follow‐up on all Danish residents, it also had limitations. Observational studies of drug effects have inherent methodological challenges, and our results should therefore be interpreted with caution. Ultimately, a randomized controlled trial would be needed to draw firm conclusions regarding ACE‐I/ARB effects on ICU admission and mortality in patients with influenza or pneumonia.
Study inclusion relied on coding of influenza and pneumonia. Although some patients may not receive a diagnosis, we believe the restriction to physician‐coded influenza and pneumonia discharge diagnoses meant that only patients with clinically relevant infections were included. The positive predictive value of pneumonia diagnoses in the patient registry is 90%. 30 Of patients diagnosed with influenza, 66% had a positive influenza test (A. Pottegård, MSc, PhD, DMSc, unpublished data, 2020). Any selection bias should be minimal because follow‐up was virtually complete, and different associations between included and nonincluded patients are not expected.
Medication use was assessed using prescriptions prior to hospitalization in a time window corresponding to the typical interval between medication dispensings. 27 Still, misclassification may occur if some patients had a sporadic use of drugs filled more than 90 days before hospitalization. Given the chronic use of the drugs included in the study, any misclassification should be minor and not associated with the outcome of interest. Any information bias is therefore expected to be towards null. We lacked in‐hospital medication data to examine the impact of prescribed and discontinued drugs during follow‐up.
Death is accurately recorded in the population registry with daily updates. 24 ICU admissions and treatments are also accurately recorded, as the patient registry has been used for financial reimbursement of hospitals, and for mandatory reporting to national quality of care databases during the study period. 26 , 31 Use of ICU admission as an outcome in observational prognostic studies is challenging. In clinical practice, ICU admission is offered to patients who are expected to have a clear prognostic benefit from invasive monitoring and treatment. 32 In addition, the patients' quality of life and functional level at home and hospital capacity may influence the decision to admit a patient to the ICU. This may explain why ICU and mortality outcomes tended to go in opposite directions for some of the associations examined in our study.
Potential confounding by medical indication for drug treatment was handled by using an active comparator in the main analysis, by using PS‐weighting including a large number of potential confounders, and by restriction to subgroups according to indication for treatment. We observed and accounted for a higher use of antihypertensive and other cardiovascular medications in ACE‐I/ARB users in our analysis. The higher use may be explained by the higher prevalence of heart diseases observed with ACE‐I/ARB use. For example, the higher prevalence of vitamin K antagonist use may be explained by the higher prevalence of atrial fibrillation in ACE‐I/ARB users compared with CCB users. On the other hand, the prevalence of eg kidney disease or dementia was lower in ACE‐I/ARB users than in CCB users, indicating cautious use of ACE‐I/ARB among patients with kidney disease and more frailty among CCB users. This may have contributed to the lower unadjusted relative risk (RR=0.88) of death in ACE‐I/ARB users compared with CCB‐users, which attenuated after PS‐weighting (RR=0.96).
Although cardiovascular and other diagnoses used in the study have documented high positive predictive values, 20 , 33 we cannot entirely rule out that our findings of a potential beneficial effect of ACE‐I/ARB use were influenced by unmeasured confounding by indication and contraindication. Healthy user bias is, however, an unlikely explanation of the findings as the captured lifestyle factors did not indicate any healthier lifestyle in ACE‐I/ARB users compared with CCB users. Finally, although our study included more than 500 000 patients, precision of risk estimates was limited in some subgroups.
In conclusion, ACE‐I/ARB users hospitalized with influenza or pneumonia had no increased risk of ICU admission and a lower mortality after controlling for confounding. Thus, our data support the current recommendations 1 , 21 to avoid discontinuation of ACE‐Is/ARBs during the COVID‐19 pandemic, unless future evidence contradicts our findings.
Sources of Funding
The study was funded by Aarhus University, Denmark. The funding source had no influence on the study.
Disclosures
Dr Christiansen, Dr Heide‐Jørgensen, Dr Rasmussen, Dr Thomsen, and Dr Sørensen have not received any personal fees, grants, travel grants, or teaching grants from companies, but the Department of Clinical Epidemiology is involved in studies with funding from various companies as research grants to (and administered by) Aarhus University. None of these studies are related to the current study. Dr Reilev, Dr Lund, Dr Pottegård, and Dr Hallas have participated in industry‐funded projects with money paid to their employer, University of Southern Denmark. None have received personal compensation of any kind, and none of these projects is related to the current study. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figure S1
(J Am Heart Assoc. 2020;9:e017297 DOI: 10.1161/JAHA.120.017297.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017297
For Sources of Funding and Disclosures, see page 14.
References
- 1. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;1824–1836. [DOI] [PubMed] [Google Scholar]
- 2. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;9:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommerstein R, Gräni C. Rapid response: Re: preventing a covid‐19 pandemic: ACE inhibitors as a potential risk factor for fatal Covid‐19. BMJ. 2020;m810 Website name: . Available at: bmj.com https://www.bmj.com/content/368/bmj.M810/rr-2. Accessed August 14, 2020.32111649 [Google Scholar]
- 4. Patel AB, Verma A. COVID‐19 and angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;1769–1770. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS‐CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV-2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;1775–1776. [DOI] [PubMed] [Google Scholar]
- 10. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, et al. Covid‐19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID‐19 and cardiovascular disease. Circulation. 2020;1648–1655. [DOI] [PubMed] [Google Scholar]
- 13. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aronson JK, Ferner RE. Drugs and the renin‐angiotensin system in covid‐19. BMJ. 2020;m1313. [DOI] [PubMed] [Google Scholar]
- 15. Sommerstein R, Kochen MM, Messerli FH, Grani C. Coronavirus disease 2019 (COVID‐19): do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9:e016509 DOI: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caldeira D, Alarcao J, Vaz‐Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta‐analysis. BMJ. 2012;9:e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, Good C, Restrepo MI, Downs JR, Frei CR, et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu A, Good C, Downs JR, Fine MJ, Pugh MJ, Anzueto A, Mortensen EM. The Association of cardioprotective medications with pneumonia‐related outcomes. PLoS One. 2014;9:e85797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin‐converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018;419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID‐19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020;745–747. [DOI] [PubMed] [Google Scholar]
- 22. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS‐CoV2: should inhibitors of the renin‐angiotensin system be withdrawn in patients with COVID‐19? Eur Heart J. 2020;1801–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, Sørensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;541–549. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christiansen CF, Møller MH, Nielsen H, Christensen S. The Danish intensive care database. Clin Epidemiol. 2016;525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pottegård A, Schmidt SA, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, et al. Angiotensin‐converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, et al. A pilot clinical trial of recombinant human angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomsen RW, Riis A, Nørgaard M, Jacobsen J, Christensen S, McDonald CJ, Sørensen HT. Rising incidence and persistently high mortality of hospitalized pneumonia: a 10‐year population‐based study in Denmark. J Intern Med. 2006;410–417. [DOI] [PubMed] [Google Scholar]
- 31. Blichert‐Hansen L, Nielsson MS, Nielsen RB, Christiansen CF, Nørgaard M. Validity of the coding for intensive care admission, mechanical ventilation, and acute dialysis in the Danish National Patient Registry: a short report. Clin Epidemiol. 2013;9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phua J, Weng L, Ling L, Egi M, Lim C‐M, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, et al. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respir Med. 2020;506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;9:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


